Antitumor Monoclonal Antibodies Enhance Cross-Presentation of Cellular Antigens and the Generation of Myeloma-specific Killer T Cells by Dendritic Cells (original) (raw)
Abstract
The mechanism of antitumor effect of monoclonal antibodies (mAbs) is not fully understood. Here we show that coating myeloma cells with anti–syndecan-1 antibody promotes cross-presentation of cellular antigens by dendritic cells (DCs) to autologous T cells from healthy donors. The tumor cells treated with anti–syndecan-1 or isotype-matched control antibody were fed to HLA-mismatched monocyte-derived immature DCs. Tumor cell–loaded mature DCs induced a strong CD8+ T cell response that was specific for the cancer-testis (C-T) antigens expressed in the tumor. The CD8+ T cells killed peptide-pulsed targets, as well as myeloma tumor cells. Importantly, mAbs-coated tumor-loaded DCs were consistently superior to DCs loaded with peptides or dying cells for eliciting tumor-specific killer T cells. This enhanced cross-presentation was not due to enhanced tumor cell uptake or to DC maturation. When mixtures of NY-Eso-1-positive and -negative myeloma cells were captured by DCs, the anti–syndecan-1 antibody had to be on the NY-Eso-1-positive cells to elicit NY-Eso-1–specific response. Cross-presentation was inhibited by pretreatment of DCs with Fcγ receptor blocking antibodies. Targeting of mAb-coated tumors to DCs may contribute to the efficacy of tumor-reactive mAb and offers a new strategy for immunotherapy.
Keywords: immunotherapy, myeloma, cancer-testis antigens, tumor immunity, Fc receptors
Introduction
MHC class I molecules are generally complexed with peptides derived exclusively from newly synthesized cytosolic proteins (1). However, CD8+ T cell responses can also be directed to exogenous cell–associated antigens derived from tumors, transplants, and virus-infected nonhematopoietic cells (2). This requires presentation of these antigens by bone marrow–derived cells (a process termed cross-presentation), with dendritic cells (DCs)*being one of the major candidate cell types. DCs can acquire antigen from dying tumor cells and elicit tumor-specific CD8+ T cell responses in vitro (3–8). Strategies to optimize cross-presentation of antigen from tumor cells are of great interest for immunotherapy of tumors.
Studies in animal tumor models have implicated both cellular and humoral responses in protective antitumor immunity (9). Interest in tumor-specific humoral immunity has been intensified by certain successes of mAbs in cancer therapy. The mechanism of antitumor effects of these antibodies is not fully understood. Direct effects on tumor cells, as well as enhancement of innate effectors (complement and antibody-dependent cytotoxicity) have been proposed (10–12). Fc receptors are required for both active and passive immunity to melanoma, (11) and the protective effect of therapeutic mAbs in some murine models (12). Together, these studies point to the importance of Fc-dependent innate effector mechanisms in the protective effects of tumor-specific antibodies. However, whether coating of tumor cells by antibodies also affects antitumor cellular immunity is not known.
Here we show that coating of tumor cells with antitumor mAbs leads to enhanced cross-presentation of tumor-derived cellular antigens and generation of tumor specific–killer T cells by DCs. This effect is dependent on Fcγ receptors (FcγRs) on the DCs, but is exerted at a step after the uptake of tumor cells by DCs.
Materials and Methods
Myeloma Cell Lines.
Myeloma cell lines were obtained from American Type Culture Collection (U266, RPMI 8226), or provided by J. Epstein, Arkansas Cancer Center, Little Rock, AR; cag, arp, ark cells). HLA A2.1 status on cell lines was assessed by serotyping. All cell lines were grown in RPMI 1640/10–20% FCS/glutamine/gentamicin.
Expression of Cancer-Testis Antigens.
The expression of a panel of cancer-testis (C-T) antigens (MAGE1, MAGE3, MAGE4, MAGE10, CT-7, LAGE-1, and NY-Eso-1) by myeloma cells was examined using RT-PCR, as described previously (13).
Generation of Dying/Antibody-Coated Tumor Cells.
Tumor cells were killed by repeated freeze thaw cycles (necrosis) or by γ irradiation (30 Gy) (apoptosis). The induction of apoptosis was monitored using staining with Annexin V-FITC. For antibody coating, tumor cells (107 cells per milliliter) were incubated with anti–syndecan-1 antibody (14) (1 μg/ml, B-B4; Serotec) or isotype (IgG1) control, for 30 min at 4°C. Syndecan-1 is a heparan sulfate proteoglycan highly expressed on myeloma cells. After antibody coating, cells were washed, irradiated with 3 Gy, and immediately added to immature DCs as live (Annexin V-negative) cells. To determine if antibody coating enhanced presentation of antigens from dying cells, tumor cells were killed either by γ-irradiation (apoptosis) or freeze thaw (necrosis) as above, and then treated with anti–syndecan-1 or isotype control antibody as described above, or left untreated, before feeding to DCs at DC/tumor ratio of 1:1.
Generation and Loading of DCs with Tumor Antigens.
DCs were generated as described previously (15), by culture of plastic adherent blood mononuclear cells, obtained from leucocyte concentrates, or whole blood of A2.1+ve healthy donors, in GM-CSF (Immunex) and IL-4 (R&D Systems). The nonadherent blood cells were used as a source of T cells. On day 5 or 6 of culture, the immature DCs were fed in 96-well plates with apoptotic, necrotic, or live antibody coated HLA A2.1-negative tumor cells at a ratio of 1:1 and then 4–12 h later, a cytokine cocktail consisting of IL-1β (10 ng/ml), IL-6 (1,000 U/ml), TNF-α (10 ng/ml), and PGE2 (1 μg/ml) was added to induce maturation (16, 17). As controls, some mature DCs were pulsed for 2 h with 1 μM HLA A2.1-restricted peptides from MAGE-3 (271–279; FLWGPRALV) and NY-Eso-1 (157–167; SLLMWITQCFL). For some experiments, DCs were pretreated with a cocktail of anti–human Fc receptor blocking anti-CD16 (clone 3G8) and CD32 (clone IV.3) antibodies (both at 10 μg/ml for 60 min; Medarex), before tumor cell feed. For some experiments, DCs were fed with a mixture of antibody-treated and untreated tumor cells, differing in the expression of tumor antigens.
