CpG Oligodeoxynucleotides Act as Adjuvants that Switch on T Helper 1 (Th1) Immunity (original) (raw)
Abstract
Synthetic oligodeoxynucleotides (ODN) that contain unmethylated CpG motifs (CpG ODN) induce macrophages to secrete IL-12, which induces interferon (IFN)-γ secretion by natural killer (NK) cells. Since these cytokines can induce T helper 1 (Th1) differentiation, we examined the effects of coadministered CpG ODN on the differentiation of Th responses to hen egg lysozyme (HEL). In both BALB/c (Th2-biased) and B10.D2 (Th1-biased) mice, immunization with HEL in incomplete Freund's adjuvant (IFA) resulted in Th2-dominated immune responses characterized by HEL-specific secretion of IL-5 but not IFN-γ. In contrast, immunization with IFA-HEL plus CpG ODN switched the immune response to a Th1-dominated cytokine pattern, with high levels of HEL-specific IFN-γ secretion and decreased HEL-specific IL-5 production. IFA-HEL plus CpG ODN also induced anti-HEL IgG2a (a Th1-associated isotype), which was not induced by IFA-HEL alone. Control non–CpG ODN did not induce IFN-γ or IgG2a, excepting lesser increases in B10.D2 (Th1-biased) mice. Thus, CpG ODN provide a signal to switch on Th1-dominated responses to coadministered antigen and are potential adjuvants for human vaccines to elicit protective Th1 immunity.
Antigen-specific CD4+ Th cell responses can be divided into two types, type 1 and type 2, based upon cytokine secretion and effector function (1–3). Type 1 responses involve Th1 cells, whose differentiation is driven by IL-12 (from macrophages) and IFN-γ (from NK cells or T cells). Th1 cells secrete cytokines such as IFN-γ, IL-2, and lymphotoxin. In turn, IFN-γ activates macrophages and enhances immunoglobulin isotype switching to IgG2a, a hallmark of Th1 immunity (4). In contrast, type 2 responses involve IL-4–dependent differentiation of Th2 cells, which produce IL-4, IL-5, IL-10, and IL-13. Type 2 responses are associated with decreased macrophage activation, since some Th2-associated cytokines depress certain macrophage functions. The Th1/Th2 model provides a useful conceptual framework for Th differentiation, and the existence of distinct type 1 and type 2 responses is clearly established, although certain aspects of the model require further investigation (5). Moreover, differential induction of type 1 or type 2 responses is required for protective immunity to certain infectious diseases, and induction of the wrong response type can increase susceptibility to infection (see Discussion). Thus, the type of response induced by a vaccine may be crucial to its efficacy.
The type of Th response generated to an administered antigen can be directed by the type of adjuvant used. Injection of antigen in CFA induces a Th1-dominated response to the antigen, while injection of antigen in IFA induces a Th2-dominated response (6). However, because of its undesirable inflammatory side effects, CFA is not suited for use in human vaccines. Since type 1 immunity plays an important role in the protective response to infection with certain microbes, it is now important to characterize other novel adjuvants that safely induce type 1 immunity and that may potentially be incorporated in future human vaccines. The recent discovery that certain DNA preparations affect cytokine expression by cells of the innate immune system suggests the possibility that DNA preparations could be used as adjuvants to influence the differentiation of Th responses.
The ability of DNA to induce expression of cytokines depends on its source and characteristics (7). In vitro, bacterial DNA induces macrophage expression of IL-12 (8) and TNF-α (9), which are not induced by mammalian DNA. Bacterial DNA also indirectly activates NK cells and stimulates their production of IFN-γ (10–12), since NK cell production of IFN-γ is triggered by IL-12 that is generated by macrophages in response to bacterial DNA (8, 13).
To define components of bacterial DNA that have immunomodulatory effects, a panel of synthetic oligodeoxynucleotides (ODN)1 was used to identify specific 6-base pair sequences that conferred activity (14). These sequences shared a CpG motif, containing a central unmethylated CpG dinucleotide preferentially flanked by two 5′ purines and two 3′ pyrimidines. CpG dinucleotides are present in bacterial DNA at the expected frequency of 1/16 bases, but they are three- to fourfold less frequent in mammalian DNA, a phenomenon known as CpG suppression (15). Also, the cytosines in CpG dinucleotides in mammalian DNA are highly methylated, whereas those in bacterial DNA are not (15). Elimination of the CpG sequence or methylation of the cytosine abrogates the stimulatory activity of ODN containing CpG motifs (CpG ODN) and bacterial DNA (9, 11, 14).
When added to splenocytes in culture, CpG ODN induce production of the Th1-associated cytokines IFN-γ and IL-12, as well as the Th2-associated cytokine, IL-6, within several hours (16). However, production of other Th2-associated cytokines, such as IL-4, IL-5, and IL-10, is not detected. The rapid production of IFN-γ is mediated by NK cells stimulated by IL-12 secretion from CpG-activated macrophages; this initial phase of IFN-γ production does not require T cells (8, 13). The induction of IFN-γ and IL-12 (which promote Th1 responses), but not IL-4 (which promotes Th2 responses), suggests that administration of CpG ODN in vivo might produce an environment favoring a Th1 immune response. Indeed, some bacterial plasmid DNA vaccines, which contain this CpG motif, cause development of antigen-specific CD4+ splenocytes that secrete IFN-γ, but not IL-4 or IL-5 (17, 18).
The effect of CpG ODN on antigen-specific T cell responses has not been previously tested. Our current experiments directly test the hypothesis that CpG ODN may serve as adjuvants to switch on Th1 responses. While immunization with hen egg lysozyme (HEL) in IFA induced a Th2-dominated response to HEL, immunization with IFA-HEL plus CpG ODN induced a strongly Th1-dominated response to HEL, as measured by production of specific IgG2a antibody and production of IFN-γ by antigen-stimulated T cells. We propose that CpG ODN function as adjuvants that switch on Th1 responses, making them important candidate adjuvants for potential use in future human vaccines.
Materials and Methods
Oligodeoxynucleotides.
