Identification of a Novel Developmental Stage Marking Lineage Commitment of Progenitor Thymocytes (original) (raw)
Abstract
Bipotent progenitors for T and natural killer (NK) lymphocytes are thought to exist among early precursor thymocytes. The identification and functional properties of such a progenitor population remain undefined. We report the identification of a novel developmental stage during fetal thymic ontogeny that delineates a population of T/NK-committed progenitors (NK1.1+/CD117+/CD44+/CD25−). Thymocytes at this stage in development are phenotypically and functionally distinguishable from the pool of multipotent lymphoid-restricted (B, T, and NK) precursor thymocytes. Exposure of multipotent precursor thymocytes or fetal liver– derived hematopoietic progenitors to thymic stroma induces differentiation to the bipotent developmental stage. Continued exposure to a thymic microenvironment results in predominant commitment to the T cell lineage, whereas coculture with a bone marrow–derived stromal cell line results in the generation of mature NK cells. Thus, the restriction point to T and NK lymphocyte destinies from a multipotent progenitor stage is marked by a thymus-induced differentiation step.
Understanding how molecular signals in developing tissues induce commitment and differentiation of stem cells is a fundamental question of developmental biology. In the immune system, the thymus provides a model system to study the mechanisms controlling tissue-specific differentiation events and lineage commitment pathways. During ontogeny, the thymus is formed when fetal liver–derived hematopoietic stem cells colonize the rudimentary thymic stroma at day 12 of fetal life, providing the necessary elements for the commitment and differentiation of stem cells into T cells (1–3). Multipotent precursors for T, NK, and B lymphocyte lineages are present in the early fetal thymus (1–5); however, it remains unclear when and how commitment and lymphocyte lineage restriction occur. These newly arrived fetal thymic lymphoid progenitor (TLP)1 cells display a c-kit+/CD44+/Thy-1lo/CD25−/CD3−/CD4−/ CD8− cell surface phenotype that is characteristic of hematopoietic stem cells (1–5).
Several reports have suggested, but not defined, the presence of a common thymic progenitor for T and NK lymphocytes within the TLP population (6–14). These studies also failed to address the possibility that NK cells derived from intravenous injection of immature thymocytes represent the outgrowth of preexisting cells with a NK phenotype within the TLP pool (6–14). To investigate these questions, we analyzed mouse day 13–15 fetal thymocytes, which contain precursors for all lymphoid lineages and have no mature α/β T or B lymphocytes (1–5).
We now report the identification of a novel T/NK-committed progenitor population of early fetal thymocytes distinguishable from the TLP subset based upon expression of the natural killer cell marker, NK1.1 (15, 16). Fetal TLPs lacking NK1.1 (FTLPs) maintain multipotency for the B, T, and NK lineages, whereas those expressing NK1.1 (fetal thymic NK1.1+ or FTNK progenitors) are committed exclusively to T and NK lymphocyte fates, and have lost B lymphopoietic potential. Furthermore, both FTLPs and fetal liver–derived hematopoietic precursors differentiate to the FTNK stage soon after entry into the thymic microenvironment. Our results identify a novel stage in fetal thymocyte differentiation that defines an important restriction point to the T and NK lymphocyte destinies and will facilitate the molecular characterization of thymic stromal signals that are responsible for lineage commitment.
Materials and Methods
Mice.
Timed-pregnant mice, C57Bl/6 and Swiss.NIH, were obtained from the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD).
Flow Cytometric Analysis and Cell Sorting.
Single-cell suspensions were stained for surface expression of various markers using FITC-, Cychrome-, APC-, PE-, or Red-613–conjugated mAbs obtained from PharMingen (San Diego, CA) or GIBCO BRL (Gaithersburg, MD), respectively, in staining buffer HBSS with 1% BSA and 0.05% NaN3). Cells were stained in 100 μl for 30 min on ice and washed twice before analysis. Stained cells were analyzed with a FACScan® flow cytometer using Lysis II software (Becton-Dickinson, Mountain View, CA); data was live-gated by size and lack of propidium iodide uptake. All plots display 10,000 events contoured at 50% log, except the control panels in Fig. 2, b and c where all events (⩽300 cells) are shown; events contained in each quadrant are given as percent of total in the upper right corner. CD24lo/CD25− day-14 thymocytes were obtained by antibody- and complement-mediated lysis. Single-cell suspensions were incubated on ice with 300 μl of culture supernatant of J11d.2 (anti-CD24) and 7D4 (anti-CD25) for 15 min, Low-Tox rabbit complement (Cedar Lane, Hornby, ON) was added at a 1/10 dilution in 3 ml medium, and cells were incubated at 37°C for 30 min. After complement-mediated lysis, viable cells were recovered by discontinuous density gradient centrifugation with Lympholyte-M (Cedar Lane). CD24lo/CD25− cells represented 4% of total day-14 fetal thymocytes, of which 10–20% show NK1.1+ staining. For cell sorting, fetal thymus single-cell suspensions were prepared and stained for FACS® as described above, except that no NaN3 was added to staining buffer. Cells were sorted using a Coulter Elite cytometer (Hialeah, FL); sorted cells were 98–99% pure, as determined by post-sort analysis. Staining with anti-NK1.1 (PK136) was not altered in the presence of FcγRII/III blocking antibody (2.4G2), and no significant staining was observed on immature precursor thymocytes derived from BALB/c mice, an NK1.1 nonexpressing strain (data not shown).
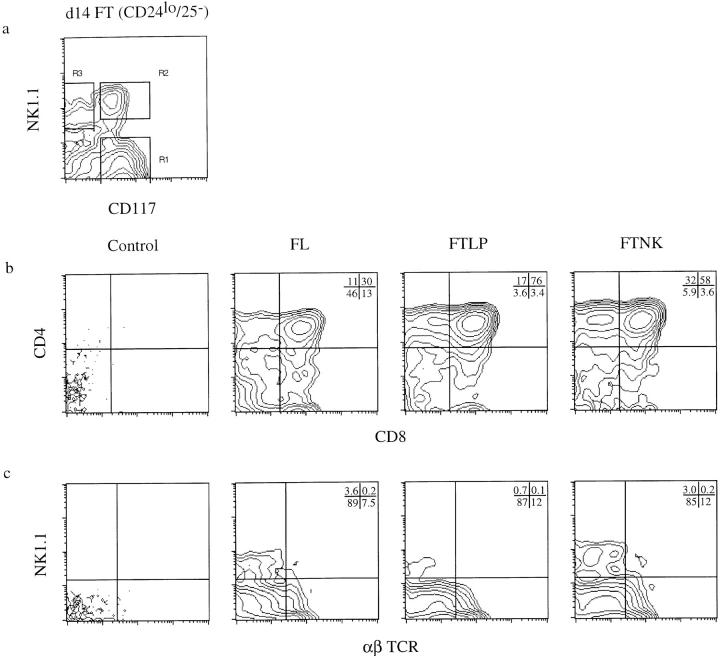
Figure 2.