Evaluation of Tumor Cell Uptake.
To evaluate phagocytosis of tumor cells by DCs, tumor cells were dyed red with PKH26 (Sigma-Aldrich) before cell death or antibody coating, and immature DCs were stained green with PKH67 (Sigma-Aldrich) before coculture with tumor for 0–16 h at 4°C or 37°C (5). Phagocytosis was monitored by flow cytometry as double-positive cells. Tumor cell uptake was also evaluated by applying tumor cell–loaded DCs to alcian blue coated slides for two color fluorescence microscopy using an Olympus epifluorescence microscope. DCs were stained with anti-CD11c and tumor cells were stained with an antibody for C-T antigen, CT-7 (a gift of A. Jungbluth, Ludwig Institute, New York, NY). The motorized stage allowed 0.5-mm optical sections with a cooled CCD camera (Hammatsu) and Metamorph software (Universal Imaging Corp.).
Stimulation of T Cells.
Antigen loaded or unpulsed DCs were cocultured in 96-well round-bottomed microtest plates with autologous nonadherent cells at a ratio of 1:30 in 0.2 ml of 5% pooled human serum. As controls, irradiated (3 Gy) antibody/isotype treated tumor cells were also used to present antigen. The culture media was supplemented with recombinant IL-2 (50 U/ml) on days 4 and 7 of culture. At 1 wk, cultures were restimulated with the identical APCs as the initial stimulation. For some experiments, DCs loaded with tumor cells were purified from the DC-tumor mixtures after initial coculture for 4 h, using anti-CD11c-coupled magnetic beads (Miltenyi Biotech). The DCs were matured before their use as APCs. For some experiments, DCs loaded with tumor cells were cultured in transwell inserts, physically separated from autologous T cells and unpulsed DCs.
Evaluation of Antigen-specific T Cell Response.
Antigen-specific T cell response against defined HLA A*0201-restricted peptide epitopes from MAGE-3 and NY-Eso-1 was determined using IFN-γ enzyme linked immunospot (ELISPOT) and cytotoxicity assays, after 2 wk of culture with tumor/peptide loaded DCs. For the ELISPOT assay, autologous mature DCs pulsed with 1 μM of specific (MAGE-3, NY-Eso-1) or irrelevant (HIV-gag) peptide were used as APCs at ratio of 1:30, and spot-forming cells were quantified after overnight culture as described previously (15). For evaluation of antigen-specific killers, T2 cells pulsed with 1 μM of specific (MAGE-3, NY-Eso-1) peptide were used as targets in a standard 5 h 51Cr release assay. Other targets were HLA A*0201 +ve (U266 cells) or –ve (ark cells) myeloma cells known to express both MAGE-3 and NY-Eso-1, and K562 cells to monitor NK cell–mediated lysis.
Results
Myeloma Cells Express Several C-T Antigens.
The expression of several members of C-T antigen family by myeloma cell lines was evaluated by RT-PCR. All cell lines tested expressed at least one member of this family (Table I).
Table I.
Expression of C-T Antigens in Myeloma Cells by RT-PCR
| C-T Antigen | |||||||
|---|---|---|---|---|---|---|---|
| Cells | NY Eso-1 | LAGE-1 | MAGE-1 | MAGE-3 | MAGE-4 | MAGE-10 | CT-7 |
| arp | − | − | +++ | +++ | − | − | − |
| ark | ++ | +++ | +++ | +++ | ++ | ++ | +++ |
| cag | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| U266 | +++ | ++ | +++ | ++ | +++ | +/− | ++ |
| RPMI8226 | +/− | + | ++ | +++ | − | − | − |
Immature DCs Acquire Cellular Antigens from Dying or Antibody-coated Tumor Cells but Inflammatory Cytokines Are Required for Full DC Maturation.
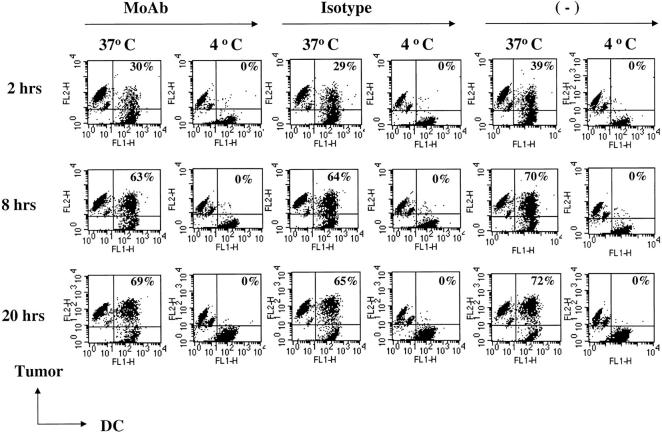
We used 2 HLA A2.1-negative cell lines (cag, arp) expressing both MAGE-3 and NY-ESO-1 (cag cells) or MAGE-3 alone (arp cells), as sources of defined C-T antigens to elicit HLA A*0201-restricted peptide-specific T cells in vitro. Both cell lines expressed high levels of syndecan-1, and binding of anti-syndecan antibody did not induce apoptosis (data not shown). The myeloma cells were induced to undergo apoptosis by high dose (30 Gy) γ irradiation, necrosis by repeated freeze thaw cycles, or treated with anti–syndecan-1 antibody/isotype, as live, low dose (3 Gy) γ-irradiated cells. Myeloma cells were then fed to immature DCs from healthy donors. Phagocytosis studies performed using tumor cells and DCs labeled with different color fluorochromes indicated that label from anti–syndecan-1, isotype control antibody treated, or untreated tumor cells was taken up by DCs upon 2–20 h of coculture at 37°C (but not at 4°C) with similar efficiency (Fig. 1) . Uptake of tumor cells was also verified using confocal microscopy after staining for tumor cells with an antibody to the CT-7 tumor antigen (data not shown). Therefore the coating with anti–syndecan-1 antibody was not required for tumor cell uptake in this system.
