The Sequential Role of Lymphotoxin and B Cells in the Development of Splenic Follicles (original) (raw)
Abstract
The transfer of lymphocytes into severe combined immunodeficiency (SCID) mice induces a series of histological changes in the spleen, including the appearance of mature follicular dendritic cells (FDCs). Studies were undertaken to clarify the role of lymphotoxin (LT) in this process. The results show that SCID mice have a small and partially differentiated white pulp containing marginal zone and interdigitating dendritic cells, but lacking FDCs. Transferred spleen cells can segregate into T and B cell areas shortly after their injection to SCID mice. This ability is dependent on signaling through LT-β receptor (LT-βR), since blocking ligand–receptor interaction in recipient SCID mice ablates the capacity of the transferred cells to segregate. A week after lymphocyte transfer, host-derived FDCs appeared in the reconstituted SCID mice. This induction of FDCs is dependent on LT-βR signaling by B cells since LT-α−/− B cells are incapable of inducing development of FDCs in SCID mice, even after cotransfer of LT-α+/+ T cells. Therefore, LT plays at least two discrete roles in splenic organization. First, it appears that LT induces the differentiation of the white pulp to create sites for lymphocyte segregation. Second, LT expression by B cells drives the maturation of FDCs and the organization of B cell follicles.
The spleen is subdivided into the red and white pulp, the latter being the lymphoid compartment (1). The histological structure of the white pulp consists of a cuff of lymphocytes lining the splenic arterioles, referred to as the periarteriolar lymphocyte sheath (PALS)1. The PALS is composed mainly of T cells (1, 2), whereas the B cells form spheroid-shaped structures named primary follicles that are located in the periphery of the PALS (composed of naive, recirculating B cells; references 3, 4). Upon immunization, these follicles transform into germinal centers (reactive to T cell–dependent antigen and also called secondary follicles; reference 5). The boundary region between the white pulp and the red pulp is called the marginal zone and is populated essentially by specialized subsets of macrophages (metallophilic and marginal zone macrophages) and a nonrecirculating population of IgD− B cells (6).
The accessory cells are also segregated within the T and B cell areas in the white pulp; interdigitating dendritic cells, which belong to a hematopoietic lineage (7), are present in the PALS (7), whereas the follicular dendritic cells (FDCs), a cellular lineage of controversial origin (8, 9) are associated with the B cell vollicles. Together with these cellular elements, there are also differences in the distribution of some extracellular matrix proteins that are found in these subanatomic sites. The T cell areas are rich in reticular fibers (10), tenascin (11), and laminin (12), whereas B cell follicles are richer in vitronectin and fibronectin (12, 13).
The cytokines of the TNF/lymphotoxin (LT) family are important in the establishment and/or maintenance of the normal splenic architecture as shown by the findings that the LT-α (14), LT-β (15), TNFR-I (16), and TNF-α (17) knockout mice all have abnormal splenic architecture, as do mice in which LT-α/β signaling is disrupted (18–20). It is not known why defects in signaling by TNF/LT create such disturbances in splenic architecture. Signaling via the LT axis between lymphocytes and their microenvironment likely contributes significantly to the organization of the spleen. In this regard, it is interesting that the FDCs, a conspicuous component of B cell follicles, are not present in any of the different TNF/LT knockout mice (14–20). SCID mice that lack mature T and B lymphocytes also lack FDCs (21–23). Interestingly, host-derived FDCs differentiate in the spleen of SCID mice after transfer of wild-type lymphocytes (21–23).
Data presented in this report show that SCID mice, devoid of any identifiable FDCs, have a rudimentary white pulp with marginal zone and areas “predetermined” to be B cell follicles. The persistence of these predetermined sites in the SCID mouse is dependent on LT expression. After the initial colonization of these predetermined areas by B cells, expression of LT-α by the B cells induces the development of mature FDCs. It appears that LT expression only by B cells, and not T cells, is crucial for the maturation of FDCs and follicles. Therefore, LT plays at least two discrete roles in splenic organization, an early role involved with maintaining the differentiation of the splenic stromal elements, and a later one, where LT-α expression by B cells and/or by non–T, non–B cell populations drives the maturation of FDCs.
Materials and Methods
Mice.
BALB/c, BALB/c scid/scid, and C57BL/6 mice were purchased from the National Cancer Institute laboratories (Bethesda, MD). C57BL/6 scid/scid and LT-α−/− (on a mixed background 129Sv × C57BL/6) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were maintained in the specific pathogen-free animal facility at Dartmouth Medical School (Lebanon, NH). SCID mice were routinely assessed for serum Ig levels and those that had levels of serum Ig in excess of 0.2 μg/ml were excluded from the study.
Transfer of Splenocytes.
In some experiments, whole spleen single cell suspensions were obtained from BALB/c, C57BL/6, or LT-α−/− mice, and 1.5–2.5 × 107 cells were transferred to BALB/c or C57BL/6 SCID mice by injection in the tail vein. In some experiments, the cellular suspension injected intravenously was enriched in B cells (at least 80% of cluster of differentiation [CD]19+ cells) by the cytotoxic elimination of the T cells after incubation with anti-Thy1 and anti-CD4 mAb followed by complement. In other experiments, a mix of purified B cells and purified T cells (at least 80% CD3+ cells obtained by panning to plates coated with goat anti–mouse Ig) obtained from different donors were used.