Sorted NK1.1+/ CD117+ (FTNK) fetal thymocytes give rise to both T and NK lymphocytes upon reconstitution of alymphoid fetal thymic lobes in vitro. (a) FACS® of CD24lo (HSA, J11d.2) and CD25− (IL-2Rα, 7D4) antibody/complement-depleted day-14 fetal thymocytes from timed-pregnant mice. Cells were live-gated for the presence or absence of NK1.1 and/or CD117; regions 1, 2, and 3 (R1, R2, and R3) indicate the gates used for isolating either NK1.1−/CD117+ (FTLP; 79.4%), NK1.1+/CD117+ (FTNK; 8.4%), and NK1.1+/ CD117− (mature NK; 3.4%) subpopulations, respectively. Two-parameter analysis of cell surface expression of b CD4 versus CD8, and c NK1.1 versus α/β TCR on thymocytes from dGuo-treated FTOCs reconstituted with day-14 fetal liver cells or sorted day-14 FTLP and FTNK fetal thymocytes. (b and c) Panels show dGuo-treated FTOCs without the addition of reconstituting cells (Control, first panel) or with the addition of day-14 fetal liver (FL, second panel), FTLP (third panel), or FTNK (fourth panel) progenitors. The above results are representative of at least four independent trials. Cell yields for each experiment using 1 × 103 precursor cells ranged from 5–15 × 104 cells/lobe; no significant difference was observed between FTLP and FTLP reconstituted lobes. Plots display 1 × 104 live-gated events, except in the control panels where all events (⩽300) are shown.
Fetal Thymic Organ Culture Reconstitution.
Sorted populations were washed twice with DMEM medium supplemented with 12% FCS, 2 mM glutamine, 10 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml gentamicin, 110 μg/ml sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mM Hepes, pH 7.4 (fetal thymic organ culture [FTOC] medium). Day-14 fetal liver (FL) single-cell suspensions were prepared in the same manner. Lymphocyte-depleted thymic lobes were prepared by culturing day-15 fetal thymic lobes from timed-pregnant Swiss mice in FTOC medium containing 1.35 mM dGuo, as previously described (17, 18). In brief, host deoxyguanosine (dGuo)-treated FTOCs were cultured for 4–6 d, dGuo-containing medium was replaced with FTOC medium for 1 d, then lobes were rinsed twice, resuspended in 10 μl medium, and placed in Terasaki plates at two lobes (one thymus) per well. A titration of 101–3 × 103 NK1.1−/CD117+ (FTLP), NK1.1+/CD117+ (FTNK), or 1–3 × 104 FL donor cells were resuspended in 20 μl medium and added to dGuo-treated alymphoid fetal thymic lobes in Terasaki plates. After adding donor cells or medium alone, the plates were inverted (hanging drop) and cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air for 24–48 h. Lobes were then transferred to FTOC for 10–12 d. Cell suspensions from reconstituted thymic lobes were analyzed by flow cytometry. Reconstituted dGuo–FTOC lymphoid cells were >98% donor-derived as determined by flow cytometric analysis for donor-specific MHC class I expression. Reconstitution of host dGuo-treated FTOC was consistently successful only if >1 × 103 FL donor cells were used, or >30 FTNK or FTLP donor cells were used. Cell yields from dGuo–FTOC reconstitution experiments with 0.3–1 × 103 FTLP or FTNK cells showed no significant difference in total thymic cellularity recovered after 12-d cultures; cell yields for each experiment using 1 × 103 precursor FTLP or FTNK cells ranged from 5–15 × 104 cells/lobe.
In Vitro OP9 Stromal Cell Line Coculture.
Sorted CD117+/ NK1.1− FL and fetal thymocytes, FTLP and FTNK, were prepared as described above. A titration of 3 × 101–4 × 103 cells were cocultured in FTOC medium (6-well/plate) for 11 d on a confluent monolayer of OP9 cells (19, 20) in the presence of IL-3, IL-6, IL-7, and stem cell factor (SCF) (50 ng/ml of each cytokine), and then stimulated with LPS (10 μg/ml) and IL-7 for 4 d. Cells and culture supernatant were then harvested for flow cytometry and ELISA analysis, respectively. ELISA analysis (21), with a sensitivity of ⩾20 ng/ml of sIgM, revealed the presence of sIgM from the supernatant of FL and FTLP but not from the FTNK cocultures. Cell yields from OP9 coculture experiments with 1–10 × 102 FTLP or FTNK cells showed a clear difference in total number of lymphocytes recovered after 7-d cultures, i.e., before LPS activation; moreover, cell yields were typically ⩾100-fold higher in FTLP/OP9 than in FTNK/OP9 cocultures after 4 d of LPS activation. This increase is due to presence of LPS responsive B-lineage cells in the FTLP/OP9 cocultures and the their absence in the FTNK/OP9 cocultures (Fig. 3).
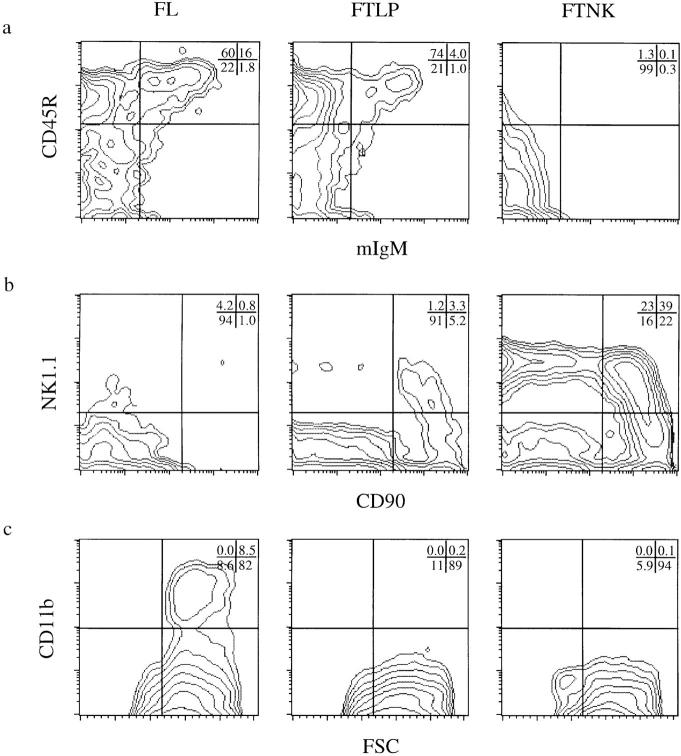
Figure 3.
