Fuc-Tvii Is Required for T Helper 1 and T Cytotoxic 1 Lymphocyte Selectin Ligand Expression and Recruitment in Inflammation, and Together with Fuc-Tiv Regulates Naive T Cell Trafficking to Lymph Nodes (original) (raw)
Abstract
To determine how the α(1,3)fucosyltransferases Fuc-TIV and Fuc-TVII, and the selectin ligands they control may contribute to the adaptive immune response, contact hypersensitivity (CHS) was characterized in mice deficient in either or both enzymes. We find a substantial CHS deficiency in Fuc-TVII−/− mice, and a complete deficiency in Fuc-TIV−/−/Fuc-TVII−/− mice. These defects are not accounted for by alterations in the number or function of epidermal Langerhans cells required for cutaneous antigen processing and presentation. By contrast, defective CHS in Fuc-TVII−/− mice or Fuc-TIV−/−/Fuc-TVII−/− mice is attributed in part to prominent, or nearly complete deficiencies, respectively, in the complement of naive T lymphocytes available in lymph nodes for antigen-dependent activation, expansion, differentiation, and dissemination. Fuc-TVII deficiency also deletes expression of E- and P-selectin ligands by Th1 and T cytotoxic 1 (Tc1) lymphocytes, annuls T cell trafficking to inflamed cutaneous sites in vivo, and thereby controls an essential component of the efferent phase of the cutaneous immune response. These observations indicate that collaborative contributions of Fuc-TIV and Fuc-TVII to L-selectin ligand synthesis, and to lymphocyte recruitment, are requisite components of the primary cellular immune response, and assign an essential role to Fuc-TVII in control of E- and P-selectin ligand expression by Th1 and Tc1 lymphocytes.
Keywords: fucosyltransferase, selectin, T lymphocyte, Th1, Tc1
Introduction
Adhesive interactions between the three members of the selectin family of cell adhesion molecules and their respective ligands mediate a proximal step in leukocyte emigration from the blood 1. Constitutive and inducible expression of E- and P-selectins by vascular endothelium initiates and sustains leukocyte rolling through engagement of specific glycoprotein counterreceptors on granulocytes, monocytes, and certain subsets of activated T lymphocytes 1 2. These leukocyte counterreceptors are active as counterreceptors only after specific and appropriate posttranslational modification 2. L-selectin mediates shear-dependent adhesive interactions between lymphocytes and glycoprotein counterreceptors on high endothelial venules (HEVs) in secondary lymphoid organs, in a process required for lymphocyte homing 1 2 3. L-selectin counterreceptors are expressed constitutively by HEVs in secondary lymphoid organs 2 3, and inducibly in sites of chronic inflammation 4, and support L-selectin–dependent adhesion only when properly glycosylated. Selectin-dependent adhesion promotes interactions between leukocytes and activational stimuli derived from the vascular wall, leading to integrin activation, and leukocyte transmigration in acute and chronic inflammation or lymphocyte recruitment to secondary lymphoid organs 1.
While the glycosylation requirements for selectin counterreceptor activity are complex and incompletely defined, modification of their glycan components with one or more α(1,3)-linked fucose residues contributes prominently to their functional activity 2. Regulated and/or lineage specific expression of the α(1,3)fucosyltransferases (Fuc-Ts) responsible for α(1,3)fucosylation may thus control expression of selectin counterreceptor activities. Two of the six known mammalian Fuc-T loci 2 5, termed Fuc-TIV and Fuc-TVII, have been implicated in the control of leukocyte and HEV selectin ligand activities by virtue of their expression patterns, the structures of the fucosylated glycans they elaborate, and from analysis of mice with induced null mutations in these loci 2 6 7.
Analyses of Fuc-TVII–deficient mice assign to this enzyme a primary though partial contribution to fucosylation-dependent E- and P-selectin counterreceptor activities on neutrophils, monocytes, and eosinophils, and to L-selectin ligand activity on the HEVs in secondary lymphoid organs 6. A role for Fuc-TIV in the synthesis of selectin ligands has been less certain because biochemical and genetic studies implicating this enzyme in selectin ligand synthesis have been conflicting 2. However, recent analysis of mice made deficient in this enzyme demonstrate that Fuc-TIV can provide a Fuc-TVII–independent contribution to the expression of neutrophil ligands for E- and P-selectin 7 8.
While these analyses imply important yet unequal roles for these two enzymes in the control of selectin counterreceptor activity on some leukocytes and by HEVs, their respective contributions to the cellular arm of the adaptive immune response have not yet been characterized. Furthermore, studies that implicate Fuc-TVII expression in determining selectin counterreceptor activities and trafficking properties of Th1 or T cytotoxic 1 (Tc1) lymphocytes are correlative 9 10 11 12 13 and a requirement for this enzyme has not yet been established through analysis of Th1 or Tc1 lymphocyte selectin counterreceptor expression or recruitment in the absence of Fuc-TVII. Similarly, a role for Fuc-TIV in the control of lymphocyte selectin counterreceptor activity is uncertain 9 13 and has not yet been explored though an analysis of T cell selectin ligand expression in the absence of Fuc-TIV. Finally, although dendritic cells (DCs) can express selectin ligands in some contexts and are postulated to contribute to DC trafficking 14 15, a role for Fuc-TIV and Fuc-VII in selectin-dependent DC trafficking has not been examined.
To address these issues, we report here an analysis of the components of the immune system that contribute to contact hypersensitivity (CHS), a form of delayed-type hypersensitivity, in mice deficient in Fuc-TIV, or in Fuc-TVII, or in both. We find a substantial yet incomplete reduction in CHS in Fuc-TVII−/− mice, and a virtual absence of CHS in mice deficient both in Fuc-TVII and Fuc-TIV. Multiple Fuc-T–specific flaws account for the defects, and correspond to faulty accumulation of naive T cells in peripheral LNs (PLNs) with attendant defects in the ability to generate memory/effector lymphocytes available for dissemination to peripheral sites, defective E- and P-selectin counterreceptor activity on Th1 and T cytotoxic 1 (Tc1) lymphocytes, and defective trafficking of effector T cells to sites of cutaneous inflammation. Considered together, these observations assign an essential role for Fuc-TVII in response to cutaneous antigenic challenge, and in the generation of E- and P-selectin counterreceptor activities by Th1 and Tc1 lymphocytes. These observations further assign an important role for Fuc-TIV in these processes, and demonstrate that both enzymes contribute to multiple, distinct components of immune surveillance.
Materials and Methods
Mice_._
Strains of Fuc-TVII−/−, Fuc-TIV−/−, and Fuc-TIV−/−/ VII−/− mice that had been backcrossed nine or more generations to the C57Bl/6J strain 8 were used in all experiments except in Fig. 6, where recombinant inbred strains 6 were used. The construction and full characterization of a recombinant inbred strain that is homozygous for a null Fuc-TVII allele in which an enhanced green fluorescent protein (EGFP) is fused to the six NH2-terminal amino acids of Fuc-TVII (Fuc-TVII(-EGFP/-EGFP) mice) will be described elsewhere. Mice were housed and used in accordance with procedures approved by the University of Michigan Committee for Use and Care of Animals.
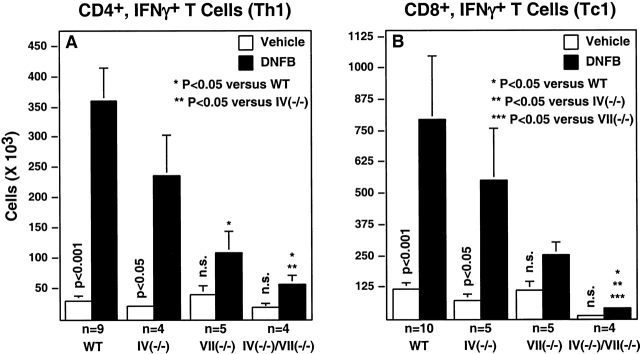
Figure 6.
In vivo expansion of Th1 and Tc1 lymphocytes. Th1 and Tc1 cells were detected in the draining PLNs of DNFB sensitized mice by cell surface staining with anti-CD4 or anti-CD8 monoclonal Abs in combination with intracellular staining for IFN-γ (Materials and Methods). Histograms depict the mean cell number (± SE) that simultaneously express CD4 and IFN-γ (A; Th1) or CD8 and IFN-γ (B; Tc1). Vertical P values derive from a Student's t test evaluation of Th1 or Tc1 cell number with or without DNFB sensitization in mice of the same genotype. Differences in Th1 or Tc1 cell number between genotypes after sensitization were analyzed exactly as done in Fig. 1.
Flow Cytometry.
All Abs and secondary reagents were obtained from BD PharMingen. Cells were analyzed using a Becton Dickinson FACScan™, or a Coulter Elite flow cytometer.
