Peripheral Expression of Jak3 Is Required to Maintain T Lymphocyte Function (original) (raw)
Abstract
The Jak family tyrosine kinase, Jak3, is involved in signaling through cytokine receptors that utilize the common γ chain (γc), such as those for IL-2, IL-4, IL-7, IL-9, and IL-15. Recent studies of Jak3-deficient mice and humans have demonstrated that Jak3 plays a critical role in B and T lymphocyte maturation and function. The T lymphocyte defects in Jak3-deficient mice include a small thymus, a decrease in peripheral CD8+ cells, an increase in the surface expression of activation markers, and a severe reduction in proliferative and cytokine secretion responses to mitogenic stimuli. To determine whether the peripheral T lymphocyte defects result from aberrant maturation in the thymus or from the absence of Jak3 protein in peripheral T cells, we generated reconstituted mice that express normal levels of Jak3 protein in the thymus but lose Jak3 expression in peripheral T cells. Jak3 expression in the thymus restores normal T cell development, including CD8+, γδ, and natural killer cells. However, the loss of Jak3 protein in peripheral T cells leads to the Jak3 −/− phenotype, demonstrating that Jak3 is constitutively required to maintain T cell function.
T lymphocyte maturation and function are regulated by a number of signal transduction pathways. During T cell development in the thymus, the progressive maturation of T cells in large part is mediated by the TCR. First, the preTCR is required to induce the differentiation of CD4− CD8− into CD4+CD8+ thymocytes and their subsequent expansion (1); at the CD4+CD8+ stage, the mature αβ TCR is required to mediate both positive and negative selection of the TCR repertoire, as well as CD4 versus CD8 lineage commitment (for review see reference 2). In addition to the TCR, signals induced by interactions of thymocytes with thymic stromal cells are critical for T cell maturation (3), as are signaling pathways induced by soluble growth factors or cytokines (4, 5). Once T cells are mature, their ability to function properly is again dependent on a number of different signaling pathways. Most notably, T cell responses are contingent on an intact TCR signaling pathway involving molecules such as Zap-70 (6–8), Lck (9), Fyn (10, 11), and Vav (12–14). Until recently, significantly less was known about the role of other signaling pathways, such as those initiated by costimulatory molecules or cytokines, in the maintenance of a functional and responsive immune system.
Recent studies utilizing gene targeting in mice have revealed the importance of the B7 ligand, CTLA-4, and the IL-2 signaling pathway in preserving the balance between resting and activated T cells. For instance, loss of expression of the costimulatory molecule CTLA-4 (15, 16), or of the α (17) or β (18) chain of the IL-2 receptor, results in dramatic imbalances in T cell function, leading to accumulation of activated T cells and, in two of the cases, to lymphocyte infiltration into many organs and premature death of the animals. In contrast with this, mice and humans lacking the IL-2 receptor γ chain (γc)1 (19–21), or the γc-associated signaling protein, Jak3 (22–26), have severe combined immunodeficiency (SCID), with defects in lymphocyte maturation as well as function. The extremely severe phenotype of γc-deficient and Jak3-deficient individuals is due to the fact that γc is also a component of the receptors for IL-4, IL-7, IL-9, and IL-15 (27–31); thus, individuals deficient in γc or Jak3 have pleiotropic defects resulting from the loss of multiple cytokine signaling pathways. Therefore, it has been impossible to assess the role of γc or Jak3 in mature peripheral T cell function, as a consequence of the fact that T cell development is defective in the absence of each of these important proteins.
Recently, we and others have generated mice lacking expression of the γc-associated signaling protein, Jak3 (24–26). The phenotype of these mice strongly resembles that seen in γc-deficient mice (19, 20). For instance, both B and T cell development is aberrant in Jak3 mutant mice. In the bone marrow, B cell maturation is blocked at the pre-B stage, resulting in few IgM+ B cells in adult Jak3-negative mice. In the thymus, Jak3-deficient mice have an unusual defect in T cell development. The thymi of the mice are extremely small (∼1–10% of normal); yet, T cell maturation appears to progress relatively normally. In spite of these small thymi, adult Jak3 −/− mice have nearly normal numbers of CD4+ (but not CD8+) T cells in their spleens (24–26), although the Jak3 −/− peripheral T cells are phenotypically and functionally abnormal (26). By surface phenotype, virtually all of the Jak3 −/− T cells resemble activated or memory cells, rather than naive, resting cells. Functionally, the Jak3 −/− T cells fail to proliferate in response to mitogenic stimuli. The most unexpected finding was the severe deficiency in IL-2 secretion by Jak3 −/− T cells stimulated through their TCR plus CD28 (26).
These data provide an interesting contrast to the phenotype of T cells in mice lacking other components of the IL-2 signaling pathway, such as IL-2 itself, or the IL-2 receptor α or β chains (17, 18, 32). Instead of being hyper activated and prone to causing autoimmune syndromes, Jak3 −/− T cells appear anergic (26). From our initial studies, the reason for the loss of T cell function in the Jak3-deficient mice was unclear. Since T cells in these mutant mice lack Jak3 protein at all stages of development, it was impossible to distinguish defects due solely to the absence of Jak3 in mature T cells from defects acquired earlier during T cell maturation. Specifically, aberrant T cell development in the thymus might be responsible for the abnormal phenotype and responsiveness of the peripheral Jak3 −/− T cells. Alternatively, T cells might be functionally normal as they develop, and may acquire the unresponsive phenotype due to the absence of specific cytokine receptor signals as mature T cells. To address this issue, we have reconstituted Jak3deficient mice with wild type Jak3 under conditions in which Jak3 is expressed in thymocytes and then lost from peripheral T cells. These studies demonstrate that all phenotypic and functional defects of Jak3 −/− T cells result from the absence or decreased expression of Jak3 in mature peripheral T cells.
