Rapid Effector Function in CD8+ Memory T Cells (original) (raw)
Abstract
The nature of the CD8+ T cells that underlie antiviral protective immunological memory in vivo is unclear. We have characterized peptide-specific CD8+ T lymphocytes directly ex vivo from peripheral blood in humans with past exposure to influenza virus, using single cell interferon γ (IFN-γ) release as a measure of effector function. In individuals in the memory state with respect to influenza virus infection, unrestimulated antigen-specific CD8+ T cells displayed IFN-γ release within 6 h of antigen contact, identifying a population of memory CD8+ T cells that exhibit effector function without needing to divide and differentiate over several days. We have quantified circulating CD8+ effector T cells specific for six different MHC class I–restricted influenza virus epitopes. Enumeration of these CD8+ T cells gives frequencies of peptide-specific T cells that correlate with, but are in general severalfold higher than, CTL precursor frequencies derived from limiting dilution analysis, indicating that this novel population of memory CD8+ T cells has hitherto been undetected by standard means. The phenotype of these cells, which persist at a low frequency long after recovery from an acute viral infection, suggests that they play a role in protective immunological memory.
After recovery from an acute viral infection, the frequency of antigen-specific CD8+ T cells is too low to permit direct analysis. Instead, assays have used antigen- experienced T cells that have been expanded by in vitro restimulation with cognate antigen, a process that may introduce quantitative and qualitative biases, particularly with respect to the activation state of the cell. It has therefore been difficult to establish, for viral infections in humans, the phenotype of antigen-specific memory T cells in their natural state. In particular, it remains uncertain whether antiviral protective immunological memory is subserved by long-lived quiescent T cells (1–3) that must divide and differentiate over several days to become effectors, or by circulating effector T cells continuously activated either by persisting antigen or by cross-reactive environmental antigens (4, 5).
We have studied individuals previously exposed to influenza virus but without acute clinical influenza, that is, individuals in the memory state with respect to influenza virus infection. Influenza infection is well suited for the study of CD8+ T cell memory. First, CD8+ CTLs are crucial for host defense against influenza virus; they are important in the clearance of intranasal virus (6, 7) and, since CTLs recognize the relatively conserved internal viral proteins (8, 9), they cross-react between viruses of different strains which evade neutralizing antibody through variation in surface glycoproteins (10, 11). Second, influenza virus–specific CTLs secrete IFN-γ which has direct effects on virus replication in infected cells (12) and may be more important in vivo for protection against influenza virus than perforin- or fas-mediated lysis (13). Examination of IFN-γ secretion by antigen-specific CD8+ T cells is therefore expected to be of at least as much relevance to protection as conventional measurements of lytic activity.
We have applied a sensitive enzyme-linked immunospot (ELISPOT)1 assay for single cell IFN-γ secretion in a novel way to detect low frequencies of uncultured influenza peptide–specific CD8+ T lymphocytes freshly isolated from peripheral blood. The ELISPOT assay detects secreted cytokine molecules in the immediate vicinity of the cell from which they are derived, while still at a relatively high concentration; each spot in the read-out represents a ‘footprint' of the original cytokine-producing cell. Quantitation of these IFN-γ spot-forming cells (SFCs) by this technique is highly sensitive; for influenza virus–specific CD8+ CTL lines and bulk cultures, the ELISPOT assay is an order of magnitude more sensitive than the 51Cr-release cytotoxicity assay for detecting low numbers of peptide-specific CTLs (data not shown). We have exploited this enhanced sensitivity to demonstrate the presence of circulating influenza virus–specific CD8+ memory T cells capable of rapid effector function, long after exposure to the virus.
Materials and Methods
Subjects.
Healthy adult volunteers were recruited from May through October, during which period influenza virus does not circulate in Britain. Subjects were HLA typed serologically by complement-mediated lymphocytotoxicity. Molecular subtyping for HLA-B27.05 and HLA-A2.01 was performed by amplification refractory mutation system PCR with sequence-specific primers as previously described (14).
Peptides.
Six MHC class I–restricted influenza epitopes were used and are listed in Table 1. Peptides were synthesized on solid phase on a semiautomated peptide synthesizer (Zinsser Analytical, Frankfurt, Germany) using f-moc chemistry; purity was confirmed by HPLC.
Table 1.
Recognition of CD8+ Influenza Epitopes by Freshly Isolated T Cells in the ELISPOT Assay for IFN-γ and Corresponding Peptide-specific T Cell Frequencies
| Protein | Sequence | MHC class I restriction | No. of responders | No. of donors tested | Range of peptide-specific IFNγ SFCs (effector frequencies)/PBMC |
|---|---|---|---|---|---|
| M1 58–66 | GILGFVFTL | HLA-A2.01 | 7 | 9 | 1/111,000–1/3,500 |
| NP 380–388 | ELRSRYWAI | HLA-B8 | 5 | 7 | 1/67,000–1/15,000 |
| NP 265–273 | ILRGSVAHK | HLA-A3 | 5 | 6 | 1/83,000–1/45,000 |
| PB1 591–599 | VSDGGPNLY | HLA-A1 | 2 | 4 | 1/100,000–1/91,000 |
| MI 128–135 | ASCMGLIY | HLA-B35 | 2 | 2 | 1/25,000–1/16,000 |
| NP 383–391 | SRYWAIRTR | HLA-B27.05 | 1 | 1 | 1/24,000 |
ELISPOT Assay for Single Cell IFN-γ Release: Detection of Antigen-specific Effectors from Freshly Isolated PBMCs.
