Regulatory CD4+CD25+ T Cells Restrict Memory CD8+ T Cell Responses (original) (raw)
Abstract
CD4+ T cell help is important for the generation of CD8+ T cell responses. We used depleting anti-CD4 mAb to analyze the role of CD4+ T cells for memory CD8+ T cell responses after secondary infection of mice with the intracellular bacterium Listeria monocytogenes, or after boost immunization by specific peptide or DNA vaccination. Surprisingly, anti-CD4 mAb treatment during secondary CD8+ T cell responses markedly enlarged the population size of antigen-specific CD8+ T cells. After boost immunization with peptide or DNA, this effect was particularly profound, and antigen-specific CD8+ T cell populations were enlarged at least 10-fold. In terms of cytokine production and cytotoxicity, the enlarged CD8+ T cell population consisted of functional effector T cells. In depletion and transfer experiments, the suppressive function could be ascribed to CD4+CD25+ T cells. Our results demonstrate that CD4+ T cells control the CD8+ T cell response in two directions. Initially, they promote the generation of a CD8+ T cell responses and later they restrain the strength of the CD8+ T cell memory response. Down-modulation of CD8+ T cell responses during infection could prevent harmful consequences after eradication of the pathogen.
Keywords: T lymphocytes, memory, bacterial infection, regulation, vaccination
Introduction
Infection of mice with Listeria monocytogenes causes a potent CD8+ T cell response as an important component of protective immunity against this pathogen (1–3). In BALB/c mice, a large fraction of CD8+ T cells is directed against few dominant listerial proteins. The most dominant CD8+ T cell epitope is listeriolysin O (LLO)* 91–99, a peptide derived from the secreted protein listeriolysin (4, 5). At the peak of the primary response, 3–4% of the CD8+ T cell population are specific for LLO91–99, and during the secondary response, LLO91–99–specific T cells reach levels as high as 15% of all CD8+ T cells (4). Listeriolysin has also been used to vaccinate mice against L. monocytogenes, and we have recently demonstrated that repeated administration of DNA containing hly, the gene encoding listeriolysin, induces a LLO91–99–specific CD8+ T cell response and protects mice against subsequent L. monocytogenes infection (6).
Under various circumstances, priming of naive CD8+ T cells requires signals from CD4+ TH cells. However, CD4+ T cell help is not essential and can be substituted by other stimuli (7). A subpopulation of CD4+ T cells can also negatively regulate immune responses (8, 9). These regulatory or suppressor T cells are CD4+D25+ and are enriched in the CD45RBlow T cell population. The mode of action of these T cells is still unclear and may include direct mechanisms via cell–cell contact or the production of inhibitory cytokines such as IL-10 or TGF-β (8, 9). Although regulatory T cells have been demonstrated in several autoimmune models, there is only limited evidence for a function of these cells during infection or vaccination. Particularly, their role in the regulation of CD8+ T cell responses is largely unknown (8, 9).
Here we analyzed the role of CD4+ T cells in the formation of memory CD8+ T cell responses after secondary L. monocytogenes infection, or after boost immunization with the peptide LLO91–99 or a DNA vaccine containing the gene for listeriolysin. Depletion of CD4+ T cells significantly enhanced memory CD8+ T cell responses, particularly after peptide and DNA immunization. Further depletion and transfer experiments demonstrated that the suppressive activity was enriched in the CD4+CD25+ T cell population. Thus, CD4+ T cells regulate a CD8+ T cell response in both directions. During primary responses, CD4+ T cells promote the generation and accumulation of specific CD8+ T cells, during memory responses, CD4+CD25+ T cells restrict the strength of the response.
Materials and Methods
Bacterial Infection of Mice.
BALB/c mice and SCID mice were bred in our facility at the Federal Institute for Health Protection of Consumers and Veterinary Medicine in Berlin, and experiments were conducted according to the German animal protection law. Mice were infected with L. monocytogenes strain EGD. For primary infection, mice received 2 × 103 bacteria intravenously. After 8–12 wk, mice were secondary infected with 105 bacteria intravenously (10).
Antibodies.
Rat Ig, anti-CD16/CD32 mAb (2.4G2), anti-CD8α mAb (YTS169), anti-CD4 mAbs (YTS191.1 and GK1.5), anti-CD25 mAb (PC61), anti-CD62L mAb (Mel-14), anti-CD152/CTL-associated antigen (CTLA-4) mAb (9H10), anti–IFN-γ mAb (clone: R4–6A2, IgG1), and anti–TGF-β mAb (2G7) were purified from rat serum or hybridoma supernatants with protein G sepharose. Antibodies were Cy5- or FITC-conjugated according to standard protocols. FITC-conjugated anti-CD25 mAb (7D4), PE-conjugated anti–TNF-α mAb (MP6-XT22, IgG1), and FITC- and PE-conjugated rat-IgG1 isotype control mAb (R3–34) were purchased from BD Biosciences.
DNA and Peptide Immunization.
The plasmid pChly was constructed by cloning the hly (listeriolysin) gene into the eukaryotic expression plasmid pCI (6). The plasmid pCMV-GM-CSF contains the gene encoding GM-CSF under the control of a CMV promoter. DNA of pChly (1.0 μg) was coprecipitated with 0.8 μg of pCMV-GM-CSF on 1.0-μm gold particles (0.5 mg). Phosphothiate-modified oligodeoxynucleotides (ODN) containing a CpG motif (ODN1760; reference 11) were synthesized by Interactiva Biotechnology. For gene gun immunization, two nonoverlapping shots per mouse were performed into freshly shaven abdominal skin using 0.5 mg DNA-coated gold particles per shot. Subsequently, 10 μg of the CpG-containing ODN were injected intradermally at the site of particle bombardment (6).
The peptide LL091–99 (GYKDGNEYI; Jerini Bio Tools) was dissolved at 2 mg/ml in PBS. The solution was emulsified with an equal volume of Freund's incomplete adjuvant. Mice were injected subcutaneously with 100 μl of the emulsion corresponding to 100 μg of peptide.