Materials and Methods
Transgenic Mice and Southern Blot Analysis.
Both wild-type and kinase-dead Jak3 cDNAs (33) were introduced into the Lck proximal promoter vector (34), a gift from R. Perlmutter. Lck–Jak3 sequences were removed from the bacterial vector DNA by cleavage with NotI, and prepared for microinjection. DNA was injected into (C57Bl/6 × C3H)F2 fertilized eggs by standard procedures (35). Pups were screened for the transgene by Southern blot analysis of EcoRI digested tail DNA probed with an 0.35-kb EcoRI-HindIII fragment of the Jak3 cDNA clone. Founders were backcrossed to C57Bl/10 mice; transgenic progeny were then crossed to Jak3 −/− mice to generate homozygous Jak3_-deficient mice heterozygous for each Lck–_Jak3 transgene.
Western Blot Analyses.
Lysates from individual thymi or spleens were prepared by generating a cell suspension, counting the cells, and lysing them at 108/ml in buffer containing 1% Triton X-100. Jak3 was immune precipitated from lysate of 1 × 107 thymocyte– cell equivalents or 2 × 107 splenocyte–cell equivalents with an anti-Jak3 monoclonal antibody specific to the carboxy-terminal 25 amino acids of murine Jak3 (33). Washed immune precipitates were fractionated by SDS-PAGE, transferred to nylon membranes, and probed with an anti-Jak3 rabbit antiserum, as described previously (26).
Flow Cytometry Analysis.
Bone marrow, thymocyte, and splenocyte cell suspensions were prepared and counted for total cellularity. For flow cytometry, 5 × 105 cells were stained with directly conjugated antibodies to CD45R (B220), CD4, CD8 (_GIBCO_BRL, Gaithersburg, MD) or biotinylated anti-IgM (PharMingen) and streptavidin–Cy-Chrome (PharMingen, San Diego, CA). For activation marker analyses, 5 × 105 splenocytes were stained with antibodies to CD4, CD8 (GIBCO-BRL), and biotinylated antiCD44 (PharMingen) or biotinylated anti-CD62L (MEL-14) (PharMingen), followed by streptavidin–FITC (PharMingen or Southern Biotech, Birmingham, AL).
Intracellular IL-2 Assays.
5 × 105 splenocytes were plated in 96-well microtiter plates previously coated with goat anti–hamster antibody (5 μg/ml) followed by anti-CD3 antibody (5 μg/ml), and cultured for 5 h in the presence of a 1/8 dilution of antiCD28 antibody hybridoma supernatant (determined to be saturating by cell surface staining). As a control, cells were cultured in media alone. To inhibit secretion of newly synthesized IL-2, stimulations were carried out in the presence of 10 μM monensin and 5 μg/ml brefeldin A (Sigma Chemical Co., St. Louis, MO). After stimulation, the cells from 4 wells were pooled and stained with PE-conjugated anti-CD4 antibody and biotinylated anti-CD44 antibodies followed by streptavidin–Cy-Chrome (PharMingen). Antibody stains were carried out in staining buffer containing 10 μM monensin. Cells were fixed in 4% paraformaldehyde for 20 min on ice, washed, and permeabilized with 0.5% saponin (Sigma) in PBS, 1% BSA, 0.05% NaN3. Cells were then stained with FITC-conjugated anti-IL-2 antibody (PharMingen) for 30 min on ice, washed twice with 0.5% saponin buffer, and analyzed by flow cytometry.
T Cell Functional Assays.
T cells were stimulated by culturing in wells coated with goat anti–hamster antibody followed by antiCD3 antibody, in the presence of anti-CD28 antibody hybridoma supernatant as described above. As a control, cells were cultured in media alone. For proliferation assays, 1 × 105 total thymocytes or total splenocytes adjusted to contain 1 × 104 CD4+ T cells per well were cultured for 48 h, then pulsed overnight with [3H]thymidine and counted. For cytokine assays, 1 × 106 total thymocytes or total splenocytes adjusted to contain 1 × 105 CD4+ T cells per well were stimulated. Supernatants from duplicate cultures were harvested at 24 h, and IL-2 and IL-3 levels were quantitated by titration on indicator cells. For IL-2, 1 U/ml corresponds to 1/50 maximal proliferation of the HT-2 indicator cells (Fig. 4 A) or 1/10 maximal proliferation of the indicator cells (Fig. 4 B). For IL-3, 1 U/ml corresponds to 1/10 maximal proliferation of the DA-1 indicator cells.
Figure 4.
Functional responses of Jak3 −/− (tgthy) T cells are restored in the thymus, but lost in the periphery. (A) Thymocytes and splenocytes from Jak3 +/−, Jak3 −/−, Jak3 −/− (tgthy+spl), and Jak3 −/− (tgthy) mice were analyzed for proliferation (top) and IL-2 (middle) or IL-3 (bottom) secretion in response to stimulation with anti-CD3 plus anti-CD28 antibodies. All data shown are from one 35-d-old Jak3 −/− (tgthy+spl) mouse and one 35-d-old Jak3 −/− (tgthy) mouse (designated B). IL-2 secretion data from splenocytes of two additional Jak3 −/− (tgthy) mice, one 25 d of age (designated A) and one 42 d of age (designated C), are also shown. All values are mean ± SD. Overall, no statistically significant differences between Jak3 +/−, Jak3 −/− (tgthy+spl), and Jak3 −/− (tgthy) thymocytes were observed for IL-2 or IL-3 secretion responses. (B) Thymocytes and splenocytes from Jak3 +/−, Jak3 −/−, and Jak3 −/− (tgkd) mice were stimulated with antibodies to CD3 plus CD28. Proliferative, IL-2 secretion, and IL-3 secretion responses are shown. All values are mean ± SD. For proliferation assays, all cell populations cultured in media alone gave responses of <500 cpm. For cytokine assays, cells cultured in media alone secreted undetectable levels of IL-2 (<0.02 U/ml) and IL-3 (<0.01 U/ml). Data are representative of 2–9 independent experiments.