96-well polyvinylidene difluoride backed plates (MAIP S 45; Millipore, Bedford, MA) were coated with 15 μg/ml of anti–IFN-γ mAb 1-D1K (Mabtech, Stockholm, Sweden) overnight at 4°C. Plates were then washed 6 times with RPMI-1640 and blocked with RPMI supplemented with l-glutamine, penicillin, and 10% heat-inactivated pooled human AB serum (R10) for 1 h. PBMCs were separated from heparinized whole blood on LYMPHOPREP (Nycomed Pharma AS, Oslo, Norway), washed 3 times, and resuspended in R10. PBMCs were added in 100 μl R10/well to the precoated plates. Input cell numbers were 5 × 105/well, in duplicate wells. For assays performed in parallel with limiting dilution analyses (LDAs), duplicate wells with 5 x 105 and 2.5 × 105 PBMCs/well were used.
Detection of peptide-specific T cells from freshly isolated PBMCs is complicated by the fact that the target cells used for peptide presentation elicit responses from T cells of other specificities. Heterologous B cell lines (BCLs) register strong responses from alloreactive T cells, whereas autologous BCLs result in potent EBV-specific responses. Allo-specific and EBV-specific responses were circumvented by using the autologous fresh PBMCs themselves to present peptide. Peptides were added to a final concentration of 2 μM. Where the cell line CIR-A2.01 was used to present the M1 58–66 peptide to fresh PBMCs, the cell line was prepulsed with a 2 μM concentration of peptide in R10 for 1 h, and then washed 3 times.
Assays were incubated for 6 h at 37°C, 5% CO2, but some experiments were run overnight (14 h) for convenience. Assays were arrested by shaking off the contents and washing 6 times with PBS 0.05% Tween 20 (Sigma Chemical Co., St. Louis, MO). Next, 100 μl of 1 μg/ml of the biotinylated anti–IFN-γ mAb 7-B6-1 biotin (Mabtech, Stockholm, Sweden) was added. After 3 h of incubation, plates were washed six times more and a 1:1,000 dilution of streptavidin alkaline phosphatase conjugate (Mabtech) was added to the wells and the plates incubated at room temperature for a further 2 h. Next, wells were again washed 6 times and 100 μl of chromogenic alkaline phosphatase substrate (Bio Rad Labs., Hercules, CA), diluted 1:25 with deionized water, was added. After 30 min, the colorimetric reaction was terminated by washing with tap water and plates were air dried.
Enumeration of IFN-γ SFCs.
Spots were counted under magnification of 20 with a stereomicroscope (Leitz GZ6; Leitz, Wetzlar, Germany). Only large spots with fuzzy borders were scored as SFCs as per convention (15). Responses were considered significant if a minimum of five SFCs were present per well, and additionally, this number was at least twice that in negative control wells.
LDAs for Peptide-specific CTL Precursors.
LDAs were carried out as described (16). In brief, influenza A virus infection of fresh PBMCs was inactivated with human serum and the cells mixed with uninfected PBMCs to give a final concentration of 10% infected cells. Replicate microcultures (24/input cell number) were set up in 96-well round-bottomed plates, with PBMC dilutions ranging from 1,562–200,000 cells/well. Cultures were restimulated with autologous, washed, irradiated, peptide-pulsed B cells on day 7 or 8 at a feeder to responder ratio of 1:3 and they received 10% Lymphocult-T (Biotest AG, Dreiech, Germany) and fresh medium then and 3–4 d later. LDAs for donors PM, JM, and AH were assayed on day 14, and LDAs for SM and WB (11/96) were assayed on day 18 after a further restimulation on day 14. Split-well analysis was performed on 51Cr-labeled peptide-pulsed and unpulsed autologous B cells. Equal aliquots of each resuspended well were assayed for cytotoxicity on respective targets. Maximum and spontaneous release was measured for each target. Wells were scored as positive using a threshold of 10% specific lysis.
For WB (9/96), an alternative methodology was used. Replicate microcultures (30/input cell number) of responder PBMCs were set up with 25,000 autologous, peptide-pulsed, washed, irradiated feeder PBMCs. Lymphocult-T was added on days 0 and 5. On day 8, split-well analysis was performed as above, but using heterologous HLA-A2.01–matched BCLs as targets. Wells were scored positive if specific lysis was greater, by three standard deviations, than spontaneous release.