In Vivo mAb Applications.
CD4+ T cells were depleted by intraperitoneal injection of 300 μg of anti-CD4 mAb (YTS191.1) at intervals of 5 d starting 3 d before infection or immunization. Efficacy of depletion was >95%. CD25+ cells were depleted by intraperitoneal injection of 250 μg of anti-CD25 mAb (PC61) at days −5, −1, 2, and 4 of immunization (efficacy: >75%). CTLA-4 and TGF-β were blocked by daily intraperitoneal injection of 250 μg of anti-CD152 mAb or 500 μg of anti–TGF-β mAb, respectively, starting at the day of immunization.
Purification of Cells and Reconstitution of SCID Mice.
Splenocytes from naive mice or mice infected 3 mo earlier with L. monocytogenes were incubated with biotinylated anti-CD25 mAb and magnetic anti-biotin micro beads (Miltenyi Biotec), and CD25+ T cells were purified with a magnetic column (autoMACS; Miltenyi Biotec). T cells bound and recovered from the column were >75% CD25+ and >90% CD4+. CD25-depleted cells were incubated with FITC-conjugated anti-CD4 mAb (GK1.5) and subsequently with anti–FITC-mAb–coated magnetic micro beads (Miltenyi Biotec). CD4+ T cells were isolated using the autoMACS and were >98% CD4+ and <0.5% CD25+.
BALB/c mice were immunized with pChly DNA, and after 7 wk, mice were CD4 T cell depleted by intraperitoneal administration of 300 μg anti-CD4 mAb (depletion efficiency: >95%). 3 d later, mice were killed, and splenocytes were adoptively transferred into SCID mice (40 × 106/mouse, intravenously). SCID mice received in addition purified CD4+CD25− T cells (12 × 106) or CD4+CD25+ T cells (4 × 106) from naive or listeria-infected mice. Immediately after cell transfer, mice were immunized with pChly DNA. Mice were analyzed 7 d after cell transfer and immunization.
Cytotoxicity Assay.
Spleen cells were incubated with 5,000 51Cr-labeled P815 target cells at the effector/target ratios indicated. Peptides were added at 10−6 M. After 4 h, supernatants were counted with a γ-counter. Each value was determined as triplicate (10). Cytotoxicity is given as percent specific lysis which was calculated with the formula: percent specific lysis = 100 × (experimental 51Cr-release − spontaneous 51Cr-release)/(detergent induced 51Cr-release − spontaneous 51Cr-release)
Tetramer Staining and Flow Cytometric Determination of Cytokine Expression.
Intracellular cytokine staining after short term in vitro restimulation was performed as described (10). Briefly, spleen cells were stimulated for 5 h with 10−6 M of the peptide LLO91–99. During the final 4 h of culture, 10 μg/ml Brefeldin A (Sigma-Aldrich) were added. Cultured cells were extracellularly stained with Cy5-conjugated anti-CD8α mAb, and intracellularly stained with FITC-conjugated anti–IFN-γ mAb and PE-conjugated anti-TNF-α mAb or FITC- and PE-conjugated isotype control mAb. Cells were analyzed using a FACSCalibur™ and the CELLQuest™ software (Becton Dickinson).
Generation of LLO91–99/H-2Kd-tetramers and analysis of cells with tetramers has been described previously (10).
Statistical Analysis.
Statistical significance of results was determined using the unpaired Student's t test. *P < 0.05; **P < 0.01.
Results
CD8+ T Cell Responses during Primary and Secondary Infection with L. monocytogenes in CD4+ T Cell–depleted Mice.
Infection of mice with L. monocytogenes causes a strong CD8+ T cell response that is crucial for protective immunity. There is also a strong induction of a CD4+ T cell response during L. monocytogenes infection, but the relevance of these cells during infection is less clear (2, 3). An important function of CD4+ T cells is to provide help for the generation of CD8+ T cell responses. To analyze this function in more detail, BALB/c mice were treated with a depleting anti-CD4 mAb and infected with L. monocytogenes. The CD8+ T cell response was analyzed with MHC class I tetramers containing the immunodominant CD8+ T cell epitope LLO91–99 (4, 10). During primary infection, depletion of CD4+ T cells caused an ∼50% reduction of the frequency of LLO91–99–specific CD8+ T cells in spleens of infected mice (Fig. 1 A). Surprisingly, depletion of CD4+ T cells during secondary infection did not diminish, but increased frequencies and numbers of listeria-specific CD8+ T cells (Fig. 1 B, and unpublished data). During both primary and secondary infection, neither the bacterial titers in spleen and liver nor the course of bacterial clearance were significantly altered by anti-CD4 mAb treatment (unpublished data).
Figure 1.