Figure 1.
Uptake of tumor cells by DCs. Myeloma cells were labeled with dye (PKH26) and either stained with anti–syndecan-1 antibody (mAb), isotype control antibody, or left unstained. Tumor cells were irradiated (3 Gy) just before coculture with dye (PKH67) labeled DCs at 4°C or 37°C, and the percent of double-positive DCs (top right of each panel) analyzed by flow cytometry.
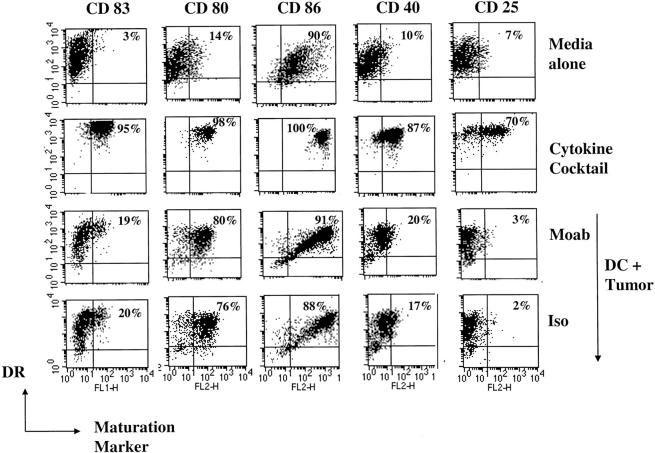
Ligation of FcγR is known to induce the maturation of mouse DCs. In our system however, the uptake of antibody treated (both anti–syndecan-1 or isotype control), or untreated tumor cells was associated with partial, but equivalent phenotypic maturation of DCs, as measured by a slight increase in the surface expression of CD83, and considerable increases in CD80 and CD86. However, upregulation of HLA-DR, CD40, and CD25 was not noted, as with DCs matured using inflammatory cytokines (Fig. 2) . However these DCs could be fully matured by the addition of inflammatory cytokine cocktail after tumor cell uptake (data not shown). Therefore the anti–syndecan-1 antibody does not detectably influence DC uptake of myeloma cells or DC maturation.
Figure 2.
Phagocytosis of mAb-coated tumor does not trigger extensive DC maturation. Monocyte-derived DCs at day 6 of culture were cultured in media alone, with inflammatory cytokine cocktail, or with tumor cells pretreated with anti–syndecan-1 or isotype control antibody. After 1 d of culture, the cells were stained with anti-HLA DR (FITC/PE) and one of the following antibodies (anti-CD80, CD86, CD83, CD40, and CD25). Percentage of HLA-DR +ve DCs expressing CD83, CD80, CD86, CD40, or CD25 (top right of each panel) were quantified by flow cytometry.
Uptake of Antibody-coated Tumor Cells Leads to Strong Cross-Presentation for Antigen-specific T Cell Responses, Including T Cells Lytic for HLA A2.1+ Myeloma Targets.
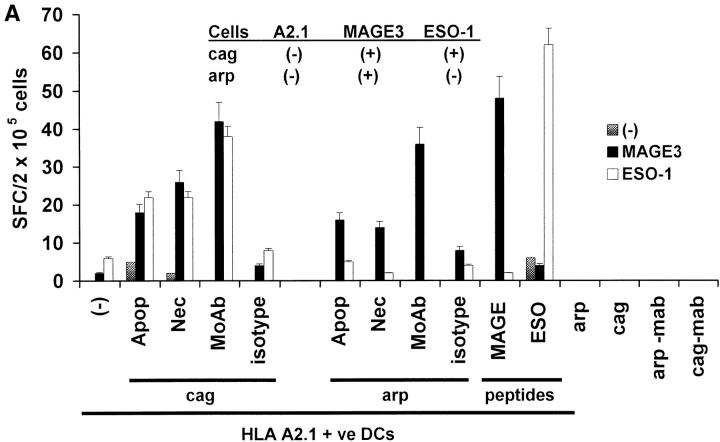
After phagocytosis and ex vivo maturation using cytokine cocktail, the tumor-loaded, peptide-pulsed, or unpulsed DCs, as well as tumor cells alone, were used as APCs to stimulate T cells in culture. No T cell reactivity to either MAGE3 or NY-Eso-1 peptide was noted at baseline before in vitro expansion in any subject (data not shown). However, DCs loaded with apoptotic-, necrotic-, or antibody-coated myeloma cells expanded C-T antigen-specific T cells using two cycles of stimulation in vitro (Fig. 3A). If the tumor expressed both MAGE-3 and NY-Eso-1 (cag cells), then both MAGE-3 and NY-Eso-1–specific T cells developed. In contrast, expansion of only MAGE-3 but not NY-Eso-1–specific T cells was seen using DCs loaded with arp cells, consistent with the pattern of antigen expression on these cells. Peptide-pulsed DCs also led to the expansion of peptide-specific T cells, while no expansion was seen using tumor cells alone. The antigen-specific T cell responses were lost after depletion with anti-CD8 antibody before the ELISPOT, indicating the CD8+ nature of the IFN-γ–secreting cells (data not shown).
Figure 3.
