Human Mannose-binding Lectin in Immunity: Friend, Foe, or Both? (original) (raw)
Abstract
Human mannose-binding lectin (MBL) recognizes a wide range of microorganisms and triggers the most ancient pathway of complement activation. However, ∼5% of individuals lack functional serum MBL and have not been found to be prone to severe infections in prospective studies. These data suggest that human MBL is largely redundant for protective immunity and may even have been subject to counter selection because of a deleterious impact.
The MBL Connection.
Long before human Toll-like receptors (TLRs) were discovered, other pathogen sensors had been described in the innate immune system. One such sensor is mannose-binding lectin (MBL), a member of the collectin family of proteins, in which collagen structures are associated with carbohydrate-recognizing lectin domains (1). The low affinity for sugars of the four to six individual subunits that make up MBL is compensated for by the high order multimerization, which generates functional complexes of sufficient avidity for microorganisms (2). MBL is a serum protein that binds to a large variety of sugar moieties expressed by many different microorganisms, ranging from viruses to parasites (3). MBL can mediate phagocytosis of microorganisms and induce inflammatory responses via as yet poorly defined receptors on phagocytes. MBL also provides a distinct, third pathway of complement activation, which appears to be the most evolutionarily ancient (4). Complement activation by MBL requires MBL-associated serine proteases (MASPs; 5). Upon MBL binding to carbohydrate-bearing pathogens, MASP-2 is activated and cleaves the C4 and C2 components of complement. The C4b2a complex in turn exerts C3 convertase activity, generating opsonic C3b fragments. But what actual role does human MBL play in host defense?
Human MBL2 Diversity.
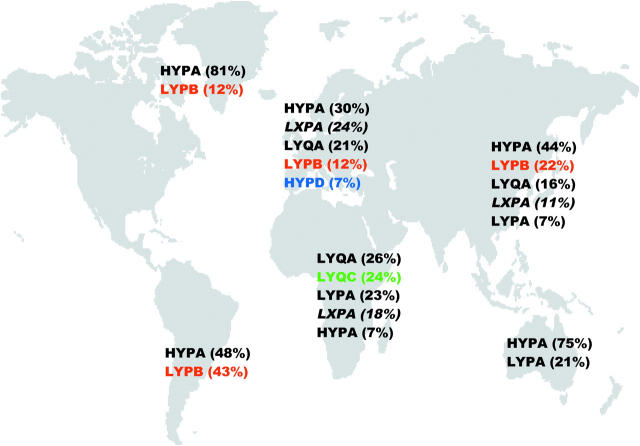
The first clue to the physiological function of MBL was obtained in 1989 (6). Super et al. (6) found that a syndrome characterized by impaired yeast opsonization and multiple infections, first reported in 1968 (7), resulted from a lack of functional serum MBL. The first pathogenic mutation in MBL2, the gene encoding MBL (MBL1 is a pseudogene) was found in 1991 (8). Three common missense mutations, affecting codons 52, 54, and 57 in the first MBL2 exon encoding the NH2-terminal part of the collagen helix, have since been found, and the corresponding alleles were designated D, B, and C, respectively (A is the wild-type allele for all three positions). The pathogenic effects of these three coding sequence mutations involve the impairment of MBL multimerization, decreasing ligand binding, and resulting in a lack of complement activation (9). Three common regulatory mutations were also identified in the MBL2 promoter and 5′ untranslated regions: H/L at position −550, X/Y at position −221, and P/Q at position +4 (10). The regulatory and coding variants are in strong linkage disequilibrium, with only seven haplotypes defined in human populations (HYPA, LYQA, LYPA, LXPA, HYPD, LYQC, and LYPB), and therefore most likely result from founder mutational events (11). Among haplotypes carrying the wild-type A allele, HYPA results in the production of high amounts of MBL, whereas LXPA is associated with the most severe defect in MBL secretion. The frequencies of the seven haplotypes vary considerably between populations (Fig. 1), reflecting the respective ages of the founder mutations, the history of human migrations, and selective environmental pressures acting on the human genome.