CHS_._
For sensitization 16, 2,4-dinitrofluorobenzene (DNFB; Sigma-Aldrich) (0.5%) in a vehicle of 4:1 acetone in olive oil was applied to shaved abdominal skin on days 0 and 1. On day 5, the right ear was treated with 0.25% DNFB (10 μl on each side), and the left ear was treated with the vehicle. Swelling was measured before and 18–24 h after treatment using an engineer's micrometer.
Langerhans Cell Migration and Function.
Epidermal Langerhans cells (LCs) were isolated from ears and characterized by flow cytometry, or were studied in epidermal sheets 17. LC migration, and the antigen presentation function of migrated LCs in a mixed lymphocyte reaction assay, were assessed according to published procedures 17 18.
Lymphocyte Activation, Expansion, and Polarization In Vivo.
Mice were sensitized with 0.5% DNFB on their ears (see Fig. 5) or shaved abdomens (see Fig. 6) on day 0 and day 1. On day 4, the mice were killed, their draining PLNs (cervical, for ear sensitization; inguinal for abdominal sensitization) were removed and were used to prepare a single cell suspension. In some experiments (see Fig. 6), the cells were cultured for 4 h at 37°C in 10 μg/ml brefeldin A, 50 ng/ml phorbol myristic acetate, and 500 ng/ml ionomycin. Lymphocytes were phenotyped using monoclonal Abs specific for cell surface markers (CD4, CD8, CD19, CD44), or by staining for intracellular IFN-γ or IL-4 19.
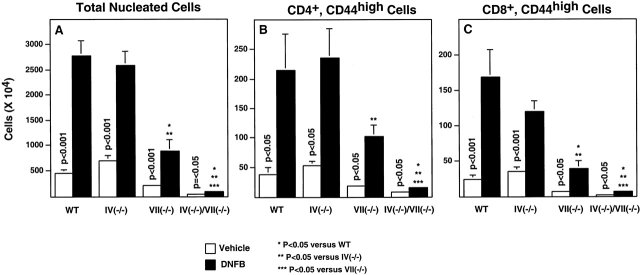
Figure 5.
Activated T cell expansion in the draining LNs after DNFB sensitization. Mice were sensitized on day 0 and day 1 and their draining LNs were removed and analyzed on day 4 (Materials and Methods). Data are average numbers (± SE), determined for between four and six mice in each group, derived from three independent experiments. Statistical analyses were done as described in the legend to Fig. 1.
Generation of Th1 and Tc1 Cells In Vitro.
CD4+ and CD8+ T cells for in vitro cytokine polarization were isolated from mesenteric LNs (MLNs) by panning. Single cell suspensions of MLN cells were diluted into complete tissue culture medium (CTCM; RPMI 1640 with 10% fetal calf serum, 50 U/ml pen-strep, 2 mM glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 0.1 mM MEM nonessential amino acids). CD4+ or CD8+ lymphocytes were enriched by staining MLN cells with a cocktail of rat mAbs against CD11b, IAdb, and CD16/32 (Fc Block; BD PharMingen), and either anti-CD8 or anti-CD4, on ice for 20 min. The cells were then washed with CTCM and panned twice on dishes coated with a goat anti–rat mAb. More than 95% of the resulting nonadherent cells were either of the CD4+ or CD8+ phenotype.
Splenic B cells were used as antigen-presenting cells for in vitro cytokine polarization procedures. Splenic leukocytes isolated with a Lympholyte M (Cedarlane) gradient were depleted of T cells by Thy 1.2 staining followed by complement-mediated lysis. The remaining cells (>93% CD19-positive) were washed with CTCM, incubated with 50 μg/ml mitomycin C for 20 min at 37°C, and then washed to remove all mitomycin C before addition to cytokine polarization cultures.
Cytokine polarization was accomplished by coculturing CD4+ (Th1) or CD8+ (Tc1) lymphocytes (106) with 4 × 106 syngeneic splenic B cells in dishes coated with anti-CD3 (mAb 2C11). Cells were cocultured for 48–72 h as follows. Th1: 50 U/ml IL-2, 1,000 U/ml IL-12 (R&D Systems) and 10 μg/ml anti–IL-4 (NALE; BD PharMingen). Th2: 50 U/ml IL-2, 10 ng/ml IL-4 (Genzyme), 10 μg/ml anti–IFN-γ (NALE; BD PharMingen). Tc1: 50 U/ml IL-2, 2,000 U/ml IL-12, and 25 μg/ml anti–IL-4. Tc2: 50 U/ml IL-2, 30 ng/ml IL-4, 50 μg/ml anti–IFN-γ.
Cells were then washed, resuspended in media containing the same cytokine cocktail, and expanded in culture for at least three additional days. Intracellular cytokine expression was evaluated by culturing cells at a concentration of 106 cells/ml in CTCM containing 50 ng/ml phorbol myristic acetate, 500 ng/ml ionomycin, and 10 μg/ ml brefeldin A for 4.0 h at 37°C.
Ab Staining for Flow Cytometry.
Cells were stained on ice in PBS with 3% FCS or in staining medium (SM; DMEM plus 0.1% BSA and 0.1% sodium azide). Cells were incubated with Fc block (1:100) for 5 min, then washed and stained for 10 min with one or more primary mAbs. Fluorochrome-based secondary reagents (SA-Cy-Chrome, SA-PE or SA-FITC) were applied after washing Ab-stained cells. P-selectin IgM and E-selectin-IgM chimeras were used as described previously 6. For simultaneous identification of cell surface antigens and intracellular cytokines, lymphocytes were stained for surface antigens using directly conjugated mAbs, washed once with SM, and fixed with Cytofix/Cytoperm solution (100 μl; BD PharMingen) for 20 min on ice. The cells were then washed and resuspended in mAbs prediluted in 1× Perm/Wash (BD PharMingen), incubated for 20 min, washed twice with Perm/Wash, resuspended in SM, and subjected to flow cytometry.
Adoptive Transfer of 5-Chloromethylfluorescein Diacetate–labeled Activated Effector Lymphocytes.
Wild-type (WT) mice served as recipients. WT, Fuc-TIV−/−, and Fuc-TVII−/− mice were used as donors; Fuc-TIV−/−/Fuc-TVII−/− mice could not be used as donors because it was not possible to isolate sufficient numbers of lymphocytes from the DNFB-activated PLNs to allow adoptive transfer (see Fig. 5). Donors and recipients were sensitized with 0.5% DNFB on their shaved abdomens on day 0 and day 1. Donors were killed on day 4, their draining PLNs were removed, single cell suspensions were prepared, and cells were labeled with 5 μM 5-chloromethylfluorescein diacetate (CMFDA; Molecular Probes) 6. Anesthetized recipients received 20 × 106 labeled cells by tail vein injection. Recipients were immediately challenged with 0.25% DNFB on the right ear, and treated with vehicle on the left ear. Ear swelling was measured 24 h later, the recipients were killed, and the dorsal portion of the epidermis and dermis from each ear were removed. Single cell suspensions of the ear tissue were prepared using a published protocol 20, and assessed for CMFDA+ by flow cytometry. Vehicle-treated ears from sensitized recipients served as a control for nonspecific trafficking of adoptively transferred CMFDA-labeled lymphocytes. Ears from sensitized and challenged mice, not injected with CMFDA-labeled cells, were analyzed in parallel to determine background fluorescence. Dead cells were excluded from the analyses using propidium iodide staining, and using appropriate forward and side scatter profiles. The ear swelling response and the influx of recipient-derived CD45+ leukocytes in the hapten-treated ears were equivalent in all recipients regardless of the genotype of donor cells injected, confirming that the recipients sustained a normal response to antigenic challenge, and indicating that adoptive transfer did not alter the course of the CHS response.
Results
CHS Is Attenuated in Fuc-TVII_−_ /− Mice, and Absent in Fuc-TIV_−_ /−/Fuc-TVII_−_ /− Mice.