ODN were purchased from Operon Technologies (Alameda, CA) or Oligos Etc. (Wilsonville, OR). ODN were phosphorothioate-modified to increase their resistance to nuclease degradation. ODN used in these studies are listed in Table 1 and their sequences are given here (CpG motifs or reversed non-CpG motifs are underlined). Sequences of ODN that were phosphorothioate-modified throughout (S ODN) are: CpG ODN 1826, TCCATGACGTTCCTGACGTT; non–CpG ODN 1745, TCCAATGAGCTTCCTGAGTCT; CpG ODN 1760, ATAATCGACGTTCAAGCAAG; non–CpG ODN 1908, ATAATAGAGCTTCAAGCAAG. Sequences of ODN phosphorothioate-modified on the ends only (S-O ODN) are: CpG ODN 1585, GGGGTCAACGTTGAGGGGGG; and non–CpG ODN 1972, GGGGTCTGTGCTTTTGGGGGG. The first two 5′ end bonds and last five 3′ end bonds of the S-O ODN are phosphorothioate-modified. Synthetic ODN were dissolved in TE (10 mM Tris, 1 mM EDTA). LPS content of ODN was <1 ng LPS/mg DNA, as measured by Limulus amebocyte assay (QCL-1000; BioWhittaker, Walkersville, MD).
Table 1.
Sequences of Synthetic ODN
| ODN | Sequence* | Motif | Backbone |
|---|---|---|---|
| ↓ ↓ ↓ ↓ | |||
| 1826 | TCCATGACGTTCCTGACGT T | CpG | S ODN |
| 1745 | TCCAATGAGCTTCCTGAGTC T | non-CpG | S ODN |
| ↓ ↓ ↓ ↓ | |||
| 1760 | ATAATCGACGTTCAAGCAAG | CpG | S ODN |
| 1908 | ATAATAGAGCTTCAAGCAAG | non-CpG | S ODN |
| ↓ ↓ | |||
| 1585 | GGGGTCAACGTTGAGGGGGG | CpG | S-O ODN |
| 1972 | GGGGTCTGTGCTTTTGGGGGG | non-CpG | S-O ODN |
Immunizations.
BALB/c and B10.D2 mice (Jackson Laboratory, Bar Harbor, ME and Harlan Sprague Dawley, Indianapolis, IN) were housed in microisolators under specific pathogen-free conditions and injected at 7–12 wk of age. HEL (Sigma Chem. Co., St. Louis, MO) was dissolved in PBS, ODN were dissolved in TE, and LPS (E. coli 0127:B8; Difco, Detroit, MI) was dissolved in PBS. ODN were added to HEL in a volume less than 10% of the final volume. HEL solutions with or without ODN or LPS were combined with IFA (GIBCO BRL, Gaithersburg, MD) at a 1:1 (vol/vol) ratio and emulsified to achieve a final HEL concentration of 1 mg/ml. CFA was prepared by suspending Mycobacterium tuberculosis H37 RA (Difco) at 4 mg/ml in IFA, and CFA was emulsified with the HEL solutions as above. Groups of three mice were injected i.p. with 200 μl of an emulsion and killed 3 wk after injection.
ELISA Assay for Antigen-specific Antibody Production.
Sera were collected from mice by tail bleed 3–4 d before sacrifice (15–18 d after immunization with HEL), then diluted 1:10 in PBS/0.2% sodium azide and stored at −20°C. For ELISA, Nunc brand 96-well immunoplates (Fisher, Pittsburgh, PA) were coated by overnight incubation at 4°C with HEL at 10 μg/ml in 0.1 M sodium bicarbonate buffer. Plates were washed and blocked with PBS with 0.05% Tween (PBST) containing 0.1% gelatin for 1–2 h at room temperature. Sera were added to the top row of each plate and serial 1:3 dilutions in PBS were then made into subsequent rows. The plates were incubated overnight at 4°C and washed. Alkaline phosphatase-conjugated detecting antibody was added in PBST/ 0.1% gelatin and incubated for 2 h at room temperature. For IgG1 and IgG2a detection, goat anti–mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, AL) was used at 1:4,000. For total Ig detection, goat anti–mouse Ig(H+L) (Southern Biotechnology Associates), specific for IgM + IgG + IgA, was used at 1:2,000. The colorimetric assay was developed with para-nitrophenyl phosphate (50 mg/ml in 2.5 M sodium bicarbonate/2.5 M magnesium chloride buffer) for 1–3 h. Absorbance at 405 nm was determined using a Beckman Bio Tek Microplate Autoreader (EL309; Beckman Instruments, Palo Alto, CA). The serum from each mouse was assayed in duplicate and the mean value was used to represent each animal. These values were used to calculate the mean and standard deviation for each group of three mice.
ELISA Spot Assay for Cytokine Production.
Splenocytes were isolated from mice 3 wk after immunization. A modified ELISA spot assay for detection of cytokine production by splenocytes has been developed in prior work (6). ELISA spot plates (Polyfiltronics, Rockland, MA) were coated with capture antibody for IFN-γ (R46A2; 4 μg/ml in PBS) or IL-5 (TRFK5; 5 μg/ml in PBS) overnight at 4°C. Plates were then washed and blocked with PBS/1% BSA for 1–2 h at room temperature. After washing, freshly isolated splenocytes were plated at 106 cells/well in serum-free medium, HL-1 (BioWhittaker), supplemented with l-glutamine and penicillin/streptomycin, in the presence or absence of HEL (100 μg/ml). In some experiments purified GK1.5 anti-CD4 antibody (American Type Culture Collection, Rockville, MD) was added at 10–30 μg/ml to block CD4 T cell function. After culture for 24 h (for IFN-γ detection) or 48 h (for IL-5 detection), cells were removed by washing with PBS and then PBST. Detecting antibody (XMG1.2-HRP, 1:400 for IFN-γ; TRFK4, 4 μg/ml for IL-5) was added in PBST/1% BSA and incubated overnight. For the IL-5 assay only, anti–IgG2a-HRP (Zymed, South San Francisco, CA) was added after washing with PBST, and the plates were incubated for 2 h at room temperature. All plates were washed with PBS before developing the colorimetric assay by the addition of 1% 3–amino-9-ethylcarbazole/ N,_N_-dimethylformamide in 0.1 M sodium acetate buffer (1:30 vol/vol) for 45–60 min. The plates were then washed with distilled water and air dried. Spots were quantitated by an image analysis program (Optimas, Bothell, WA).
Results
Coadminstered CpG ODN Induce Production of HEL-specific IgG2a (a Th1-associated Isotype).