For both methods, where the single hit kinetics of the Poisson model were fulfilled, with negligible lysis of unpulsed targets and a straight line relationship, the regression line was calculated by the maximum likelihood method. The peptide-specific CTL precursor frequency was estimated from the initial responder cell number at which 37% of the wells were negative for cytotoxicity.
Characterization of Effectors by Specific Cell Depletion Studies.
CD4+ and CD8+ T cells were depleted by 30 min of incubation with anti-CD4 or anti-CD8 mAbs conjugated to ferrous beads (DYNABEADS M-450; Dynal, Oslo, Norway) in 500 μl R10 on ice. After dilution in R10, the conjugate-coated cells were removed by a magnet (Dynal). The DYNABEADS used here reliably deplete >99% of the target cell population. Vβ17+ TCR-bearing T lymphocytes were depleted by incubating PBMCs with the murine mAb E17.5F3 (Human T Cell Receptor Monoclonal Antibody Workshop, IX International Congress of Immunology, San Francisco, CA, 1995) for 30 min on ice, washing twice and then incubating with goat anti–mouse IgG mAb conjugated to ferrous beads (Dynal) for a further 30 min followed by magnetic depletion. Depletion of CD45RO+ PBMCs was carried out as above except that the first mAb was the murine anti-CD45RO mAb UCHL1 (DAKO, Glostrup, Denmark). Depletion of the CD45RO+ and Vβ17 TCR-bearing cells was confirmed on a FACS® (Becton Dickinson, Mountain View, CA).
Results
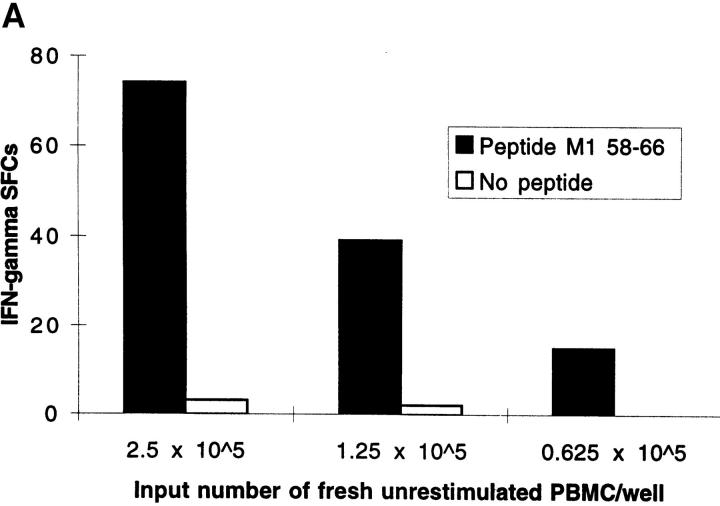
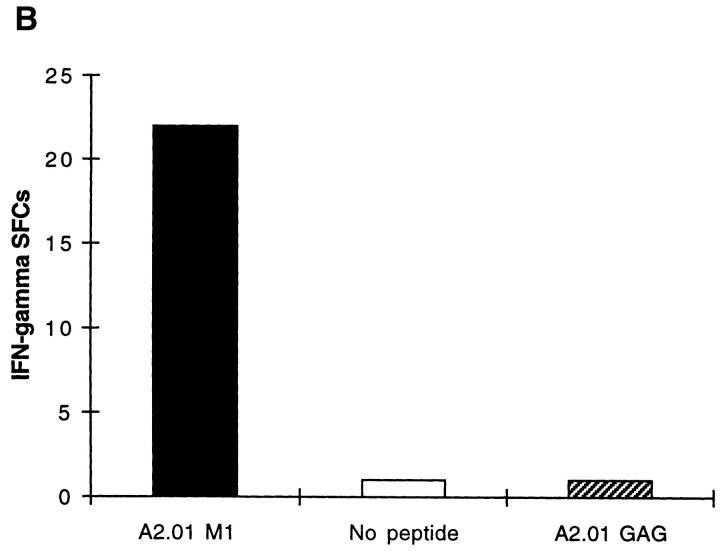
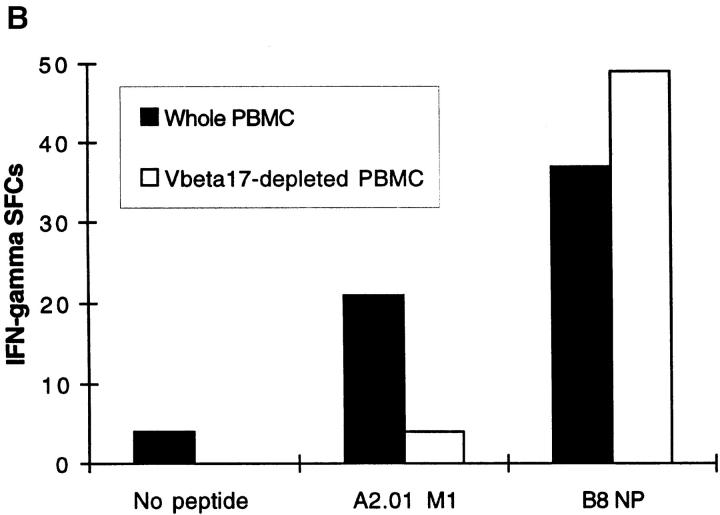
Freshly Isolated Influenza Peptide-specific CD8 + T Cells from Donors Without Active Influenza Infection Display Effector Function Within 6 h of Antigen Contact. Unrestimulated CD8+ T cells, freshly isolated from the peripheral blood of donors in the memory state with respect to influenza virus, secreted IFN-γ within 6 h of contact with HLA class I–restricted influenza peptide epitopes. Fig. 1 A quantitates CD8+ T cells that display effector function within 6 h of exposure to antigen; T cells specific for the HLA-A2.01– restricted influenza matrix epitope, M1 58-66, were detected in PBMCs freshly isolated from donor SM with HLA-A2.01. The number of IFN-γ SFCs did not increase when PBMCs were incubated in the ex vivo ELISPOT assay for periods of time progressively longer than 6 h, up to 40 h (data not shown). Negative controls in the ELISPOT assay were wells with PBMCs but no peptide or irrelevant peptides from infectious agents with which the donor was not infected. These never elicited a response as, for example, in Fig. 1 B. Using six well-defined HLA class I–restricted influenza epitopes, we studied a panel