Sorted fetal thymic NK1.1+/CD117+ (FTNK) progenitors give rise to NK cells and fail to generate B cells upon OP9 bone marrow stromal cell line coculture in vitro. Flow cytometric analysis of cell surface expression of (a) CD45R (B220) versus IgM, (b) NK1.1 versus CD90 (Thy-1), and (c) CD11b (Mac-1) versus forward size scatter (FSC) on sorted day-14, OP9-cocultured FL, FTLP, and FTNK cells. Cells were cocultured on confluent OP9 monolayer in the presence of IL-3, IL-6, IL-7 and SCF for 11 d, then stimulated with LPS and IL-7 for an additional 4 d before analysis.
Results and Discussion
Identification of NK1.1+/CD117+ Cells in the Fetal Thymus.
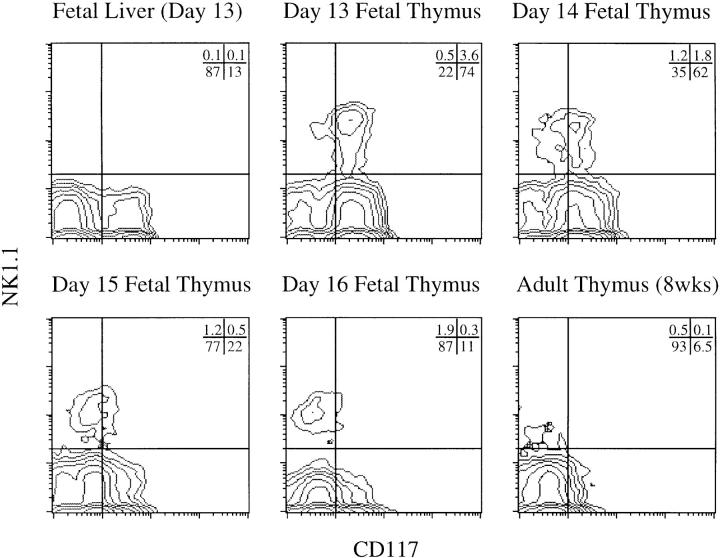
Analysis of mouse day-13–16 fetal thymocytes, showed a small percentage of NK1.1+ lymphocytes (4–6%) as early as day 13 of gestation (Fig. 1). We were surprised to detect NK1.1+ cells in the fetal thymus, because NK1.1+ thymocytes were previously reported to be absent early in thymic ontogeny (6, 13, 14); however, an earlier report by Koo et al. (22) showed evidence for NK1.1 expression on early fetal thymocytes. Perhaps, the fact that NK1.1+ cells represent ⩽2% of total day-15 fetal thymocytes may explain why some investigators failed to notice this subset during thymic ontogeny (6, 13, 14). We further analyzed fetal thymocytes for expression of the SCF receptor, c-kit (CD117), which is characteristic of hematopoietic precursors in the fetal liver, bone marrow, and thymus (1–3, 14, 23–27). CD117 is expressed on the majority of day 13/14 NK1.1+ thymocytes but on very few NK1.1+ thymocytes later in ontogeny or in the adult thymus (Fig. 1). Significant expression of NK1.1 was not detectable among CD117+ fetal liver (FL) cells (Fig. 1), suggesting that it may be induced during or after migration to the thymus.
Figure 1.
Identification of a novel NK1.1+/CD117+ (c-kit) fetal thymocyte population during thymic ontogeny. Two-parameter flow cytometric analysis of cell surface expression of NK1.1 versus CD117 on fetal thymocytes from timed-pregnant C57BL/6 mice (days 13, 14, 15, 16 of gestation), fetal liver cells (day 13 of gestation), and thymocytes from adult mice (8 wk old). NK1.1 and CD117 are coexpressed predominantly early in thymocyte differentiation.
The most immature progenitor thymocytes are CD117+ cells that have not yet expressed the IL-2 receptor α-chain (CD25) and bear low levels of Thy-1 (CD90) and heat-stable antigen (HSA; CD24) (1–3). We purified these progenitors by depleting day 14–15 thymocytes of CD25+ and CD24hi cells. Within this immature CD90lo/CD24lo/CD25− thymocyte pool, NK1.1 expression was evident on a higher percentage of the cells (10–20%) (Fig. 2 a; data not shown). Analysis for several other cell surface markers revealed a composite phenotype that is comparable to that of previously described early progenitor thymocytes (1–3, 6, 13, 14), demonstrating that this TLP population is not homogenous. Rather, we identify a population with the cell surface phenotype: NK1.1+/CD117+/CD44+/CD16+/CD32+/ CD90lo/CD24lo/CD25−/CD3−/CD4−/CD8−, termed fetal thymic NK1.1+ (FTNK) progenitors. These cells display markers characteristic of thymic progenitor cells as well as the NK1.1 molecule (NKR–P1C) (15, 16) of NK cells. A similar finding was recently observed in early immature human thymocytes, in which a small subset of CD34+/CD117+ thymocytes were shown to express a different member of the NKR–P1 gene family, NKR–P1A (28). Thus, the expression of NKR–P1 genes by early immature thymocytes appears to be a common feature during mouse and human thymic development.
NK1.1+/CD117+ Fetal Thymocytes Serve as Precursors for Both T and NK Cells.
To test whether FTNK progenitors are indeed a novel population of lymphoid precursor cells, we isolated FTNK cells from day-14 fetal thymocytes (the population of CD117+, CD90lo, CD24lo, and CD25− cells that express NK1.1) by antibody- and complement-mediated lysis followed by FACS® (Fig. 2 a, R2 gate; sorted populations were 98–99% pure; data not shown). FTNK cells were tested for precursor potential by a 24-h incubation with host fetal thymic lobes depleted of lymphocytes with dGuo, followed by FTOC for 10 d (17, 18). Reconstituted thymic lobes were analyzed by flow cytometry.
dGuo-depleted FTOCs that were not reconstituted with precursors remained devoid of T lymphocytes (Fig. 2 b, Control) (17, 18), whereas nondepleted FTOCs typically gave rise to both immature CD4/CD8 double-positive (DP) and mature CD4 and CD8 single-positive (SP) T lymphocytes (data not shown) (17, 18). dGuo-depleted thymic lobes reconstituted with FL cells or FTLP cells that lack NK1.1 expression (FTLPs: NK1.1−/CD117+/CD90lo/ CD24lo/CD25− cells; Fig. 2 a, R1 gate) resulted in the generation of DP and SP T lymphocytes (Fig. 2 b) (4, 5). Sorted FTNK cells (NK1.1+/CD117+/CD90lo/CD24lo/ CD25− cells; Fig. 2 a, R2 gate) also had potent reconstituting ability, giving rise to DP and mature CD4 and CD8 SP T lymphocytes (Fig. 2 b). Thus, both FTNK and FTLP thymocytes display T cell precursor potential. Moreover, reconstitution experiments revealed that the precursor potential of both populations titrated to a similar dilution (⩾30 cells/lobe; data not shown), ruling out the possibility that a minor admixture of CD117+/NK1.1− cells accounts for the reconstitution of thymic lobes by FTNK cells. Furthermore, NK1.1+ fetal thymocytes lacking CD117 expression (Fig. 2 a, R3 gate), corresponding to a mature NK phenotype (Carlyle, J.R., A.M. Michie, and J.C. Zúñiga-Pflücker, manuscript in preparation), failed to reconstitute dGuoFTOCs (data not shown), in accord with prior evidence that CD117 expression correlates with precursor activity (14, 23–25, 27).