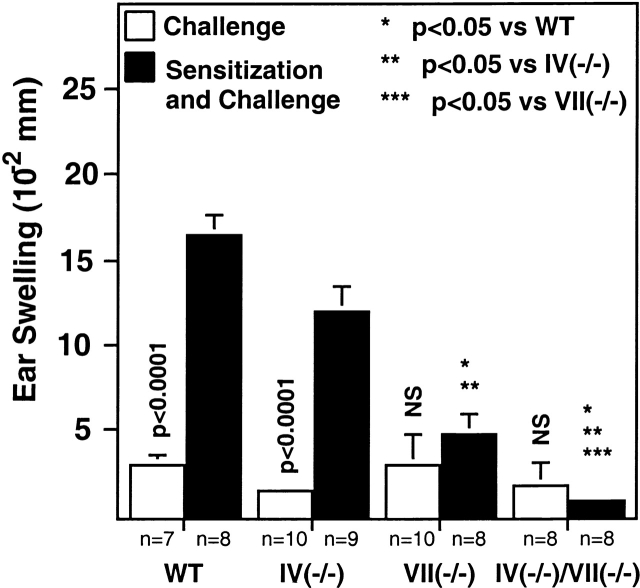
Fuc-TIV−/− mice sustain a slight decrement, relative to WT mice, in ear swelling after sensitization and challenge with the hapten DNFB, but this decrement does not reach statistical significance (Fig. 1). By contrast, Fuc-TVII−/− mice exhibit a significant reduction in response to DNFB sensitization and challenge (Fig. 1). Fuc-TVII−/− mice retain a significant response, however, and this is clearly Fuc-TIV dependent, as the doubly deficient mice do not respond to sensitization and challenge with DNFB (Fig. 1). Fuc-TVII therefore makes a prominent contribution to the immune response to a cutaneous antigen. The contribution by Fuc-TIV is subtle when Fuc-TVII expression is normal, and Fuc-TIV accounts for virtually all the response retained in the absence of Fuc-TVII.
Figure 1.
CHS. Mice were sensitized with 0.5% DNFB on the abdomen on day 0 and day 1, then challenged on the ear on day 4 with 0.25% DNFB on the ear (Sensitization and Challenge). Swelling in response to nonspecific irritation by DNFB was measured after a single application of 0.25% DNFB to nonsensitized mice (Challenge). The numbers of mice used to generate each experimental data point are shown below the histogram, and are derived from two independent experiments. Mean ear swelling (± SE) is plotted. Vertical P values derive from a Student's t test evaluation of ear swelling with or without sensitization in mice of the same genotype. Differences in ear swelling between genotypes after sensitization and challenge were analyzed using the Kruskal-Wallis test and post-hoc Mann-Whitney U test. Differences of statistical significance are noted with asterisks.
LC Number, Migration, and Antigen Presentation Activity Are Not Altered in Fuc-T Deficiency.
LCs are absolutely required for a CHS response 21 22. E- and P-selectin ligands are expressed by human LCs, and are implicated in LC recruitment to the skin 14 15 23 24. We therefore sought to determine if the Fuc-T–dependent deficiencies in CHS may be accounted for by deficits in localization of LCs to the epidermis, in LC emigration from the skin to regional PLNs, or in their antigen presentation capability. The number of LCs in the epidermis does not vary among the four strains (Table ), nor does the distribution of these cells within the epidermis, or their morphology (Fig. 2). These observations indicate that neither Fuc-TIV nor Fuc-TVII are required for populating the epidermis with LCs, and indicate that Fuc-T–dependent deficiencies in CHS cannot be accounted for by an inability of LCs to localize to the epidermis, or to differences in their distribution within this tissue.
Table 1.
Epidermal LCs
| Genotype | Percentage of LC | LC |
|---|---|---|
| WT | 1.75 ± 0.83 | 450 ± 115 |
| Fuc-TIV−/− | 1.45 ± 0.55 | 370 ± 111 |
| Fuc-TVII−/− | 1.96 ± 0.67 | 460 ± 110 |
| Fuc-TIV−/−/VII−/− | 2.08 ± 0.58 | 587 ± 264 |
Figure 2.
Distribution of epidermal LCs. Epidermal sheets were isolated from the dorsal portion of the ear of WT and Fuc-T–deficient mice, stained with DEC-205, and examined by fluorescence microscopy (Materials and Methods; original magnification: ×500).
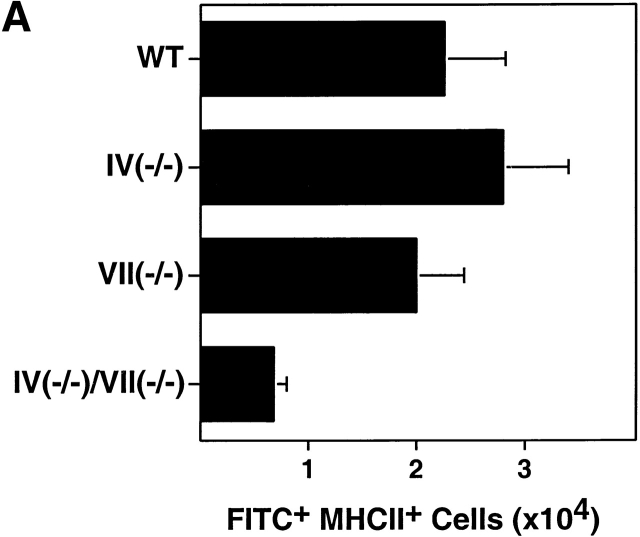
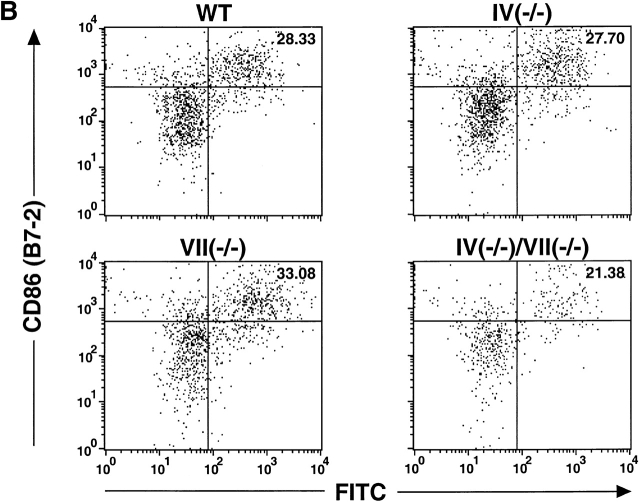
To assess migration of epidermal LCs to draining LNs, ears were painted with FITC to label, activate, and incite migration of LCs 17 18. MHC class IIbrightFITC+ cells (representing skin-derived LCs 18, as confirmed by light microscopic examination of Wrights stained cytospin preparations of the sorted cells; data not shown), were recovered from the draining LNs and quantitated 18–24 h later. An apparent migration decrement was observed only in Fuc-TIV−/−/Fuc-TVII−/− mice, though this decrement does not reach statistical significance (Fig. 3 A), and the small size, hypocellularity, and perturbed cellular complement of Fuc-TIV−/−/Fuc-TVII−/− PLNs (8; and below) may prevent an accurate assessment of LC migration efficiencies relative to the other strains. In each Fuc-T null strain, newly migrated LCs prominently express the T cell costimulatory molecule CD86 (Fig. 3 B), and were found to be equivalently potent to newly migrated WT LCs on a cell-to-cell basis in their capacity to stimulate naive allogeneic T cells in an in vitro mixed leukocyte reaction assay (data not shown). These observations indicate that the LC activation, migration, and antigen presentation axis is largely intact in the absence of Fuc-TIV and/or Fuc-TVII, and is therefore not accountable for the CHS deficits observed in Fuc-T–deficient mice, notwithstanding the possibility that a modest defect in LC migration may exist in Fuc-TIV−/−/Fuc-TVII−/− mice.
Figure 3.
LC migration. FITC was applied to ear pinnae, and draining cervical nodes were removed 18–24 h later. Single cell suspensions were made from the PLNs, were stained with Abs specific for MHC class II and CD86, and were then subjected to flow cytometry analysis (Materials and Methods). (A) Quantitation LCs recently migrated to PLNs. The number of FITC+, MHC class IIbright cells in the draining PLNs were calculated for each genotype. Data are mean values of LCs per draining PLN (± SE) obtained in four independent experiments. Mean values are not significantly different as judged by the Kruskal-Wallis test (P = 0.06). (B) FACS® analysis of LCs recently migrated to a draining PLN. Representative two-parameter histograms of viable (based on forward and side scatter parameters) MHC class IIbright cells. The number in the upper right hand quadrant is the percentage of MHC class IIbright cells that are both FITC+ and CD86+.
Partial and Virtually Complete Deficits of Naive T Cells in the PLNs of Fuc-TVII−/− and Fuc-TIV−/−/VII−/− Mice, Respectively.
Homeostasis in LN cellularity, and corresponding cellular immune function, including response to cutaneous antigenic challenge, is regulated prominently by L-selectin–dependent lymphocyte trafficking to secondary lymphoid organs 25 26 27 28. As Fuc-TIV and Fuc-TVII both contribute to L-selectin ligand synthesis by PLN HEVs and to lymphocyte homing 6 8, we sought to determine if their deficiency alters the number and phenotype of T lymphocytes in a manner that contributes to defective CHS in Fuc-T–deficient mice.