Reagents and Antibodies.
LT-βR–Ig is a fusion protein that contains the extracellular domain of the murine LT-βR fused to hinge second and third domains of the constant heavy chain of the human IgG1 (24). mAbs specific for MAdCAM-1 biotin (MECA-367 clone), CD19 FITC (1D3 clone), and CD3 PE (145-2C11 clone) were obtained from PharMingen (Torreyana, CA). The follicular dendritic cell (FDC) markers FDC-M1 and FDC-M2 (25) rat mAbs were conjugated to FITC or biotin in our laboratory. The hybridomas producing anti-CD11c (N418, a hamster mAb donated by R. Steinman, The Rockefeller University, New York), anti-CD4 (GK1.5 rat mAb; American Type Culture Collection, Rockville, MD), anti-CD8 (53.6.72 rat mAb; American Type Culture Collection), and anti-B7.2 (GL-1 rat mAb; American Type Culture Collection) were grown as ascites fluid and then purified over DEAE anion exchange HPLC and conjugated to FITC, biotin (Sigma Chemical Co., St. Louis, MO) or Cy5 (Amersham Corp., Arlington Heights, IL) in our laboratory.
Evaluation of the Splenic Architecture: Analysis Using Viable Thick Sections and Image Analysis.
Spleens were harvested and embedded in 4% agarose in HBSS. The embedded tissue was sectioned in a vibrating microtome (Microcut H12; Energy Beam Sciences, Agawam, MA). The spleen sections (500 μm thick) were incubated overnight at 4°C with the corresponding mAbs conjugated to FITC, biotin, PE, or Cy5, all at a concentration between 10 and 20 μg/ml in PBS/1% BSA/0.02% sodium azide. Mouse IgG1 was used to block Fc binding. After washing, the sections were incubated for 3 h with Streptavidin FITC or Streptavidin PE (Southern Biotechnology Assoc., Birmingham, AL). After washing again, the samples were mounted in slides with PBS/azide/10% glycerol and analyzed on the confocal microscope (BioRad 1024; BioRad Labs., Hercules, CA). This technology offers a number of attractive assets. First, virtually all antibodies that stain by flow cytometry also stain with this method. Second, it appears that the sensitivity of detection may be superior to other standard immunohistological methods. Finally, simultaneous, three-color immunohistological analysis is possible.
Results
In the Absence of FDCs, T and B Cells Can Segregate in the Spleen.
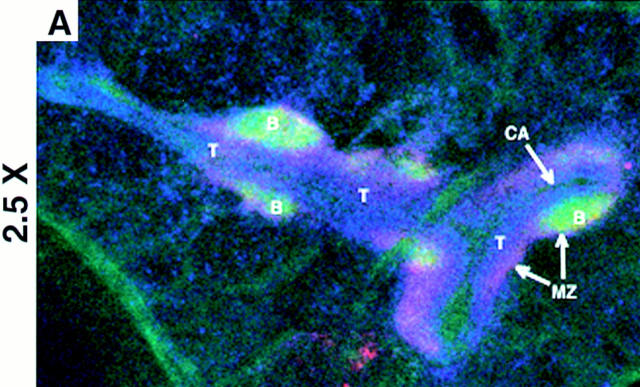
Studies were undertaken to evaluate if T and B cells could segregate in the spleen before the appearance of FDCs. To test this hypothesis, BALB/c SCID mice (devoid of FDCs) were injected intravenously with 1.5–2.5 × 107 syngeneic spleen cells from wild-type donors. The next day, the homing pattern of the infused lymphocytes in the host SCID mice was analyzed by confocal image analysis. Studies were performed through immunohistological analysis of viable, thick sections of the spleens. In contrast to conventional immunohistochemistry, this method is executed without the use of fixatives, and permits greater sensitivity and simultaneous, multicolor analysis. As is shown in Fig. 1 (longitudinal cut of the white pulp) and in Fig. 2 C (transversal cut), the reconstituted SCID mice had a detectable number of CD4+/CD8+ T cells (blue) and CD19+ B cells (green) that segregated in a similar manner to that of wild-type mice. The T cells were concentrated around the arterioles forming the PALS, and the CD19+ B cells appeared to form follicular structures in the periphery of the PALS (Fig. 1 and Fig. 2 C). The transferred lymphocytes which formed follicular structures were B220+, IgD+ phenotype (data not shown). These follicles and PALS (Fig. 1 and Fig. 2 C) were smaller than those from a wild-type BALB/c (Fig. 2 A), which may reflect the limited number of lymphocytes in the reconstituted SCID. As expected, the control, noninjected SCID lacked detectable B and T cells (Fig. 2 B).
Figure 1.
Normal segregation of B and T cell areas in SCID 1 d after reconstitution with wild-type lymphocytes. A section is shown at low magnification (A) and high magnification (B–D) of spleen from SCID that received intravenous wild-type syngeneic lymphocytes. The section is stained with anti-CD19 FITC (green), anti–MAdCAM-1 biotin + Streptavidin PE (red) anti-CD4 Cy5, and anti-CD8 Cy5 (blue). A and B show the triple staining, C shows the red and blue signals, and D shows the green and blue signals. These micrographs are representative of at least three different experiments with two animals per group per experiment. Objectives: ×2.5 (A) and ×20 (B–D), zoom 1.5–2.5. B, B cell follicles; T, T cell areas; MZ, marginal zone; CA, central arteriole.