The progeny of FTLP as well as FTNK cells expressed high levels of α/β T cell receptors and expressed IL-2 mRNA after concanavalin A stimulation, indicating a mature T cell phenotype (Fig. 2 c; data not shown). Thus, despite bearing the NK1.1 marker, FTNK cells display a cell surface phenotype similar to TLPs and serve as precursors to conventional T cells. In addition, both FTLP and FTNK cells gave rise to NK1.1+/TCR− as well as a few NK1.1+/ TCR+ thymocytes, with the former population representing conventional NK cells and the latter probably corresponding to the recently described CD1-restricted and IL-4–producing subset of T cells (29, 30). Thus, FTNK as well as FTLP thymocytes contain cells with T and NK precursor potential.
NK1.1+/CD117+ Fetal Thymocytes Fail to Serve as Precursors for B-lymphoid or Myeloid Lineage Cells.
We and other investigators have proposed that TLPs display a multipotent lymphoid precursor potential, which includes the ability to give rise to T, B, and NK lineages but not to myeloid lineage cells (1–5, 14, 31, 32). We applied an in vitro model system to test whether the FTLP or FTNK subsets of TLPs possess B-lymphoid or myeloid differentiation potential. Sorted CD117+/NK1.1− day-14 FL cells cocultured with the bone marrow–derived stromal cell line OP9 (19, 20) predominantly differentiated into functional B cells, as determined by IgM surface expression on B220+ (CD45R) cells and IgM secretion after induction with LPS and IL-7 (Fig. 3 a; data not shown) (21, 33). FL cells cocultured with OP9 also gave rise to a myeloid, Mac-1+ (CD11b), population (Fig. 3 c). A small population of NK1.1+ cells was also detected from FL cells cocultured on OP9 (Fig. 3 b). Thus, the OP9 cell line supports in vitro B, myeloid, and NK cell differentiation (19, 20), whereas α/β TCR-bearing T lymphocytes were not detected (Fig. 3; data not shown).
We next tested the differentiation potential of FTLP thymocytes after coculture with OP9 cells. As reported for TLPs in vivo (4, 5, 14, 31, 32), day-14 FTLPs showed a potent ability to give rise to functional B lymphocytes in vitro (Fig. 3 a), expressed membrane and secreted IgM, and gave rise to a small percentage of NK1.1+ lymphocytes (Fig. 3 b). However, unlike FL cells, FTLPs lack myeloid potential, as demonstrated by their inability to differentiate into CD11b+ cells (Fig. 3 c). These findings support the notion that the earliest fetal thymic progenitor population contains a multipotent lymphoid-restricted precursor (1, 2, 4, 5, 14, 31, 32). However, our failure to detect myeloid lineage cells derived from day-14 FTLPs is not consistent with work from groups that used day-12 fetal thymocytes as a source of progenitor cells (34, 35). The discrepancy between these results may be due to the immaturity of the day-12 fetal thymic microenvironment, as the full capacity of the fetal thymus to support thymopoiesis does not develop until day 13–14 (36). Restriction of myeloid potential may be a very rapid event upon entry of a multipotent precursor into a mature thymic microenvironment, but may not occur efficiently in the day-12 fetal thymic rudiment.
Both FTLP and FTNK progenitors displayed T and NK cell lineage precursor potential in FTOC reconstitution assays (see Fig. 2, b and c); however, no detectable B cell precursor potential was evident when FTNK cells were cocultured with OP9, as shown by the lack of CD45R+ cells expressing surface or secreted forms of IgM (Fig. 3 a; data not shown). As expected, FTNKs also lacked myeloid potential, as demonstrated by the absence of CD11b+ cells upon coculture with OP9 cells (Fig. 3 c). Despite the inability of FTNK progenitors to serve as precursors for B cells after OP9 coculture, these cells showed a strong precursor potential for NK1.1+ lymphocytes (Fig. 3 b). Thus, the inability to give rise to B cells is not due to an incapacity of FTNK cells to grow on OP9; rather, it demonstrates a T and NK lineage commitment by FTNK cells, which in the absence of a proper thymic microenvironment results in the generation of NK cells (37). Parallel assays in which sorted FTNK cells were used for both FTOC reconstitution and OP9 coculture resulted in the generation of T and NK cells in FTOC, but failed to give rise to B cells in OP9 coculture. Furthermore, the absence of B lymphocytes in the FTNK/OP9 cocultures, at up to 4,000 cells in culture, demonstrates that their ability to give rise to T and NK cells in the thymus does not come from an admixture of FTLP cells, which are clearly capable of B lymphopoiesis in OP9 cocultures, with as few as 30 cells in culture (data not shown). Thus, our results identify a novel population of CD117+ fetal thymocytes that express the NK1.1 surface marker and serve as committed bipotent precursors for mature α/β T lymphocytes and NK cells, whereas CD117+ thymocytes lacking NK1.1 expression can act as multipotent precursors for the T, B, and NK lymphocyte lineages.
NK1.1+/CD117+ Fetal Thymocytes Represent an Early Developmental Stage in Thymocyte Differentiation.