WT and Fuc-TIV−/− PLNs do not differ in total cellularity, and have essentially identical numbers of CD19+ B cells, CD3+ T cells (Fig. 4 A), and T cells with a memory/effector (CD4+CD44highCD45RBlow) or naive (CD4+ CD44lowCD45RBhigh) phenotype (29 30; Fig. 4 A). These observations indicate that any contribution by Fuc-TIV to maintaining homeostasis in lymphocyte numbers in PLNs is too subtle to detect with these methods, or is fully masked by Fuc-TVII–dependent processes.
Figure 4.
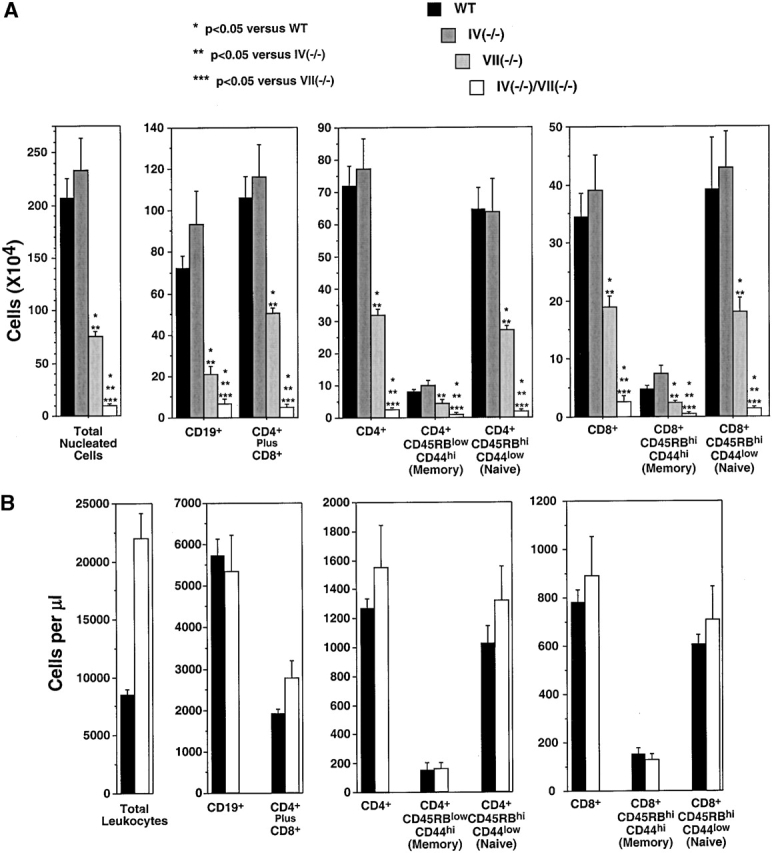
Quantitative and phenotypic analysis of peripheral node and blood lymphocytes in WT and Fuc-T–deficient mice. Lymphocytes isolated from peripheral nodes (A) or blood leukocytes (B) were stained with Abs against the antigens indicated below each panel, and were subjected to flow cytometry analysis (Materials and Methods). The number of cells of each indicated phenotype was calculated from the fraction of cells with the indicated cell surface phenotype. Data are average numbers (± SE) determined for 7 to 10 mice of each genotype. Statistical analyses were done as described in the legend to Fig. 1.
By contrast, the substantial reduction in total cellularity observed in Fuc-TVII−/− PLNs (6; Fig. 4 A) is accounted for by reductions of 70, 63, and 46%, respectively, in CD19+ B lymphocytes, CD4+ lymphocytes, and CD8+ lymphocytes. Striking reductions are also observed in naive CD4+ T lymphocytes (58% reduction) and naive CD8+ T lymphocytes (54% reduction; Fig. 4 A), as are significant reductions in T cells of the memory/effector phenotype (Fig. 4 A). These observations disclose that Fuc-TVII is thus responsible for a major contribution to the maintenance of cellularity of B cells, naive T cells, and memory/effector cells in PLNs.
The partial reduction in B cells, naive T cells, and memory/effector T cells in Fuc-TVII−/− PLNs implies a residual ability to recruit such cells to these organs, and aligns with the Fuc-TIV–dependent residual L-selectin ligand expression and lymphocyte homing activities assigned by analysis of Fuc-TIV−/−/Fuc-TVII−/− mice 8. Indeed, in Fuc-TIV−/−/VII−/− nodes, a 95% reduction in cellularity, relative to WT PLNs (Fig. 4 A), is accounted for by a 91% decrease in B lymphocyte content, profound deficiencies of CD4+ and CD8+ populations (decreased 97 and 92%, respectively; Fig. 4), selective losses of CD4+ and CD8+ cells of the naive phenotype (reductions of 97 and 96%, respectively), and significant decrements in memory/effector cells (Fig. 4 A).
Reduced accumulation of B lymphocytes and naive or memory/effector T cells are not accounted for by decreases in the number available for recruitment, as essentially equivalent numbers of these cells are present in the peripheral blood of WT and Fuc-TIV−/−/Fuc-TVII−/− mice (Fig. 4 B), nor by deficiency of L-selectin, as this molecule is expressed at normal levels on all three Fuc-T–deficient strains 6 8. By contrast, Fuc-TVII contributes prominently but incompletely to the synthesis of L-selectin ligands on PLN HEVs, and to lymphocyte homing 6. Partial reductions in the accumulation of B and T cells in Fuc-TVII−/− mice are thus likely consequent to partial deficits in L-selectin–dependent lymphocyte homing processes that recruit these cells to PLNs 28 31. We also infer that the profound decrement in B lymphocyte content and nearly complete absence of naive and memory/effector T cells in Fuc-TIV−/−/VII−/− PLNs result from the loss of Fuc-TIV–dependent L-selectin ligands that contribute to residual L-selectin–dependent recruitment in Fuc-TVII−/− mice 8.
Hapten-dependent Lymphocyte Expansion In Vivo Requires Fuc-TIV and Fuc-TVII.
To determine how each Fuc-T may contribute to hapten-dependent generation of memory/effector T cells in vivo, we examined lymphocyte expansion (reflecting increases in recruitment [32, 33] and proliferation 34), activation, and differentiation in PLNs draining the site of cutaneous DNFB sensitization. Sensitization elicited large and significant increases in cellularity in WT, Fuc-TIV−/−, and Fuc-TVII−/− PLNs, relative to mice treated with vehicle only (Fig. 5 A). A significant increase was also observed in Fuc-TIV−/−/VII−/− PLNs after sensitization, but the absolute number of cells in vehicle-treated mice or after sensitization was extremely low (Fig. 5 A). CD19+ B lymphocytes contribute substantially to the expanded cell populations in the PLNs, as do CD3+ T cells (Table ). T and B cells account for >90% of the cells in the nodes, except in doubly deficient mice, where the relative larger fraction of non-B and non-T cells is accounted for by stromal cell elements, DCs, and Gr-1+ myeloid lineage cells (data not shown).
Table 2.
B and T Cells in PLNs Before and After DNFB Sensitization
| Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| WT | IV−/− | VII−/− | IV−/−/VII−/− | |||||
| VEH | DNFB | VEH | DNFB | VEH | DNFB | VEH | DNFB | |
| % CD19 | 21 | 47 | 19 | 44 | 18 | 37 | 29 | 39 |
| % CD3 | 73 | 47 | 74 | 48 | 74 | 56 | 40 | 39 |
In response to DNFB sensitization, activated (CD44high) CD4+ and CD8+ T cells increase 5.8- and 7.1-fold, respectively, in WT PLNs, and 4.5- and 3.4-fold, respectively, in Fuc-TIV−/− PLNs, and their absolute numbers do not vary between the two strains (Fig. 5B and Fig. C). Fuc-TIV thus does not contribute significantly to the expansion of PLN T cells after cutaneous antigenic stimulation, or any such contribution is masked by Fuc-TVII–dependent contributions. In Fuc-TVII−/− mice, activated CD4+ and CD8+ T cells increase 5.7- and 6.4-fold, respectively, after DNFB sensitization, though the absolute number of such cells is significantly decreased, relative to WT and Fuc-TIV−/− mice (Fig. 5B and Fig. C). By contrast, there is a profound reduction in the absolute numbers of activated CD4+ and CD8+ T cells recovered from DNFB-sensitized Fuc-TIV−/−/Fuc-TVII−/− draining PLNs, though 2.1- and 4.1-fold increments are observed. Fuc-TVII thus contributes significantly, but not exclusively, to increases in the number of activated CD4+ and CD8+ lymphocytes in PLNs after antigen challenge. These results further imply that Fuc-TIV is responsible for the significant numbers of T lymphocytes recovered from Fuc-TVII−/− PLNs after DNFB sensitization, and for corresponding increments in activated CD4+ and CD8+ T lymphocyte recovery observed in these mice.
Fuc-T Deficiency Yields Decrements in Accumulation of IFN-γ–expressing CD4 and CD8+ Lymphocytes In Vivo, but Is Permissive for Normal Th1 and Tc1 Polarization In Vitro.