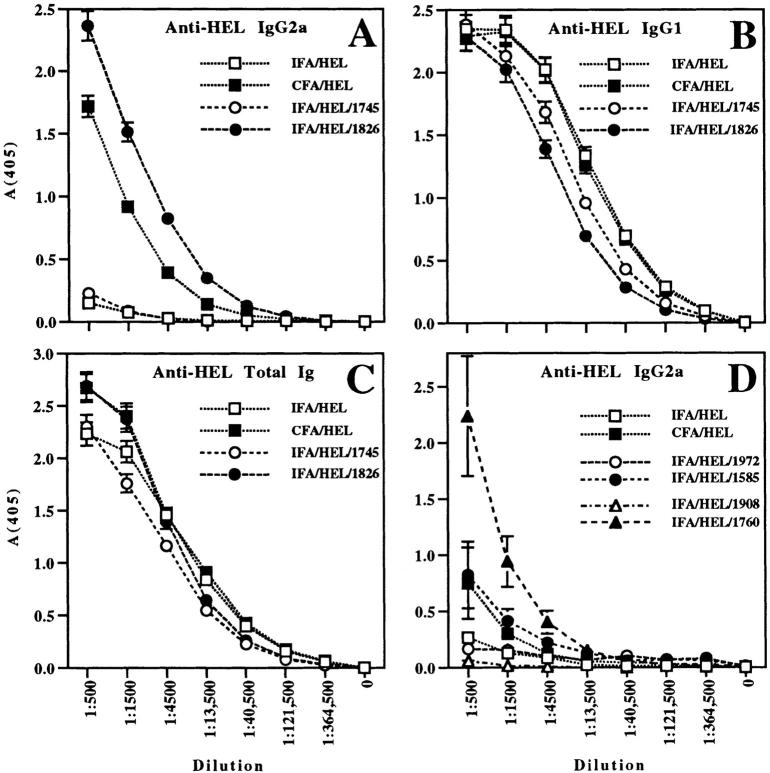
BALB/c mice were injected i.p. with 200 μg of HEL in the following adjuvants: CFA, IFA, IFA with CpG ODN 1826, or IFA with a similar ODN lacking the CpG motif, ODN 1745. ODN 1826 and ODN 1745 are phosphorothioate-modified for the entire length of the backbone (S ODN; see Table 1), which greatly increases resistance to nuclease degradation (19). Based on preliminary dose titration studies, ODN were initially used at 100 μg/mouse. Sera were collected 15–18 d after immunization and assayed for anti-HEL Ig (total or specific isotype) by ELISA. Consistent with previous results demonstrating that IFA induces a Th2 response while CFA induces a Th1 response to antigen (6), mice injected with IFA-HEL did not produce detectable IgG2a responses (Fig. 1 A). In contrast, mice injected with CFA-HEL produced high levels of IgG2a. The addition of non–CpG ODN 1745 to the IFA-HEL protocol did not induce IgG2a production. However, immunization with IFA-HEL-CpG ODN 1826 altered the isotype profile of the antibody response, causing a marked increase in anti-HEL IgG2a. Furthermore, in three independent experiments, the production of HEL-specific IgG2a was consistently higher in mice treated with IFA-HEL-CpG ODN 1826 than in mice treated with CFA-HEL.
Figure 1.
Th1-associated antigen-specific IgG2a responses are induced by immunization of BALB/c mice with IFA-HEL-CpG ODN but not IFA-HEL-non–CpG ODN. (A–C). Mice were injected i.p. with CFA-HEL (a control for a Th1-dominated response), IFA-HEL (a control for a Th2-dominated response), or IFA-HEL with 100 μg of CpG ODN 1826 or non–CpG ODN 1745. Sera were collected from mice 15–18 d after injection and assayed by ELISA for: (A) anti-HEL IgG2a, an isotype associated with Th1-dominated responses; (B) anti-HEL IgG1; and (C) anti-HEL total Ig response. A–C represent data from a single experiment representative of three similar experiments. (D) BALB/c mice were immunized as above, except that 30 μg of CpG ODN 1585, non–CpG ODN 1972, CpG ODN 1760, or non–CpG ODN 1908 was used for each mouse. Anti-HEL IgG2a antibodies were detected by serum ELISA. Data shown in D are representative of three similar experiments.
Despite the changes in IgG2a responses, similar levels of anti-HEL IgG1 or total anti-HEL Ig were produced by all immunizations (Fig. 1, B and C). Thus, immunization with IFA-HEL or IFA-HEL–non-CpG ODN 1745 was successful and sufficient to generate an antibody response to HEL, with both anti-HEL IgG1 and total anti-HEL Ig levels comparable to those seen with CFA-HEL or IFA-HEL-CpG ODN 1826. Although the IgG1 isotype has been linked to Th2 responses, our data demonstrate that IgG1 can also be observed in Th1-dominated responses and, at least in this system, cannot be used to accurately assess Th differentiation. We conclude that the increased IgG2a production associated with IFA-HEL-CpG ODN 1826, like that caused by CFA-HEL, represents a selective induction of this isotype, i.e., a qualitative switch in the relative levels of antibody isotypes produced rather than a simple enhancement of all anti-HEL isotypes.
To confirm the role of the CpG motif, we also examined the effects of two additional pairs of CpG and non– CpG ODN. CpG ODN 1760 and a related non-CpG control, ODN 1908, are S ODN (Table 1). CpG ODN 1585 and a related non-CpG control, ODN 1972, are phosphorothioate-modified on the ends only (S-O ODN; see Table 1). ODN 1760 and ODN 1826 share a common CpG motif, GACGTT. Immunization of BALB/c mice with HEL in IFA with or without 30 μg of each ODN showed that both CpG ODN (ODN 1760 and ODN 1585) induced anti-HEL IgG2a antibodies, which were not induced by the non–CpG ODN (ODN 1908 and ODN 1972) (Fig. 1 D). The S ODN 1760 induced significantly higher levels of anti-HEL IgG2a than CFA or the S-O ODN 1585 (which is more nuclease-sensitive than ODN 1760). Anti-HEL IgG1 and total anti-HEL Ig responses were similar in all groups (data not shown), again indicating that all of the immunizations generated anti-HEL antibody responses of similar overall magnitude. We conclude that antigen-specific IgG2a antibodies are induced by CpG ODN, suggesting that CpG ODN induce a Th1-dominated response to coadministered protein antigen.
Coadministration of CpG ODN Induces Th1-dominated Antigen-specific Cytokine Responses.