During early development, thymic progenitors transiently express various markers associated with activated mature T cells (38, 39) and NK cells (6, 13). These include lymphokine receptors such as CD25, several adhesion molecules such as intercellular adhesion molecules, very late activation antigens, and CD44, and other function-associated molecules such as CD16/32 (1–3, 6, 38, 39). It would appear that the NK1.1+ phenotype also corresponds to a temporary stage that T lymphocytes pass through on their way to maturity. To test this, we purified day-14 FTLP and FL cells by FACS® and followed their developmental progression to determine whether these isolated precursors could give rise to FTNK cells in reconstituted dGuo–FTOCs. Indeed, both FL and FTLP precursors directly give rise to substantial populations of FTNK cells within 48 h after transfer into FTOCs (Fig. 4). FTLPs appear to differentiate and reach the FTNK stage with faster kinetics than FL-derived progenitors (Fig. 4). This finding is in agreement with previous reports showing that fetal thymus–derived precursors show a faster reconstitution kinetics than FL-derived progenitors (2, 40). The appearance of FTNK cells peaks by day 4 after thymic reconstitution with FTLPs (Fig. 4; 83%), and declines by day 6. It is at these later timepoints that FTNK cells gain CD25 expression (Fig. 5 b; 37% of CD117+ gated cells coexpress NK1.1+/CD25+), and later lose NK1.1 expression as irrevocable commitment to the T cell lineage occurs and the CD25+/CD117+/NK1.1− stage is reached (Fig. 5 b) (4, 5). Again, the temporal kinetics of CD25+ expression by FTNK cells and loss of CD117 expression by developing thymocytes is more accelerated in dGuo–FTOCs reconstituted with FTLPs than with FL-derived progenitors (Fig. 5, a and b). Fig. 5 b also shows that by day 6 of reconstitution both precursor cell types give rise to CD117−/ CD25−/NK1.1+ cells, presumably pre-NK cells, while few CD117−/CD25+/NK1.1− (pre-T cells) are detected. These cells develop at later timepoints (data not shown). Finally, Fig. 5 b shows that nonreconstituted dGuo–FTOCs (control FTOC) remain devoid of lymphocytes. Thus, when purified FTLPs, which make up 4% of total day-14 fetal thymus, or FL cells are reintroduced to the thymic microenvironment a synchronized progression through the FTNK stage is clearly observed (Figs. 4 and 5), which occurs before the CD117+/ CD25+ stage of T cell development.
Figure 4.
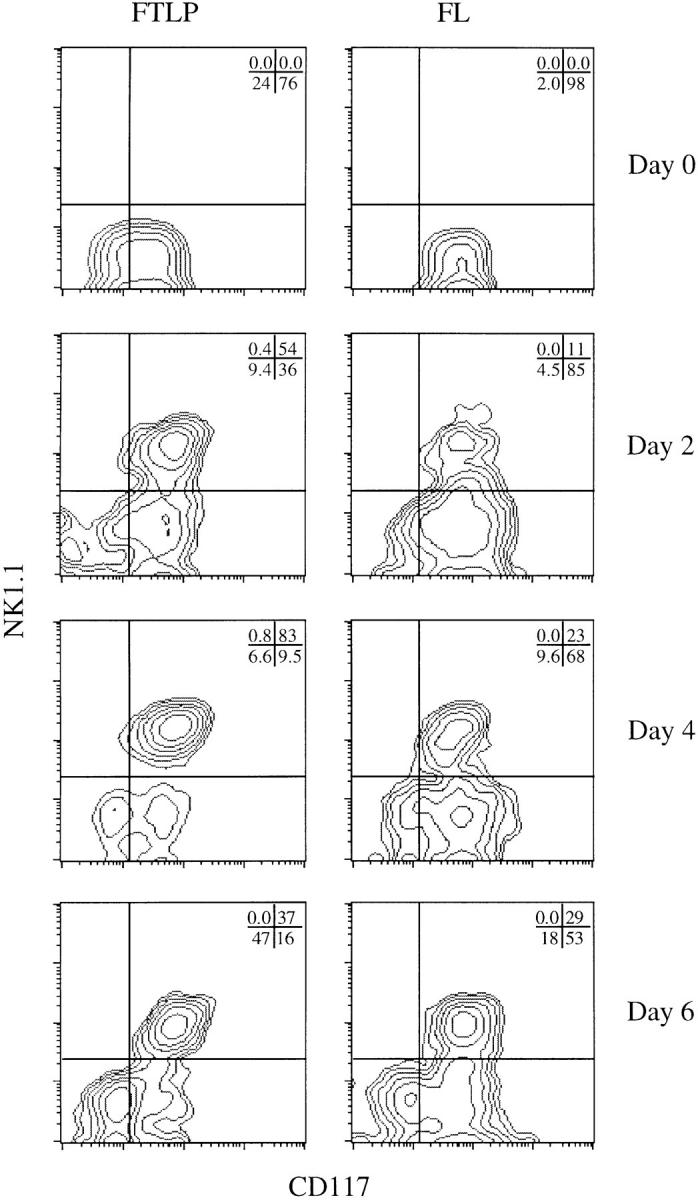
Temporal generation of FTNK cells from FTLP and FL precursors after in vitro transfer into fetal thymi. Sorted (NK1.1−/CD117+/ CD90lo/CD24lo/CD25−) day-14 fetal thymic lymphoid progenitor (FTLP) and fetal liver (FL)–derived precursors were transferred into dGuo-depleted fetal thymic lobes, cultured for 24 h in a hanging drop configuration, and then cultured in standard FTOC for an additional 1, 3, or 5 d. Flow cytometric analysis of cell surface expression of NK1.1 versus CD117 shows that NK1.1 expression is induced on CD117+ precursors (FTNK stage) as early as 2 d after exposure to thymic stroma, peaks by day 4, and declines by day 6 with the appearance of NK1.1−/CD117− cells. 3 × 104 sorted cells per dGuo-treated fetal thymus lobe (three lobes/timepoint) were used; the above results are representative of at least four independent trials. Cell yields ranged from 1–5 × 103, 0.5–1 × 104, and 1–3 × 104 cells/lobe at days 2, 4, and 6, respectively. Plots display 1 × 104 live-gated events.
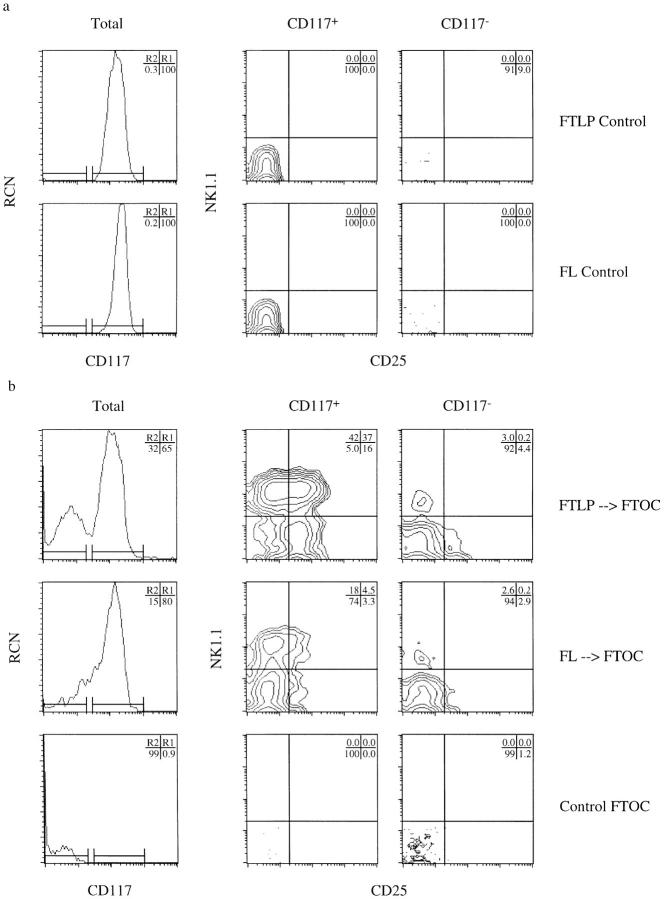
Figure 5.