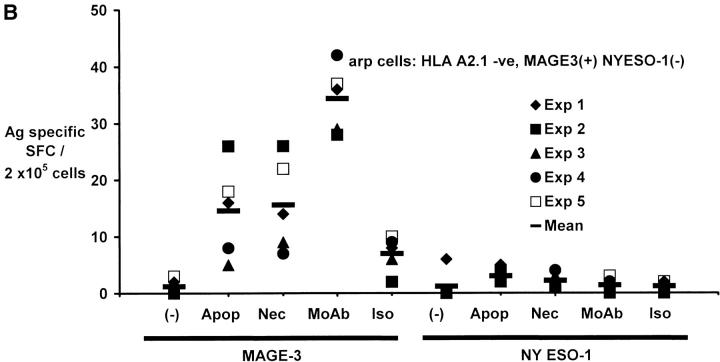
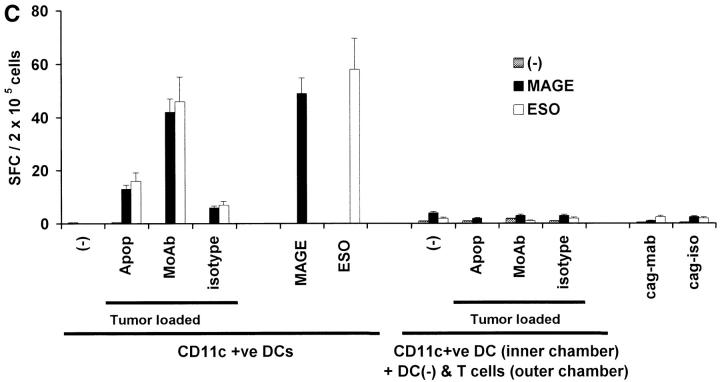
Generation of cancer testis antigen-specific IFN-γ–producing T cells by DCs. (A) Generation of MAGE-3 or NY-Eso-1 specific T cells by peptide-pulsed or tumor cell–loaded DCs. HLA A2.1 −ve myeloma cells (cag, arp) were killed either by γ-irradiation (30 Gy) (apoptosis), freeze thaw cycles (necrosis), or coated with anti–syndecan-1 antibody (mAb)/isotype control, irradiated (3 Gy), and added to HLA A2.1+ ve immature DCs as live (annexin V-negative) cells. DCs were matured using cytokine cocktail. Autologous T cells were stimulated with mature peptide-pulsed or tumor cell–loaded DCs, or tumor cells alone. After two stimulations, the number of HLA A*0201-restricted MAGE3/NY-Eso-1 peptide specific IFN-γ–producing cells were quantified with an ELISPOT assay, using peptide pulsed autologous DCs as APCs. (B) Summary of MAGE3/NY-Eso-1–specific T cells elicited in five experiments, using DCs loaded with dying (apoptotic/necrotic) or antibody-treated (anti–syndecan-1 mAb/isotype) myeloma cells (arp cells, MAGE3+, NY-Eso-1 –ve). (C) Stimulation using CD11c+ DCs from DC-tumor cocultures. CD11c+ DCs were purified using magnetic beads from 4-h cocultures of HLA A2.1 +ve DCs with dying HLA A2.1−ve myeloma (cag) cells (apoptosis/necrosis, as described earlier); or coated with anti–syndecan-1 antibody/isotype. These DCs were then matured using cytokine cocktail and used as APCs to either directly stimulate autologous T cells, or in transwell cultures separated from T cells and unpulsed DCs. After two stimulations, HLA A*0201-restricted MAGE-3 or NY-Eso-1 peptide-specific T cells were quantified by ELISPOT using autologous peptide-pulsed DCs as APCs. Data are representative of two similar experiments.
In experiments with five consecutive donors, the generation of tumor-specific T cells was the greatest using DCs loaded with antibody-coated tumor cells (5/5 donors), higher than the responses observed using DCs loaded with only dying cells (3/5 experiments; Fig. 3 B). Thus antibody coating of tumor cells enhances the cross-presentation of cellular antigens after tumor cell uptake by DCs.
To examine the possibility that contaminating cells in the DC-tumor cocultures led to T cell expansion, we purified CD11c+ DCs from the DC-myeloma mixtures by positive selection, and then used CD11c+ or CD11c−ve fractions as APCs. The CD11c purified DCs expanded antigen specific T cells (Fig. 3 C). No T cell expansion was seen with the CD11c −ve fraction (data not shown). To examine if soluble factors/peptides generated in these cultures could lead to T cell expansion, we cultured these tumor cell–loaded DCs in transwell inserts, physically separated from mixtures of T cells and unpulsed DCs. No expansion of antigen-specific T cells was seen in these cultures. In summary, activation of tumor-specific T cells requires direct contact of T cells with the tumor-loaded DCs (Fig. 3 C).