We used a modified ELISA spot assay (see Materials and Methods) to assess recall antigen-specific IFN-γ secretion as a measure of Th1 memory cells induced after immunization with HEL. Three weeks after immunization of BALB/c mice with HEL in adjuvant, splenocytes were isolated, incubated in vitro with or without HEL and assayed for IFN-γ production. Upon restimulation with HEL, splenocytes from mice immunized with IFA-HEL showed little or no antigen-specific production of IFN-γ (Fig. 2 A), as expected (6). In contrast, splenocytes from mice immunized with CFA-HEL showed antigen-specific production of IFN-γ, demonstrating that CFA-HEL induced a Th1 response to HEL in these mice, as previously observed (6). Prior studies using isolated spleen-derived CD4+ T cells (with irradiated BALB/c-scid splenocytes as antigen-presenting cells) have shown that antigen-specific cytokine secretion measured by this assay is mediated by CD4+ T cells (Yip, H., A. Karulin, M. Tary-Lehmann, P. Heeger, R. Trezza, T. Forsthuber, and P.V. Lehmann, manuscript submitted for publication).
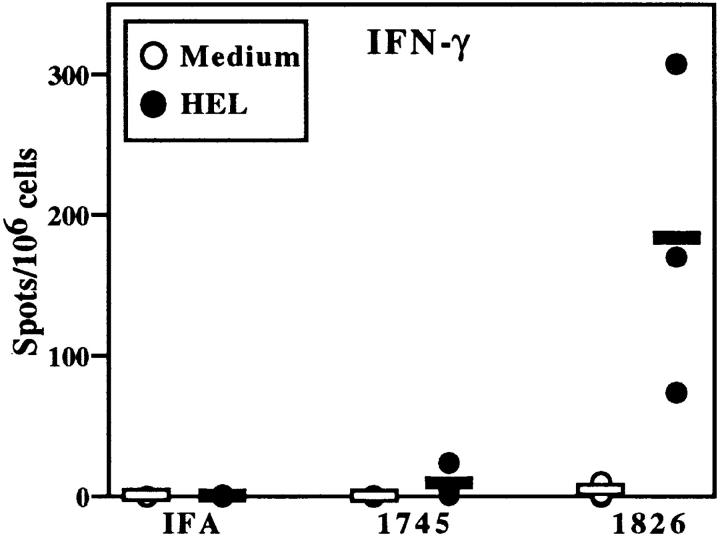
Figure 2.
CpG ODN enhance HEL-specific IFN-γ production by BALB/c splenocytes. Mice were immunized as in Fig. 1 with 100 μg ODN/mouse in A and 30 μg ODN/mouse in panel B. After 3 wk, splenocytes were isolated and incubated with HEL (closed circles) or medium alone (open circles). ELISA spot assay was performed and spots were quantitated by a computerized image analysis program. Each point represents the mean number of spots per well for one mouse (assayed in duplicate); horizontal bars indicate the mean of points for each group of mice. Similar results were observed in five independent experiments with CpG- and non–CpG ODN in BALB/c mice.
The results obtained with ODN established an important role for the CpG motif in determining Th differentiation. Immunization with IFA-HEL-non–CpG ODN 1745 did not enhance antigen-specific IFN-γ production over that observed with IFA-HEL. In contrast, immunization with IFA-HEL-CpG ODN 1826 strongly induced the production of antigen-specific cells secreting IFN-γ (Figs. 2 A and 3). Immunization with IFA-HEL-CpG ODN 1826 produced two- to fourfold more antigen-specific IFN-γ secretion than observed with CFA-HEL (Fig. 2 A and data not shown).
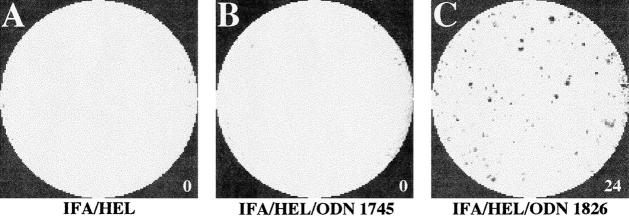
Figure 3.
ELISA spot assessment of IFN-γ production by splenocytes from immunized BALB/c mice. Pictures show representative images of ELISA spot wells from the experiment shown in Fig. 2 A. The number of spots, as quantitated by an image analysis program, is indicated next to each well. Each well contained HEL (100 μg/ml) and 106 splenocytes isolated from mice immunized with IFA-HEL (A), IFA-HEL-non–CpG ODN 1745 (B) or IFA-HEL-CpG ODN 1826 (C).
To determine the dose range for effective Th1 adjuvant activity of CpG ODN 1826, BALB/c mice were injected i.p. with 200 μg HEL in IFA, together with 0, 10, 30, or 100 μg ODN 1826. Antigen-specific serum Ig levels were assayed as above. Production of anti-HEL IgG2a was strongly enhanced in mice treated with as little as 10 μg ODN 1826, while specific production of total Ig and IgG1 was not affected by ODN 1826 at any dose (data not shown). ELISA spot analysis of splenocytes from these mice showed strong induction of IFN-γ by 30 or 100 μg ODN 1826 and lesser enhancement with only 10 μg (data not shown). Thus, Th1-directing adjuvant activity of CpG ODN is seen with doses as low as 10 μg in BALB/c mice.