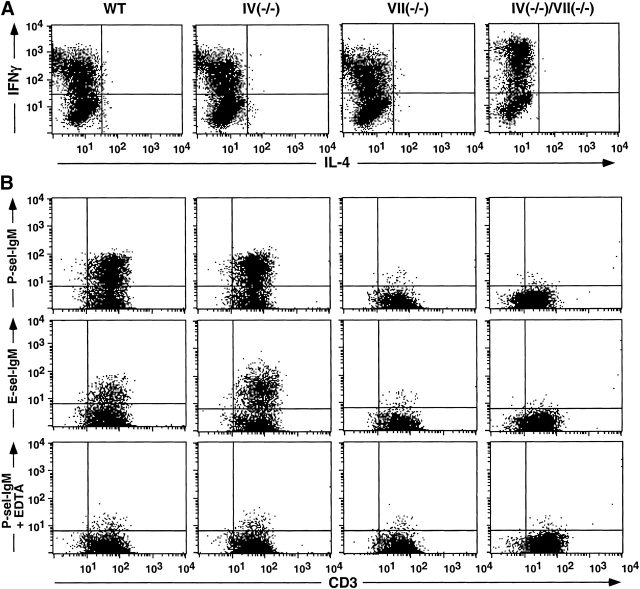
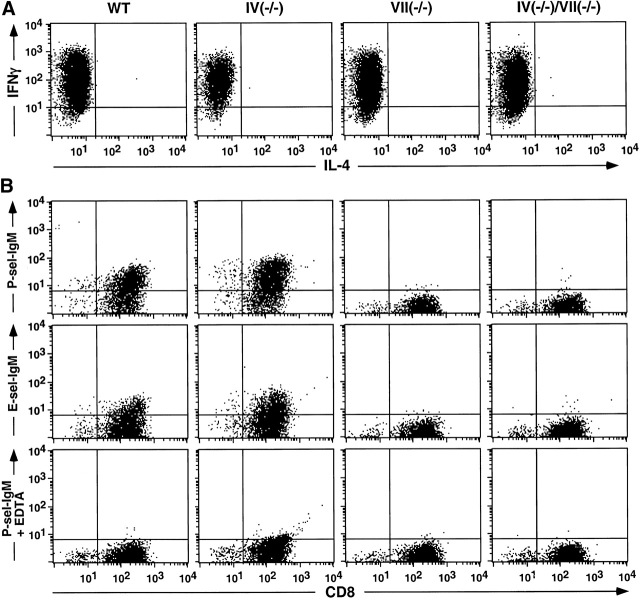
Th1 and Tc1 lymphocytes, defined in part by their characteristic IFN-γ expression properties 35, are generated in draining PLNs after antigen sensitization, traffic via the circulation to the skin, and contribute to the cutaneous inflammatory response during elicitation/challenge 35 36 37. Th1 and Tc1 progenitors most likely derive from naive T lymphocytes recruited to PLNs primarily by L-selectin–dependent mechanisms (discussed in reference 11). As Fuc-TIV and Fuc-TVII contribute to L-selectin–dependent lymphocyte recruitment 6 8, we sought to determine if their deficiency altered the accumulation or generation of such IFN-γ–expressing lymphocytes in a way that contributes to defective CHS in the Fuc-T–deficient mice. The numbers of these cells were equivalently low in the PLNs of nonsensitized WT and all Fuc-T–deficient strains (Fig. 6). Significant increases in these cells are observed in WT and Fuc-TIV−/− mice after DNFB sensitization (Fig. 6A and Fig. B). By contrast, in Fuc-TVII−/− mice and the doubly deficient mice, sensitization did not significantly increase the number of such IFN-γ–positive CD4+ or CD8+ lymphocytes. Graded, genotype-specific decrements in the absolute number of these cells recovered from the PLNs of the sensitized mice were also observed, and are most apparent and significant in the doubly deficient mice (Fig. 6). These decrements imply that Fuc-TIV and Fuc-TVII contribute independently to hapten-dependent accumulation of Th1 and Tc1 cells in vivo. To exclude the possibility that the Fuc-Ts also or instead contribute to properties intrinsic to Th1 and Tc1 precursors that allow them to proceed through the polarization process, WT- and Fuc-T–deficient naive T cells were characterized for their ability to differentiate into Th1 or Tc1 cells in vitro. These experiments show that naive CD4+ or CD8+ T cells isolated from each Fuc-T null strain respond to cytokine polarization by differentiating into cells with a Th1 (IFN-γ+IL-4−) or Tc1 (IFN-γ+IL-4−) phenotype (Fig. 7 A and 8 A). These observations imply that defective generation of Th1 and Tc1 cells in Fuc-TVII−/− and Fuc-TIV−/−/VII−/− mice in response to haptenic sensitization (Fig. 6) is due to a deficiency in the number of naive Th1 cell and Tc1 cell progenitors available for cytokine-dependent polarization, and not to an intrinsic differentiation defect. In vitro polarization towards the Th2 (CD4+IFN-γ−IL-4+) and Tc2 (CD8+IFN-γ−IL-4+) phenotypes is also retained in the absence of Fuc-TIV and/or Fuc-TVII (data not shown), indicating that neither enzyme is required for these alternative differentiation pathways.
Figure 7.
Selectin ligand expression by Th1 cells in WT and Fuc-T–deficient mice. CD4+ T cells were isolated from mesenteric nodes and cultured in vitro under Th1 polarization conditions (Materials and Methods). Cells were then subjected to flow cytometry analysis to confirm Th1 polarization by assessing expression of intracellular IFN-γ and IL-4 (A), or to assess selectin ligand expression using P-selectin-IgM or E-selectin-IgM chimeras (B). Divalent calcium dependence observed for binding of the P-selectin-IgM chimera (B, bottom) was also observed for the E-selectin-IgM chimera (data not shown). Data shown are representative of two or more independent experiments. Vertical and horizontal lines in the histograms define the fluorescence intensities below which 99% of the cells fall when analyzed using a nonbinding isotype control for AntiCD3, or observed with either selectin chimera used in the presence of 5 mM EDTA.
Th1 and Tc1 Lymphocytes Are Deficient in E- and P-Selectin Ligand Activities in the Absence of Fuc-TVII.
E- and P-selectin ligand activities are expressed by a substantial fraction of the cells in populations of lymphocytes expressing the Th1 or Tc1 phenotype 9 11 12 13. Correlative and indirect evidence associates Fuc-TVII and expression of E- and P-selectin ligands on Th1 and Tc1 cells 9 11 12 13 but a requirement for Fuc-TVII in E- or P-selectin ligand expression by Th1 or Tc1 cells has not been established, and a definitive role for Fuc-TIV in T cell selectin ligand expression remains unexplored. To directly determine if Fuc-TIV and/or Fuc-TVII are essential to T cell selectin ligand expression, E- and P-selectin ligand expression was assessed on Th1 and Tc1 cells generated from each Fuc-T null strain. Representative two-parameter histograms demonstrate that the selectin-IgM chimeras bind equivalently to WT and Fuc-TIV−/− Th1 cells (Fig. 7 B), and Tc1 cells (Fig. 8 B). By contrast, the E-and P-selectin-IgM chimeras do not bind to Th1 and Tc1 cells generated from either of the Fuc-TVII–deficient strains (Fig. 7 B and 8 B). Lack of selectin ligand expression does not reflect inefficient polarization, faulty activation, or defective expression of PSGL-1, a major glycoprotein carrier of Th1 cell E- and P-selectin ligands 38, as Fuc-TVII−/− and Fuc-TIV−/−/VII−/− lymphocytes do not differ from WT cells in expression of IFN-γ (35; Fig. 7 A and 8 A), or CD25 and PSGL-1 (data not shown). Fuc-TVII is therefore essential to the synthesis of functional E- and P-selectin ligands on Th1 and Tc1 lymphocytes, whereas any contribution by Fuc-TIV to E- or P-selectin counterreceptor activities on these cells, either in the presence or in the absence of Fuc-TVII is negligible, or is too subtle to detect with the flow cytometry–based assay. As reported by others 9 12 13 39 40 41, WT Th2 and Tc2 cells do not express ligands for E- or P-selectins, nor do Th2 and Tc2 cells generated from the three Fuc-T–deficient strains (data not shown).
Figure 8.
Selectin ligand expression by Tc1 cells in WT and Fuc-T–deficient mice. CD8+ T cells were isolated from mesenteric nodes and cultured in vitro under Tc1 polarization conditions (Materials and Methods). Cells were then subjected to flow cytometry analysis to confirm Tc1 polarization by assessing expression of intracellular IFN-γ and IL-4 (A), or to assess selectin ligand expression using P-selectin-IgM or E-selectin-IgM chimeras (B). Divalent calcium dependence observed for binding of the P-selectin-IgM chimera (B, bottom) was also observed for the E-selectin-IgM chimera (data not shown). Data shown are representative of two or more independent experiments.