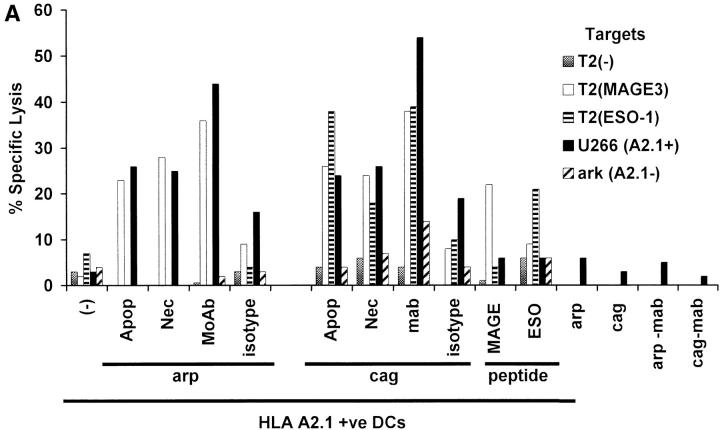
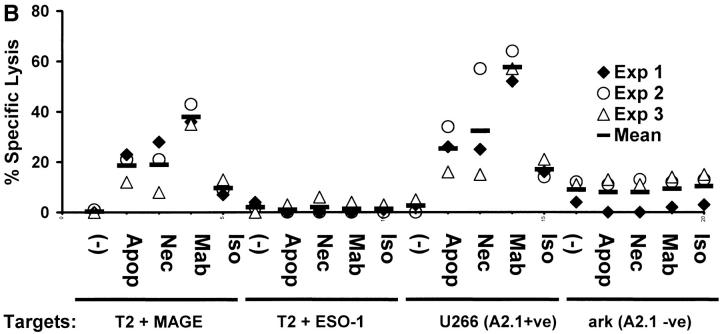
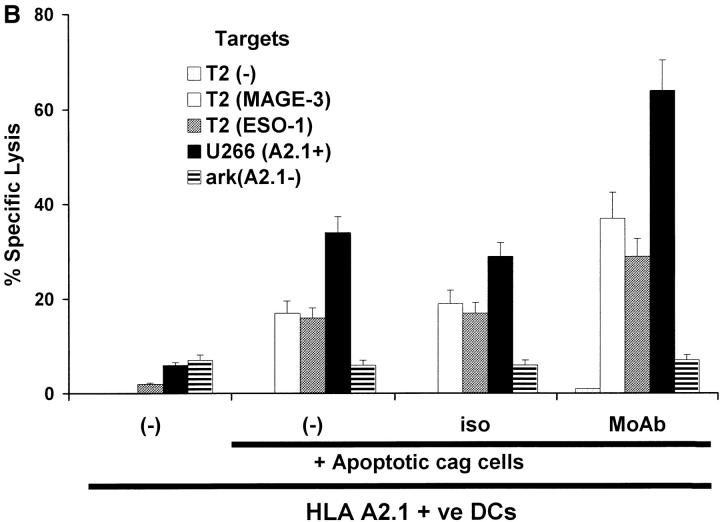
T cells elicited using mAb tumor–loaded DCs efficiently killed not only peptide pulsed targets, but also killed tumor cells HLA matched to DCs (Fig. 4A and B). In contrast, T cells elicited using peptide-pulsed DCs only killed peptide pulsed targets, but not HLA A2.1+ myeloma cells expressing these antigens (Fig. 4 A). The killing of tumor cells was inhibited by preincubation of targets with anti-MHC I, but not anti-MHC II (data not shown), consistent with the response being due to CD8+ T cells and specific for MHC class I binding peptides. Again, the generation of tumor-specific killer T cells was higher and more consistent using DCs loaded with antibody- coated cells (3/3 donors) than using dying cell loaded DCs (2/3 donors; Fig. 4 B). Thus DCs loaded with antibody-coated tumor cells are superior to peptide pulsed DCs or DCs loaded with dying cells to stimulate tumor-specific killer T cells.
Figure 4.
Generation of tumor-specific killer T cells. (A) Generation of killer T cells using peptide-pulsed or tumor cell–loaded DCs. T cells from experiment in Fig. 3 A were tested for killing of T2 cells pulsed with 10 μM HLA A*0201-restricted MAGE3/ NY-Eso-1 peptide or HLA A*0201-positive (U266) or -negative (ark) myeloma cells, at a E/T ratio of 20:1, with a 5 h 51Cr release assay. Lysis of K562 cells (as control) was <10% (data not shown). (B) Summary of experiments on three donors using DCs loaded with dying (apoptotic/necrotic) or antibody-treated (anti–syndecan-1 mAb/isotype) myeloma cells (arp cells: MAGE-3 +ve, NY-Eso-1 –ve).
mAbs also Lead to Enhanced Cross-Presentation of Antigen from Tumor Cells that Have Been Induced to Undergo Apoptosis.
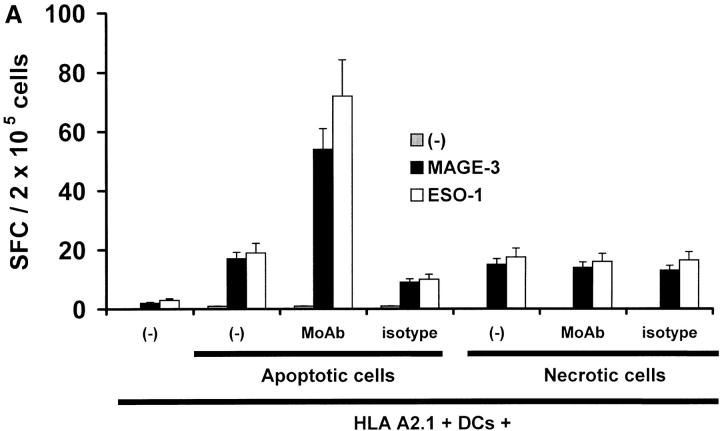
In the experiments described above, we cocultured DCs with live antibody-coated tumor cells after low dose (3 Gy) irradiation. However, several studies have shown that DCs can cross-present antigen from apoptotic or necrotic tumor cells. Therefore, we next examined if antibody coating also enhanced cross-presentation of antigen from dying cells. We first confirmed the expression of syndecan-1 on tumor cells rendered apoptotic after γ-irradiation, by double staining for Annexin V and CD138 (data not shown). Next, we fed DCs with apoptotic or necrotic tumor cells that had been stained with anti–syndecan-1 antibody, isotype control, or left unstained. These DCs were then matured using an inflammatory cytokine cocktail and used as APCs to stimulate autologous T cells. The DCs that were fed with antibody-coated apoptotic tumor cells expanded antigen-specific T cells more efficiently relative to DCs fed with apoptotic cells alone (Fig. 5A). No enhancement of presentation of necrotic cells was observed by antibody-mediated targeting, which is likely due to the lack of effective binding of the antibody to necrotic fragments. T cells expanded using DCs loaded with antibody-coated apoptotic cells were also superior at killing peptide-pulsed or tumor cell targets (Fig. 5 B). Thus antibody coating of tumor cells also leads to enhanced cross-presentation of dying cells by DCs.