Figure 1.
Distribution of the seven MBL haplotypes in different indigenous populations from Africa (Kenya and Mozambique; references 10 and 11), Europe (Denmark; references 11 and 38), Asia (Japan; reference 39), Australia (reference 40), Greenland (references 10 and 11), and South America (Argentina; reference 11). Only haplotypes with a frequency >5% are shown (with the actual frequency in brackets). Those carrying a coding sequence mutation (B, C, or D) are indicated in color, and the functionally deficient haplotype (LXPA) is indicated in italics. Of the three haplotypes encoding missense mutations, LYQC is typically found in sub-Saharan Africans, HYPD mainly in Caucasians, and LYPB has spread from Europeans to native Americans via Asian populations following historical migrations, whereas all three of these haplotypes are lacking in indigenous Australian populations (reference 40). Overall, haplotypes associated with missense (HYPD, LYQC, and LYPB) or regulatory (LXPA) deleterious mutations are very common, with frequencies exceeding 30% in many areas worldwide.
MBL Deficiency in Man.
We will refer to the ∼5% individuals that are homozygous or compound heterozygous for any of the three missense mutations as “MBL deficient,” keeping in mind that this definition underestimates the actual number of deficient individuals because certain regulatory mutations are also detrimental. Clearly, MBL deficiency is not a primary immunodeficiency in the classical, Mendelian sense of the term, as its clinical penetrance is extremely low. It may, however, have a substantial impact on protective immunity at the population level, as susceptibility to many common infectious diseases is under complex genetic control. A number of hospital-based association studies have indeed suggested that MBL deficiency is more common in patients with infectious diseases (12, 13). MBL-deficient patients seemed to be more susceptible not only to severe diseases caused by encapsulated bacteria, but also to a broad range of viral, fungal, and parasitic illnesses. However, many of these associations were weak, preliminary, or contradictory, and await confirmation. For example, a case control study suggested that MBL deficiency was weakly associated with invasive pneumococcal disease in Britain (14), but another study in Denmark reached a different conclusion (15). Perhaps the most convincing study dealt with meningococcal infection, showing a greater risk of invasive disease in MBL-deficient children in both hospital- and community-based case control studies (16).
Prospective Studies.
In the first prospective population-based study reported, MBL-deficient children aged 6–17 mo, but not younger or older children, were found to have an increased risk of acute, generally benign, respiratory disease (17). A second population-based longitudinal study, reported in this issue of the JEM, deals with over 9,000 adults, with 24 and 8 yr of follow-up information on morbidity and mortality, respectively (18). Remarkably, MBL-deficient adults displayed neither higher morbidity nor higher mortality caused by infections than adult controls. This study does not strictly contradict the former population-based study in children (17) or the previous hospital-based association studies, as it would not have enough power to detect the impact of MBL deficiency on specific infectious diseases (12, 13). However, this important study clearly indicates that MBL deficiency is not a major risk factor for infectious diseases in adult Scandinavians and this conclusion can probably be extended to other Caucasians at least. This is consistent with the observation that the distributions of the three MBL2 mutant coding alleles are in Hardy-Weinberg equilibrium worldwide, indicating that there is currently no preferential selective pressure on any genotypic combination.
MBL Deficiency in the Mouse.
Although human MBL deficiency was first reported in 1989, the existence of two functional MBL genes in the mouse, Mbla and Mblc, delayed the generation of MBL-deficient animals. MBL-A knockout mice were found to be resistant to both Candida albicans and Plasmodium yoelii (19). Surprisingly, mice lacking MBL-A but with sufficient quantities of MBL-C had higher survival rates in a septic peritonitis model (20). This suggests that MBL-A is redundant and may even be detrimental to mouse defences. The generation of double knockout MBL-deficient mice is reported in this issue of the JEM (21). The double knockout mice, but neither of the two single knockout mice, are susceptible to intravenous inoculation with Staphylococcus aureus (21). However, the susceptibility of MBL-deficient mice to intravenously injected S. aureus is modest: at 48 h, when all MBL-deficient mice had died, half of the wild-type mice had also died of infection. Moreover, although the dose of S. aureus used for inoculation was very high, the bacterial load in most tissues analyzed varied by only about one and a half orders of magnitude. It would thus be interesting to challenge MBL-deficient animals with other microorganisms, including those that seem to threaten MBL-deficient patients, such as Neisseiria meningitidis and Streptococcus pneumoniae, but also, and perhaps more importantly, with natural rodent-tropic pathogens. In any event, these data indicate that murine MBL is involved in, and that MBL-A and MBL-B are mutually redundant for, the control of high inoculums of S. aureus introduced intravenously.