of donors in the memory state for influenza virus infection. Out of 29 individuals tested with epitopes restricted by their own HLA class I alleles, 22 had detectable influenza peptide–specific T cells freshly isolated from peripheral blood, that rapidly secreted IFN-γ on exposure to cognate peptide (Table 1). The proportion of donors responding to these influenza virus epitopes in the ex vivo ELISPOT is considerably higher than the proportion that respond to the same epitopes in 51Cr-release cytotoxicity assays with bulk culture CTLs. Experiments were performed at final peptide concentrations of 2 μM, but responses were still readily detectable when peptide concentrations were titrated down to 0.02 μM (data not shown).
Figure 1.
(A) Freshly isolated, unrestimulated, peptide-specific, memory CD8+ T cells secrete IFN-γ within 6 h of antigen contact. Enumeration of IFN-γ SFCs in a 6 h ELISPOT assay performed with freshly isolated PBMC from donor SM (class I HLA haplotype: A2.01, A24; B44, B14). The HLA-A2.01–restricted peptide M1 58–66 was used at a final concentration of 2 μM. Mean values from duplicate wells are shown. (B) Irrelevant peptides do not induce IFN-γ SFCs. Using fresh PBMCs from HIV-uninfected donor SC (class I HLA haplotype: A2.01, A28; B7, B14) in a 6 h ELISPOT assay, quantitation of IFN-γ SFCs shows a response specific for the influenza HLA-A2.01–restricted peptide M1 58–66 compared with no response to the HLA-A2.01–restricted peptide from the HIV GAG protein, GAG 77-85 (SLYNTVATL).
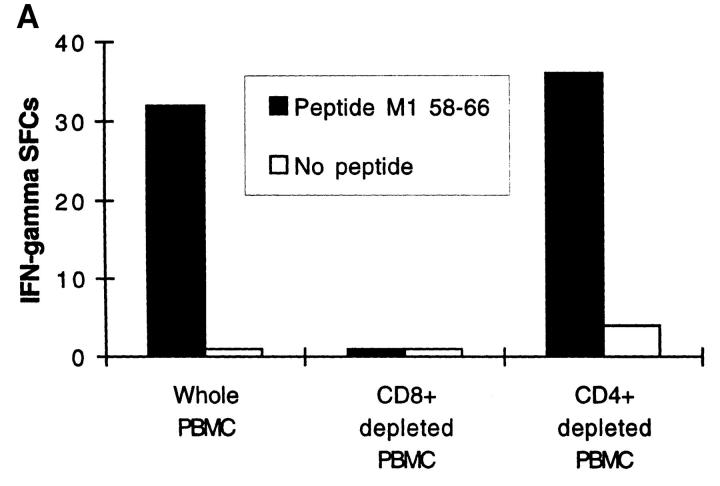
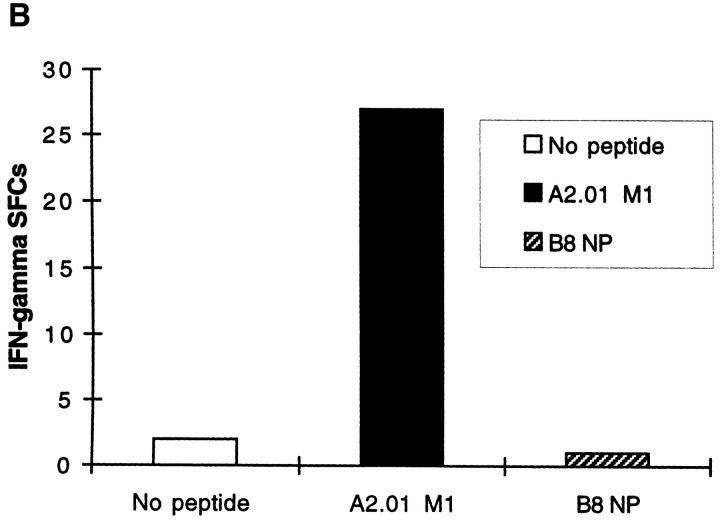
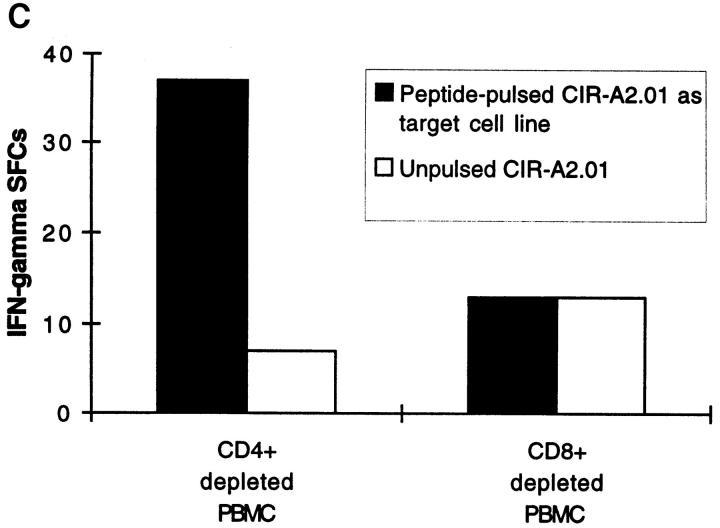
Peptide-specific Effector T Cells Detected by Ex Vivo ELISPOT Are CD8 + and HLA Class I Restricted. Depletion of CD8+ T cells from fresh PBMCs completely abrogated the influenza peptide–specific response (Fig. 2 A). Conversely, depletion of CD4+ cells did not diminish the number of IFN-γ SFCs, indicating that neither CD4+ cells nor their cytokine products were required for the acquisition or deployment of effector function by the freshly isolated peptide-specific CD8+ T cells (Fig. 2 A). Immediate effector responses were only detected to influenza epitopes restricted by the HLA class I alleles present in the particular donor being tested; influenza peptides restricted by HLA class I molecules not present in the donor never resulted in IFN-γ SFCs, as illustrated in Fig. 2 B. To confirm that the HLA-A2.01–restricted matrix epitope, for example, was presented through HLA-A2.01, we used the class I–reduced cell line, CIR-A2.01, which expresses only HLA-A2.01 at a significant level, prepulsed with the peptide M1 58-66, to present this epitope to fresh PBMCs. Despite high backgrounds representing allo-responses to non-A2.01 MHC molecules expressed at a low level on CIR-A2.01, peptide-specific responses to the HLA-A2.01–restricted influenza virus matrix epitope were detected (Fig. 2 C).