Figure 5.
Effects of antitumor antibodies on cross presentation of cellular antigens from dying tumor cells. (A) HLA A2.1−ve myeloma cells were killed either by γ-irradiation (apoptosis) or freeze thaw (necrosis), and either left untreated, or coated with anti–syndecan-1 antibody (mAb) or isotype. HLA A2.1+ DCs were fed with tumor cells, matured with cytokine cocktail and then used to stimulate autologous T cells. After two stimulations, generation of HLA A*0201-restricted MAGE-3 or NY-Eso-1 peptide-specific T cells was quantified in a 16 h ELISPOT using autologous peptide-pulsed mature DCs as APCs. (B) T cells from experiment in A were tested for killing of T2 cells pulsed with HLA A*0201-restricted MAGE3/NY-Eso-1 peptide or HLA A*0201-positive (U266) or -negative (ark) myeloma cells, at a E/T ratio of 20:1, with a standard 5 h 51Cr release assay.
Antibody Enhanced Cross-Presentation Depends on Fc Receptors, but at a Step Beyond Tumor Cell Uptake.
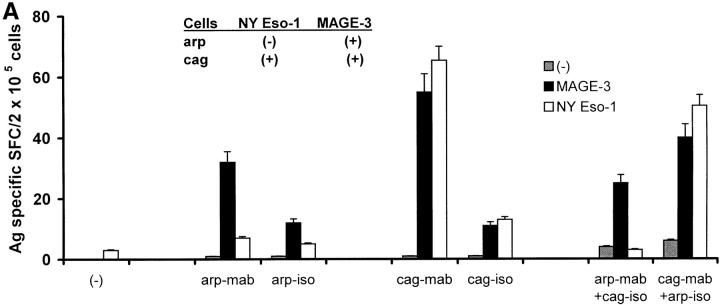
To examine the role of antibody and FcγR in the cross-presentation of antigens by DCs, DCs were first fed with a mixture of antigen (NY-Eso-1)-positive (cag cells) and negative (arp cells) tumors. The tumor cells were internalized similarly with or without antibody coating, implying substantial direct recognition of tumor cells by DCs. However, NY-Eso-1–specific T cells were efficiently expanded in these cultures only if the NY-Eso-1-positive tumor cells (cag cells) were coated with anti–syndecan-1 antibody (Fig. 6A). Therefore, antibody coating directly promotes the cross-presentation of tumor cells.
Figure 6.
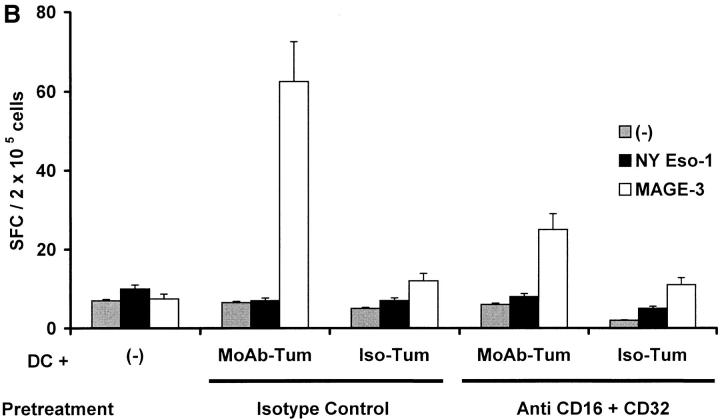
Cross-presentation requirements beyond tumor cell uptake. (A) Antibody enhances cross-presentation only when coating the tumor antigen-expressing myeloma cell. arp (NY-Eso-1−ve) and cag (NY-Eso-1 +ve) cells were treated with anti–syndecan-1 or isotype control antibody and fed to HLA A2.1 +ve DCs, either alone, or together, at DC/tumor ratio of 1:1. Tumor cell–loaded DCs (after maturation with cytokine cocktail), were used to stimulate autologous T cells. After two stimulations, the number of peptide-specific T cells was quantified using peptide-pulsed DCs as APCs in an ELISPOT assay. (B) FcγR blocking antibodies decrease cross-presentation. DCs were pretreated with anti-FcγR blocking antibodies (CD16+CD32) or with isotype controls, before feeding tumor cells treated with anti-syndecan (mAb-Tum) or isotype (Iso-Tum) control antibody, as described in the legend to Fig. 3 A. Antigen-specific T cells were quantified after two stimulations, in an ELISPOT assay using peptide-pulsed DCs as APCs.
Preincubation of DCs with a cocktail of Fc receptor blocking antibodies (anti-CD16 plus CD32) also significantly inhibited the generation of antigen-specific IFN-γ–producing T cells after uptake of tumor cells (Fig. 6 B), although the uptake of tumor cells was not blocked (data not shown). Likewise, the generation of antigen-specific killer T cells was also inhibited in these cultures (data not shown). Culture of DCs and T cells in heat-inactivated serum to inactivate complement, did not inhibit the generation of tumor-specific T cells (data not shown). Thus enhanced cross-presentation of mAb-coated tumor cells is dependent on Fc receptor mediated interactions, that act at a step subsequent to standard opsonization or uptake.
Discussion
Cross-presentation of cellular antigens is of special interest in tumor immunology, as tumors themselves are poor APCs (2). Recent studies have shown that DCs can acquire cellular antigen from dying tumor cells and expand tumor reactive CD8+ killer T cells in vitro (3–8). Our studies now demonstrate that cross-presentation of cellular antigens by human DCs is enhanced by mAb-mediated targeting. DCs, positively selected after just 4-h coculture with live mAb treated tumor cells, are able to present tumor antigen with superior efficiency than DCs loaded with dying cells or defined peptides.