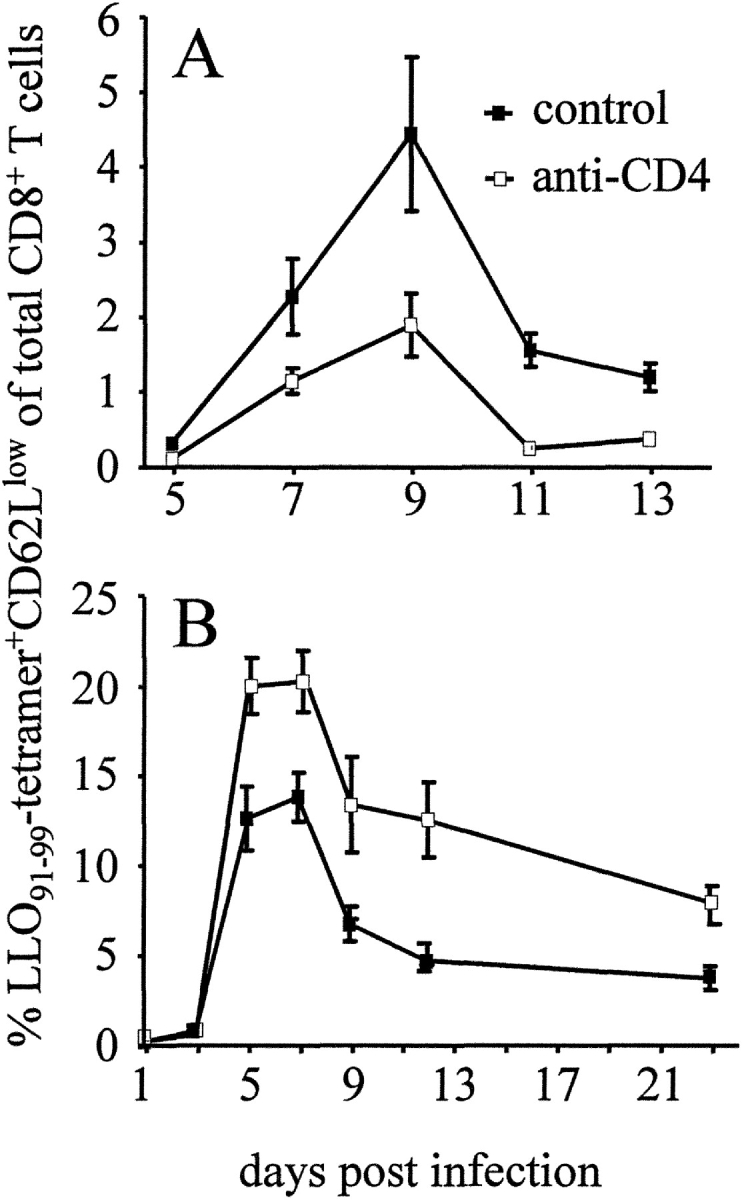
LLO91–99–specific CD8+ T cell response during primary and secondary infection with L. monocytogenes (A) Primary infection: mice infected with L. monocytogenes were left untreated (control) or received anti-CD4 mAb (anti-CD4). (B) Secondary infection: mice were infected and after 60 d challenged with L. monocytogenes. During challenge infection, mice were left untreated or received anti-CD4 mAb. At the indicated days, spleen cells were stained with Cy5-conjugated anti-CD8α mAb, FITC-conjugated anti-CD62L mAb, and PE-labeled LLO91–99-tetramers, and analyzed by flow cytometry after the addition of propidium iodide. Figures show percent values of live CD62Llowtetramer+ cells of total CD8+ cells. Data represent mean ± SD of three mice per group and time point. Experiments in A and B are representative of two or three experiments, respectively.
CD4+ T Cell Depletion Enhances Memory CD8+ T Cell Responses after Peptide or DNA Immunization.
Protection against secondary listeriosis is predominantly mediated by CD8+ T cells, but CD4+ T cells participate as well in the response against L. monocytogenes (3). Thus, the lack of listeria-specific CD4+ T cells could result in higher bacterial titers and delayed bacterial clearance. Consequently, the enhanced CD8+ T cell response could be due to an increase in antigen load. Although we did not observe a significant difference in bacterial titers during secondary infection between control mice and anti-CD4 mAb-treated mice, we cannot formally exclude that the enhanced CD8+ T cell response reflects compensatory mechanisms. To circumvent this problem, mice were infected with L. monocytogenes, and secondary CD8+ T cell responses were analyzed following specific DNA or peptide immunization. For DNA immunization, the plasmid pChly containing the gene for listeriolysin (hly) under the control of a eukaryotic promoter was applied with a gene gun (6). The peptide LLO91–99 was given subcutaneously in incomplete Freund's adjuvant (Fig. 2) . Prior to treatment, LLO91–99–specific CD8+ T cells comprised 0.1–0.2% of CD8+ T cells. Both DNA and peptide immunization increased frequencies of LLO91–99–specific CD8+ T cells. Depletion of CD4+ T cells drastically enlarged the frequencies and numbers of these T cells. In all experiments, CD4+ T cell depletion caused at least a 10- to 20-fold enhancement of the LLO91–99–specific CD8+ T cell response (Fig. 2, and unpublished data). DNA immunization was also used to analyze the effect of CD4+ T cell depletion in a setting independent from infection. Mice were prime/boost immunized with pChly DNA. 50 d after priming, frequencies of LLO91–99 specific-CD8+ T cells were close to the detection levels of the tetramer assay (unpublished data and Table I). Boost with the same DNA construct resulted in frequencies of ∼1.5% at day 7 after immunization. Depletion of CD4+ T cells during the boost markedly enhanced frequencies (up to 40%) and numbers of LLO91–99–specific CD8+ T cells (Figs. 3 A and 4 A).
Figure 2.
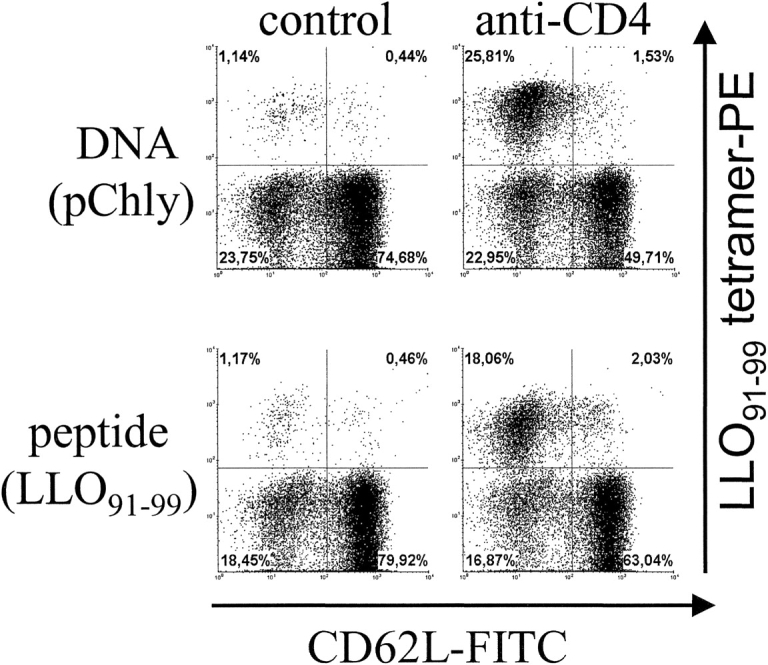
Response against peptide and DNA boost-immunization of _L. monocytogenes_–primed mice after CD4+ T cell depletion. Mice were infected with L. monocytogenes. After 100 d, mice were immunized either with the peptide LLO91–99 in incomplete Freund's adjuvant subcutaneously or with pChly DNA using the gene gun. Groups of mice were left untreated (control) or were injected with anti-CD4 mAb during the boost immunization. On day 7, spleen cells were stained and analyzed by flow cytometry. Dot plots depict CD62L and LLO91–99-tetramer staining of viable CD8α gated cells. The experiment shown is representative of two independent experiments with three individually analyzed mice per group.