Results and Discussion
We considered two possible explanations for the phenotypic and functional defects in peripheral Jak3 −/− T cells. First, Jak3 −/− T cells might be abnormal due to defects resulting from aberrant T cell development in the thymus; alternatively, Jak3 −/− T cells might be functionally and phenotypically normal when they leave the thymus, and might acquire their defects due to the absence of Jak3 in the periphery. To investigate the stage of development at which Jak3 −/− T cells acquired their phenotypic and functional defects, we utilized a transgenic reconstitution system. The wild-type Jak3 cDNA (33) was placed under control of the Lck proximal promoter (34). This vector has been used in numerous transgenic lines to express both cell surface and signal transduction proteins in thymocytes; in some cases, the transgene-encoded protein is also expressed in peripheral T cells, and in other cases, transgene expression is restricted to thymocytes (36–42). One of our transgenic lines expressed the Jak3 protein in both thymocytes and peripheral T cells, and therefore can serve as a positive control (hereafter referred to as tgthy+spl). A second line was also identified in which Jak3 was expressed in thymocytes, but was lost in peripheral cells over time (hereafter referred to as tgthy).
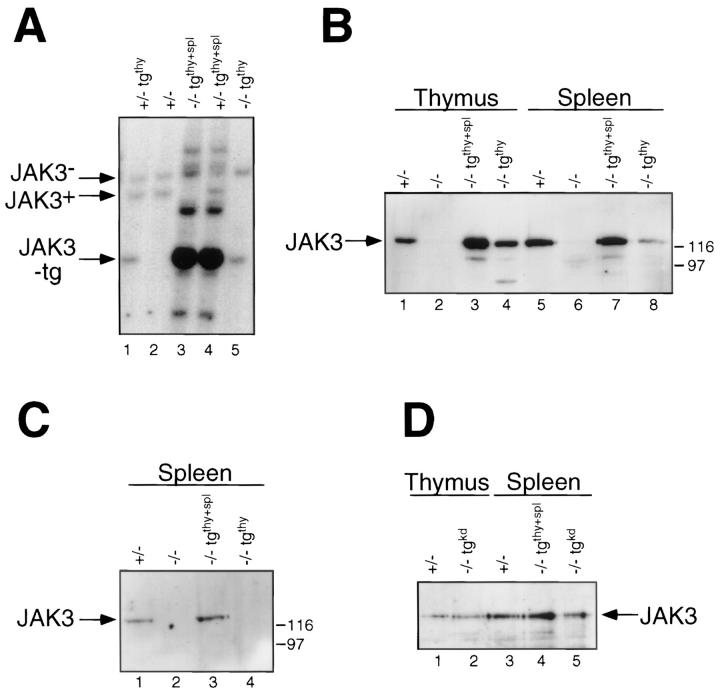
Both transgenic lines were crossed to the Jak3 −/− mice, to generate mice homozygous for the Jak3 mutation and heterozygous for one of the transgenes (Fig. 1 A). Analysis of Jak3 protein levels indicated that both transgenic lines expressed substantial amounts of Jak3 in thymocytes, while only the Jak3 −/− (tgthy+spl) line expressed levels of Jak3 comparable to wild type in the spleen (Fig. 1 B). In the Jak3 −/− (tgthy) line, Jak3 protein is barely detectable in spleen cells of a very young mouse (25 d) and becomes undetectable in older mice (Fig. 1 B and C); in contrast, no decrease in the levels of Jak3 protein was observed in the spleens of Jak3 −/− (tgthy+spl) mice up to 120 d of age (Fig. 1 D). These findings strongly suggest that Jak3 is constitutively expressed in peripheral T cells in the Jak3 −/− (tgthy+spl) mice, as the majority of T cells in a 4-mo-old mouse are not recent thymic emigrants, but cells that have been out of the thymus for several months. In contrast, the loss of detectable Jak3 protein from the spleen cells of older Jak3 −/− (tgthy) mice suggests that, in this transgenic line, the peripheral Jak3 protein observed in young animals is the residue of thymic Jak3 expression. However, we cannot rule out the possibility that the difference in detectable Jak3 expression between these two transgenic lines results from a difference in the dose of expression of the transgenes.
Figure 1.
Reconstituted expression of Jak3 in Jak3 −/− mice with Lck–Jak3 transgenes. (A) Southern blot of EcoRI-digested tail DNA from mouse pups indicating the genotyping of the endogenous Jak3 locus (Jak3 +/−, lanes 1, 2, 4; and Jak3 −/−, lanes 3 and 5), as well as the wild-type Jak3 transgenes (tgthy+spl, lanes 3 and 4; and tgthy, lanes 1 and 5). The blot was probed with an 0.35-kb EcoRI–HindIII fragment of the Jak3 cDNA clone (33). (B, C, D) Protein immunoblots of Jak3 immunoprecipitates from thymocytes and splenocytes of the indicated mice. Lysates were from Jak3 −/− (tgthy+spl) and Jak3 −/− (tgthy) mice at 25 d of age (B) or 33 d of age (C). In (D), lysates were from a Jak3 −/− (tgthy+spl) mouse at 120 d of age and a Jak3 −/− (tgkd) mouse at 49 days of age. Jak3 was immunoprecipitated using an antiJak3 monoclonal antibody (33) from lysate of 1 × 107 thymocytes or 2 × 107 splenocytes. The membranes were probed with anti-Jak3 rabbit antiserum (26).