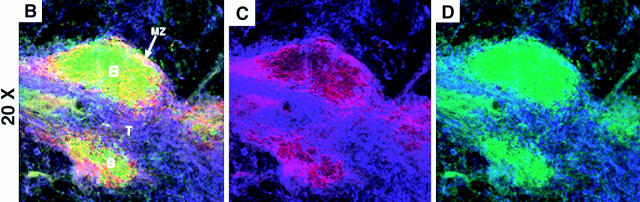
Figure 2.
Phenotype of the white pulp from SCID mice. Sections are shown of wild-type (left column), SCID (center column), and SCID mice that received syngeneic splenocytes the previous day (right column). Sections were stained as indicated with αCD19 FITC (B cell marker), αMAdCAM-1 biotin + Streptavidin PE (marginal sinus lining cells marker), αCD4 Cy5 and αCD8 Cy5 (T cell markers), αCD11c FITC (IDC marker), αB7.2 biotin + Streptavidin PE (stains brightly macrophages of the marginal zone), and αFDC-M1 FITC and αFDC-M2 biotin + Streptavidin PE (FDC markers). The yellow color indicates colocalization in the same area of green and red, not discriminating between single and double positive cells at the resolution used. These micrographs are representative of at least three different experiments with two animals per group per experiment. Objective: ×2.5, zoom 1.5–2.5. B, B cell follicles; T, T cell areas; MZ, marginal zone; CA, central arteriole.
The White Pulp from SCID Mice Have a Differentiated Marginal Zone and Segregated Interdigitating Cells, but Lack Differentiated FDCs.
Attempts were made to identify the elements in the white pulp from SCID mice that are involved in guiding the segregation of the transferred T and B lymphocytes. Within the spleen of control SCID mice, the white pulp was delimited by bands of staining for MAdCAM-1 (stains the marginal sinus cells; reference 6; Fig. 2, B and E) and B7.2 (brightly stains the marginal zone macrophages; reference 26; Fig. 2 H).
FDCs, identified by the specific markers FDC-M1 and FDC-M2, were not detected in splenic sections prepared from SCID mice (Fig. 2 K), but were found associated with B cell follicles in wild-type mice (Fig. 2 J). This absence of FDCs in the spleen of SCID mice has been previously reported (21–23). Using the markers FDC-M1 and FDC-M2, FDCs continued to be absent one day after transfer of wild-type lymphocytes (Fig. 2 L). Therefore, the lack of mature FDCs did not prevent the transferred B cells from organizing into primary IgD+ B cell follicles in the reconstituted SCID (Fig. 1 and Fig. 2 C). Interestingly, such follicles tended to locate in particular areas where the staining for the marginal zone marker MAdCAM-1 was a wide band instead of a narrow rim delimiting the white pulp (Fig. 1 B and 2 C) as indicated by the yellowish color of the B cell follicles (Fig. 1 B and 2 C). It must be noted that a yellow color indicates colocalization in the same area of the green (B cells) and the red (MAdCAM-1) stainings, not discriminating between single and double positive cells at the resolution used. The separate analysis of the patterns of staining for MAdCAM-1 (Fig. 1 C) and CD19 (Fig. 1 D) allowed us to conclude that MAdCAM-1 staining was not present in B lymphocytes.
In contrast to the absence of FDCs, interdigitating dendritic cells (IDCs) identified by the marker CD11c, were detected in SCID mice and located within the area delimited by the marginal zone markers forming a cuff around the central arterioles (Fig. 2, E–I). There was also a rim of CD11c+ cells running by the outside of the marginal zone markers (Fig. 2, E–I). Interestingly, CD11c+ cells (green signal, Fig. 2, D–I) tended to avoid the discrete areas in which the distribution of MAdCAM-1 and B7.2 was a wide band instead of a narrow line (red signal, Fig. 2, D–I). Taken together, IDCs are present in the spleen from nonmanipulated SCID (Fig. 2, E and H) and wild-type mice (Fig. 2, D and G), and concentrate in the periarteriolar area (Fig. 2, D–I).
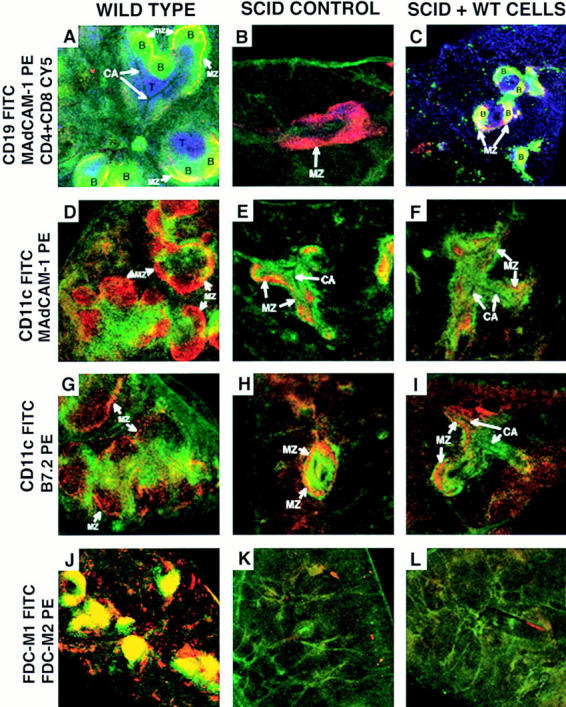
Blocking of LT-βR Signaling Disturbs the Segregated Homing of B and T Lymphocytes after Short-term Transfer to SCID.