Table I.
Anti-CD4 mAb Treatment Does Not Induce LLO91–99–specific CD8+ Memory T Cell Proliferation in the Absence of Antigen Stimulation
| PercentLLO91–99-tetramer+ of CD8+ cells | LLO91–99-tetramer+CD8+ cells/spleen | |
|---|---|---|
| Naive | <0.02 | <1,000 |
| + anti-CD4 mAb | <0.02 | <1,000 |
| L. monocytogenes | 0.06 ± 0.01 | 1,700 ± 100 |
| + anti-CD4 mAb | 0.07 ± 0.04 | 2,700 ± 1,600 |
| Gene gun | 0.09 ± 0.04 | 3,900 ± 2,800 |
| + anti-CD4 mAb | 0.10 ± 0.03 | 3,400 ± 900 |
Figure 3.
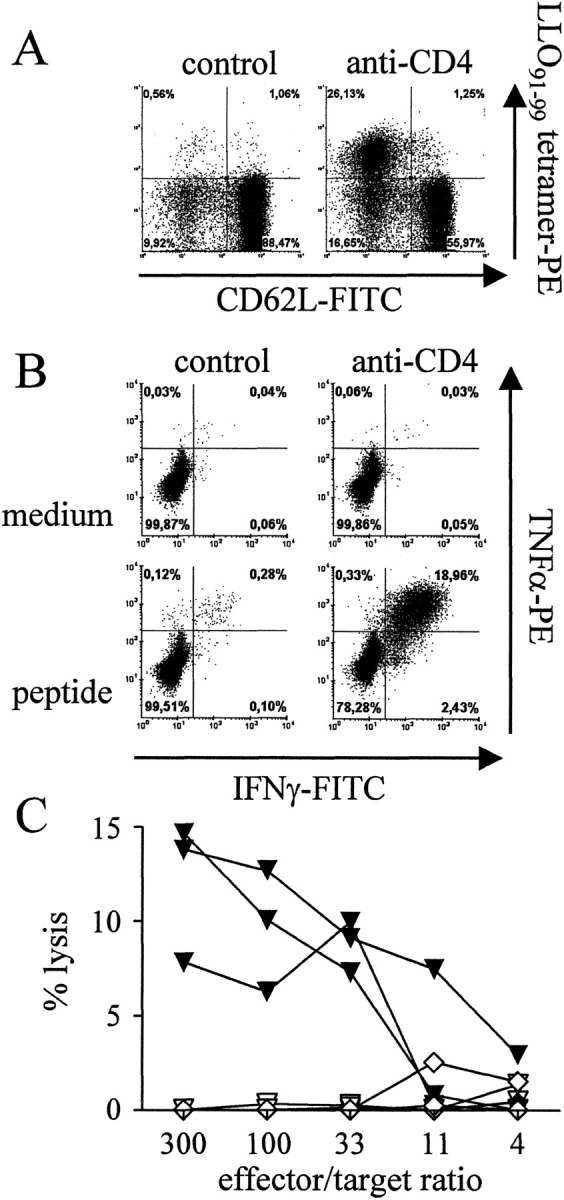
Effector functions of LLO91–99–specific CD8+ T cells after prime/boost DNA immunization. Mice were immunized with pChly using the gene gun. After 50 d, a boost immunization with the same DNA was performed. Mice were left untreated (control) or were treated with anti-CD4 mAb during the boost immunization. At day 7, mice were killed. (A) Spleen cells were stained and analyzed by flow cytometry. Dot plots depict CD62L and LLO91–99-tetramer staining of viable CD8-gated T cells and figures represent percent values calculated for CD8+ T cells only. (B) Spleen cells were cultured for 5 h with or without the peptide LLO91–99 and stained extracellularly for CD8 and intracellularly for IFN-γ and TNF-α or with isotype control mAbs. Dot blots show CD8-gated cells, and figures give percent values calculated for CD8+ T cells only. Values for isotype controls were below 0.05% (data not depicted). Dot blots in A and B show corresponding results from the same mice. (C) Spleen cells from untreated (diamonds) or anti-CD4 mAb-treated mice (triangles) were incubated for 4 h with target cells with (filled symbols) or without LLO91–99 (open symbols). After 4 h, lysis of target cells was determined. C shows results from three individually analyzed mice per group. Experiments in A–C are representative for at least two independent experiments with three individually analyzed mice per experimental group in each experiment.
In contrast to the strong effect of anti-CD4 mAb treatment on the memory response after secondary DNA or peptide immunization, this treatment had only limited consequences after primary immunization. With both immunization protocols, we obtained only very low frequencies of LLO91–99–specific CD8+ T cells after a single immunization. 9 d after a primary immunization, frequencies were close to the detection limit of our assays (0.05–0.10% LLO91–99-tetramer+CD62Llow cells or IFN-γ–producing cells of CD8+ spleen cells). Anti-CD4 mAb treatment did not significantly alter these responses (unpublished data).