Figure 2.
(A) Influenza virus–specific CD8+ memory T cells with rapid effector function are CD8+. Freshly isolated PBMCs from donor SC were tested against the influenza HLA-A2.01–restricted peptide M1 58–66 at a concentration of 2 μM in a 14 h ELISPOT assay before or after depletion of CD4+ or CD8+ cells. Input cell numbers were 5 × 105/ well predepletion, and mean values from duplicate wells are shown. (B and C) Unrestimulated influenza virus–specific memory CD8+ T cells are HLA class I restricted. (B) Peptide epitopes restricted by HLA class I alleles not present in the donor, do not elicit IFN-γ SFCs. Freshly isolated PBMCs from donor SC (class I HLA haplotype: A2.01, A28; B7, B14) were tested in an ELISPOT assay in the absence of peptide and in the presence of the influenza HLA-A2.01–restricted peptide M1 58–66 or the irrelevant influenza HLA-B8-restricted peptide NP 380–388, each at a concentration of 1 μM. (C) Freshly isolated CD8+ T cells recognize the HLA-A2.01–restricted peptide M1 58–66 presented by HLA-A2.01 on the surface of the cell line CIR-A2.01. Fresh PBMCs from donor WB (HLA class I haplotype: A1, A2; B7, B8) were depleted of CD4+ or CD8+ cells; after depletion, 3 × 105 cells were plated out per well, along with 2 × 104 CIR-A2.01 cells prepulsed with 2 μM of the influenza HLA-A2.01–restricted peptide M1 58–66 and washed three times. Control wells had equal numbers of peptide-unpulsed CIR-A2.01.
Influenza Peptide-specific CD8+ Effector T Cells Freshly Isolated from Peripheral Blood Were Not Detectable by Conventional 51Cr-release CTL Assays.
During acute influenza infection, expanded antigen-specific CD8+ effector T cells can be detected by 51Cr-release cytotoxicity assays performed with fresh uncultured PBMCs (Klenerman, P., M. Callan, and A.J. McMichael, unpublished observations). After recovery, however, such ‘fresh killing' is not observed. Our results indicate that this may not be because effector CD8+ T cells are absent, but rather because their numbers have declined to a very low frequency. ELISPOT assays with freshly isolated PBMCs from donor WB consistently detected effector CD8+ T cells specific for the HLA-A2.1- restricted matrix epitope M1 58–66 at eight different time points, whereas parallel 51Cr-release cytotoxicity assays with the same PBMCs failed to give measurable specific lysis on several occasions, even at effector/target ratios of 100:1 (data not shown).