The results presented above show that wild-type lymphocytes transferred into SCID mice segregate into B cell follicles and T cells areas in the absence of mature FDCs. To evaluate if LT plays any role in creating these predetermined sites, SCID mice were injected with 200 μg of LT-βR–Ig or control polyclonal human IgG1. 6 d later, the SCID mice received 2.5 × 107 wild-type spleen cells intravenously. The following day, the spleens were harvested, immunostained, and analyzed by confocal microscopy. The results show (Fig. 3) an absence in CD4+/CD8+ T cell and CD19+ B cell segregation in the white pulp of the SCID mice pretreated with LT-βR–Ig (Fig. 3, A and B). In contrast, in the SCID mice treated with the control protein, there was a normal segregation with B cells organizing follicle-like structures on the periphery of a T cell area (Fig. 3, C and D). The expression of the marginal zone markers MAdCAM-1 and B7.2 was downregulated in the SCID mice treated with LT-βR–Ig (not shown) confirming the activity of the fusion protein (20). The administration of another fusion proteins does not affect marginal zone markers (21).
Figure 3.
The blocking of LT-βR signaling disturbs the segregated homing of B and T cells after short-term transfer to SCID. SCID mice received 200 μg intraperitoneally of the fusion protein LT-βR–Ig (A and B) or polyclonal human Ig as control (C and D). 1 wk later they were injected intravenously with wild-type (A and C) or LT-α−/− spleen cells (B and D). The next day the spleens were harvested and analyzed by confocal microscopy. Sections are stained with αCD19 FITC, αCD4 Cy5, and αCD8 Cy5. These micrographs are representative of at least three different experiments with two animals per group per experiment. Objective: ×20, zoom 1.5–2.5. B, B cell follicles; T, T cell areas; CA, central arteriole.
To determine if T/B cell segregation was mediated through LT-α expressed on the transferred lymphocytes or the recipient stroma, the following studies were performed. Splenic lymphocytes obtained from LT-α−/− mice were used as donor cells for the transfer experiments to SCID mice. 1 d later, both the LT-α−/− and wild-type B and T cells segregated into follicles and into PALS, respectively (Fig. 3, B and D). This suggested that LT-α expression on the donor lymphocytes was not essential for segregation. It is worth noting that the splenic architecture of the donor LT-α−/− mice is very disturbed, lacking marginal zone and B/T cell segregation. Therefore, these results suggest that elements of the white pulp that are present in SCID mice are adequate to direct the normal short-term homing of LT-α−/− cells.
The Appearance of Mature FDCs in Reconstituted SCID Mice 1 wk after Lymphocyte Transfer Is Blocked by LT-βR–Ig.
Studies have shown that FDCs develop after lymphocyte transfer into SCID mice (21–23). In wild-type and in reconstituted SCID mice, FDC-M1 and FDC-M2 expression appear closely associated with CD19+ staining. The origin of the emerging FDCs was also confirmed. Spleen cells from CB6F1 (BALB/c × C57BL/6 F1) were transferred into BALB/c SCID mice and it was observed that the T and B lymphocytes expressed the donor MHC class I haplotype H-2b,d, whereas the FDCs were of host origin (single positive H-2d; data not shown). Thus, spleen cells induced the appearance of host-derived FDCs in SCID mice as has been previously reported (23).
It was next determined whether LT-βR–Ig treatment interferes with the differentiation of FDCs in reconstituted SCID mice. To this end, SCID mice were coinjected with 2.5 × 107 wild-type spleen cells and 200 μg of LT-βR–Ig. 2 wk later, the spleen sections were stained and analyzed by confocal microscopy. In reconstituted SCID mice treated with LT-βR–Ig (Fig. 4, B, D, and F), there was minimal expression of FDC-M1 and FDC-M2 codistributed with CD19+ B cells. In addition to the FDC clusters, FDC-M2 stains an unidentified stromal component on the red pulp of the spleen (27). Furthermore, LT-βR–Ig treatment prevented the B cells from organizing into follicle-like structures and they appeared to concentrate in a “ring” of B cells around the PALS (Fig. 4, B, D, and F). Cells transferred to control human Ig-treated SCID induced defined B cell follicles and FDCs (Fig. 4, A, C, and E). Treatment with OX-40–Ig (not shown) and LFA3–Ig (21) fusion proteins did not affect FDC induction.
Figure 4.