A lymphopenic environment can induce homeostatic T cell proliferation in the absence of antigen stimulation (12). To analyze whether the expansion of the antigen-specific CD8+ T cell population was due to a homeostatic proliferation after CD4+ T cell depletion, mice were either infected or DNA immunized, and after 2 mo treated with anti-CD4 mAb without any further challenge. 10 d after anti-CD4 mAb injection, mice were analyzed (Table I). At this time point, frequencies and numbers of memory CD8+ T cells were close to the detection level of our assays. 2 mo after both L. monocytogenes infection and DNA immunization, anti-CD4 mAb treatment did not induce a significant expansion of the LLO91–99–specific CD8+ T cell population. This result indicates that in our experimental model the expansion of the specific memory CD8+ T cell population after CD4+ T cell depletion depended on antigen challenge and was not due to homeostatic proliferation. In accordance with this result is the observation that, in contrast to the strong enhancement of the memory CD8+ T cell response following CD4+ T cell depletion, the total CD8+ T cell population is not enlarged significantly (unpublished data).
CD8+ T Cells Activated in the Absence of CD4+ T Cells Are Functional Effector Cells.
To determine whether the enlarged LLO91–99–specific CD8+ T cell population after CD4+ T cell depletion consisted of functional CD8+ effector T cells, mice were primed and boosted with a DNA vaccine. During boost immunization, mice were treated with anti-CD4 mAb. After 7 d, spleen cells were either stained with tetramers (Fig. 3 A) or restimulated in vitro for 5 h with the peptide LLO91–99, and analyzed for IFN-γ and TNF-α production (Fig. 3 B). Frequencies of LLO91–99-tetramer+ T cells and of cytokine producing CD8+ T cells were similar after CD4+ T cell depletion. In addition, cells were isolated and directly incubated for 4 h with LLO91–99–loaded target cells in a 51Cr-release assay (Fig. 3 C). Antigen-specific cytotoxicity was only detected in cells from anti-CD4 mAb-treated mice. In contrast to the tetramer and intracellular cytokine staining, the cytotoxicity assay does not provide information on activities of individual cells. Therefore, we cannot make any statement on cytotoxic functions of individual cells, yet our experiments demonstrate that after in vivo antigen restimulation, anti-CD4 mAb treated mice contain a population of cells with specific cytotoxic capabilities. Hence, LLO91–99–specific CD8+ T cells generated in absence of CD4+ T cells were functional effector cells in terms of IFN-γ and TNF-α secretion, and at least a subpopulation of these cells expresses specific cytotoxicity.
Inhibition of Memory CD8+ T Cell Responses by CD25+ Cells.
Increasing evidence suggests that a subpopulation of CD4+ T cells can suppress immune responses (8, 9). Although a distinct surface phenotype of regulatory T cells has not been defined as yet, these cells are enriched in the CD4+CD25+ T cell population (8, 9). Regulatory T cells are further associated with the expression of CTLA-4/CD152 and TGF-β (13–16). Mice were primed and boosted by DNA immunization, and the role of different cell populations and molecules during the secondary CD8+ T cell response was analyzed by administration of antibodies that either deplete positive cell populations or block the function of the recognized proteins (Fig. 4) . Depletion of CD25+ T cells markedly enhanced the numbers of LLO91–99-tetramer–reactive CD8+ T cells and of CD8+ T cells responding to LLO91–99 stimulation with IFN-γ production, although this increase was less pronounced as compared with that after anti-CD4 mAb treatment. In contrast, mAbs against CD152 or TGF-β did not significantly alter the secondary CD8+ T cell response (Fig. 4).
Figure 4.
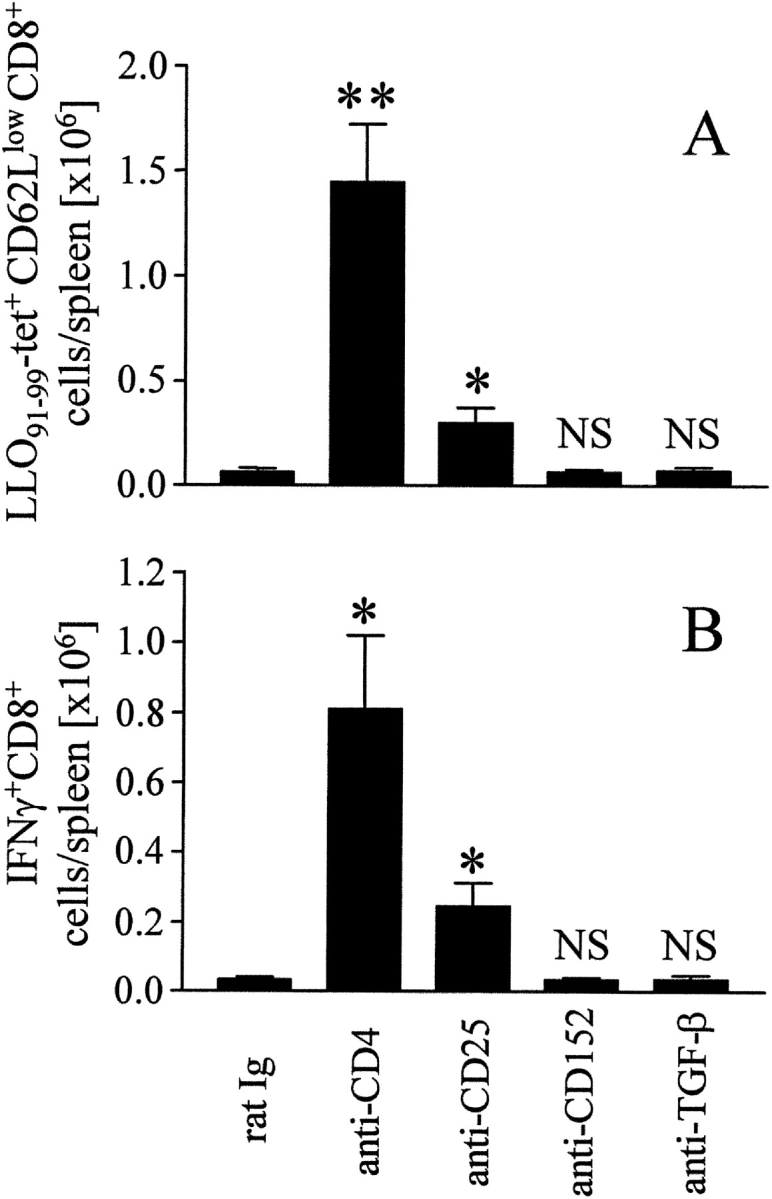
Inhibition of memory CD8+ T cell responses by CD4+ and CD25+ cells BALB/c mice were immunized with pChly using the gene gun, and after 35 d, a boost immunization with the same DNA was performed. During boost immunization, mice were treated with purified rat Ig or the mAb indicated. On day 7, spleen cells were analyzed with LLO91–99-tetramers (A) and for IFN-γ production after incubation with LLO91–99 (B), as described in Fig. 3. Without peptide stimulation, we observed <4,000 IFN-γ+CD8+ T cells/spleen in all samples analyzed (data not depicted). The experiment shown is representative of three similar experiments and represents mean ± SD of three individually analyzed mice per group. Differences to rat Ig treatment: *P < 0.05; **P < 0.01; NS, P > 0.05.