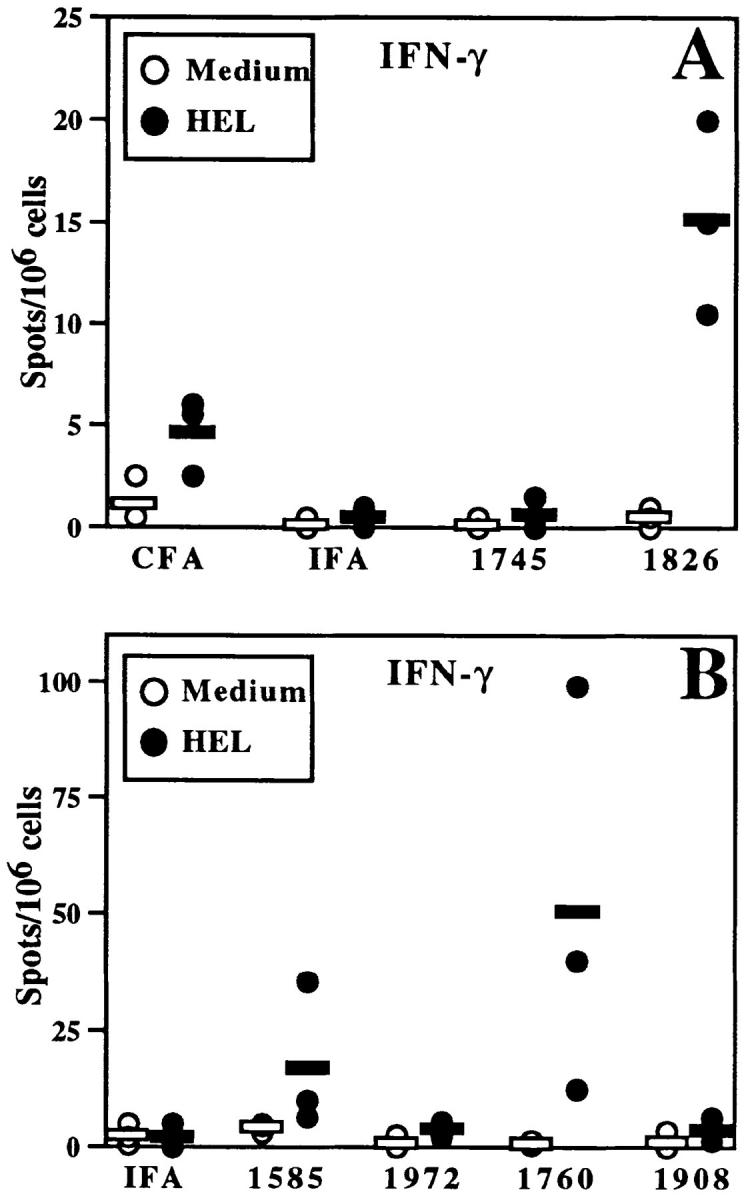
To confirm that the Th1 adjuvant activity of CpG ODN 1826 was specific to the CpG motif, other CpG and non– CpG ODN were examined for effects on the differentiation of the Th response to HEL. As demonstrated in Fig. 2 B, immunization with both of the additional CpG ODN (ODN 1760 and ODN 1585) increased the number of cells secreting IFN-γ in response to secondary stimulation with HEL, while little or no increase was seen with the non– CpG ODN (ODN 1972 and ODN 1908). Consistent with the pattern of IgG2a induction, the S ODN 1760 appeared to have a greater effect than the S-O ODN 1585 on increasing numbers of IFN-γ–secreting cells. Thus, the number of cells secreting IFN-γ is enhanced by CpG ODN but not by non–CpG ODN, supporting the induction of Th1-dominated responses by CpG ODN. Furthermore, the addition of anti-CD4 antibody (GK1.5 at 10–30 μg/ml) during the in vitro antigen stimulation blocked CpG ODN-enhanced, HEL-specific IFN-γ secretion (data not shown), confirming that the CpG ODN-enhanced production of IFN-γ was T cell–dependent in this system.
To assess Th2 differentiation, ELISA spot analysis was similarly performed to detect splenocytes producing IL-5 (Fig. 4). Immunization with IFA-HEL induced cells that secreted IL-5 in response to restimulation with HEL, consistent with a Th2-dominated response. In contrast to the results with IFA-HEL, little or no HEL-specific IL-5 secretion was seen in mice immunized with CFA-HEL, consistent with a Th1-dominated anti-HEL response in these mice. HEL-specific IL-5 secretion was observed after immunization with IFA-HEL-non–CpG ODN (e.g., ODN 1745 and ODN 1972), although immunization with IFA-HEL-non–CpG ODN 1745 produced somewhat lower levels of IL-5 than observed with IFA-HEL. In contrast, greater reduction in HEL-specific IL-5 secretion was observed after immunization with CpG ODN (ODN 1826, ODN 1760 and ODN 1585). Thus, the addition of CpG ODN induced a switch from a Th2-dominated response to a Th1-dominated response, as manifested by a decrease in Th2-associated cytokine secretion as well as the induction of Th1-associated cytokine secretion.
Figure 4.
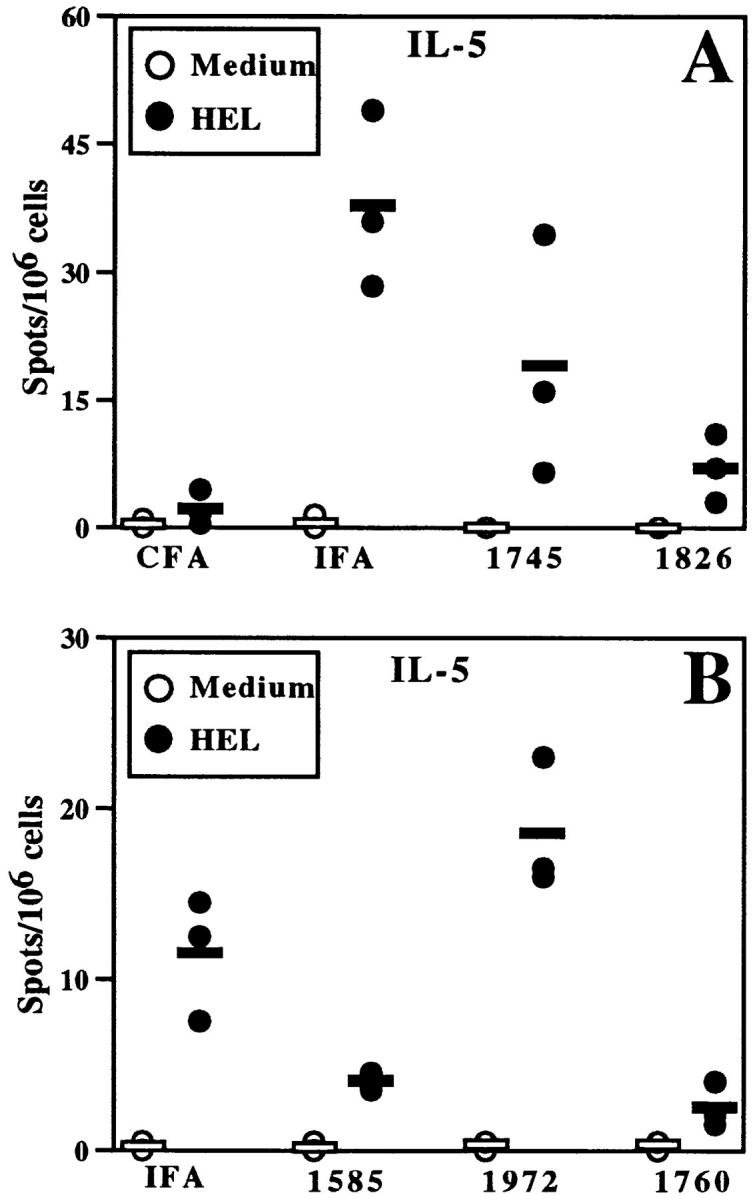
CpG ODN decrease HEL-specific IL-5 production by BALB/c splenocytes. Mice were immunized as in Fig. 2 (30 μg ODN/mouse), and splenocytes were harvested for in vitro restimulation with or without HEL. ELISA spot analysis was performed for IL-5. The data are representative of five similar experiments with CpG- and non–CpG ODN in BALB/c mice.