The Frequency of Peptide-specific CD8+ T Cells Enumerated by Ex Vivo ELISPOT Is Higher than the Corresponding CTL Precursor Frequency Derived by LDA.
Expressing the enumerated IFN-γ SFCs as a proportion of the input number of fresh PBMCs gives a measure of the frequency of circulating peptide-specific CD8+ effectors in peripheral blood (Table 1). We investigated whether the antigen-specific CD8+ T cells enumerated by the ex vivo ELISPOT assay are detectable by LDA. Table 2 shows the results for several subjects using three different influenza virus epitopes; for each individual, the ELISPOT assay and LDA were performed in parallel on PBMCs from the same blood sample. In all but one individual, the number of specific CD8+ T cells detected by ELISPOT assay is severalfold higher than the CTL precursor frequency generated by the corresponding LDA. For donor AH, the ELISPOT assay and LDA give similar frequencies. For the remaining five pairs of assays, performed in four individuals, there is a correlation between the IFN-γ SFCs in the ELISPOT and the precursor frequencies estimated by LDA (r [correlation coefficient] = 0.99), with the ELISPOT for IFN-γ consistently detecting higher numbers of peptide-specific CD8+ T cells than LDA. The LDA methodology used is based on that of Lehner et al. (16) who measured CTL precursor frequencies for the HLA-A2.01–restricted matrix epitope M1 58–66 in five healthy donors. The range of CTL precursor frequencies by LDA for this epitope in the three HLA-A2.01 positive donors we have studied here is 1/59,000 to 1/250,00, which is very similar to the range of 1/54,000 to <1/ 250,000 obtained by Lehner et al. (16).
Table 2.
Peptide-specific T Cell Frequencies Enumerated by IFN-γ ELISPOT and Corresponding Precursor Frequencies by LDA for Influenza Epitopes in a Series of Individuals
| Donor | Epitope tested | HLA class I restriction | Sequence | Effector frequency in PBMCs by ELISPOT | Precursor frequency in PBMCs by LDA |
|---|---|---|---|---|---|
| WB (9/96)* | M1 58–66 | HLA-A2.01 | GILGFVFTL | 1/15,000 | 1/100,000 |
| WB (11/96)‡ | M1 58–66 | HLA-A2.01 | GILGFVFTL | 1/111,000 | 1/250,000 |
| SM‡ | M1 58–66 | HLA-A2.01 | GILGFVFTL | 1/6,000 | 1/59,000 |
| JM | M1 58–66 | HLA-A2.01 | GILGFVFTL | 1/77,000 | 1/333,000 |
| PM | NP 380–388 | HLA-B8 | ELRSRYWAI | 1/43,000 | 1/200,000 |
| AH | NP 265–273 | HLA-A3 | ILRGSVAHK | 1/45,000 | 1/42,000 |
The Majority of Influenza-specific CD8+ Effectors Express CD45RO.
Antigen-experienced T cells express CD45RO, a marker of memory phenotype (17). Fresh PBMCs stained with anti-CD45RO antibody were magnetically depleted; this maneuver greatly reduced the number of peptide-specific IFN-γ SFCs, commensurate with the 67% depletion of CD45RO+ cells measured by FACS® analysis (Fig. 3 A). Similar results were also obtained with CD8+ cells specific for other epitopes.
Figure 3.
(A) The majority of effectors detected in the ex vivo ELISPOT assay bear the memory-associated cell-surface marker CD45RO. Fresh PBMCs from donor SD were depleted of CD45RO+ cells and plated out at 5 × 105/well with or without the HLA-B27.05–restricted peptide NP 383– 391. FACS® analysis confirmed 67% depletion of CD45RO+ CD8+ T cells from PBMCs. (B) All detectable CD8+ effectors specific for the A2.01-restricted peptide M1 58–66 bear TCRs with the Vβ17 gene segment. The ELISPOT assay was performed with freshly isolated whole PBMCs or PBMCs depleted of Vβ17+ T cells; FACS® analysis confirmed that the CD8+ Vβ17+ T cells initially present among whole PBMCs were depleted by 90%. Cells were seeded at 5 × 105/well in the presence of the HLA-A2.01–restricted peptide M1 58–66 or the HLA-B8–restricted peptide NP 380–388. PBMCs were from donor WB (HLA class I haplotype: A1, A2.01; B7, B8).