Is There a Contradiction between Mice and Men?
The two papers published in this issue seem to convey contradictory information (18, 21). This may provide yet another example of greater redundancy in the human immune system compared with the mouse (22). To mention only a couple of examples, patients with genetic defects in the IL-12/23–IFN-γ cytokine signaling circuit are particularly susceptible to infection with Mycobacteria and Salmonella, whereas the corresponding knockout mice have a much broader phenotype (23–25). Likewise, patients deficient in IRAK-4, an essential component of TLR and IL-1 receptor signaling, are susceptible to only a small subset of pyogenic bacteria, S. aureus and S. pneumoniae in particular, which is at odds with the requirement of the TLR–Il-1R-IRAK pathway for immunity to a broad range of pathogens in mouse (26, 27). Many factors may account for such discrepancies between human and mouse studies, largely reflecting the differences between natural and experimental infections (22). Animals are generally inoculated with mutants of laboratory strains derived from human-tropic microbes, whereas humans are infected by coevolving natural pathogens. Moreover, the routes of experimental infections often bypass mucosal or cutaneous immunity, which are known to influence subsequent systemic responses. Finally, the “wild-type” controls of knockout animals are only wild-type at the locus knocked out in the mutant strain, as their homozygous genomes carry a variety of mutant genes involved in immunity to infection (28). Susceptibility of the MBL-deficient mouse may thus partly reflect the inherent limitations of experimental studies. In the ecosystem of human and microbial life, there is more immunological redundancy and MBL deficiency is likely to be of less importance.
The Impact of MBL Deficiency Is Mild.
There is, in fact, no profound contradiction between the two studies published in this issue. In mice and humans, the MBL genotype influences the complex (i.e., non-Mendelian) inheritance of protective immunity to infection. For example, MBL-deficient mice were susceptible to intraperitoneal inoculation of S. aureus only in the presence of concomitant neutropenia (21), consistent with the higher risk of infection in MBL-deficient patients undergoing chemotherapy in two of three studies (29–31). The susceptibility of MBL-deficient mice to intravenously inoculated S. aureus (21) suggests, in turn, that human MBL deficiency may favor staphylococcal septicemia, which occurs in a small fraction of patients with a transcutaneous intravenous central line. Patients with MBL deficiency and other well-characterized immunodeficiencies, such as common variable immune deficiency (32), have a higher risk of infectious diseases. The impact of MBL deficiency in mice and men is thus very modest and its phenotypic expression is favored by concomitant inherited or acquired risk factors. Further suggesting that the MBL–MASP-2 pathway is largely redundant in host defense, a common amorphic missense mutation was recently identified in human MASP2 (33). More studies are required to define the contribution of MASP-2 deficiency to protective immunity in mice and men.
Is MBL Deficiency Beneficial?
The spread of deleterious MBL2 alleles worldwide suggests in itself that human MBL is merely a redundant and residual vestige of the most ancient pathway of complement activation. A globally neutral, or to an even lesser extent a mildly beneficial contribution of MBL to host protection, would not account for the observed frequency of MBL deficiency. Instead, the MBL2 mutations may have exerted a beneficial impact on host defense during certain epidemics (34). Their impact may have been strong, as amorphic common mutations that do not markedly impair immunity may even confer a strictly Mendelian protection against infection. For example, a regulatory mutation in the gene encoding the Duffy antigen chemokine receptor confers resistance to Plasmodium vivax and accounts for the expansion of the mutant allele in endemic areas (35). The worldwide expansion of a few MBL2 mutant alleles thus suggests that MBL is not only redundant for protective immunity in most circumstances, but also deleterious to our species in some circumstances. For example, MBL may favor autoimmune processes or intracellular infections, such as tuberculosis and leishmaniasis (13, 34).