As a negative control, Jak3 −/− mice were also reconstituted with a kinase-dead Jak3 construct driven by the Lck proximal promoter (hereafter referred to as tgkd) (Fig. 1 D). For these experiments, we utilized a Jak3 cDNA carrying a mutation in the codon for the conserved lysine residue present in all protein kinase domains (43). Substitution of Arg for Lys at this position (residue 851) eliminates all detectable tyrosine kinase activity of Jak3 (33). The kinasedead Jak3 protein was expressed in both thymocytes and peripheral T cells at levels comparable to those found in the Jak3 +/− control (Fig. 1 D).
Jak3 −/− (tgthy+spl), Jak3 −/− (tgthy), and Jak3 −/− (tgkd) mice were analyzed to determine the reconstitution of both the B and T cell lineages. Flow cytometry analysis of bone marrow cells indicated that no reconstitution of B cell development had occurred in any of these lines, as assessed by the lack of CD45R (B220)+ IgM+ cells (Fig. 2 A). Staining of bone marrow cells with antibodies to CD43 and CD45R (B220) also indicated that the block in B cell development observed in the Jak3 −/− mice is not corrected with any of the Lck promoter–driven Jak3 transgenes (data not shown). Analysis of B cells in the spleen demonstrated a reduced level of CD45R (B220)+ IgM+ cells in the Jak3 −/− mice expressing either wild-type or kinase-dead Jak3 transgenes compared with the Jak3 +/− control (Fig. 2 A).
Figure 2.

Both wild-type Jak3 transgenes reconstitute T cell, but not B cell, development in Jak3 −/− mice. (A) The bone marrow, thymus, and spleen cells of Jak3 +/−, Jak3 −/−, Jak3 −/− (tgkd), and 35-d-old Jak3 −/− (tgthy+spl) and Jak3 −/− (tgthy) mice were stained with the indicated antibodies and analyzed by flow cytometry. Staining is shown on a logarithmic scale of fluorescence intensity. Numbers in the quadrants indicate subpopulation percentages. The dot plots are representative of average staining profiles, although some Jak3 −/− individuals had greatly increased CD4+/CD8+ ratios in the thymus and spleen. (B) The total cellularity of bone marrow, thymus, and spleen of mice analyzed in these experiments is indicated. For each organ, Jak3 +/−, lane 1; Jak3 −/−, lane 2; Jak3 −/− (tgthy+spl), lane 3; Jak3 −/− (tgthy), lane 4; and Jak3 −/− (tgkd), lane 5 are shown. Note the reconstitution of normal thymocyte cellularity by both wild-type, but not the kinase-dead, Jak3 transgenes. Data shown are representative of greater than six independent experiments.
Both transgenic lines expressing wild type Jak3 in the thymus were capable of completely reconstituting the defects in T cell maturation. Thymi from Jak3 −/− (tgthy+spl) and Jak3 −/− (tgthy) mice were reconstituted to the normal number of cells (Fig. 2 B). In addition, the increased CD4+/ CD8+ ratio often observed in the thymi of Jak3 −/− mice was not observed in thymuses of either line of Jak3 −/− mice reconstituted with wild-type Jak3. Staining of spleen cells from the Jak3 −/− (tgthy+spl) and Jak3 −/−(tgthy) mice also demonstrated the recovery of normal T cell maturation (Fig. 2 A). In particular, the Jak3 −/− mice generally lack peripheral CD8+ T cells, a defect that is corrected in both of these reconstituted lines. In contrast, the kinase-dead Jak3 could not reconstitute thymus cellularity or CD8+ cell development (Fig. 2 A and B). Another feature of the Jak3 −/− mice is that they lack γδ T cells and NK cells (25); in addition, peripheral lymph nodes are nearly undetectable (24–26). The Jak3 −/− mice reconstituted with wild type Jak3 have normal numbers of CD4−CD8− γδ TCR+ cells in their thymus and normal numbers of CD4−TCR−NK1.1+ cells in their spleen; however, they still lack lymph nodes (data not shown).