Sorted NK1.1−/ CD117+ progenitors predominantly differentiate into T lineage–committed precursors upon exposure to thymic stroma in vitro. Three-parameter flow cytometric analysis of cell surface expression of CD117, NK1.1, and CD25 on sorted (NK1.1−/CD117+/ CD90lo/CD24lo/CD25−) day-14 fetal thymic lymphoid progenitor (FTLP) and fetal liver (FL)–derived precursor cells. Panels show relative cell number (RCN) versus CD117 expression on total cells, and NK1.1 versus CD25 expression gated on CD117+ (R1) and CD117− (R2) cell populations, respectively, from sorted precursors either (a) before (Control), or (b) 6 d after transfer into dGuo-depleted fetal thymi (FTOC). Upregulation of CD25 expression, marking commitment to the T lineage, occurs on the majority of NK1.1+/CD117+ thymocytes within 6 d after exposure to thymic stroma.
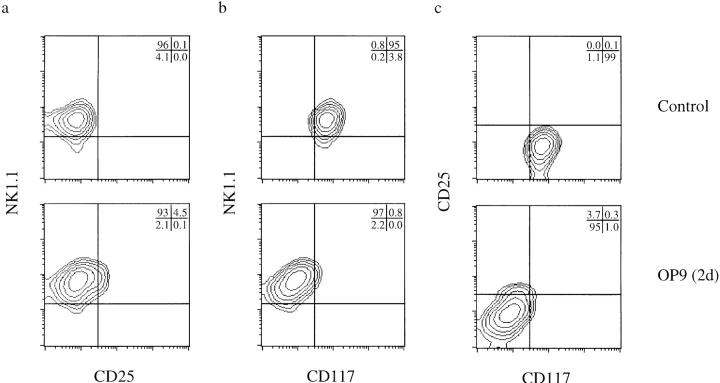
To delineate further this developmental stage, we purified FTNK progenitors by FACS® and analyzed changes in their phenotype using defined in vitro culture conditions that promote commitment to the NK lineage (OP9 cocultures; Fig. 3). Isolated FTNK progenitors cultured in medium containing the cytokines IL-3, IL-6, IL-7, and SCF to maintain viability (5), retain their phenotype after short-term (48 h) culture (Fig. 6, Control). In contrast, a similar exposure of purified FTNKs to the OP9 bone marrow– derived stromal cell line stimulates commitment to the NK cell lineage, as indicated by the rapid loss of CD117 expression with the retention of NK1.1 expression (Fig. 6, OP9). On the other hand, during reconstitution of dGuo– FTOCs, not only is CD117 expression retained on FTNK cells but CD25 expression is induced (see Fig. 5 b). In contrast, FTNK cells cultured in isolation or on OP9 cells remained NK1.1+ and did not progress to the CD25+ stage of thymocyte development (Fig. 6).
Figure 6.
Sorted NK1.1+/ CD117+ (FTNK) progenitors rapidly differentiate into NK lineage–committed precursors upon exposure to OP9 bone marrow stromal cells in vitro. Three-parameter flow cytometric analysis of cell surface expression of (a) NK1.1 versus CD25, (b) NK1.1 versus CD117, and (c) CD25 versus CD117 on sorted, cultured FTNK progenitors. FTNK progenitors were generated in vitro as in Fig. 4 (FTLP; day 2); these cells were then sorted (NK1.1+/CD117+/ CD90lo/CD24lo/CD25−) and cultured in vitro for an additional 2 d with or without OP9 cells. Panels show cells cultured in medium plus cytokines (IL-3, IL-6, IL-7, SCF) alone (Control), or upon coculture with OP9 bone marrow stromal cells plus cytokines (OP9). After transfer onto OP9 bone marrow stromal cells, NK1.1 expression is maintained, in the absence of CD25 upregulation, while CD117 expression is rapidly downregulated, marking NK lineage commitment.
Our findings suggest that the FTNK stage represents a developmental crossroad during thymocyte differentiation, where in the thymus most thymocytes will be guided to enter the T lineage pathway and express CD25 (see Fig. 5), while a minor fraction will enter the NK lineage, lose CD117 expression, and fail to express CD25 (Figs. 5 and 6). The generation of small numbers of mature NK cells in FTOCs (see Fig. 2 c) and during thymic ontogeny in vivo (see Fig. 1) may result from the occasional or rare failure of the local thymic microenvironment to support efficiently T lineage commitment and differentiation. In such instances, the thymus would still be capable of supporting NK cell maturation. Stromal influences, thymic or other, appear to be necessary or at least sufficient for NK cell maturation, as cells at the bipotent FTNK stage fail to mature into NK cells when cultured without stromal cells in the presence of IL-3, IL-6, IL-7, and SCF for up to 8 d in vitro (data not shown). Cytokines, such as IL-12, IL-15, and perhaps IL-2, may play a role in the differentiation to the NK lineage (41–44), while it appears that the cytokines TNF-α and IL-1 are required for T lineage commitment and differentiation (4). The identification of the FTNK stage of thymocyte development marks an important step towards the elucidation of the molecular signals responsible for mediating commitment to T and NK lineages.
Our results demonstrate the existence of a stage in early thymocyte differentiation during which progenitors transiently express some hallmarks of NK lymphocytes; a stage that defines commitment towards the T/NK lineages with the concomitant loss of B lineage potential. Thymocytes at this stage are phenotypically similar to the TLP population, but can be distinguished from TLP cells and mature NK cells based upon expression of NK1.1 and CD117, respectively. Hence, some or all of the previously reported NK cells derived from intravenous injections of populations of TLP thymocytes (6, 13, 14) are likely due to the differentiation and outgrowth of the FTNK population or from preexisting mature NK cells (Carlyle, J.R., A.M. Michie, and J.C. Zúñiga-Pflücker, manuscript in preparation). FTNK cells that retain their NK phenotype but lose CD117 expression would also lose their ability to differentiate into T cells and therefore possess an NK-restricted potential. While this remains to be demonstrated at the clonal level, we propose that NK1.1− FTLPs represent the earliest thymic progenitors that are multipotent for the B, T, and NK lineages and rapidly give rise to FTNK precursor cells, which represent restricted bipotent T/NK precursor cells.