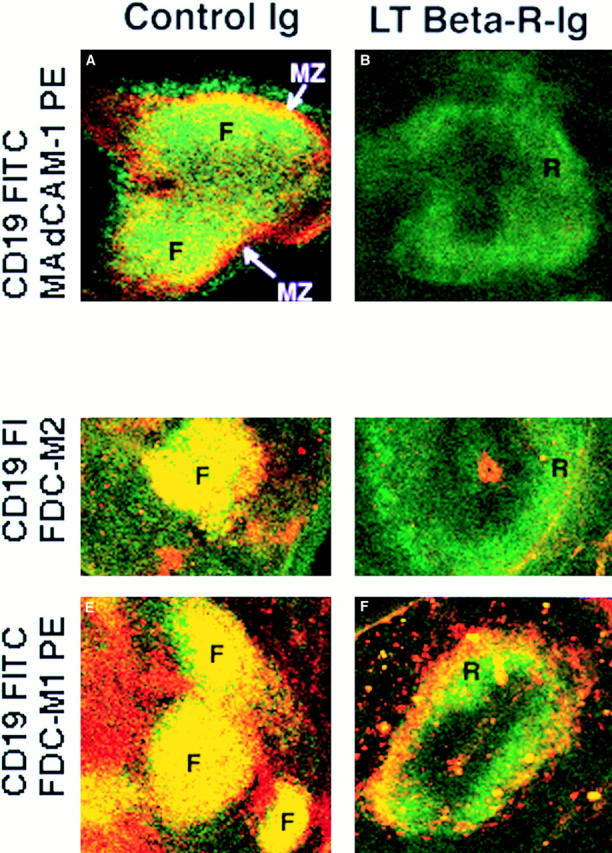
B cells segregate abnormally and fail to induce differentiation of FDCs 1 wk after transfer of syngeneic cells to SCID mice treated with LT-βR–Ig. SCID mice received intravenous injection of syngeneic splenocytes plus fusion protein LT-βR–Ig (B, D, and F) or polyclonal human Ig (A, C, and E). 1 wk later, spleen sections were stained as indicated with αCD19 FITC and αMAdCAM-1 biotin + Streptavidin PE (A and B), αCD19 FITC and αFDC-M1 biotin + Streptavidin PE (C and D), and αCD19 FITC and αFDC-M2 biotin + Streptavidin PE (E and F). The yellow color indicates colocalization in the same area of green and red, not discriminating between single and double positive cells at the resolution used. These micrographs are representative of at least three different experiments with two animals per group per experiment. Objective: ×2.5, zoom 1.5–2.5. F, follicle-like B cell area; R, ring-like B cell area; MZ, marginal zone.
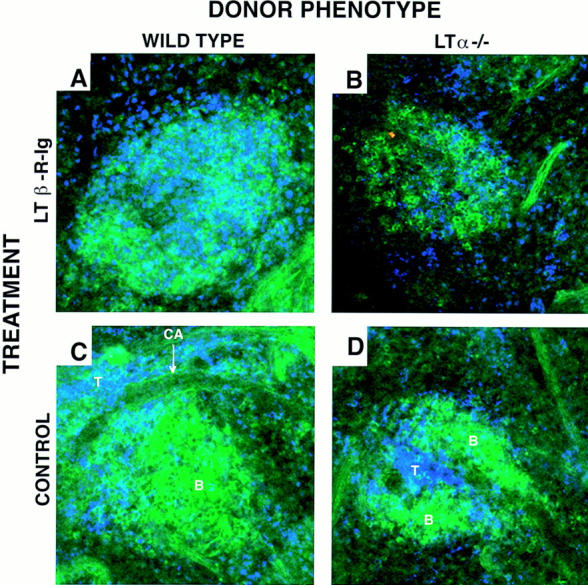
Interactions between LT-α+/+ B Cells and FDC Precursors Are Essential for FDC Development.
The fact that FDCs developed in SCID mice after lymphocyte reconstitution in a LT-dependent fashion suggested that expression of LT-α on the transferred cells induced the appearance of FDCs. To test this hypothesis, spleen cells or mixtures of T and B cells that were either from wild-type mice or LT-α−/− mice were tested for their capacity to restore splenic lymphoid organization. Spleen cells from LT-α−/− mice were transferred to C57BL/6 SCID mice, and 2 wk later, the splenic architecture and presence of FDCs was assessed. As is shown in Fig. 5, A–C, CD19+ B cells form abnormal ring-like structures that surround the PALS. With reference to the induction of FDC development, the expression of FDC-M1 was negative and FDC-M2 was low in the SCID mice reconstituted with LT-α−/− spleen cells (Fig. 5, A and B), in contrast with wild-type reconstituted SCID (Fig. 5, J and K). The residual staining for FDC-M2 colocalized in the ring-like structures where the CD19+ cells concentrated. Interestingly, the abnormal splenic architecture of the donor LT-α−/− mice does not include these ring-like structures, but an absence of segregation into B and T cell areas (data not shown). A population of MAdCAM-1+ cells was situated lining the outside of the rings of B cells (Fig. 5, C, F, I, and L), which indicates that the marginal zone is not disturbed as happens in the LT-βR-Ig–treated mice.
Figure 5.