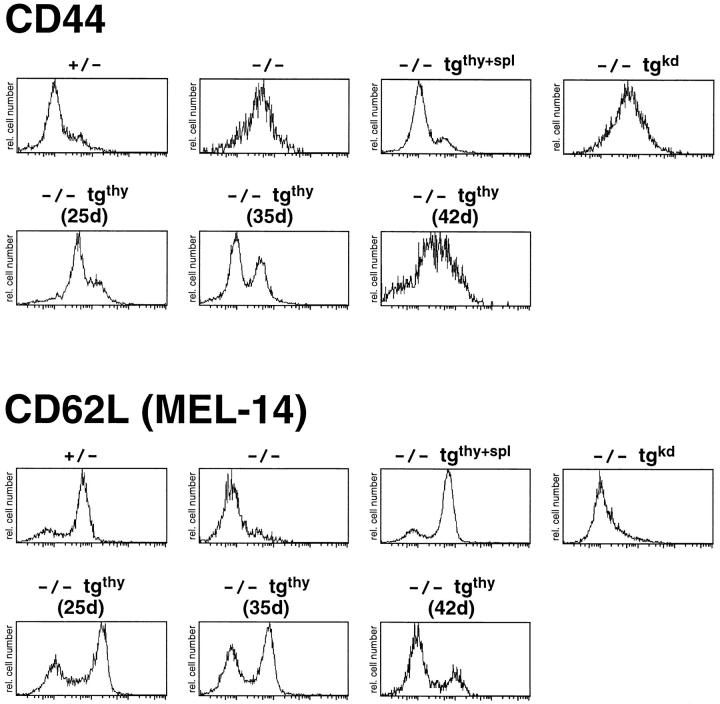
The reconstituted Jak3 −/− mice were examined for the surface phenotype and function of their T cells. Splenic T cells were stained with antibodies to CD4, CD8, and a panel of activation markers. Analysis of CD44 and CD62L (MEL-14) levels on gated CD4+ T cells indicate that Jak3 −/− T cells resemble activated or memory T cells, expressing high levels of CD44 and low levels of CD62L (Fig. 3). The T cells in the Jak3 −/− (tgthy+spl) mice are completely restored to normal, appearing indistinguishable from control (Jak3 +/−) T cells at all ages analyzed (Fig. 3; data not shown). In contrast, the splenic CD4+ T cells from the Jak3 −/− (tgkd) mice are indistinguishable from Jak3 −/− T cells (Fig. 3). Most interestingly, CD4+ T cells from Jak3 −/− (tgthy) mice have a cell surface phenotype that appears to correlate with peripheral Jak3 protein expression (Fig. 3). In the youngest Jak3 −/− (tgthy) mouse shown (25 d of age), where Jak3 protein is still detectable in the spleen (see Fig. 1 B), the majority of CD4+ T cells are CD44lo and CD62Lhi. In an older mouse (35 d of age), where Jak3 protein is no longer detectable in the spleen (see Fig. 1 C), two populations of T cells can be seen. In an even older mouse (42 d of age), most of the T cells in the Jak3 −/− (tgthy) mouse are CD44hi and CD62Llo, and resemble the Jak3 −/− T cells. This gradual appearance of phenotypically aberrant peripheral T cells, which correlates with the age of the mice, indicates that Jak3 protein is constitutively required to maintain a normal population of resting T cells. In total, nine Jak3 −/− (tgthy) mice and eleven Jak3 −/− (tgthy+spl) mice have been analyzed; in all cases, the peripheral T cells from the Jak3 −/− (tgthy+spl) mice resembled wild-type T cells, whereas the peripheral T cells from the Jak3 −/− (tgthy) mice had a surface phenotype that roughly correlated with the age of the mice. However, some variation in the precise age at which the vast majority of Jak3 −/− (tgthy) peripheral T cells acquired the Jak3 −/− surface phenotype was observed, most likely owing to variations in the loss of Jak3 protein from these cells. Overall, a comparable pattern of activation marker expression is observed on splenic CD8+ T cells in all mice analyzed (data not shown).
Figure 3.
Splenic CD4+ T cells from Jak3 −/− (tgthy) mice acquire increasing numbers of phenotypically activated T cells as the mice age. Splenocytes from Jak3 +/−, Jak3 −/−, Jak3 −/− (tgthy+spl), Jak3 −/− (tgkd), and three Jak3 −/− (tgthy) mice of different ages were stained with antibodies to CD4, CD8, and CD44 or CD62L (MEL-14). The staining of CD44 (top) and CD62L (bottom) on gated CD4+ T cells is shown on a logarithmic scale of fluorescence intensity. All histograms are directly comparable except the staining of the 25-d-old Jak3 −/− (tgthy) mouse, which was performed with a different lot of streptavidin-FITC, resulting in a brighter overall level of CD44 and CD62L fluorescence. Data shown are representative of greater than six independent experiments.
To address whether expression of the wild-type or kinase-dead Jak3 transgenes had reconstituted the function of Jak3 −/− T cells, thymocytes and splenic T cells from the Jak3 −/− (tgthy+spl), the Jak3 −/− (tgthy), and the Jak3 −/− (tgkd) mice were assessed for proliferation and cytokine production in response to TCR and CD28 costimulation. A set of data, representative of a total of nine independent experiments performed with different sets of mice, is shown in Fig. 4. Jak3 −/− thymocytes are substantially reduced in their proliferative response to CD3 plus CD28 stimulation. As expected, thymocytes from both lines reconstituted with wild type Jak3, but not with kinase-dead Jak3, have restored proliferative capacity (Fig. 4, A and B). The incomplete reconstitution of proliferative capacity with wild type Jak3 most likely results from the failure of the transgenes to be induced following activation. The massive induction of the endogenous Jak3 gene observed after T cell activation (44) may be essential to sustain a vigorous proliferative response. To test the thymocytes for their cytokine secretion responses, supernatants from anti-CD3 plus antiCD28-stimulated thymocytes were harvested and assayed for the presence of IL-2 and IL-3. Jak3 −/− thymocytes make substantially less IL-2 and IL-3 than the control (Jak3 +/− ) thymocytes (Fig. 4). As expected, thymocytes from both Jak3 −/− (tgthy+spl) and Jak3 −/− (tgthy) mice (Fig. 4 A), but not the Jak3 −/− (thykd) mice (Fig. 4 B), are restored in their ability to produce IL-2 and IL-3 in response to stimulation. These data demonstrate that expression of wild type Jak3 in thymocytes restores the functional capacity of Jak3 −/− cells to secrete cytokines when stimulated.