Medical Implications.
What are the clinical implications of 15 yr of research in the field of MBL deficiency? Importantly, there is currently no reason for clinicians to diagnose MBL deficiency in individual patients with infectious diseases. However, there is a need to evaluate further the contribution of MBL deficiency to specific infections and to undertake longitudinal population-based cohort studies in different regions of the world. This is important because the possibility of therapeutic use of recombinant MBL has emerged in recent years (36). There is clearly no rationale for the use of MBL in patients proficient in this molecule, but is there even a rationale for giving MBL to MBL-deficient patients? Recombinant MBL is expected to be safe and nonimmunogenic, but it might favor autoimmune reactions and intracellular infections, and possibly elicit antibodies against MBL allotypes. Even if well tolerated, it would be difficult to identify the patients most likely to benefit from recombinant MBL treatment. There seems to be no clinical justification and it would not appear economically worthwhile to treat children with benign, self-healing acute respiratory disease with MBL (17). Could such treatment help MBL-deficient children with more severe infections? Experiments in mice have shown that recombinant MBL can efficiently prevent infection (21), but could it help to the cure infection? In any event, before any therapeutic trial, human studies are required to define more accurately the target patients.
Conclusion.
Human MBL deficiency clearly does not confer a Mendelian susceptibility to infection on individuals. In addition, the worldwide expansion of deleterious MBL2 haplotypes suggests that these may even have been beneficial at the population level and that balancing selection may have acted on this gene throughout its evolutionary history. Studies of sequence diversity together with the definition of linkage disequilibrium levels at this locus would help to elucidate the underlying genetic and biological forces leading to the observed patterns. Nevertheless, the MBL2 gene may contribute, in specific circumstances of impaired immunity, to the complex genetic control of protective immunity to infection. In most circumstances, other components of the immune system can compensate for the lack of MBL. The available prospective data do not suggest that MBL operates as an “ante-antibody” mechanism throughout life (37). Instead, they suggest that MBL physiologically plays a minor yet detectable role in children between 6 and 18 mo of age, who no longer have maternal antibodies but are not yet able to mount an antibody response to the carbohydrate antigens of encapsulated bacteria (6, 17).
Acknowledgments
We would like to thank Lluís Quintana-Murci for critical reading.
References
- 1.Holmskov, U., S. Thiel, and J.C. Jensenius. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547–578. [DOI] [PubMed] [Google Scholar]
- 2.Garred, P., F. Larsen, H.O. Madsen, and C. Koch. 2003. Mannose-binding lectin deficiency–revisited. Mol. Immunol. 40:73–84. [DOI] [PubMed] [Google Scholar]
- 3.Jack, D.L., and M.W. Turner. 2003. Anti-microbial activities of mannose-binding lectin. Biochem. Soc. Trans. 31:753–757. [DOI] [PubMed] [Google Scholar]
- 4.Fujita, T., M. Matsushita, and Y. Endo. 2004. The lectin-complement pathway-its role in innate immunity and evolution. Immunol. Rev. 198:185–202. [DOI] [PubMed] [Google Scholar]
- 5.Schwaeble, W., M.R. Dahl, S. Thiel, C. Stover, and J.C. Jensenius. 2002. The mannan-binding lectin-associated serine proteases (MASPs) and MAp19: four components of the lectin pathway activation complex encoded by two genes. Immunobiology. 205:455–466. [DOI] [PubMed] [Google Scholar]