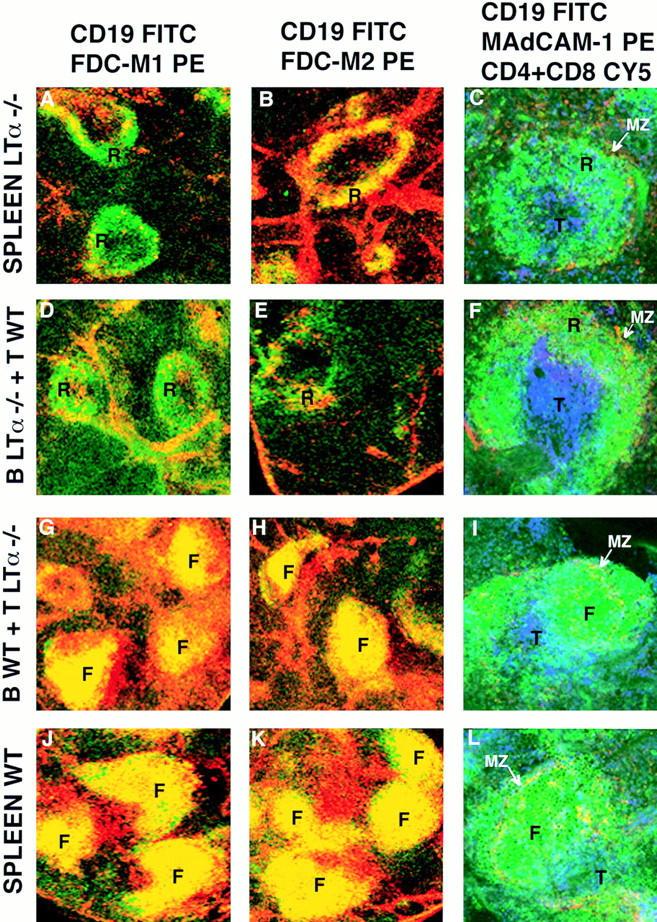
LT-α−/− B cells failed to induce FDCs and segregate abnormally after transfer into SCID mice. SCID mice were injected intravenously with wild-type spleen cells (A–C), wild-type B cells plus LT-α−/− T cells (D–F), wild-type T cells plus LT-α−/− B cells (G–I), or LT-α−/− spleen cells (J–L). 2 wk later, spleen sections were stained as indicated with αCD19 FITC and αFDC-M1 biotin + Streptavidin PE (A, D, G, and J), αCD19 FITC and αFDC-M2 biotin + Streptavidin PE (B, E, H, and K), and αMAdCAM-1 biotin + Streptavidin PE, αCD19 FITC, αCD4 Cy5, and αCD8 Cy5 (C, F, I, and L), and analyzed by confocal microscopy. A yellow color indicates colocalization in the same area of green and red staining, not discriminating between single and double positive cells at the resolution used. These micrographs are representative of at least three different experiments with two animals per group per experiment. Objective: ×2.5, except the right column: ×20; zoom 1.5–2.5. T, T cell area; F, follicle-like B cell area; R, ring-like B cell area; MZ, marginal zone.
To identify the cell type required for the induction of FDC development and splenic organization in SCID mice, cotransfer experiments of LT-α−/− and/or LT-α+/+ T and B cells were performed. Cotransfer of T cell–depleted, LT-α−/− spleen cells (composed of 80–87% B cells) plus B cell–depleted LT-α+/+ spleen cells (composed of 75–85% T cells) showed (Fig. 5, D–F) that the cotransfer of LT-α+/+ T cells did not support the normal organization, e.g., the B cells were in ring-like structures (Fig. 5, D–F) and FDCs were not differentiated (Fig. 5, D–E). On the contrary, SCID mice reconstituted with T cell–depleted LT-α+/+ spleen cells plus B cell–depleted LT-α−/− spleen cells showed normal T/B segregation and induction of FDCs (Fig. 5, G–I and Table 1). These experiments suggest that FDC induction and long-term maintenance of normal splenic architecture requires LT-α signaling by B cells and/or by non–T, non–B cell populations. The presence of LT-α expressing T lymphocytes is not sufficient to induce FDCs.
Table 1.
Splenic Architecture 2 wk after Reconstitution of SCID Mice
| Donor genotype | Host treatment | Splenic architecture | FDC |
|---|---|---|---|
| WT spleen | None | Follicles | Yes |
| WT spleen | LT-βR–Ig | Rings | No |
| WT B + LTα−/− T cells | None | Follicles | Yes |
| WT T + LTα−/− B cells | None | Rings | No |
| LT-α−/− spleen | None | Rings | No |
Discussion
The data presented in this study show that B lymphocytes can segregate from T cells in the absence of mature FDCs, and B cells induce the appearance of FDCs through the expression of LT-α. Although it has been assumed that FDCs create a nucleation site for B cell follicle formation, the data presented suggest that sites for B cell follicle formation exist before the maturation of FDCs. Furthermore, an intimate interaction of B cells with host-derived FDC precursors induces FDC development. Both of these steps of splenic organization appear to rely on the function of LT-α. That is, the disruption of LT-βR signaling in SCID mice appeared to erase the predetermined B cells “sites” destroying the ability of B cells to segregate upon entry into the spleen and prevent splenic organization induced by LT-α–bearing B cells. A summary of these results is presented in Table 2.
Table 2.