Inhibition of Memory CD8+ T Cell Responses by CD4+ CD25+ Cells in a Transfer Model.
So far, our assumption that CD4+CD25+ T cells suppress CD8+ memory T cell responses is based on antibody depletion experiments. However, antibody treatment, particularly anti-CD4 mAb treatment, could have effects beyond CD4+ T cell depletion, which we cannot fully exclude. Therefore, we assessed the role of CD4+CD25+ T cells for memory CD8+ T cell responses in SCID mice that were immunized after reconstitution with defined T cell populations. SCID mice received CD4-depleted cells from mice that had been DNA immunized 5 wk earlier. Groups of these mice received purified CD25+ T cells (>90% CD4+) or purified CD4+CD25− T cells in addition. Purified T cells were derived either from naive mice, or from mice that had been infected with L. monocytogenes 3 mo before transfer. After reconstitution, SCID mice were DNA immunized. 7 d later, LLO91–99-tetramer–reactive T cells and IFN-γ production after LLO91–99 stimulation were determined (Fig. 5) . In SCID mice reconstituted with CD4-depleted cells from previously immunized mice, DNA immunization caused strong proliferation of LLO91–99–specific CD8+ T cells. Addition of CD4+CD25− T cells from either naive or L. monocytogenes infected mice did not significantly alter expansion of these CD8+ T cell populations. In contrast, cotransfer of CD4+CD25+ T cells markedly impaired the LLO91–99–specific CD8+ T cell response, and this inhibition was observed with CD4+CD25+ T cells derived from both naive and _L. monocytogenes_–infected mice.
Figure 5.
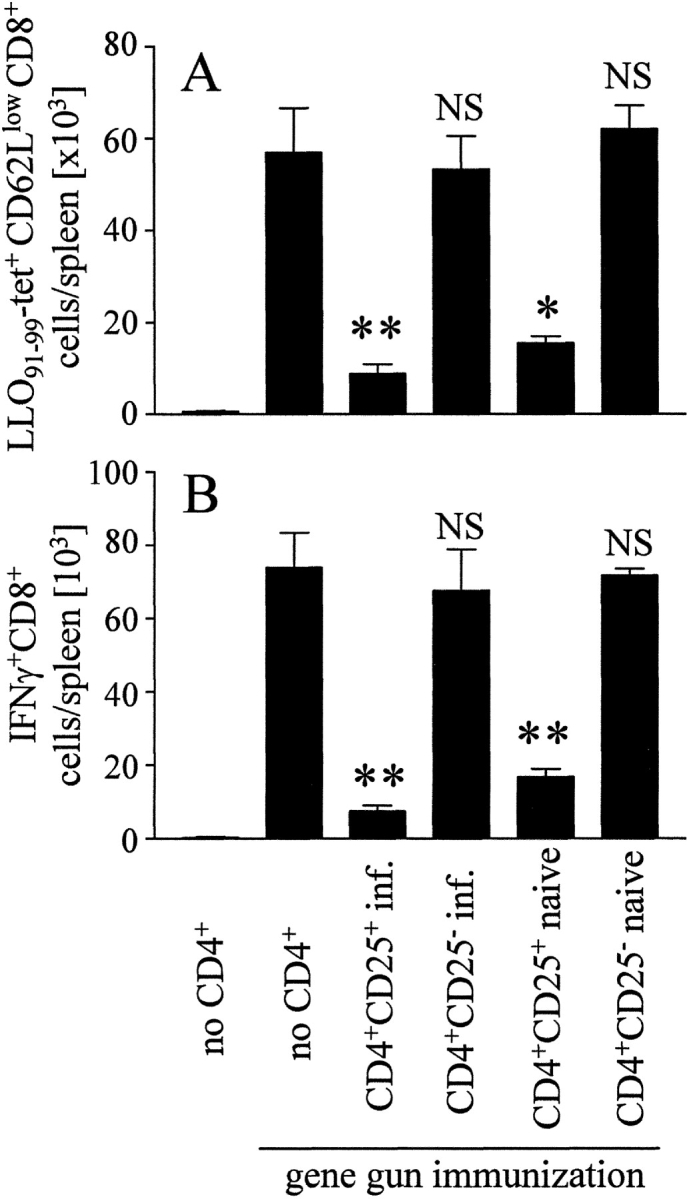
Inhibition of memory CD8+ T cell responses by CD4+CD25+ cells in a transfer model SCID mice received CD4-depleted cells from mice previously immunized by DNA vaccination. Groups of mice received in addition purified CD4+CD25− and CD4+CD25+ cells from either naive or _L. monocytogenes_–infected mice. Immediately after cell transfer, mice were DNA immunized with pChly. On day 7, spleen cells were analyzed with LLO91–99-tetramers (A) and for IFN-γ production after incubation with LLO91–99 (B), as described in Fig. 3. Without peptide stimulation we observed <1,500 IFN-γ+CD8+ T cells/spleen in all samples analyzed (data not depicted). The experiment shown represents mean ± SD of three individually analyzed mice per group and is representative for three independent experiments. Differences to gene gun treated mice that received only CD4+ T cell–depleted cells: *P < 0.05; **P < 0.01; NS, P > 0.05.