T Cell Recruitment to Sites of Cutaneous Inflammation Is Fuc-TVII Dependent.
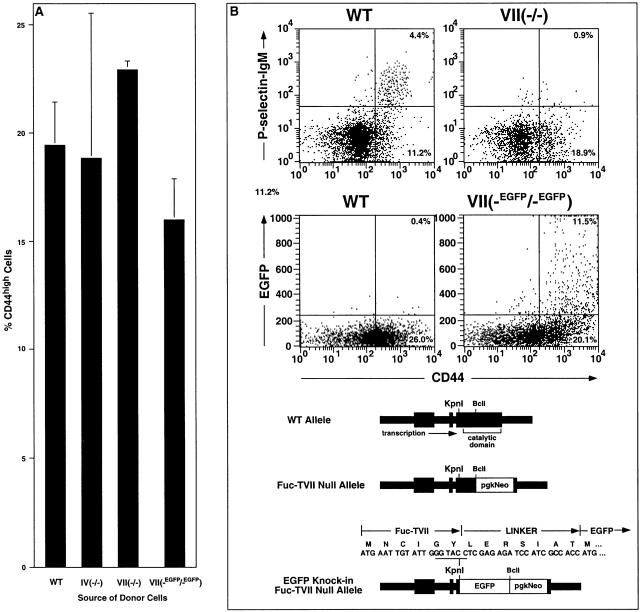
In the elicitation phase of CHS, E- and P-selectins are implicated in the recruitment of cytokine-polarized CD4+ and CD8+ effector T cells to the cutaneous site of inflammation 12 39 40 41. To address the contributions of Fuc-TIV and Fuc-TVII to this process in vivo, adoptive transfer was used to assess trafficking of activated Fuc-T–deficient T cells to a cutaneous site inflamed by hapten challenge (Fig. 9). Donor cells in these experiments derive from the PLNs of DNFB-sensitized WT, Fuc-TIV−/−, or Fuc-TVII−/− mice (Materials and Methods). Characterization of such donor cell populations indicate that they are equivalent in the fraction of activated (CD44hi) cells (Fig. 9 A) that encompass the CD4+ and CD8+ effector cell populations eligible for selectin ligand–dependent trafficking. The fraction of these CD44hi cells that express E- or P-selectin ligands is equivalent in WT and Fuc-TIV−/− donor populations, whereas there is a substantial deficiency of selectin ligand–positive cells in the Fuc-TVII−/− donor population (Fig. 9 B). To confirm that this deficiency is not accounted for by absence of a specific population of CD44hi effector cells that ordinarily express the Fuc-TVII locus and would presumably be most eligible for trafficking, we examined donor-equivalent PLN cells from a DNFB-sensitized strain of Fuc-TVII−/− mice where an EGFP gene has been “knocked-in” to the Fuc-TVII locus (Fuc-TVII(-EGFP/-EGFP)) mice; Materials and Methods), in an arrangement where EGFP expression reports expression of the defective Fuc-TVII locus (Fig. 9 C). The CD44hi fraction in these cells is equivalent to the other three strains (Fig. 9 A), though they remain selectin ligand deficient (data not shown). However, the CD44hi fraction in these Fuc-TVII−/− cells is populated by EGFP-positive cells to a degree equivalent to the fraction of CD44hi cells that are selectin ligand positive in WT or Fuc-TIV−/− strains (Fig. 9B and Fig. C). This observation indicates that in Fuc-TVII deficiency, an essentially normal complement of effector cells eligible for trafficking remains in DNFB-sensitized donor cells, even though such cells no longer express selectin ligands.
Figure 9.
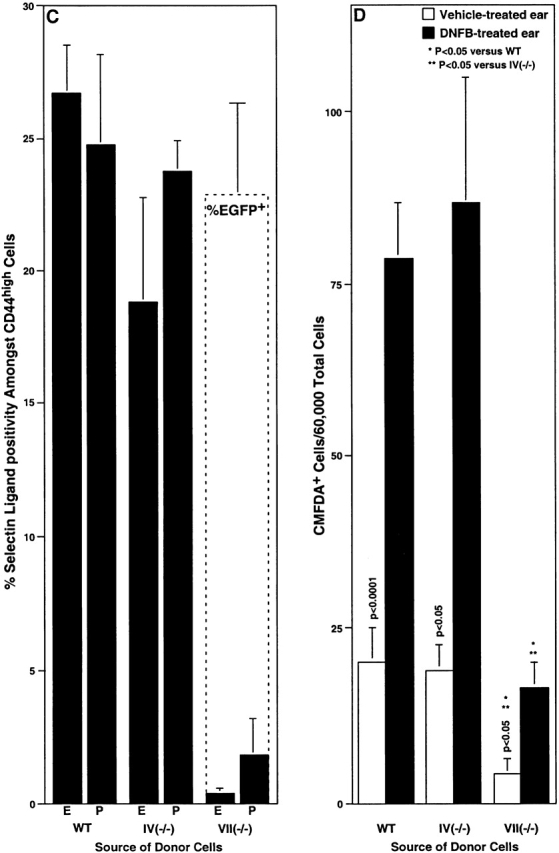
Recruitment of activated T cells into inflamed skin in WT and Fuc-T–deficient mice. Lymphocytes were isolated from the PLNs of mice previously sensitized with DNFB, and were used in flow cytometry analyses (A–C), or were labeled with CMFDA and used in adoptive transfer experiments (D) (Materials and Methods). (A) The fraction of activated (CD44high) lymphocytes (mean ± SE) determined by flow cytometry. (B) Expression of P-selectin ligands or the Fuc-TVII locus (reported by EGFP) by flow cytometry in WT, Fuc-TVII−/−, and Fuc-TVII(-EGFP/-EGFP) mice. The top pair of representative two parameter flow cytometry histograms show CD44 expression level versus P-selectin-IgM chimera binding in lymphocytes prepared from DNFB-sensitized WT or Fuc-TVII−/− mice. The bottom pair of representative two parameter flow cytometry histograms show CD44 expression level versus green fluorescence in lymphocytes prepared from DNFB-sensitized WT or Fuc-TVII(-EGFP/-EGFP) mice. Numbers in the top right and top left quadrants are the percentage of all viable cells in the node that fall within that quadrant. Schematic depictions of the WT Fuc-TVII locus (reference 6), the null Fuc-TVII locus (reference 6), and the Fuc-TVII null/EGFP knock-in locus (Materials and Methods) in Fuc-TVII(-EGFP/-EGFP) mice are at the bottom. DNA and protein sequences in the region encompassing the initiation codon of the Fuc-TVII-EGFP fusion protein are above the Fuc-TVII null/EGFP knock-in schematic. (C) The fraction of CD44high cells in WT, Fuc-TIV−/−, or Fuc-TVII−/− cells that are positive for E-selectin-IgM or P-selectin-IgM chimera binding (mean ± SE) determined by flow cytometry (black bars), or the fraction of Fuc-TVII(-EGFP/-EGFP) cells (see B) that are green fluorescent (dashed box overlying the Fuc-TVII−/− bars; mean ± SE). (D) Adoptive transfer. 20 × 106 CMFDA-labeled cells were administered by tail vein injection to DNFB sensitized WT recipients. Recipients were immediately challenged with DNFB on one ear, and were treated with vehicle on the other ear. 24 h later, the number of CMFDA-positive donor cells in the dermis and epidermis combined, for each ear, was enumerated by flow cytometry (mean ± SE). Differences in CMFDA+ cell accumulation between genotypes were analyzed as in Fig. 1.
Such donor cell populations derived from WT or Fuc-TIV−/− mice accumulate to equivalently low levels in vehicle-treated recipient ears, and to significantly and equivalently higher levels in DNFB-challenged ears (Fig. 9 D). Fuc-TIV deficiency thus is not accompanied by defective lymphocyte trafficking to inflamed cutaneous sites. By contrast, Fuc-TVII−/−–derived donor lymphocytes accumulate to significantly lower levels in DNFB-challenged ears, corresponding to a fivefold reduction, relative to WT or Fuc-TIV−/− donor cells (Fig. 9 D). Deficiency of selectin ligand expression by Fuc-TVII−/− effector T lymphocytes therefore accompanies defective recruitment of these cells to sites of cutaneous inflammation. Accumulation of Fuc-TVII−/− donor cells in vehicle treated ears is also significantly deficient, relative to WT or Fuc-TIV−/− donor cell trafficking to vehicle-treated ears (Fig. 9 D). To the extent that vehicle-treated ears are reflective of a relatively noninflamed state, this latter observation implies the existence of constitutive Fuc-TVII–dependent recruitment process that may populate the skin with selectin ligand–positive T lymphocytes. It remains to be determined if Fuc-TIV–dependent selectin ligand expression by Fuc-TVII−/− donor cells accounts for the small amount of their apparent residual trafficking to DNFB-challenged ears observed in these experiments.