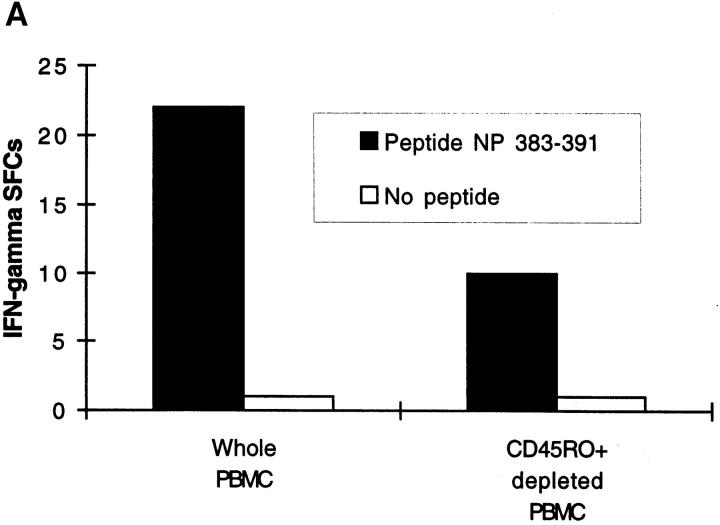
The Ex Vivo Response of Unrestimulated CD8+ T Lymphocytes to the HLA-A2.1–restricted M1 58–66 Epitope Is Dominated by T Cells Bearing Vβ17+ TCRs.
M1 58–66-specific CTL clones and lines show marked conservation for usage of the Vβ17 gene segment in their TCRs (16, 18). Depletion of fresh PBMCs stained with an anti-Vβ17 antibody abrogated the ex vivo ELISPOT response to the HLA-A2.1–restricted epitope M1 58–66, indicating that usage of the Vβ17 gene segment is highly conserved amongst circulating uncultured M1 58-66–specific CD8+ effectors (Fig. 3 B). In contrast, the HLA-B8–restricted response to the nucleoprotein epitope NP 380–88 in the same donor was unaffected.
Discussion
Using a sensitive ex vivo assay, we have directly characterized unrestimulated low frequency antigen-specific CD8+ memory T cells freshly isolated from peripheral blood. In individuals in the memory state with respect to influenza virus infection, we have demonstrated that influenza-specific memory CD8+ T cells circulate in a state wherein they can display effector function within 6 h of antigen contact. The fact that IFN-γ release within 6 h was triggered by exposure to cognate peptide alone and in the absence of exogenous cytokines, suggests that these CD8+ T cells are capable of immediate effector function in their natural state in vivo. This novel population of CD8+ T cells thus does not conform to the conventional view of memory T cells which require restimulation to divide and differentiate to become effectors (1). The identification of influenza-specific CD8+ effector T cells in our donors is remarkable given that the donors have all been exposed to influenza in the past but none had acute influenza at the time of venesection and most have not experienced clinical influenza for several years. This does not exclude the possibility of a more recent subclinical infection, but even this would have been at least 5 mo before venesection, since influenza virus stopped circulating in this region by early April 1996 (as per records of viral isolates, Virology Department, Public Health Laboratory, John Radcliffe Hospital, Oxford, UK) and most subjects were studied from September through mid-November. Thus, long after an acute viral infection in humans with a nonpersistent virus, we have identified memory CD8+ T cells capable of rapid effector activity which are, in a functional sense, in a relatively activated state.
During acute (19) and some persistent (20) viral infections, the frequency of circulating antigen-specific CD8+ effectors is markedly raised and lytic activity can be demonstrated in freshly isolated PBMCs (19). However, after recovery, such activated effector cells are not detectable in humans. Murine studies using peripheral (mucosal, cutaneous, and solid organ) rather than intravenous routes of challenge with cytopathic and noncytopathic viruses have demonstrated that antiviral protective immunity in vivo depends upon circulating activated CTLs, capable of rapid effector function as measured ex vivo in CTL assays with freshly isolated PBMCs and in vivo by cytokine-mediated foot pad swelling (4, 21, 22). These cells are similar to the expanded populations of CD8+ effector T cells found during acute viral infections; their continued presence in a relatively activated state at a low frequency after recovery is thought to reflect ongoing low-level stimulation by persisting antigen (21). We provide evidence for an analogous population of CD8+ T cells long after recovery from an acute viral infection in humans.
Influenza virus is cytopathic and causes disease by replicating (with a life cycle of 3–6 h) and causing tissue damage at its site of entry, the nasopharyngeal mucosa. In this situation, cellular antiviral protective immunity in vivo would require circulating, influenza-specific CD8+ T cells capable of rapid effector function; this is the phenotype displayed by the influenza-specific CD8+ T cells characterized here, and we propose that these cells subserve protective immunological memory to influenza virus infection. The almost immediate release of IFN-γ by these lymphocytes would have rapid antiviral effects (12) acting in a paracrine fashion on both infected and uninfected cells at mucosal surfaces. Confirmation of this hypothesis will require the demonstration of a protective association of the presence of specific CD8+ effectors during the next influenza pandemic with an antigenically shifted virus that circumvents humoral immunity.