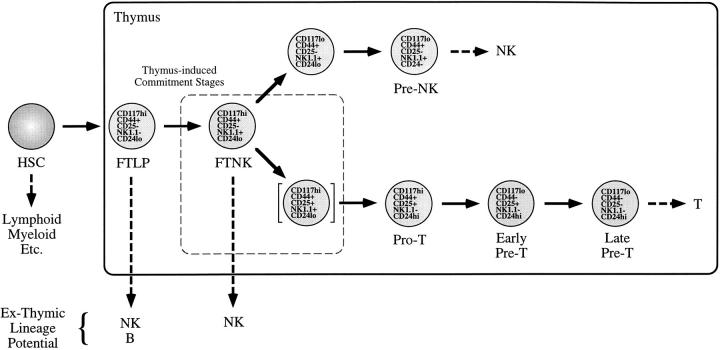
The FTNK stage, as shown in Fig. 7, may represent one of the earliest phenotypic changes that occurs after hematopoietic progenitors enter the thymus. Our data suggests that the expression of NK1.1 by early precursor thymocytes denotes their loss of B cell potential and, therefore, commitment to the T or NK lineages. The stage shown in brackets represents a transition stage (see Fig. 6; CD117+/NK1.1+/ CD25+) that would be indicative of precursor thymocytes progressing to the pro-T cell stage (1–5), but may still display some NK cell potential (5). Our findings are the first to delineate and characterize a separate phenotypic stage for progenitor thymocytes that possess a T/NK-restricted precursor function, which is distinguishable from the multipotent TLP population and free of preexisting mature NK cells. Thus, Fig. 7 provides a new paradigm for T cell development and defines a novel thymocyte developmental stage within the full context of thymocyte differentiation.
Figure 7.
Model of lineage commitment and differentiation events in the mouse fetal thymus. Upon entry into the fetal thymus, multipotent progenitors rapidly commit to the lymphoid lineages (FTLP stage), restricting other hematopoietic potentials including that of the myeloid lineage. Soon after thymic immigration, a thymus-induced differentiation step marked by expression of NK1.1 (FTNK stage) signifies commitment to the T and NK lineages, with the concomitant loss of B lymphoid potential. FTNK progenitors that undergo a second thymus-induced commitment step, marked by expression of CD25, lose NK1.1 and commit to the T cell lineage (_Pro_-T stage). FTNK cells that do not undergo the second thymus-induced differentiation event lose CD117 expression and become NK lineage–committed precursors (Pre-NK stages).
Our findings allow us to redefine which fetal thymocytes possess a lymphoid-restricted multipotent reconstitution potential (Fig. 7). In the adult thymus, progenitor cells with a similar reconstitution potential have been characterized by their CD44+/CD117+/CD4lo phenotype. These CD4lo precursors have been shown to serve as progenitors for not only T, NK, and B cells, but also for a novel subset of thymus-derived dendritic cells (1, 31, 32, 45). However, dendritic cell potential is not restricted to the CD4lo stage but is maintained up to the CD44+/CD117+/CD25+ stage of thymocyte development (31, 45). Thus, we predict that FTNK as well as FTLP cells would also serve as progenitors for thymic dendritic cells, as both the FTNK and FTLP developmental stages occur before the induction of CD25 expression (Figs. 5 and 7). Moreover, it appears that the FTNK stage of thymocyte development is not restricted to fetal thymocyte development, as FACS®-purified CD4lo progenitor cells from adult mouse thymus also contain a subset of NK1.1+ cells; these cells comprise 4% of CD4lo progenitors and display a CD44+/CD117+/CD4lo/NK1.1+ phenotype (data not shown). Thus, the expression of NKR– P1 genes by early immature thymocytes appears to be a common feature during both fetal and adult development in the mouse as well as the human thymus (28).
Taken together, our identification of FTNK cells in the thymus and the recent description of mature NK1.1+ αβ T cells indicate that T and NK lymphocytes may be closely linked from their earliest differentiation steps and throughout their maturation. Identification of the restriction point in which B cell potentiality is lost, whereas that for T or NK fate is maintained, will facilitate for the molecular characterization of thymic signals that control this event.
Acknowledgments
We thank Drs. R. Germain, N. Iscove, M. Julius, P. Ohashi, R. Phillips, and P. Poussier for discussions and critically reading the manuscript, and C. Smith for technical assistance with cell sorting. We are also grateful to Dr. T. Honjo for providing the OP9 bone marrow stromal cell line.
Footnotes
J.R. Carlyle is supported by a studentship from the Medical Research Council of Canada (MRC). J.C. Zúñiga-Pflücker is supported by a scholarship from the MRC. This work was funded by grants from the MRC and the National Cancer Institute of Canada.
1 Abbreviations used in this paper: dGuo, deoxyguanosine; DP, double positive; FL, fetal liver; FTLP, fetal thymic lymphoid progenitor; FTNK, fetal thymic NK1.1+; FTOC, fetal thymic organ culture; HSA, heat-stable antigen; SCF, stem cell factor; TLP, thymic lymphoid progenitor.