Together, these results indicate that CpG ODN directed Th1 differentiation in the T cell responses to coadminstered antigen. Relative to immunization with IFA-HEL, immunization with IFA-HEL-CpG ODN increased HEL-specific IFN-γ production by splenocytes, decreased HEL-specific IL-5 production by splenocytes and increased IgG2a anti-HEL titers. Furthermore, the Th1 adjuvant activity of CpG ODN for both antigen-specific antibody and cytokine production was significantly greater than that of an established Th1 adjuvant, CFA.
CpG ODN Direct Th1-dominated Responses in Th1-biased (B10.D2) Mice as well as Th2-biased (BALB/c) Mice.
Strains of mice differ in genetic bias toward the development of Th1- or Th2-dominated Th responses. Earlier publications have demonstrated that BALB/c mice are Th2-biased, while B10.D2 mice are more Th1-biased (20). To explore the impact of varying Th1/Th2 bias on the effect of CpG ODN, B10.D2 mice were injected i.p. with IFA-HEL, with or without 30 μg CpG ODN 1826 or non–CpG ODN 1745, and splenocytes were subsequently isolated for ELISA spot analysis. Immunization with IFA-HEL-CpG ODN 1826 produced a very high level of HEL-specific IFN-γ production, while IFN-γ was not produced after immunization with IFA-HEL alone (Fig. 5). Again, CpG ODN 1826 induced levels of HEL-specific IFN-γ production that exceeded even those seen after immunization with CFA-HEL, and augmentation of IFN-γ production, albeit at lower levels, was seen in B10.D2 mice treated with as little as 3 μg of ODN 1826 in IFA-HEL (data not shown). Immunization with IFA-HEL plus either of the two other CpG ODN, ODN 1760, and ODN 1585, also induced HEL-specific secretion of IFN-γ (data not shown). Immunization with the non–CpG ODN 1745 and 1908 (30 μg dose) induced HEL-specific production of IFN-γ by splenocytes from B10.D2 mice, but at a minimal level (Fig. 5 and data not shown), while the other non–CpG ODN, ODN 1972, did not induce IFN-γ (data not shown). Thus, CpG ODN had strong Th1 adjuvant activity in Th1-biased as well as Th2-biased mice, while non–CpG ODN induced little or no Th1 differentiation, as assessed by antigen-specific secretion of IFN-γ.
Figure 5.
Induction of HEL-specific IFN-γ responses by CpG ODN in B10.D2 mice. B10.D2 mice were immunized as in Fig. 1, except that ODN were used at 30 μg per mouse. Three weeks after immunization, HEL-specific production of IFN-γ by splenocytes was measured by ELISA spot assay as in Fig. 2. The data shown are representative of three similar experiments.
The effects of CpG ODN on IFN-γ responses were paralleled by changes in IgG2a levels in B10.D2 mice. Again, immunization with IFA-HEL-CpG ODN 1826 (3, 10, or 30 μg ODN) induced high titers of anti-HEL IgG2a, and similar results were seen with the other CpG ODN, ODN 1760, and ODN 1585 (only the 30 μg dose was assessed, data not shown). Mice treated with 30 μg non–CpG ODN 1745 also produced anti-HEL IgG2a, though the levels were not as high as in mice treated with ODN 1826, but the other non–CpG ODN (ODN 1908 and ODN 1972) did not induce IgG2a production. Anti-HEL total Ig and IgG1 levels were similar under all of these conditions (data not shown). These results confirm that CpG ODN enhance Th1-associated antigen-specific IgG2a responses in both Th1- and Th2-biased mouse strains.
Discussion
Because it is highly effective in inducing both cellular and humoral immunity, CFA has been an important model adjuvant (21). Furthermore, CFA has been shown to induce Th1-dominated immune responses (6). However, due to its inflammatory side effects, CFA cannot be used in humans. Thus, the discovery and characterization of adjuvants that promote Th1 cell–mediated immunity is currently an important area in vaccine development. Our results establish that CpG ODN are excellent candidate adjuvants for vaccines to induce Th1 immunity.
CFA is prepared by mixing two components, IFA (mineral oil) and nonviable Mycobacterium tuberculosis. As an adjuvant, CFA has been proposed to provide two crucial functions. First, it creates a local antigen depot (by entrapment of antigen in the mineral oil emulsion) which allows for prolonged regional antigenic stimulation. This function is also provided by IFA. Second, CFA contains immunomodulatory substances derived from Mycobacterium tuberculosis that stimulate immune responses and promote Th1 differentiation. It is possible that the adjuvant activity of CFA may be due in part to mycobacterial DNA, as M. bovis DNA sequences have been shown to be immunostimulatory (22, 23). Thus, a general strategy for the development of type 1 vaccine adjuvants may be to provide both an antigen depot and an immunomodulatory function that promotes Th1 differentiation.
Our studies address the ability of CpG ODN to modulate the differentiation of Th responses. In these experiments, ODN were mixed with IFA, which itself establishes an antigen depot but does not promote Th1 differentiation (future vaccines using CpG ODN may include alternative components such as biodegradable oils to optimize vaccine safety and efficacy). Mice that were immunized with IFA-HEL developed both humoral and Th2 cellular immune responses, consistent with prior observations (6). However, the addition of CpG ODN to this system induced strong Th1-dominated responses. Th1-dominated responses were induced with as little as 3 μg CpG ODN in B10.D2 mice and 10 μg CpG ODN in BALB/c mice.