Since the freshly isolated influenza-specific CD8+ T cells we have characterized are present at such low frequencies, it is not possible to test whether they can directly lyse target cells since this is only measurable when they constitute 0.1–1% of the population. However, the frequency of virus-specific CD8+ T cells reaches this value in HIV-infected patients and here there is a close correlation between the frequency of effector IFN-γ SFCs and percentage-specific lysis in fresh ex vivo CTL assays across a range of epitopes (Lalvani, A., G. Ogg, and A.J. McMichael, unpublished observations). Further indirect evidence that the cells defined here may be cytotoxic is provided by the experiment illustrated in Fig. 3 B which shows that all the freshly isolated IFN-γ–secreting CD8+ T cells specific for the HLA-A2.01–restricted peptide M1 58–66 bear Vβ17+ TCRs. It is known that CTL lines and clones specific for the M1 58– 66 influenza epitope are dominated by TCRs incorporating the Vβ17 gene segment and the magnitude of peptide-specific lysis correlates with the proportion of CD8+ T cells bearing Vβ17+ TCRs (16, 18). Therefore, it seems likely that a proportion of the M1 58–66-specific IFN-γ SFCs are CTLs, and Fig. 3 B also confirms that Vβ17 restriction of the M1 58–66 response is not merely a bias introduced by in vitro restimulation since it also defines the fresh ex vivo response to this epitope.
The frequency of antigen-specific T cells enumerated by ex vivo ELISPOT is generally severalfold higher than that calculated from LDA, the traditional method for quantitating CD8+ T cells. This suggests that the LDA detects only a subset of the specific CD8+ T cells quantitated by the ELISPOT. This may be because the LDA measures only those CTL precursors with a capacity to proliferate on antigenic stimulation in vitro (20); a proportion of the effectors detected by the ELISPOT assay probably lack this proliferative potential. It would appear that these novel CD8+ memory T cells capable of rapid effector function have thus been previously overlooked by standard techniques based on in vitro stimulation and proliferation.
Acknowledgments
We thank the subjects who donated blood; John Kurtz for records of influenza virus isolates in Oxford Regional Health Authority; and Pilar Degano and Susan Daenke for helpful discussions. A. Lalvani, S. Hambleton, R. Brookes, and A.J. McMichael are supported by the Medical Research Council of Great Britain and A.V.S. Hill is a Wellcome Trust Principal Research Fellow.
Footnotes
1
Abbreviations used in this paper: ELISPOT, enzyme-linked immunospot; LDA, limiting dilution analysis; NP, nucleoprotein; SFC, spot-forming cell.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (Wash DC) 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J, Tough DF. Lymphocyte lifespan and memory. Science (Wash DC) 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 4.Kuendig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJL, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oehen S, Waldner H, Kuendig TM, Hengartner H, Zinkernagel RM. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMichael AJ, Gotch FM, Noble GR, Beare AS. Cytotoxic T cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Askonas BA. Biological properties of an influenza virus specific killer T cell clone. J Exp Med. 1981;154:225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend A, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 9.Gotch FM, McMichael AJ, Smith GL, Moss B. Identification of the virus molecules recognized by influenza-specific cytotoxic T lymphocytes. J Exp Med. 1987;165:408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMichael AJ. Cytotoxic T lymphocyte specific for influenza virus. Curr Top Microbiol Immunol. 1994;189:75–91. doi: 10.1007/978-3-642-78530-6_5. [DOI] [PubMed] [Google Scholar]
- 11.Parker CE, Gould KG. Influenza A virus: a model for antigen presentation to cytotoxic T lymphocytes. Semin Virol. 1996;7:61–73. [Google Scholar]
- 12.Morris AG, Lin YL, Askonas BA. Immune interferon release when a cloned cytotoxic T cell line meets its correct influenza-infected target cell. Nature (Lond) 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaegi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 14.Krausa PM, Brywka M, III, Savage D, Hui KM, Bunce M, Ngai JLF, Teo DLT, Ong YW, Barouch D, Allsopp CEM, et al. Genetic polymorphism within HLA-A*02: significant allelic variation revealed in different ethnic populations. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 15.Klinman DM. ELISPOT assay to detect cytokine- secreting murine and human cells. Curr Prot Immunol. 1994;6.19:1–8. doi: 10.1002/0471142735.im0619s10. [DOI] [PubMed] [Google Scholar]
- 16.Lehner PJ, Wang ECY, Moss PAH, Williams S, Platt K, Friedman SM, Bell JI, Borysiewicz LK. Human HLA-A201–restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the Vβ17 gene segment. J Exp Med. 1995;181:79–91. doi: 10.1084/jem.181.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 18.Moss PA, Moots RJ, Rosenberg WM, Rowland-Jones SJ, Bodmer HC, McMichael AJ, Bell JI. Extensive conservation of α and β chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci USA. 1993;88:8987–8990. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callan MF, Steven N, Krausa P, Wilson JDK, Moss PAH, Gillespie GM, Bell JI, Rickinson AB, McMichael AJ. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the HIV-1–specific CTL response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinkernagel RM, Bachmann MF, Kuendig TM, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, Kuendig TM, Hengartner H, Zinkernagal RM. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without “memory T cells?” . Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]