Splenic T cells from the reconstituted Jak3 −/− mice were also assessed for their ability to respond to TCR plus CD28 stimulation. Proliferative responses of Jak3 −/− splenic T cells are virtually absent compared with Jak3 +/− control cells; in addition, IL-2 secretion by Jak3 −/− T cells is also substantially reduced (Fig. 4, A and B). T cells from Jak3 −/− (tgkd) mice are indistinguishable from Jak3 −/− cells for both proliferative and cytokine secretion responses (Fig. 4 B). The splenic T cells from both the Jak3 −/− (tgthy+spl) mice and the Jak3 −/− (tgthy) mice have a limited capacity to proliferate in response to TCR plus CD28 stimulation (Fig. 4 A). Most likely, this is due to the fact that maximal proliferative responses depend on both the initial level of Jak3 protein, as well as the amount of Jak3 protein that can be induced after stimulation. Accordingly, we find that proliferative responses in the two transgenic lines are not consistent between experiments, and do not correlate well with the observed levels of Jak3 protein in resting peripheral T cells. Thus, we include these proliferative responses to demonstrate that reconstituting Jak3 expression does restore some proliferative capacity to the Jak3 −/− cells, even though quantitative conclusions from these data are not possible.
As expected, reconstitution of the Jak3 −/− mice with the (tgthy+spl) transgene restored normal IL-2 secretion from stimulated splenic T cells (Fig. 4 A). In contrast, IL-2 secretion by the Jak3 −/− (tgthy) splenic T cells varied between individuals, and correlated with the surface phenotype of the T cells (see Fig. 3). In the Jak3 −/− (tgthy) mouse in which the vast majority of cells resembled normal resting T cells, IL-2 secretion was normal (Fig. 4, −/−tgthy-A). In comparison, splenic T cells from a Jak3 −/− (tgthy) mouse in which ∼40–50% of the splenic T cells were CD44hi and CD62Llo, secreted substantially less IL-2 when stimulated (Fig. 4, −/−tgthy-B). Finally, in a Jak3 −/− (tgthy) mouse in which the vast majority of cells were CD44hi and CD62Llo, splenic T cells were severely defective in secreting IL-2 (Fig. 4, −/−tgthy-C). Analysis of IL-3 secretion supports these basic conclusions (Fig. 4). These data indicate that sustained Jak3 expression is required in peripheral T cells to maintain the resting surface phenotype and T cell function.
The above data suggested that the loss of Jak3 protein from peripheral T cells of Jak3 −/− (tgthy) mice results in the gradual increase of phenotypically activated CD4+ T cells and the reduced capacity of these cells to synthesize cytokines when stimulated. To test whether the loss of cytokine production was associated with the change in activation marker surface expression, we assessed the functional capability of CD44lo and CD44hi CD4+ T cells separately. For these experiments, T cells were stimulated with anti-CD3 plus anti-CD28 for 5 h and the cytoplasmic IL-2 levels of CD44lo and CD44hi CD4+ T cells were measured by flow cytometry. This substantially shorter assay for IL-2 production also addresses the possibility that the decreased cytokine secretion we observed from stimulated Jak3 −/−, Jak3 −/− (tgthy), and Jak3 −/− (tgkd) peripheral T cells might result from increased apoptosis of these cells compared with Jak3 +/− T cells over the course of the usual 24-h stimulation period. As shown in Fig. 5, 19.0% of CD44lo and 11.0% of CD44hi CD4+ splenic T cells from the Jak3 +/− mouse had detectable levels of intracytoplasmic IL-2 after 5 h of stimulation. In contrast, CD3 plus CD28 stimulation did not result in any significant IL-2 production by CD44lo T cells from Jak3 −/− mice, whereas 2.9% of CD44hi T cells stained positive for intracellular IL-2. The IL-2 profiles of stimulated CD4+ T cells from three different Jak3 −/− (tgthy) mice demonstrated that the percentage of IL-2+ cells decreases as the number of phenotypically activated CD44hi T cells increases. These data support the conclusion that peripheral T cells in Jak3 −/− (tgthy) mice acquire the activated surface phenotype and lose responsiveness in parallel, and that the functional defects in these cells are not due to increased apoptosis.
Figure 5.
Functional deficiencies of Jak3 −/− and Jak3 −/− (tgthy) T cells are confirmed by intracytoplasmic IL-2 staining. Splenocytes from a Jak3 +/−, a Jak3 −/−, and three Jak3 −/− (tgthy) mice were cultured for 5 h in medium alone or with anti-CD3 plus anti-CD28 antibodies. Cells were harvested, stained with antibodies to CD4, CD44, and IL-2. CD44 staining on freshly gated CD4+ cells is shown at left; dot plots of IL-2 versus CD44 staining of gated CD4+ cells cultured in either medium alone or stimulated with CD3 plus CD28 are shown at right. CD44 stainings are shown on a logarithmic scale of fluorescence intensity; IL-2 staining is shown on a linear scale. Data are representative of three independent experiments.