As a direct measure of Th1 differentiation, we monitored the number of splenocytes secreting IFN-γ after immunization and in vitro stimulation with HEL by ELISA spot assay. This assay has been shown to detect cytokine secretion by individual CD4+ T cells (Yip, H., A. Karulin, M. Tary-Lehmann, P. Heeger, R. Trezza, T. Forsthuber, and P.V. Lehmann, manuscript submitted for publication). Also, addition of a blocking anti-CD4 mAb to the splenocytes during in vitro stimulation with HEL decreased HEL-specific production of IFN-γ by >80% (data not shown), confirming that the production of IFN-γ was dependent on antigen-specific CD4+ T cells. We also measured IFN-γ production by standard ELISA and found the same pattern of results as obtained by the ELISA spot assay (data not shown).
The addition of CpG ODN as adjuvants produced levels of IFN-γ that surpassed even the levels observed with CFA. Two of the non–CpG ODN (ODN 1745 and ODN 1908) induced IFN-γ secretion, but only at minimal levels and only in Th1-biased B10.D2 mice. ODN 1745 also induced production of antigen-specific IgG2a, although ODN 1908 did not. The slight CpG-like effects of ODN 1745 may be due in part to the presence of TG dimers in a context that provides a weak analogue of a CpG motif. Other studies have shown that weak induction in vitro of other CpG-like effects by ODN 1745 (B cell proliferation, secretion of IL-6, TNF-α, and IL-12) are eliminated in analogues of ODN 1745 that lack TG dimers (Krieg, A.M., unpublished observations). In addition, weak CpG-like effects could be triggered by the DNA backbone of non–CpG ODN (e.g. ODN 1745 and ODN 1908), since the phosphorothioate backbone of modified ODN has some intrinsic immunostimulatory properties (7, 24). Despite minor activities of some non–CpG ODN, the CpG ODN were vastly superior for inducing Th1-dominated immune responses in both Th1- and Th2-biased mouse strains. These observations indicate that the CpG motif provides a strong and reliable stimulus for Th1 differentiation in animals of differing genetic background.
We also assessed the differentiation of Th responses indirectly by the isotype profile of antigen-specific antibody responses. CpG ODN induced levels of antigen-specific IgG2a that surpassed even those achieved with CFA. Total anti-HEL Ig and anti-HEL IgG1 levels induced by CpG ODN did not differ appreciably from those induced by IFA or CFA, indicating that all of the immunization protocols used in this study were successful and effective for generation of anti-HEL Ig. Thus, the increased production of IgG2a induced by CpG ODN and CFA represents a qualitative switch in Ig isotype production from an IFA-induced Th2-influenced pattern to a Th1-influenced pattern.
We assessed the differentiation of Th2 responses by antigen-specific IL-5 production. Although IL-4 is also produced during Th2 responses, there can be differences in the production and source of these two cytokines under some circumstances. Even in the context of a Th1 response, antigen-stimulated T cells can induce IL-4 (but not IL-5) secretion by non–T cells (Karulin and Lehmann, unpublished observations). Other groups have reported that treatment with IL-12 at the time of immunization can induce antigen-specific IL-4 production by splenocytes (25), again demonstrating that IL-4 can be detected alongside markers of Th1 responses. Therefore we chose IL-5 secretion as a clear, specific marker for Th2 differentiation in our studies, i.e., a cytokine whose antigen-triggered secretion could be attributed to antigen-specific T cells. The differentiation of cells secreting IL-5 was monitored by the ELISA spot assay. We also tested the supernatants from similar incubations for IL-5 by standard ELISA, which revealed the same pattern of results (data not shown).
ELISA spot analysis demonstrated that HEL-specific IL-5 secretion was induced by immunization with IFA-HEL but was absent after immunization with CFA-HEL. Immunization with IFA-HEL-CpG ODN produced significantly lower levels of IL-5 secretion than observed with IFA-HEL. Thus, in addition to enhancing Th1 differentiation, CpG ODN appear to at least partially switch off Th2 differentiation to produce Th1-dominated immune responses. Moreover, these studies examined Th differentiation after a single immunization. It is possible that repeated administration of antigen with CpG ODN would produce an even more polarized Th1 response.
The experiments shown here monitored IgG2a levels at 15-18 d after immunization and T cell responses at three weeks after immunization. Additional experiments also examined cytokine responses upon antigenic restimulation of T cells at 4 and 6 wk after immunization, as well as IgG2a levels at 3–4 d before these times. At all of these time points the exact same pattern of results was consistently found. For example, CpG ODN produced enhanced IFN-γ and IgG2a responses in all experiments at these later time points (data not shown). These results indicate that the pattern of T cell differentiation induced by a single immunization with CpG ODN remains stable for at least 6 wk.
The possibility that the effects of CpG ODN were due to contaminating LPS was excluded by the following observations. First, LPS levels in the ODN preparations were very low (<<1 ng LPS/mg DNA). Second, we immunized with IFA-HEL plus LPS at 1 ng/mouse (an amount 10–100-fold higher than the maximum amounts contributed by the highest ODN doses) and failed to see any increase in Th1-associated results (IFN-γ secretion and induction of IgG2a) or decrease in Th2-associated IL-5 secretion (data not shown).
One concern for DNA adjuvants, as with all adjuvants, is the potential for toxicity. The administration of bacterial DNA or stimulatory ODN can induce TNF-α release and fatal shock in mice that have been previously sensitized with D-galactosamine (26), and CpG DNA can prime mice for the Shwartzmann reaction (12). With regard to ODN adjuvants, mice given repeated high doses of CpG ODN develop a dose-dependent splenomegaly and can develop other toxicity related to excessive immune stimulation, including death (27). However, significant toxicity has not been observed at the low doses of ODN used for adjuvant function in our current studies, where we observed no significant changes in mouse appearance, behavior, and body weight (measured at multiple points throughout the experiment) or spleen weight (measured at the time of sacrifice). We also noted that spleens from mice injected with CFA were more difficult to remove, presumably due to post-inflammatory peritoneal fibrosis, while spleens from mice injected with CpG ODN appeared normal. Additional studies of spleen and lymph node sizes at earlier time points after administration of CpG ODN alone, at the doses used here, revealed mild splenomegaly and hyperplasia of draining lymph nodes that was reversible within 10–14 d. Mice injected with a single dose of up to 1 mg of the S ODN used in these studies (100-fold higher than the effective adjuvant dose) showed no apparent systemic toxicity or change in feeding, grooming, physical activity or behavior. We conclude that CpG ODN provide potent adjuvant activity at doses that produce no dangerous toxicity.