Splenic Architecture 1 d after Reconstitution of SCID Mice
| Donor genotype | Host treatment | B/T segregation | FDC |
|---|---|---|---|
| Wild type | Control IgG | Normal | No |
| LT-α−/− | Control IgG | Normal | No |
| Wild type | LT-βR–Ig | Disturbed | No |
| LT-α−/− | LT-βR–Ig | Disturbed | No |
Data presented show that B cells (either from LT-α+/+ or LT-α−/− mice) segregated into follicle-like structures 1 d after their transfer into SCID mice. It is not known what specific components in the spleens of SCID mice guided the segregation of transferred T and B cells. Whatever these components may be, it is clear that blocking of LT-α function in the SCID mice with LT-βR–Ig destroyed these sites. SCID mice have a small but partially differentiated white pulp with a marginal zone and periarteriolar area occupied by IDCs. Areas within the SCID spleen that express higher levels of marginal zone markers (MAdCAM-1) are avoided by the IDCs. These sites appear to be the areas to which the transferred B cells segregate. MAdCAM-1 expression in the SCID mice is also destroyed by LT-β–Ig treatment, as has been shown in wild-type mice (19, 20) or in LT-β–Ig transgenic mice (18). It must be emphasized that the spleens of SCID mice are devoid of mature FDCs (21–23) as judged by the absence of FDC-M1 and FDC-M2 expression, as well as by the absence of immune complex retention (21–23). In addition, the use of other FDC-specific mAbs (28) have indicated that mature FDCs were absent in SCID mice (data not shown). It is possible that FDC precursors present in the spleen of SCID mice chemoattract or bind B cells (28, 29); although they do not, as yet, express FDC markers. Alternatively, B cell segregation into follicles after short-term transfer could be mediated through secreted chemokines and/or adherence to extracellular matrix proteins. The fact that B cells from LT-α−/− mice segregate into follicular structures implies that direct LT-βR signaling by B lymphocytes is not necessary for short-term homing. Furthermore, the fact that homing of B cells can be disturbed after 1 wk of treatment of recipient SCID mice with LT-βR–Ig suggests that such hypothetical chemokines or extracellular matrix proteins must have a relatively rapid turnover and/or their secretion is dependent on LT-βR signaling. Taken together, these findings support the hypothesis that LT-βR–LT-α/β interactions by nonlymphoid cells is necessary to establish the architecture to permit short-term homing of B lymphocytes.
MAdCAM-1+ cells located in discrete sites of the white pulp of SCID mice may mark the relevant cell population important in guiding the selective homing of B cells. Interestingly, the expression of MAdCAM-1 is dependent on LT-α since blocking with LT-βR–Ig or genetic loss of LT-β (15) eliminates MAdCAM-1 expression and dismantles the integrity of the splenic marginal zone (18–20). The functional significance of marginal zone MAdCAM-1 expression (29) in the spleen is unclear. Splenocytes lack overt expression of the ligand for MAdCAM-1 (α4β7 integrin; references 30, 31), which is expressed preferentially on lymphocytes that traffic in the mucosal circuit (Peyer's patches, intraepithelial lymphocytes, lamina propria lymphocytes; reference 31). Furthermore, the administration of a blocking anti–MAdCAM-1 mAb does not appear to prevent homing of lymphocytes in a normal spleen, whereas it inhibits the entry into Peyer's patches (29). Therefore, it is unlikely that MAdCAM-1 is directly responsible for initial B cell segregation. However, experiments aimed to exclude this possibility are underway by testing the ability of the anti–MAdCAM-1 Ab to interfere with short-term segregation of B cells in SCID mice. Because of the lack of lymphocytes in the SCID spleen, LT-α expression in nonlymphoid cells (32) or NK cells (32) must be responsible for maintaining MAdCAM-1 expression. That is surprising since expression of LT-α/β and LT-α3 is described as restricted to activated lymphocytes (33–35).
Having targeted to sites destined to be B cell follicles, LT-α expression on B cells induces follicular organization and FDC development. A direct role for B cells in FDC development has been previously implied by the findings that FDCs are absent in the lymphoid organs of SCID mice (21–23) as well as from mice deprived of B cells (36). It has also been shown that purified B cells obtained after exhaustive elimination of T cells are sufficient to induce FDCs in SCID mice (23), although there is a report to the contrary (21). The fact that FDC development occurs normally ∼1 wk after lymphocyte transfer to SCID mice and requires LT-βR signaling suggests that LT-α expression by B cells may be critical to induce FDC differentiation. This indeed appears to be the case since B cells from LT-α−/− mice are incapable of inducing development of FDCs in SCID mice. The requirement for LT-α expression on B cells was observed even in the presence of LT-α–expressing T cells and within a white pulp expressing LT-α.