These studies of Jak3 −/− mice expressing wild-type or kinase-dead Jak3 from the Lck proximal promoter have demonstrated several important features about the role of Jak3 in T cell maturation and function. First, the failure of the kinase-dead Jak3 gene to reconstitute any of the T cell defects in the Jak3 −/− mice indicates that Jak3 kinase activity is essential for all the functions of Jak3 assessed in these experiments. For instance, the small thymus size in the Jak3 −/− mice is presumed to result from the absence of IL-7 receptor signaling (4, 5). As the kinase-dead Jak3 protein is likely to be fully functional in binding to γc (45, 46), the phosphorylation of Jak3 by Jak1 in response to IL-7 binding might have been hypothesized to restore partial function to the IL-7 receptor. A similar situation might also have occurred in mature thymocytes or peripheral T cells in response to IL-2 binding. Yet no thymocyte expansion in vivo, or T cell proliferation in response to TCR stimulation in vitro, was observed in tgkd-reconstituted Jak3 −/− mice. This requirement for Jak3 kinase activity is in direct contrast with the ability of kinase-dead Jak1 to reconstitute IFN-γ-inducible gene expression in Jak1− cells (47).
Second, the simultaneous reconstitution of normal numbers of αβ TCR+ thymocytes, γδ TCR+ thymocytes, and NK cells indicates that the Lck proximal promoter is active in these three lineages of lymphocytes or in a common precursor to these three cell types. Because we failed to reconstitute B cell development in the bone marrow in these mice, we conclude that the wild-type Jak3 transgenes are not expressed in a bone marrow progenitor cell that gives rise to both B and T lymphocytes. Therefore, these data substantiate a close lineage relationship between T lymphocytes and NK cells (48, 49), and suggest the existence of a common αβ T cell–γδ T cell–NK cell progenitor that cannot give rise to B lymphocytes. We do not know whether the expression of the Lck promoter–driven Jak3 genes initiates in the thymus, or in an earlier bone marrow–derived progenitor cell, as we cannot detect any expression of the Jak3 transgenes in bone marrow (data not shown).
The tgthy-reconstituted Jak3 −/− mice provide a system for distinguishing the roles of Jak3 during T cell development from Jak3 function in peripheral T cells. The normal level of Jak3 expression in thymocytes from these mice corrects virtually all the detectable defects of Jak3 −/− thymocytes. In Jak3 −/− (tgthy) mice of all ages, thymocyte numbers are increased to normal, the production of peripheral CD4+ and CD8+ T cells is restored to normal, and cytokine secretion by thymocytes in response to stimulation is restored. Nonetheless, peripheral T cells in these tgthyreconstituted Jak3 −/− mice slowly acquire all the defects of Jak3 −/− T cells. Although the precise kinetics of this effect vary between individual animals, the Jak3 −/− (tgthy) splenic T cells eventually become phenotypically and functionally deficient in an identical manner to the Jak3 −/− T cells; in all mice analyzed this process appears complete by 5–6 wk of age. These results demonstrate that the maintenance of normal levels of Jak3 protein in mature peripheral T cells is essential to preserve the continued function of these cells. This requirement is met in the tgthy+spl-reconstituted Jak3 −/− mice, which retain normal T cell phenotype and function at all ages analyzed.
In addition to the disappearance of proliferative ability, the loss of Jak3 protein in peripheral T cells leads to the acquisition of a memory cell phenotype and the loss of cytokine secretion capacity. Interestingly, this phenotype strongly resembles that observed in patients with a moderate combined immunodeficiency disease (XCID). The XCID defect is a point mutation in the cytoplasmic tail of γc that diminishes, but does not abolish, Jak3 binding (45). XCID patients have substantial numbers of T cells, but those T cells are deficient in proliferative responses, have an activated/memory phenotype, and are impaired in IL-2 secretion after stimulation (50). Thus, individuals with an impaired Jak3–γc interaction have sufficient Jak3 function to generate T cells in the thymus, unlike the SCID patients completely lacking Jak3 or γc expression; yet the peripheral phenotype of these T cells mimics that seen in our Jak3 −/− mice reconstituted with the tgthy transgene.
Currently, we have not yet identified the biochemical mechanism responsible for the aquisition of a memory cell phenotype and the loss of cytokine secretion capacity by the Jak3 −/− (tgthy) peripheral T cells. One possible mechanism is suggested by the studies of Nakajima et al. using γc − mice (51). Their studies indicate that the mutant T cells may be receiving activation signals from self- or environmental antigens; owing to their loss of IL-2 receptor signaling, the T cells then may be driven into a state of anergy in response to these activation events (52, 53). However, our intracellular IL-2 staining data indicate that even phenotypically naive T cells lose the capacity to secrete IL-2 when they lose Jak3 expression. This impaired ability of phenotypically normal Jak3-deficient cells to secrete cytokines when stimulated also suggests the possibility of a previously unsuspected role for Jak3 in TCR or CD28 signaling. In fact, both of these mechanisms may be involved and may contribute together to the numerous T cell defects observed in the Jak3-deficient mice.
Acknowledgments
We thank Roger Perlmutter for the Lck proximal promoter vector; Karen Sporny for technical assistance; and Charles Sagerström, Deborah Yelon, and Stephanie Heyeck for critical reading of the manuscript.
Footnotes
This work was supported by the American Cancer Society (L.J. Berg) and the National Institutes of Health (L.J. Berg). D.C. Thomis is a SmithKline Beecham Pharmaceuticals Fellow of the Life Sciences Research Foundation.