Enhanced cross-presentation of antibody-coated tumor cells is dependent on FcγR-mediated interactions and inhibited by preincubation of DCs with FcγR blocking antibodies. Increased presentation is not simply explained by increased phagocytosis of mAb-treated tumor cells. Thus, the uptake of tumor cells treated with isotype control antibody is comparable to the cells treated with anti-syndecan, yet the former are presented poorly under identical conditions. Indeed, when DCs are fed with a mixture of antigen-positive and -negative cells, efficient generation of antigen-specific T cells occurs only when the cells that express the antigen are coated with the mAb.
Although uptake of antigen as immune complexes or FcγR targeted liposomes delivers a maturation signal to murine DCs, we were unable to show a difference in phenotypic maturation between DCs fed with antibody-treated or untreated tumor cells (18, 19). Taken together, our data suggest that the enhanced cross-presentation of antibody-coated tumor cells by DCs is primarily due to enhanced efficiency of cross-presentation for antigen via FcγR. Thus while DCs may be efficient at phagocytosing myeloma cells, efficient cross-presentation of the phagocytosed antigen by DCs may depend on the specific receptors involved in tumor antigen uptake. This result is reminiscent of the findings of Albert et al., who found that monocytes could phagocytose apoptotic cells as well as immature DCs, and expressed high levels of MHC I, but only DCs could cross-present the cell-associated antigen (20). Enhancement of cross-presentation observed here may be due to novel pathways of antigen processing for FcR-mediated uptake (21) or FcR-mediated signaling (22). Indeed, several studies have shown efficient FcγR-mediated uptake and presentation of immune complexes in murine and human APCs including DCs (18, 23–28).
Whole (either autologous or allogeneic) tumor cells are an attractive source of antigen to load DCs simultaneously with multiple tumor-specific epitopes for immunotherapy. Our findings that DCs loaded with antibody-coated cells are superior to both peptide-pulsed, as well as dying cell-loaded DCs, provides a novel and potentially more effective antigen loading strategy for DC immunotherapy and the generation of tumor-specific killer T cells for adoptive transfer. T cells elicited using peptide-pulsed DCs killed peptide pulsed targets, but not tumor cells. Lack of tumor cell killing by some (specially MAGE-3) peptide-specific T cells may relate to the lack of processing and presentation of this epitope by tumor cells (29). Importantly, T cells elicited using mAb-tumor loaded DCs were superior to peptide-loaded DCs not only for killing of peptide-pulsed targets but also lysed HLA-matched tumor cells. The mechanism for eliciting superior antigen-specific lytic effectors after mAb-tumor loaded DCs is not known, and may be due to the elicitation of tumor-specific CD4+ T cells, the polyepitope nature of the response or the unique efficiency of FcγR-mediated intracellular antigen processing in DCs, as compared with loading with preprocessed peptide (21, 25, 30).
Antitumor effects of mAbs in cancer are thought to be mediated by direct effects on cancer cells, as well as enhancement of innate effectors. Binding of antibodies to tumors cells can sensitize them for complement or antibody-dependent cytotoxicity (10). However the impact of this therapy on T cell immunity is unclear. In some tumor models, both CD8+ T cells and functional FcγRs were shown to be important for mAb-mediated eradication of established tumor (31, 32). Increase in T cell infiltration at the tumor site has also been observed in some human mAb trials, however specificity of the T cell response was not determined (33). Our findings suggest that mAb therapy may also promote the induction of antigen-specific CD8+ T cell responses. These data may also allow the application of mAbs to settings wherein such therapy may not be clinically feasible. For example, selective targeting of anti–syndecan-1 to myeloma cells in vivo is limited by the fact that this molecule is expressed on nonhematopoietic cells. Our data now suggest a strategy for ex vivo targeting of this molecule on myeloma tumor cells to DCs.
Acknowledgments
The authors thank Dr. Ralph Steinman for many helpful suggestions and critical reading of the manuscript, and Drs. Lloyd Old and Jeffrey Ravetch for helpful discussions.
This work was supported in part by an investigator award from the Cancer Research Institute and grants from National Institutes of Health (CA81138) to M.V. Dhodapkar; MO-RR00102 to the Rockefeller GCRC and the Rockefeller University Clinical Scholars program (to K.M. Dhodapkar).
Footnotes
*
Abbreviations used in this paper: C-T, cancer-testis; DC, dendritic cell; FcγR, Fcγ receptor.