Discussion
CD4+ T cells supported but were not essential for the generation of specific CD8+ T cells in experimental listeriosis of mice. TH cell–independent generation of CD8+ T cell responses has been described before, and it has been suggested that signals associated with infection or inflammation can bypass the need for CD4+ T cell help (7, 17). L. monocytogenes infection causes a strong inflammation in spleen and liver. It is, therefore, not unexpected that the absence of CD4+ TH cells causes only a minor impairment of the CD8+ T cell response in this model.
Depletion of CD4+ T cells during the secondary anti-listeria response did not impair the specific CD8+ T cell response, which is consistent with less restricted activation requirements of memory T cells. Rather, depletion of CD4+ T cells enlarged the specific CD8+ T cell population indicating that CD4+ T cells inhibited the secondary CD8+ T cell response. As it has been demonstrated that CD4+ T cells contribute to protection against L. monocytogenes (3), we cannot formally exclude that the enhanced CD8+ T cell response in anti-CD4 mAb-treated mice was caused by compensatory mechanisms. However, the enhanced response in the absence of CD4+ T cells was even more pronounced when memory CD8+ T cell responses were induced by specific peptide or DNA immunization. These conditions virtually exclude differences in antigen load and persistence, or strength of inflammation, and strongly imply suppression of memory CD8+ T cell responses by CD4+ T cells.
In various autoimmune models, T cells with suppressive functions are highly enriched in the CD4+CD25+ T cell population (8, 9). Our depletion and transfer experiments are consistent with these observations. Depletion of CD25+ cells enhanced the secondary CD8+ T cell response, and reciprocally, adoptive transfer of CD25+ T cells inhibited the response. Compared with the anti-CD4 mAb treatment, the anti-CD25 mAb treatment was less efficient, whereas adoptive transfer of suppression was only achieved with CD25+ T cells. Incomplete depletion of CD25+ T cells in anti-CD25 mAb treated mice could explain this discrepancy (we achieved >75% depletion of CD25+ cells compared with >95% depletion of CD4+ cells). As CD8+ T cells up-regulate CD25 expression upon activation, it is also possible that the anti-CD25 mAb-treatment directly affected the LLO91–99–specific CD8+ T cell population. However, an enlarged memory CD8+ T cell response was also observed when the anti-CD25 mAb was applied before the boost immunization (unpublished data). Finally, although there is a concentration of the suppressive function in the CD4+CD25+ cell population during transfer experiments, we cannot exclude an additional subset of regulatory T cells amongst the CD4+CD25− T cell population, as has been described before (9, 18). Regulatory T cells have been associated with the expression of CD152/CTLA-4, and membrane bound or secreted TGF-β, and TGF-β is a potential mediator of suppression (13–16). In our system, treatment of mice with blocking anti-CD152 mAb or anti–TGF-β mAb did not alter memory CD8+ T cell responses. As we applied both mAb in doses that have been proven effective in other experimental models analyzing suppressive T cells (19, 20), we can largely exclude absence of response due to inadequate experimental procedures. Our results, therefore, indicate a mode of action for regulatory CD4+CD25+ T cells that, in our model, is largely independent from CTLA-4 and TGF-β.
Transfer of T cells into lymphopenic mice induces antigen-independent homeostatic proliferation of these cells (12). It is therefore possible that a similar induction of proliferation might occur in LLO91–99–specific memory CD8 T cells in our assays. However, we did not observe significant expansion of the specific memory CD8+ T cell population after CD4+ T cell depletion without antigen stimulation. Furthermore, there was no expansion of this cell population after transfer into SCID mice unless these mice were DNA immunized. These results strongly argue for a direct suppressive function of CD4+ T cells on the memory CD8+ T cell response and against homeostatic processes, although we cannot exclude a low rate of homeostatic proliferation of the analyzed memory CD8+ T cell population, which might be below the detection level of our assays. More recently, Murakami et al. demonstrated the involvement of CD25+CD4+ T cells in the control of CD8+ memory T cell homeostasis (21). In the experiments described, memory CD8+ T cells were defined by the CD44highIL-2Rβhigh surface phenotype and not by the expression of a TCR involved in a prior immune response (21). The different cell populations analyzed in their and in our study could explain the controversial results.
The mechanisms that activate regulatory T cells, and the antigens recognized by these cells remain unknown (22). In our experiments, memory CD8+ T cells were activated by a DNA vaccine encoding the gene for listeriolysin, or the peptide LLO91–99. Listeriolysin harbors only negligible CD4+ T cell epitopes for BALB/c mice (5), and it is highly unlikely that the 9-mer peptide LLO91–99 is recognized by CD4+ T cells. Therefore, we assume that the antigens recognized by regulatory T cells and by the regulated T cells are unrelated. Consistent with this assumption, CD25+ T cells from naive and _L. monocytogenes_–infected mice exhibited similar suppression in the adoptive transfer model.