1 Abbreviations used in this paper: γc, IL-2 receptor γ chain; XCID, moderate combined immunodeficiency disease.
References
- 1.Fehling HJ, Krotkova A, Saintruf C, von Boehmer H. Crucial role of the pre-T cell receptor alpha gene in development of alpha–beta but not gamma–delta T cells. Nature (Lond) 1995;378:419–422. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 2.Blackman M, Kappler J, Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science (Wash DC) 1990;248:1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- 3.Anderson G, Jenkinson EJ, Moore NC, Owen JJT. MHC class II positive epithelium and mesenchyme cells are both required for T cell development in the thymus. Nature (Lond) 1993;362:70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 4.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R. Lymphopenia in interleukin (IL)-7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 7.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo W-L, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 8.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science (Wash DC) 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 9.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 10.Stein PL, Lee H-M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 11.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:571–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 12.Tarakhovsky A, Turner M, Schaal S, Mee PJ, Duddy LP, Rajewsky K, Tybulewicz VLJ. Defective antigen receptor–mediated proliferation of B and T cells in the absence of Vav. Nature (Lond) 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 13.Fischer K-D, Zmuldzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+CD8+thymocytes. Nature (Lond) 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Alt FW, Davidson L, Orkin SH, Swat W. Defective signalling through the T-and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature (Lond) 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 15.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fata multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science (Wash DC) 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 17.Willerford DM, Chen JZ, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Sirnard JJL, Ohashi PS, Griesser H, Taniguchi T, Paige CJ, Mak TW. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science (Wash DC) 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 19.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, Paul WE, Katz SI, Love PE, Leonard WJ. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 20.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 22.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Javad M, Aman, Migone T-S, Noguchi M, Markert ML, Buckley RH, O'Shea JJ, Leonard WJ. Mutation of JAK3 in a patient with SCID: essential role of JAK3 in lymphoid development. Science (Wash DC) 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 23.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O'Shea JJ, Vezzoni P, Notarangelo LD. Mutations of JAK3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature (Lond) 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 24.Nosaka T, van Deursen JMA, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking JAK3. Science (Wash DC) 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in JAK3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 26.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking JAK3. Science (Wash DC) 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 27.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen S, Park LS, Cosman D, Anderson D. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur Mol Biol Organ) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S-I, Sugamura K. Functional participation of the IL-2 receptor γ chain in IL-7 receptor complexes. Science (Wash DC) 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 29.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science (Wash DC) 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 30.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-4 receptor. Science (Wash DC) 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science (Wash DC) 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 32.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature (Lond) 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 33.Gurniak CB, Berg LJ. Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood. 1996;87:3151–3160. [PubMed] [Google Scholar]
- 34.Chaffin KE, Beals CR, Forbush KA, Wilkie TM, Simon MI, Perlmutter RM. Dissection of thymocyte signaling pathways by in vivo expression of pertussin-toxin ADP ribosyltransferase. EMBO (Eur Mol Biol Organ) J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan, B., F. Constantini, and E. Lacy. 1986. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 36.Garvin AM, Abraham KM, Forbush KA, Peet R, Farr AG, Perlmutter RM. Disruption of thymocyte development by SV40 T antigen. Int Immunol. 1990;2:173–180. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- 37.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Delayed thymocyte development induced by augmented expression of p56lck. J Exp Med. 1991;173:1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Thymic tumorigenesis induced by overexpression of p56lck. Proc Natl Acad Sci USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberola-Ila J, Forbush KA, Seger R, Krebs Eg, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature (Lond) 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 40.Allen JM, Forbush KA, Perlmutter RM. Functional dissection of the Lck proximal promoter. Mol Cell Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooke MP, Abraham KM, Forbush KA, Perlmutter RM. Regulation of T cell receptor signaling by a src family protein tyrosine kinase (p59fyn) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- 42.Teh H-S, Garvin AM, Forbush KA, Carlow DA, Davis CB, Littman DR, Perlmutter RM. Participation of CD4 coreceptor molecules in T cell repertoire selection. Nature (Lond) 1991;349:241–243. doi: 10.1038/349241a0. [DOI] [PubMed] [Google Scholar]
- 43.Hanks SK, Quinn AM, Hunter T. The protein tyrosine kinase family: conserved features and deduced phylogeny of the catalytic domains. Science (Wash DC) 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen Y-Q, Lal BK, Lloyd AR, Kelvin KJ, Staples JE, Ortaldo JR, O'Shea JJ. Molecular cloning of L-JAK, a Janus family protein tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA. 1994;91:6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O'Shea JJ, Leonard WJ. Interaction of IL-2Rβ and γcchains with Jak1 and Jak3: implications for XSCID and XCID. Science (Wash DC) 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn B, Ihle JN, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science (Wash DC) 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 47.Briscoe J, Rogers NC, Witthuhn BA, Watling D, Harpur AG, Wilks A, Stark GA, Ihle JN, Kerr IM. Kinase-negative mutants of Jak1 can sustain interferonγ-inducible gene expression but not an antiviral state. EMBO (Eur Mol Biol Organ) J. 1996;15:799–899. [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez MJ, Muench MO, Roncarolo MG, Lanier LL, Phillips JH. Identification of a common T/natural killer cell progenitor in human fetal thymus. J Exp Med. 1994;180:569–576. doi: 10.1084/jem.180.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanier LL, Spits H, Phillips JH. The developmental relationship between NK cells and T cells. Immunol Today. 1992;13:392–395. doi: 10.1016/0167-5699(92)90087-N. [DOI] [PubMed] [Google Scholar]
- 50.Brooks EG, Schmalstieg FC, Wirt DP, Rosenblatt HM, Adkins LT, Lookingbill DP, Rudloff HE, Rakusan TA, Goldman AS. A novel X-linked combined immunodeficiency disease. J Clin Invest. 1990;86:1623–1631. doi: 10.1172/JCI114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis . J Exp Med. 1997;185:189–195. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- 53.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, Ritz J, Nadler LM. Prevention of T cell anergy by signaling through the γcchain of the IL-2 receptor. Science (Wash DC) 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]