In the context of vaccine development, the ability to direct Th1 or Th2 differentiation of antigen-specific immune responses has significant implications for therapy of various infectious and autoimmune diseases (28). In the case of certain infectious diseases, Th1-dominated immune responses are protective, while Th2-dominated responses are associated with disease susceptibility. For example, in murine leishmaniasis, Th2-biased mouse strains (e.g., BALB/c) make IL-4–dominated responses to parasite antigens and are susceptible, whereas mice that mount Th1-dominated responses involving IFN-γ secretion (e.g., C57BL/6) are resistant (29–31). Moreover, susceptible mice can be made resistant by administration of IL-12 to enhance Th1 immunity or antibody blockade of IL-4 (32–34), and resistant mice can be made sensitive by blockade of IL-12 (35). In similar circumstances in humans, the ability to direct vaccine-induced immunity towards Th1 responses will dictate the success of vaccination. Thus, CpG ODN may be useful as adjuvants to induce protective Th1 immunity.
In other circumstances the utility of CpG ODN may lie in their potential ability to redirect pathogenic Th2 responses to less harmful Th1 responses. For example, Th2-dominated responses appear to cause allergy, and recent data suggest that administration of CpG ODN may prevent or even reverse ongoing allergic reactions, presumably by redirecting a Th2-dominated response to allergen (which promotes IgE synthesis) to a Th1-dominated response (Kline, J., T. Businga, T. Waldschmidt, J. Weinstock, and A.M. Krieg, manuscript submitted for publication). Similarly, autoimmune diseases that are potentially Th2-associated, such as systemic lupus erythematosis, may be amenable to such Th1 therapy. Th1 therapy, however, is potentially associated with the danger of inducing Th1-mediated pathology, such as certain Th1-associated autoimmune diseases (e.g., experimental allergic encephalomyelitis and type I insulin dependent diabetes mellitus) (36).
The CpG motif has been proposed to act as a danger signal that warns of bacterial infection and activates immune defenses (37). Similarly, one function of therapeutic adjuvants may be to identify vaccine antigens as dangers to which the immune system should respond. Thus, a danger signal provided by CpG ODN may provide potent adjuvant function. Our studies demonstrate that CpG ODN are extremely effective as adjuvants to induce Th1-dominated immune responses without significant toxicity. This property makes CpG ODN attractive as candidate adjuvants for potential use in human vaccines for the prevention or treatment of a wide range of infectious diseases and immune disorders.
Acknowledgments
We thank Rob Fairchild, Neil Greenspan, Richard Trezza, and Hualin Yip for technical advice and helpful discussion. John France provided technical assistance.
This work was supported by National Institutes of Health grants (AI35726, CA70149, and AI34343) to C.V. Harding, NIH grant DK48799 to P.V. Lehmann, and grants from the NIH (AR42556 and CA66570) and Department of Veterans Affairs to A.M. Kreig. R.S. Chu was supported by an NIH Medical Scientist Training Program grant (5T32 GM07250-21).
Footnotes
1 Abbreviations used in this paper: HEL, hen egg lysozyme; ODN, oligodeoxynucleotides; PBST, PBS with Tween; S ODN, phosphorothioate-modified ODN; S-O ODN, ODN with partial phosphorothioate modification.
The first two authors contributed equally to this work.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature (Lond) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 5.Kelso A. Th1 and Th2 subsets: paradigms lost? . Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 6.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science (Wash DC) 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 7.Pisetsky DS. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 8.Chace, J.H., N.A. Hooker, K.L. Midlenstein, A.M. Krieg, and J.S. Cowdery. 1997. Bacterial DNA-induced NK cell IFN-γ production is dependent on macrophage secretion of IL-12. Clin. Immunol. Immunopathol. In press. [DOI] [PubMed]
- 9.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 10.Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983–997. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 11.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 12.Cowdery JS, Chace JH, Yi A-K, Krieg AM. Bacterial DNA induces NK cells to produce IFN-γ in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 13.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-γ production by stimulation of interleukin-12 and tumor necrosis factor-α. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 14.Krieg AM, Yi A-K, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B cell activation. Nature (Lond) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 15.Bird AP. CpG-rich islands and the function of DNA methylation. Nature (Lond) 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 16.Klinman DM, Yi A-K, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M-D, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science (Wash DC) 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 18.Raz E, Tighe H, Sato Y, Dudler JA, Roman M, Swain SL, Spiegelberg HL, Carson DA. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein CA, Subasinghe C, Shinozuka K, Cohen JS. Physiochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ada, G., and A. Ramsay 1997. Immunopotentiation and the selective induction of immune responses. In Vaccines, Vaccination and the Immune Response. G. Ada and A. Ramsay, editors. Lippincott-Raven, Philadelphia. 122–136.
- 22.Tokunaga T, Yano O, Kuramoto E, Kimura Y, Yamamoto T, Kataoka T, Yamamoto S. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovisBCG induce interferons and activate natural killer cells. Microbiol Immunol. 1992;36:55–66. doi: 10.1111/j.1348-0421.1992.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce INF and augment INF-mediated natural killer activity. J Immunol. 1992;148:4072–4076. [PubMed] [Google Scholar]
- 24.Monteith, D.K., S.P. Henry, R.B. Howard, S. Flournoy, A.A. Levin, C.F. Bennett, and S.T. Crooke. 1997. Immune stimulation—a class effect of phosphorothioate oligodeoxynucleotides in rodents. Anticancer Drug Design. In press. [PubMed]
- 25.Bliss J, Van Cleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas AK, Wolf SF. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 26.Sparwasser T, Miethke T, Lipford G, Borschert K, Hæcker H, Heeg K, Wagner H. Bacterial DNA causes septic shock. Nature (Lond) 1997;386:336–337. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 27.Sarmiento UM, Perez JR, Becker JM, Narayanan R. In vivo toxicological effects of rel A antisense phosphorothioates in CD-1 mice. Antisense Res Dev. 1994;4:99–107. doi: 10.1089/ard.1994.4.99. [DOI] [PubMed] [Google Scholar]
- 28.Finkelman FD. Relationships among antigen presentation, cytokines, immune deviation, and autoimmune disease. J Exp Med. 1995;182:279–282. doi: 10.1084/jem.182.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 30.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. . J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sypek JP, Chung CL, Mayor SEH, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 36.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 37.Krieg AM. Lymphocyte activation by CpG dinucleotide motifs in prokaryotic DNA. Trends Microbiol. 1996;4:73–77. doi: 10.1016/0966-842X(96)81515-0. [DOI] [PubMed] [Google Scholar]