Discussion
Several distinct α(1,3)fucosyltransferases can construct sialylated, fucosylated molecules capable of contributing to the activities of counter-receptors for E-, P-, and L-selectins 1 2. Their expression patterns and gene ablation studies implicate two (Fuc-TVII and/or Fuc-TIV) in the physiological control of selectin ligand activity on neutrophils, monocytes, and HEVs 6 7 8. However, a role for Fuc-TVII in determining expression of Th1 and Tc1 cell E- and P-selectin ligands has been inferred only through correlative associations between its expression and selectin ligand display, and a requirement for either Fuc-TVII or Fuc-TIV in this process has not been established. Furthermore, requirements have not been established for either enzyme in the control of selectin ligand–dependent LC recruitment and migration, in effector T cell trafficking in vivo, or generally in the support of cell-mediated immunity in vivo. We address each of these issues by analyzing the components of the CHS response in mice deficient in Fuc-TIV, Fuc-TVII, and both Fuc-TIV and Fuc-TVII. These analyses disclose that both fucosyltransferases contribute essentially to the adaptive immune response by regulating selectin-dependent T lymphocyte migration.
During the afferent phase of CHS, LCs are required for antigen acquisition and processing, for delivery of processed antigen to naive T cells in draining LNs, and for events leading to T cell activation and expansion 21 22 42. Selectin ligands are indirectly implicated in recruitment of LC precursors from the blood, as blood-derived DCs express E- and P-selectin ligands 15 23, engage in selectin-dependent tethering and rolling on endothelium in noninflamed skin, and can be recruited to inflamed cutaneous sites 15. LCs themselves express the sialyl Lewis X determinant 14 and cutaneous lymphocyte antigen (CLA; reference 24), surrogate markers for selectin ligand activity 2. However, we find normal numbers of resident epidermal LC in the absence of Fuc-TIV and/or Fuc-TVII, and observe that emigration and the general function of LCs are intact in the absence of Fuc-TIV or Fuc-TVII. A possible modest reduction in LC emigration is observed in Fuc-TIV−/−/Fuc-TVII−/− mice. This could be a direct result of selectin ligand loss by the LCs, or an indirect consequence of the perturbed PLN microenvironment in these mice, and may contribute modestly to the CHS defect in these mice. Notwithstanding this caveat about LC migration in the doubly deficient mice, these results imply that any contribution by selectin ligands to populating noninflamed epidermis with LCs, or to LC function generally, must be determined by other fucosyltransferases, or by selectin ligands that are fucosyltransferase independent. Whether either enzyme controls DC recruitment in the context of cutaneous inflammation remains to be determined.
In Fuc-TVII−/− mice, and in Fuc-TIV−/−/Fuc-TVII−/− mice, substantial, or profound deficiencies are observed, respectively, in the PLN content of B cells, memory T cells, and naive T cells. Fuc-TIV and Fuc-TVII collaboratively contribute to the synthesis of L-selectin ligands on PLN HEVs, and to lymphocyte homing to PLNs 6 8. L-selectin is required for homing of naive T cells to PLNs 26 28, contributes to B cell homing 31, and in humans is implicated in the homing of CCR7+ central memory T cells 43. The numbers of peripheral blood B cells, naive T cells, and memory T cells available for homing are normal in Fuc-TVII−/− and Fuc-TIV−/−/Fuc-TVII−/− mice, and their L-selectin expression levels are normal 6 8. These considerations infer that defects in HEV-borne L-selectin ligands, and corresponding defects in L-selectin–dependent lymphocyte adhesion and homing thus account for the paucity of such cells in these animals. Reductions in accumulation of activated CD4+ and CD8+ T cells, including Th1 and Tc1 cells, in Fuc-TVII−/− and Fuc-TIV−/−/VII−/− PLNs in response to DNFB sensitization are clearly not consequent to an intrinsic defect in the ability to become activated or cytokine polarized, but instead likely reflect a quantitative deficiency of cells available to respond to this hapten. Such quantitative reductions in cells available for antigen-dependent activation and proliferation are reminiscent of L-selectin−/− mice 25 26 27, and of mice treated with Abs to L-selectin 28, where CHS defects are assigned to an inability to populate PLNs with naive T cells. The partial and nearly complete deficiencies, respectively, in the content of PLN naive T cells in Fuc-TVII−/− or doubly deficient mice likely contribute to their partial or complete defects in CHS response by severely (Fuc-TVII−/− mice) or profoundly diminishing (doubly deficient mice) the number of hapten-specific memory T cells generated during sensitization that are available for distribution to the skin and other nonlymphoid tissues 37 44 45.
Recruitment of hapten-primed T lymphocytes to the cutaneous site of hapten challenge also provides a primary contribution to the CHS response. Recruitment of activated T cells to inflammatory sites requires E- and P-selectins expressed at such sites and selectin ligands expressed by Th1 and Tc1 cells 38 39 40 41 46 47. Prior studies correlate inducible expression of Fuc-TVII with differentiation of T cells to Th1 or a Tc1 phenotype and their expression of E- and/or P-selectin ligands 9 11 12 13, though a requirement for Fuc-TVII in control of selectin ligand expression in these polarized cell types, in vitro, or in vivo, or in T cell trafficking to inflamed sites in vivo was not addressed. Our observations demonstrate that Fuc-TVII is an essential concomitant to normal E- and P-selectin ligand expression by Th1 and Tc1 cells generated in vitro or in vivo, and is required for normal recruitment of hapten-sensitized T cells to cutaneous sites of hapten challenge. These defects will thus contribute to the faulty CHS response in Fuc-TVII−/− mice by diminishing recruitment of memory/effector T cells, at least in the context of haptenic challenge. The CMFDA-labeled cells used in the adoptive transfer experiments (Fig. 9) are largely activated CD4+ and CD8+ T cells (Fig. 5). Nonetheless, as unfractionated LN suspensions were used in these studies, additional work will be necessary to define the efficiency with which each cell type traffics in this model, and how Fuc-TVII deficiency may diminish or delete such trafficking. Absence of T cell selectin ligand expression in these mice may also contribute to their defective CHS responses by disallowing dissemination of hapten-specific memory T cells to skin and other peripheral tissues after hapten sensitization, although a requirement for the selectins and their ligands in distributing antigen-specific memory cells to peripheral tissues 44 45 remains to be established.
Inducible expression of Fuc-TIV is inconsistently observed in association with Th1 polarization 9 13. Our observations indicate that Fuc-TIV deficiency in the context of a normal Fuc-TVII locus does not detectably diminish Th1 and Tc1 cell selectin ligand expression or trafficking. It remains to be determined if Fuc-TIV directs residual E- or P-selectin ligand expression by activated, cytokine-polarized T cells, as it does in neutrophils 8, if this accounts for the small amount of trafficking by Fuc-TVII−/− donor cells to the cutaneous site of hapten challenge in our adoptive transfer experiments, and if this in turn permits the residual CHS response observed in Fuc-TVII−/− mice.
Neutrophil recruitment is also required for the CHS response 48, in part by promoting recruitment of CD8+ T cells after hapten challenge 19 37, and presumably by release of inflammatory mediators. Recruitment of neutrophils to the skin in response to nonspecific irritation is normal in Fuc-TIV−/− mice, partially defective in Fuc-TVII−/− mice, and fully deleted in Fuc-TIV−/−/Fuc-TVII−/− mice 8, indicating that defective neutrophil recruitment may also contribute to Fuc-TIV– and Fuc-TVII–dependent defects in the CHS response. However, the relative roles played by defective neutrophil and T cell recruitment in the efferent phase of CHS in these mice, as in mice with defects in the selectins or PSGL-1 46 47 49 50, are not addressed by these observations and remain to be defined.
Acknowledgments
We thank Linda Bradley for her critical comments on this manuscript. This work was supported in part by National Institutes of Health Grant 1 P01 CA71932 (to J.B. Lowe).
J.B. Lowe is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper: CHS, contact hypersensitivity; CMFDA, 5-chloromethylfluorescein diacetate; CTCM, complete tissue culture medium; DC, dendritic cell; DNFB, 2,4-dinitrofluorobenzene; EGFP, enhanced green fluorescent protein; HEV, high endothelial venule; LC, Langerhans cell; MLN, mesenteric LN; PLN, peripheral LN; Tc, T cytotoxic; WT, wild-type.