- 6.Super, M., S. Thiel, J. Lu, R.J. Levinsky, and M.W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 2:1236–1239. [DOI] [PubMed] [Google Scholar]
- 7.Miller, M.E., J. Seals, R. Kaye, and L.C. Levitsky. 1968. A familial, plasma associated defect of phagocytosis: a new cause of recurrent bacterial infections. Lancet. ii:60–63. [Google Scholar]
- 8.Sumiya, M., M. Super, P. Tabona, R.J. Levinsky, T. Arai, M.W. Turner, and J.A. Summerfield. 1991. Molecular basis of opsonic defect in immunodeficient children. Lancet. 337:1569–1570. [DOI] [PubMed] [Google Scholar]
- 9.Larsen, F., H.O. Madsen, R.B. Sim, C. Koch, and P. Garred. 2004. Disease-associated mutations in human mannose-binding lectin compromise oligomerisation and activity of the final protein. J. Biol. Chem. In press. [DOI] [PubMed] [Google Scholar]
- 10.Madsen, H.O., P. Garred, S. Thiel, J.A. Kurtzhals, L.U. Lamm, L.P. Ryder, and A. Svejgaard. 1995. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155:3013–3020. [PubMed] [Google Scholar]
- 11.Madsen, H.O., M.L. Satz, B. Hogh, A. Svejgaard, and P. Garred. 1998. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J. Immunol. 161:3169–3175. [PubMed] [Google Scholar]
- 12.Eisen, D.P., and R.M. Minchinton. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37:1496–1505. [DOI] [PubMed] [Google Scholar]
- 13.Turner, M.W. 2003. The role of mannose-binding lectin in health and disease. Mol. Immunol. 40:423–429. [DOI] [PubMed] [Google Scholar]
- 14.Roy, S., K. Knox, S. Segal, D. Griffiths, C.E. Moore, K.I. Welsh, A. Smarason, N.P. Day, W.L. McPheat, D.W. Crook, et al. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 359:1569–1573. [DOI] [PubMed] [Google Scholar]
- 15.Kronborg, G., N. Weis, H.O. Madsen, S.S. Pedersen, C. Wejse, H. Nielsen, P. Skinhoj, and P. Garred. 2002. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J. Infect. Dis. 185:1517–1520. [DOI] [PubMed] [Google Scholar]
- 16.Hibberd, M.L., M. Sumiya, J.A. Summerfield, R. Booy, and M. Levin. 1999. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Meningococcal Research Group. Lancet. 353:1049–1053. [DOI] [PubMed] [Google Scholar]
- 17.Koch, A., M. Melbye, P. Sorensen, P. Homoe, H.O. Madsen, K. Molbak, C.H. Hansen, L.H. Andersen, G.W. Hahn, and P. Garred. 2001. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 285:1316–1321. [DOI] [PubMed] [Google Scholar]
- 18.Dahl, M., A. Tybjaerg-Hansen, P. Schnorr, and B. Nordestgaard. 2004. A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J. Exp. Med. 199:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, S.J., G. Gonzalez-Aseguinolaza, and M.C. Nussenzweig. 2002. Disseminated candidiasis and hepatic malarial infection in mannose-binding-lectin-A-deficient mice. Mol. Cell. Biol. 22:8199–8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi, K., J. Gordon, H. Liu, K.N. Sastry, J.E. Epstein, M. Motwani, I. Laursen, S. Thiel, J.C. Jensenius, M. Carroll, et al. 2002. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 4:773–784. [DOI] [PubMed] [Google Scholar]
- 21.Shi, L., K. Takahashi, J. Dundee, S. Shahroor-Karni, S. Thiel, J. Jensenius, F. Gad, M. Hamblin, K. Sastry, and R. Ezekowitz. 2004. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199:1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casanova, J.L., and L. Abel. 2004. The human model: a genetic dissection of immunity to infection in natural conditions. Nat. Rev. Immunol. 4:55–66. [DOI] [PubMed] [Google Scholar]