References
- 1.Schubert, U., L.C. Anton, J. Gibbs, C.C. Norbury, J.W. Yewdell, and J.R. Bennink. 2000. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 404:770–774. [DOI] [PubMed] [Google Scholar]
- 2.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. [DOI] [PubMed] [Google Scholar]
- 3.Berard, F., P. Blanco, J. Davoust, E.M. Neidhart-Berard, M. Nouri-Shirazi, N. Taquet, D. Rimoldi, J.C. Cerottini, J. Banchereau, and A.K. Palucka. 2000. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 192:1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nouri-Shirazi, M., J. Banchereau, D. Bell, S. Burkeholder, E.T. Kraus, J. Davoust, and K.A. Palucka. 2000. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J. Immunol. 165:3797–3803. [DOI] [PubMed] [Google Scholar]
- 5.Subklewe, M., C. Paludan, M. Tsang, K. Mahnke, R. Steinman, and C. Munz. 2001. Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8+ killer T cells. J. Exp. Med. 193:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenne, L., J.F. Arrighi, H. Jonuleit, J.H. Saurat, and C. Hauser. 2000. Dendritic cells containing apoptotic melanoma cells prime human CD8+ T cells for efficient tumor cell lysis. Cancer Res. 60:4446–4452. [PubMed] [Google Scholar]
- 7.Hoffmann, T.K., N. Meidenbauer, G. Dworacki, H. Kanaya, and T.L. Whiteside. 2000. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 60:3542–3549. [PubMed] [Google Scholar]
- 8.Herr, W., E. Ranieri, W. Olson, H. Zarour, L. Gesualdo, and W.J. Storkus. 2000. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4+ and CD8+ T lymphocyte responses. Blood. 96:1857–1864. [PubMed] [Google Scholar]
- 9.Houghton, A.N., J.S. Gold, and N.E. Blachere. 2001. Immunity against cancer: lessons learned from melanoma. Curr. Opin. Immunol. 13:134–140. [DOI] [PubMed] [Google Scholar]
- 10.Houghton, A.N., and D.A. Scheinberg. 2000. Monoclonal antibody therapies-a ‘constant’ threat to cancer. Nat. Med. 6:373–374. [DOI] [PubMed] [Google Scholar]
- 11.Clynes, R., Y. Takechi, Y. Moroi, A. Houghton, and J.V. Ravetch. 1998. Fc receptors are required in passive and active immunity to melanoma. Proc. Natl. Acad. Sci. USA. 95:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clynes, R.A., T.L. Towers, L.G. Presta, and J.V. Ravetch. 2000. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6:443–446. [DOI] [PubMed] [Google Scholar]
- 13.Scanlan, M.J., N.K. Altorki, A.O. Gure, B. Williamson, A. Jungbluth, Y.T. Chen, and L.J. Old. 2000. Expression of cancer-testis antigens in lung cancer: definition of bromodomain testis-specific gene (BRDT) as a new CT gene, CT9. Cancer Lett. 150:155–164. [DOI] [PubMed] [Google Scholar]
- 14.Wijdenes, J., W.C. Vooijs, C. Clement, J. Post, F. Morard, N. Vita, P. Laurent, R.X. Sun, B. Klein, and J.M. Dore. 1996. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br. J. Haematol. 94:318–323. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar, M.V., R.M. Steinman, M. Sapp, H. Desai, C. Fossella, J. Krasovsky, S.M. Donahoe, P.R. Dunbar, V. Cerundolo, D.F. Nixon, and N. Bhardwaj. 1999. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Invest. 104:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonuleit, H., U. Kuhn, G. Muller, K. Steinbrink, L. Paragnik, E. Schmitt, J. Knop, and A.H. Enk. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142. [DOI] [PubMed] [Google Scholar]
- 17.Feuerstein, B., T.G. Berger, C. Maczek, C. Roder, D. Schreiner, U. Hirsch, I. Haendle, W. Leisgang, A. Glaser, O. Kuss, et al. 2000. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods. 245:15–29. [DOI] [PubMed] [Google Scholar]
- 18.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machy, P., K. Serre, and L. Leserman. 2000. Class I-restricted presentation of exogenous antigen acquired by Fcγ receptor-mediated endocytosis is regulated in dendritic cells. Eur. J. Immunol. 30:848–857. [DOI] [PubMed] [Google Scholar]
- 20.Albert, M.L., S.F. Pearce, L.M. Francisco, B. Sauter, P. Roy, R.L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez, A., A. Regnault, M. Kleijmeer, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell. Biol. 1:362–368. [DOI] [PubMed] [Google Scholar]
- 22.Amigorena, S., and C. Bonnerot. 1999. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin. Immunol. 11:385–390. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fanger, N.A., D. Voigtlaender, C. Liu, S. Swink, K. Wardwell, J. Fisher, R.F. Graziano, L.C. Pfefferkorn, and P.M. Guyre. 1997. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fcγ RI (CD64) expressed on human blood dendritic cells. J. Immunol. 158:3090–3098. [PubMed] [Google Scholar]
- 25.Hamano, Y., H. Arase, H. Saisho, and T. Saito. 2000. Immune complex and Fc receptor-mediated augmentation of antigen presentation for in vivo Th cell responses. J. Immunol. 164:6113–6119. [DOI] [PubMed] [Google Scholar]
- 26.Ukkonen, P., V. Lewis, M. Marsh, A. Helenius, and I. Mellman. 1986. Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J. Exp. Med. 163:952–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manca, F., D. Fenoglio, G. Li Pira, A. Kunkl, and F. Celada. 1991. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J. Exp. Med. 173:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace, P.K., K.Y. Tsang, J. Goldstein, P. Correale, T.M. Jarry, J. Schlom, P.M. Guyre, M.S. Ernstoff, and M.W. Fanger. 2001. Exogenous antigen targeted to FcγRI on myeloid cells is presented in association with MHC class I. J. Immunol. Methods. 248:183–194. [DOI] [PubMed] [Google Scholar]
- 29.Valmori, D., U. Gileadi, C. Servis, P.R. Dunbar, J.C. Cerottini, P. Romero, V. Cerundolo, and F. Levy. 1999. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J. Exp. Med. 189:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amigorena, S., D. Lankar, V. Briken, L. Gapin, M. Viguier, and C. Bonnerot. 1998. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J. Exp. Med. 187:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyall, R., L.V. Vasovic, R.A. Clynes, and J. Nikolic-Zugic. 1999. Cellular requirements for the monoclonal antibody-mediated eradication of an established solid tumor. Eur. J. Immunol. 29:30–37. [DOI] [PubMed] [Google Scholar]
- 32.Vasovic, L.V., R. Dyall, R.A. Clynes, J.V. Ravetch, and J. Nikolic-Zugic. 1997. Synergy between an antibody and CD8+ cells in eliminating an established tumor. Eur. J. Immunol. 27:374–382. [DOI] [PubMed] [Google Scholar]
- 33.Vadhan-Raj, S., C. Cordon-Cardo, E. Carswell, D. Mintzer, L. Dantis, C. Duteau, M.A. Templeton, H.F. Oettgen, L.J. Old, and A.N. Houghton. 1988. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: induction of inflammatory responses at tumor sites. J. Clin. Oncol. 6:1636–1648. [DOI] [PubMed] [Google Scholar]