Regulatory T cells have been shown to suppress activation of CD8+ T cells in vitro (23). In these studies, suppression affected proliferation and IFN-γ production of CD8+ T cells and was independent of the antigen recognized by the CD8+ T cells. In different tumor models, depletion of CD4+ T cells has been shown to enhance CD8+ T cell–mediated tumor rejection (24, 25). Gunn et al. demonstrated that CD4+ T cell depletion improved tumor rejection after therapeutic tumor vaccination (19). Rejection was also enhanced following anti–TGF-β mAb and anti-CD25 mAb treatment. In a different therapeutic tumor vaccination model, depletion of CD4+ T cells enhanced the efficacy of the vaccine (20). Anti-CD25 mAb treatment alone only slightly improved vaccine efficacy, but anti-CD25 mAb and anti–CTLA-4 mAb synergized in promoting tumor rejection accompanied by enhanced frequencies of tumor reactive CD8+ T cells. Although these studies demonstrate that priming and effector functions of CD8+ T cells are regulated by CD4+ T cells, regulation of memory responses was not addressed in these studies (19, 20). To our knowledge our results describe for the first time control of memory CD8+ T cell responses by regulatory CD4+ T cells in vivo.
In summary, our results reveal that CD4+ T cells substantially suppress CD8+ memory T cell responses. This phenomenon was particularly obvious after secondary DNA and peptide immunization and weakly evident during secondary L. monocytogenes infection. DNA and peptide immunization are relatively weak stimulators of CD8+ T cell responses. In contrast, L. monocytogenes induces a strong CD8+ T cell response and is accompanied by inflammation of the infected organs. Therefore, the CD8+ T cell response after L. monocytogenes infection should be less receptive to restrictions by suppressive CD4+ T cells than the response to DNA or peptide immunization. Furthermore, enhanced proliferation of CD8+ T cells in the absence of CD4+ T cells depended on antigen stimulation. The clearance of L. monocytogenes after secondary infection but also of peptide or DNA vaccine–induced proteins is, therefore, still a limiting factor for the CD8+ memory T cell response. The situation should be different for repeated or chronic infections. Lack of counter-regulation could cause exaggerated CD8+ T cell responses with severe consequences such as immunopathology or paralysis of the immune system. Thus, control of secondary CD8+ T cell responses by CD4+ T cells has to be considered an important element of the immune response after infection or vaccination.
Acknowledgments
The authors thank Drs. Eric Pamer, Dirk Busch, Jim Allison, and Yvonne Paterson for providing material, and Manuela Stäber for her excellent technical assistance.
M. Kursar was supported by the Graduiertenkolleg 276/2, and this work will be part of his PhD thesis.
Footnotes
*
Abbreviations used in this paper: CTLA-4, CTL-associated antigen; LLO, listeriolysin O.
References
- 1.Kaufmann, S.H.E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129–163. [DOI] [PubMed] [Google Scholar]
- 2.Mittrücker, H.-W., A. Köhler, and S.H.E. Kaufmann. 2000. Substantial in vivo proliferation of CD4+ and CD8+ T lymphocytes during secondary Listeria monocytogenes infection. Eur. J. Immunol. 30:1053–1059. [DOI] [PubMed] [Google Scholar]
- 3.Ladel, C.H., I.E. Flesch, J. Arnoldi, and S.H.E. Kaufmann. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153:3116–3122. [PubMed] [Google Scholar]
- 4.Busch, D.H., I.M. Pilip, S. Vijh, and E.G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infections. Immunity. 8:353–362. [DOI] [PubMed] [Google Scholar]
- 5.Geginat, G., S. Schenk, M. Skoberne, W. Göbel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877–1884. [DOI] [PubMed] [Google Scholar]
- 6.Fensterle, J., L. Grode, J. Hess, and S.H.E. Kaufmann. 1999. Effective DNA vaccination against listeriosis by prime/boost inoculation with the gene gun. J. Immunol. 163:4510–4518. [PubMed] [Google Scholar]
- 7.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi, S. 2000. Regulatory T cells: Key controllers of immunologic self tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 9.Shevach, E.M. 2000. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18:423–449. [DOI] [PubMed] [Google Scholar]
- 10.Mittrücker, H.-W., M. Kursar, A. Köhler, R. Hurwitz, and S.H.E. Kaufmann. 2001. Role of CD28 for the generation and expansion of antigen-specific CD8+ T lymphocytes during infection with Listeria monocytogenes. J. Immunol. 167:5620–5627. [DOI] [PubMed] [Google Scholar]
- 11.Chu, R.S., O.S. Targoni, A.M. Krieg, P.V. Lehmann, and C.V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas, A.A., and B. Rocha. 2000. Population biology of lymphocytes: the fight for survival. Annu. Rev. Immunol. 18:83–111. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T.W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridge, J.P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dentritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–477. [DOI] [PubMed] [Google Scholar]
- 18.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 6:389–400. [DOI] [PubMed] [Google Scholar]
- 19.Gunn, G.R., A. Zubair, C. Peters, Z.K. Pan, T.C. Wu, and Y. Paterson. 2001. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 167:6471–6479. [DOI] [PubMed] [Google Scholar]
- 20.Sutmuller, R.P., L.M. van Duivenvoorde, A. van Elsas, T.N. Schumacher, M.E. Wildenberg, J.P. Allison, R.E. Toes, R. Offringa, and C.J. Melief. 2001. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami, M., A. Sakamoto, J. Bender, J. Kappler, and P. Marrack. 2002. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc. Natl. Acad. Sci. USA. 99:8832–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo, C.A., and E.M. Shevach. 2001. Control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J. Immunol. 167:1137–1140. [DOI] [PubMed] [Google Scholar]
- 24.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128–3133. [PubMed] [Google Scholar]
- 25.Nagai, H., I. Hara, T. Horikawa, M. Oka, S. Kamidono, and M. Ichihashi. 2000. Elimination of CD4+ T cells enhances anti-tumor effect of locally secreted interleukin-12 on B16 mouse melanoma and induces vitiligo-like coat color alteration. J. Invest. Dermatol. 115:1059–1064. [DOI] [PubMed] [Google Scholar]