- 23.Newport, M.J., C.M. Huxley, S. Huston, C.M. Hawrylowicz, B.A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941–1949. [DOI] [PubMed] [Google Scholar]
- 24.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J.F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, et al. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956–1961. [DOI] [PubMed] [Google Scholar]
- 25.Casanova, J.L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581–620. [DOI] [PubMed] [Google Scholar]
- 26.Picard, C., A. Puel, M. Bonnet, C.L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 299:2076–2079. [DOI] [PubMed] [Google Scholar]
- 27.Puel, A., C. Picard, C.L. Ku, A. Smahi, and J.L. Casanova. 2004. Inherited disorders of NF-kappaB-mediated immunity in man. Curr. Opin. Immunol. 16:34–41. [DOI] [PubMed] [Google Scholar]
- 28.Casanova, J.L., E. Schurr, L. Abel, and E. Skamene. 2002. Forward genetics of infectious diseases: immunological impact. Trends Immunol. 23:469–472. [DOI] [PubMed] [Google Scholar]
- 29.Peterslund, N.A., C. Koch, J.C. Jensenius, and S. Thiel. 2001. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 358:637–638. [DOI] [PubMed] [Google Scholar]
- 30.Mullighan, C.G., S. Heatley, K. Doherty, F. Szabo, A. Grigg, T.P. Hughes, A.P. Schwarer, J. Szer, B.D. Tait, L. Bik To, et al. 2002. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 99:3524–3529. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick, D.C., L.A. McLintock, E.K. Allan, M. Copland, T. Fujita, N.E. Jordanides, C. Koch, M. Matsushita, H. Shiraki, K. Stewart, et al. 2003. No strong relationship between mannan binding lectin or plasma ficolins and chemotherapy-related infections. Clin. Exp. Immunol. 134:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullighan, C.G., S.E. Marshall, and K.I. Welsh. 2000. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand. J. Immunol. 51:111–122. [DOI] [PubMed] [Google Scholar]
- 33.Stengaard-Pedersen, K., S. Thiel, M. Gadjeva, M. Moller-Kristensen, R. Sorensen, L.T. Jensen, A.G. Sjoholm, L. Fugger, and J.C. Jensenius. 2003. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N. Engl. J. Med. 349:554–560. [DOI] [PubMed] [Google Scholar]
- 34.Garred, P., M. Harboe, T. Oettinger, C. Koch, and A. Svejgaard. 1994. Dual role of mannan-binding protein in infections: another case of heterosis? Eur. J. Immunogenet. 21:125–131. [DOI] [PubMed] [Google Scholar]
- 35.Tournamille, C., Y. Colin, J.P. Cartron, and C. Le Van Kim. 1995. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat. Genet. 10:224–228. [DOI] [PubMed] [Google Scholar]
- 36.Summerfield, J.A. 2003. Clinical potential of mannose-binding lectin-replacement therapy. Biochem. Soc. Trans. 31:770–773. [DOI] [PubMed] [Google Scholar]
- 37.Super, M., and R.A. Ezekowitz. 1992. The role of mannose-binding proteins in host defense. Infect. Agents Dis. 1:194–199. [PubMed] [Google Scholar]
- 38.Steffensen, R., S. Thiel, K. Varming, C. Jersild, and J.C. Jensenius. 2000. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods. 241:33–42. [DOI] [PubMed] [Google Scholar]
- 39.Matsushita, M., H. Miyakawa, A. Tanaka, M. Hijikata, K. Kikuchi, H. Fujikawa, J. Arai, S. Sainokami, K. Hino, I. Terai, et al. 2001. Single nucleotide polymorphisms of the mannose-binding lectin are associated with susceptibility to primary biliary cirrhosis. J. Autoimmun. 17:251–257. [DOI] [PubMed] [Google Scholar]
- 40.Turner, M.W., L. Dinan, S. Heatley, D.L. Jack, B. Boettcher, S. Lester, J. McCluskey, and D. Roberton. 2000. Restricted polymorphism of the mannose-binding lectin gene of indigenous Australians. Hum. Mol. Genet. 9:1481–1486. [DOI] [PubMed] [Google Scholar]