References
- 1.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Zúñiga-Pflücker JC, Lenardo MJ. Regulation of thymocyte development from immature progenitors. Curr Opin Immunol. 1996;8:215–224. doi: 10.1016/s0952-7915(96)80060-4. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 4.Zúñiga-Pflücker JC, Jiang D, Lenardo MJ. Requirement for TNF-α and IL-1α in fetal thymocyte commitment and differentiation. Science (Wash DC) 1995;268:1906–1909. doi: 10.1126/science.7541554. [DOI] [PubMed] [Google Scholar]
- 5.Moore TA, Zlotnik A. T-cell lineage commitment and cytokine response of thymic progenitors. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- 6.Rodewald H-R, Moingeon P, Lucich JL, Dosiou C, Lopez P, Reinherz EL. A population of early fetal thymocytes expressing FcγRII/III contains precursors of T lymphocytes and natural killer cells. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez MJ, Muench MO, Roncarolo MG, Lanier LL, Phillips JH. Identification of a common T/natural killer cell progenitor in human fetal thymus. J Exp Med. 1994;180:569–576. doi: 10.1084/jem.180.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galy A, Travis M, Chen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 9.Moretta L, Ciccone E, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: origin, clonality, specificity, and receptors. Adv Immunol. 1994;55:341–380. doi: 10.1016/s0065-2776(08)60513-1. [DOI] [PubMed] [Google Scholar]
- 10.Moretta L, Ciccone E, Poggi A, Mingari MC, Moretta A. Origin and functions of human natural killer cells. Int J Clin Lab Res. 1994;24:181–186. doi: 10.1007/BF02592459. [DOI] [PubMed] [Google Scholar]
- 11.Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654–2670. [PubMed] [Google Scholar]
- 12.Sanchez MJ, Spits H, Lanier LL, Phillips JH. Human natural killer cell committed thymocytes and their relation to the T cell lineage. J Exp Med. 1993;178:1857–1866. doi: 10.1084/jem.178.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodewald H-R. Pathways from hematopoietic stem cells to thymocytes. Curr Opin Immunol. 1995;7:176–187. doi: 10.1016/0952-7915(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki Y, Gyotoku J, Ogawa M, Nishikawa S, Katsura Y. Characterization of c-kit positive intrathymic stem cells that are restricted to lymphoid differentiation. J Exp Med. 1993;178:1283–1293. doi: 10.1084/jem.178.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan JC, Turck J, Niemi EC, Yokoyama WM, Seaman WE. Molecular cloning of the NK1.1 antigen, a member of the NKR–P1 family of natural killer cell activation molecules. J Immunol. 1992;149:1631–1635. [PubMed] [Google Scholar]
- 16.Giorda R, Trucco M. Mouse NKR–P1. A family of genes selectively coexpressed in adherent lymphokine-activated killer cells. J Immunol. 1991;147:1701–1708. [PubMed] [Google Scholar]
- 17.Jenkinson EJ, Franchi L, Kingston R, Owen JJT. Effects of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982;12:583. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson EJ, Owen JJT. T cell differentiation in thymus organ culture. Semin Immunol. 1990;2:51–58. [PubMed] [Google Scholar]
- 19.Nakano T, Kodam H, Honjo T. Generation of lympho-hematopoietic cells from embryonic stem cells in culture. Science (Wash DC) 1994;265:1098. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 20.Nakano T. Lymphohematopoietic development from embryonic stem cells in vitro. Semin Immunol. 1995;7:197–203. doi: 10.1016/1044-5323(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 21.Cumano A, Dorshkind K, Gillis S, Paige CJ. The influence of S17 stromal cells and interleukin 7 on B cell development. Eur J Immunol. 1990;20:2183–2189. doi: 10.1002/eji.1830201006. [DOI] [PubMed] [Google Scholar]
- 22.Koo GC, Peppard JR, Hatzfeld A. Ontogeny of Nk-1+ natural killer cells. I. Promotion of Nk-1+cells in fetal, baby, and old mice. J Immunol. 1982;129:867–871. [PubMed] [Google Scholar]
- 23.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfrey DI, Zlotnik A, Suda T. Phenotypic and functional characterization of c-kit expression during intrathymic T cell development. J Immunol. 1992;149:2281. [PubMed] [Google Scholar]
- 25.deVries P, Brasel KA, McKenna HJ, Williams DE, Watson JD. Thymus reconstitution by c-kit–expressing hematopoietic stem cells purified from adult mouse bone marrow. J Exp Med. 1992;176:1503–1509. doi: 10.1084/jem.176.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissman IL. Stem cells, clonal progenitors, and commitment to the three lymphocyte lineages: T, B, and NK cells. Immunity. 1994;1:529–531. doi: 10.1016/1074-7613(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 27.Rodewald H-R, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 28.Poggi A, Costa P, Morelli L, Cantoni C, Pella N, Spada F, Biassoni R, Nanni L, Revello V, Tomasello E, et al. Expression of human NKRP1A by CD34+immature thymocytes: NKRP1A-mediated regulation of proliferation and cytolytic activity. Eur J Immunol. 1996;26:1266–1272. doi: 10.1002/eji.1830260613. [DOI] [PubMed] [Google Scholar]
- 29.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+CD4+CD8−thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 30.Bendelac A. Mouse NK1+T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 31.Ardavin C, Wu L, Chung-Leung L, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature (Lond) 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Scollay R, Egerton M, Pearse M, Spangrude GJ, Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature (Lond) 1991;349:71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- 33.Cumano A, Paige CJ. Enrichment and characterization of uncommitted B-cell precursors from fetal liver at day 12 of gestation. EMBO (Eur Mol Biol Organ) J. 1992;11:593–601. doi: 10.1002/j.1460-2075.1992.tb05091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peault B, Khazaal I, Weissman IL. In vitro development of B cells and macrophages from early mouse fetal thymocytes. Eur J Immunol. 1994;24:781–784. doi: 10.1002/eji.1830240345. [DOI] [PubMed] [Google Scholar]
- 35.Hattori N, Kawamoto H, Katsura Y. Isolation of the most immature population of murine fetal thymocytes that includes progenitors capable of generating T, B, and myeloid cells. J Exp Med. 1996;184:1901–1908. doi: 10.1084/jem.184.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amagai T, Itoi M, Kondo Y. Limited development capacity of the earliest embryonic murine thymus. Eur J Immunol. 1995;25:757–762. doi: 10.1002/eji.1830250320. [DOI] [PubMed] [Google Scholar]
- 37.Brooks CG, Georgiou A, Jordan RK. The majority of immature fetal thymocytes can be induced to proliferate to IL-2 and differentiate into cells indistinguishable from mature natural killer cells. J Immunol. 1993;151:6645–6656. [PubMed] [Google Scholar]
- 38.Zúñiga-Pflücker JC, Schwartz HL, Lenardo MJ. Gene transcription in differentiating immature T cell receptornegthymocytes resembles antigen-activated mature T cells. J Exp Med. 1993;178:1139–1149. doi: 10.1084/jem.178.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenberg EV, Chen D, Diamond RA, Dohadwala M, Novak TJ, White PM, Yang-Snyder JA. Acquisition of mature functional responsiveness in T cells: programming for function via signaling. Adv Exp Med Biol. 1991;292:71–83. doi: 10.1007/978-1-4684-5943-2_9. [DOI] [PubMed] [Google Scholar]
- 40.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8−triple negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 41.Brunda MJ. Interleukin-12. J Leukoc Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 42.Leclercq G, Debacker V, De Smedt M, Plum J. Differential effects of interleukin 15 and interleukin 2 on differentiation of bipotential T/natural killer progenitor cells. J Exp Med. 1996;184:325–336. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plum J, De Smedt M, Leclercq G, Verhasselt B, Vandekerckhove B. Interleukin-7 is a critical growth factor in early human T-cell development. Blood. 1996;88:4239–4245. [PubMed] [Google Scholar]
- 44.Manoussaka M, Georgiou A, Rossiter B, Shrestha S, Toomey JA, Sivakumar PV, Bennett M, Kumar V, Brooks CG. Phenotypic and functional characterization of long-lived NK cell lines of different maturational status obtained from mouse fetal liver. J Immunol. 1997;158:112–119. [PubMed] [Google Scholar]
- 45.Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]