References
- Vestweber D., Blanks J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- Lowe J.B. Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int. 1997;51:1418–1426. doi: 10.1038/ki.1997.194. [DOI] [PubMed] [Google Scholar]
- Hemmerich S., Rosen S.D. Carbohydrate sulfotransferases in lymphocyte homing. Glycobiology. 2000;10:849–856. doi: 10.1093/glycob/10.9.849. [DOI] [PubMed] [Google Scholar]
- Rosen S.D. Endothelial ligands for L-selectin. Am. J. Pathol. 1999;155:1013–1019. doi: 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T., Ikehara Y., Togayachi A., Kaneko M., Hiraga T., Sasaki K., Narimatsu H. Expression cloning and characterization of a novel murine 1,3-fucosyltransferase, mFuc-TIX, that synthesizes the Lewis x (CD15) epitope in brain and kidney. J. Biol. Chem. 1998;273:26729–26738. doi: 10.1074/jbc.273.41.26729. [DOI] [PubMed] [Google Scholar]
- Maly P., Thall A.D., Petryniak B., Rogers C.E., Smith P.L., Marks R.M., Kelly R.J., Gersten K.M., Cheng G., Saunders T.L. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Weninger W., Ulfman L.H., Cheng G., Lowe J.B., von Andrian U.H. Leukocyte rolling in non-inflamed skin venulesrole of selectins and alpha(1,3)-fucosyltransferase-IV and -VII in cutaneous immune surveillance. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- Homeister J.W., Thall A.D., Petryniak B., Maly P., Rogers C.E., Smith P.L., Kelly R.J., Gersten K.M., Askari S.W., Cheng G. The α(1,3)fucosyltransferases Fuc-TIV and Fuc-TVII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Wagers A.J., Waters C.M., Stoolman L.M., Kansas G.S. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on α1,3-fucosyltransferase VII gene expression. J. Exp. Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs R.N., Craig R.A., Natsuka S., Chang A., Cameron M., Lowe J.B., Stoolman L.M. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J. Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander J.M., Visintin I., Janeway C.A., Medzhitov R. α(1,3)-Fucosyltransferase VII and α(2,3)-Sialyltransferase IV are up-regulated in activated CD4 T cells and maintained after their differentiation into Th1 and migration into inflammatory sites. J. Immunol. 1999;163:3746–3752. [PubMed] [Google Scholar]
- Lim Y.-C., Henault L., Wagers A.J., Kansas G.S., Luscinskas F.W., Lichtman A.H. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J. Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- van Wely C.A., Blanchard A.D., Britten C.J. Differential expression of alpha3 fucosyltransferases in Th1 and Th2 cells correlates with their ability to bind P-selectin. Biochem. Biophys. Res. Commun. 1998;247:307–311. doi: 10.1006/bbrc.1998.8786. [DOI] [PubMed] [Google Scholar]
- Ross E.L., Barker J.N.W.N., Allen M.H., Chu A.C., Groves R.W., MacDonald D.M. Langerhans' cell expression of the selectin ligand, sialyl Lewis x. Immunology. 1994;81:303–308. [PMC free article] [PubMed] [Google Scholar]
- Robert C., Fuhlbrigge R.C., Kieffer J.D., Ayehunie S., Hynes R.O., Cheng G., Grabbe S., von Andrian U.H., Kupper T.S. Interaction of dendritic cells with skin endotheliuma new perspective on immunosurveillance. J. Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phauphak P., Moorhead J.W., Claman H.N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J. Immunol. 1974;112:115–123. [PubMed] [Google Scholar]
- Shreedhar V., Moodycliffe A.M., Ullrich S.E., Bucana C., Kripke M.L., Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11:625–636. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- Macatonia S.E., Edwards A.J., Knight S.C. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509–514. [PMC free article] [PubMed] [Google Scholar]
- Xu H., DiIulio N.A., Fairchild R.L. T cell populations primed by hapten sensitization in contact hypersensitivity are distinguished by polarized patterns of cytokine productioninterferon γ-producing (Tc1) effector CD8+ T cells and interleukin (IL)-4/IL-10–producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.K., Hammerberg C., Yoshida Y., Bata-Csorgo Z., Cooper K.D. Identification and quantification of interferon-γ producing T cells in psoriatic lesionslocalization to both CD4+ and CD8+ subsets. J. Invest. Dermatol. 1998;111:1072–1078. doi: 10.1046/j.1523-1747.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Toews G., Bergstresser P.R., Streilein J.W. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J. Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- Silberg-Sinaken I., Thornbecke G.J., Baer R.L., Rosenthal S.A., Berezowsky V. Antigen-bearing Langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell. Immunol. 1976;25:137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Strunk D., Egger C., Leitner G., Hanau D., Stingl G. A skin homing molecule defines the Langerhans cell progenitor in human peripheral blood. J. Exp. Med. 1997;185:1131–1136. doi: 10.1084/jem.185.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner S., Lenz A., Reider D., Fritsch P., Schuler G., Romani N. Expression of maturation-/migration-related molecules on human dendritic cells from blood and skin. Immunobiology. 1998;98:568–587. doi: 10.1016/S0171-2985(98)80079-X. [DOI] [PubMed] [Google Scholar]
- Xu J., Grewal I.S., Geba G.P., Flavell R.A. Impaired primary T cell responses in L-selectin-deficient mice. J. Exp. Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones M.L., Ord D.C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D.J., Tedder T.F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Catalina M.D., Carroll M.C., Arizipe H., Takashima A., Estess P., Siegelman M.H. The route of antigen entry determines the requirement for L-selectin during immune responses. J. Exp. Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L.M., Watson S.R., Swain S.L. Entry of Naive CD4 T cells into peripheral lymph nodes requires L-selectin. J. Exp. Med. 1994;180:2401–2406. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.T., Yin X.-M., Vitetta E.S. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J. Immunol. 1990;144:3288–3295. [PubMed] [Google Scholar]
- Bradley L.M., Duncan D.D., Tonkonogy S., Swain S.L. Characterization of antigen-specific CD4+ effector T cells in vivoimmunization results in a transient population of Mel-14-, CD45RB- helper cells that secrete interleukin 2(IL-2), IL-3, IL-4, and interferon γ. J. Exp. Med. 1991;174:547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.L.K., Steeber D.A., Zhang X.-Q., Tedder T.F. Intrinsic differences in L-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J. Immunol. 1998;160:5113–5511. [PubMed] [Google Scholar]
- Drayson M.T. The entry of lymphocytes into stimulated lymph nodes. The site of selection of alloantigen-specific cells. Transplantation. 1986;41:745–751. doi: 10.1097/00007890-198606000-00016. [DOI] [PubMed] [Google Scholar]
- Hoke D., Mebius R.E., Dybdal N., Dowbenko D., Gribling P., Kyle C., Baumhueter S., Watson S.R. Selective modulation of the expression of L-selectin ligands by an immune response. Curr. Biol. 1995;5:670–678. doi: 10.1016/s0960-9822(95)00132-1. [DOI] [PubMed] [Google Scholar]
- Meltzer M.S., Nacy C.A. Fundamental Immunology. Raven Press, ; New York: 1989. [Google Scholar]
- Mosmann T.R., Subash S. The expanding universe of T-cell subsetsTh1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Cher D.J., Mosmann T.R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Th1 clones. J. Immunol. 1987;138:3688–3694. [PubMed] [Google Scholar]
- Gocinski B.L., Tigelaar R.E. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J. Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- Hirata T., Merrill-Skoloff G., Aab M., Yang J., Furie B.C., Furie B. P-selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrup F., Vestweber D., Borges E., Lohning M., Brauer R., Herz U., Renz H., Hallmann R., Scheffold A., Radbruch A., Hamann A. P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Tietz W., Allemand Y., Borges E., von Laer D., Hallmann R., Vestweber D., Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- Borges E., Tietz W., Steegmaier M., Moll T., Hallmann R., Hamann A., Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J. Exp. Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Marzo A.L., Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Reinhardt R.L., Khoruts A., Merica R., Zell T., Jenkins M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Subramaniam M., Saffaripour S., Watson S.R., Mayadas T.N., Hynes R.O., Wagner D.M. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin-deficient mice. J. Exp. Med. 1995;181:2277–2282. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staite N.D., Justen J.M., Sly L.M., Beaudet A.L., Bullard D.C. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- Dilulio N.A., Engeman T., Armstrong D., Tannenbaum C., Hamilton T.A., Fairchild R.L. Groalpha-mediated recruitment of neutrophils is required for elicitation of contact hypersensitivity. Eur. J. Immunol. 1999;29:3485–3495. doi: 10.1002/(SICI)1521-4141(199911)29:11<3485::AID-IMMU3485>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Labow M.A., Norton C.R., Rumberger J.M., Lombard-Gillooly K.M., Shuster D.J., Hubbard J., Bertko R., Knaack P.A., Terry R.W.H.M.L., Kontgen F. Characterization of the E-selectin-deficient micedemonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Catalina M.D., Estess P., Siegelman M.H. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammationparticipation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]