It has been reported that deficiencies in other cytokines of the family TNF/LT produce defects in FDC development and splenic B/T cell segregation (14–20). There is a loss of splenic follicles in the absence of LT-βR signaling (18–20) and in TNFRI (16) and TNF (17) knockouts. The normal follicular organization of B cells in the spleens of these mice is replaced by a ring-like distribution surrounding the location that would be the marginal zone in a normal spleen. However, the B cells present in the “rings” are not marginal zone μhighδ− B cells (6), but are of the μ+δ+ follicular phenotype (17, 19). Therefore, although B cells appear to assemble into follicles shortly after transfer, it appears that in the absence of mature FDCs, B cells ultimately condense around the PALS to form rings. Interestingly, the LT-α−/− spleen cells transferred to SCID organize in ring-like structures surrounding the PALS 2 wk after transfer. That is, they organize differently than in the spleen of the donor LT-α−/− mice (14) and in a very similar manner to the splenic architecture of the LT-βR–treated mice (20). One explanation for this difference is that the SCID have a LT-α–expressing splenic stroma that can support a partially restored splenic architecture. This is consistent with data reported by Fu et al. (37), which show that after wild-type LT-α−/− splenocyte cross-transfer, the splenic architecture shows the phenotype of the host. So, wild-type splenocytes transferred to irradiated LT-α−/− mice organize in a nonsegregated structure (37). This supports the hypothesis that in a wild-type splenic stroma, there are elements dependent on LT-α signaling that can guide the segregated homing of B lymphocytes. However, expression of LT-α in the B cells themselves is necessary for the maturation of normal primary B cell follicles and FDCs. The LT-α−/− mice seem to have a deeper disturbance in the splenic architecture with absence of segregation between B and T cells and no clear border between red and white pulp.
In summary, LT-α plays a critical function in sculpturing the architecture of the spleen. First, expression of LT-α by non–T, non–B cells in spleens of SCID mice induce sites that can mediate the segregation of mature T and B cells. Treatment of SCID mice with LT-βR–Ig erases these sites leading to a loss in T/B cell segregation. Second, LT-α has to be expressed on B cells to induce FDC differentiation. Expression of LT-α on T cells appears ineffective at inducing FDC differentiation, leading to the conclusion that a direct interaction between B cells and FDC precursors, or alternatively B cells in cooperation with a non-FDC lineage, is essential for FDC development.
Footnotes
1 Abbreviations used in this paper: CD, cluster of differentiation; FDC, follicular dendritic cell; IDC, interdigitating dendritic cell; LT, lymphotoxin; PALS, periarteriolar lymphocyte sheath.
Confocal microscopy was done at Dartmouth Medical School in The Herbert C. Englert Cell Analysis Laboratory, which was established by a grant from the Fannie E. Rippel Foundation and is supported in part by the Core grant of the Norris Cotton Cancer Center (CA 23108). This work was supported by grants from the National Institutes of Health (AI-26296 and AI-37075) to R.J. Noelle.
References
- 1.Cormack, D.H. 1987. Introduction to Histology. J.B. Lippincot Company, Philadelphia. 10:255–261.
- 2.Paul, W.E. 1993. Fundamental Immunology. Raven Press, NY. 165–168.
- 3.Goodnow CC, Cyster JG, Hartley SB, Bell SE, Cooke MP, Healy JI, Akkaraju S, Rathmell JC, Pogue SL, Shokat KP. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 4.Kuppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO (Eur Mol Biol Organ) J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 6.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra CD, Kamperdijk EW, Dopp EA. The ontogenic development of the follicular dendritic cell. An ultrastructural study by means of intravenously injected horseradish peroxidase. Cell Tissue Res. 1984;236:203–206. doi: 10.1007/BF00216532. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ, Grouard G, de Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–179. doi: 10.1016/s0074-7696(08)62508-5. [DOI] [PubMed] [Google Scholar]
- 10.Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- 11.Chilosi M, Lestani M, Benedetti A, Montagna L, Pedron S, Scarpa A, Menestrina F, Hirohashi S, Pizzolo G, Semenzato G. Constitutive expression of tenascin in T-dependent zones of human lymphoid tissues. Am J Pathol. 1993;143:1348–1355. [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg TK, van der Ende M, Dopp EA, Kraal G, Dijkstra CD. Localization of β1 integrins and their extracellular ligands in human lymphoid tissues. Am J Pathol. 1993;143:1098–1110. [PMC free article] [PubMed] [Google Scholar]
- 13.Castanos-Velez E, Biberfeld P, Patarroyo M. Extracellular matrix proteins and integrin receptors in reactive and non-reactive lymph nodes. Immunology. 1995;86:270–278. [PMC free article] [PubMed] [Google Scholar]
- 14.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 15.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 16.Le Hir M, Bluethmann H, Kosco-Vilbois MH, Muller M, di Padova F, Moore M, Ryffel B, Eugster HP. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks, and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger R, Browning JL, Michie SA, van Ewijk W, McDevitt HO. Disrupted splenic architecture, but normal lymph node development in mice expressing a soluble lymphotoxin-β receptor-IgG1 fusion protein. Proc Natl Acad Sci USA. 1996;93:13102–13107. doi: 10.1073/pnas.93.23.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay F, Majeau GR, Lawton P, Hochmann PS, Browning JL. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur J Immunol. 1997;27:2033–2042. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- 21.Kapasi ZF, Burton GF, Shultz LD, Tew JG, Szakal AK. Induction of functional follicular dendritic cell development in severe combined immunodeficiency mice. Influence of B and T cells. J Immunol. 1993;150:2648–2658. [PubMed] [Google Scholar]
- 22.Yoshida K, van den Berg TK, Dijkstra CD. The functional state of follicular dendritic cells in severe combined immunodeficient (SCID) mice: role of the lymphocytes. Eur J Immunol. 1994;24:464–468. doi: 10.1002/eji.1830240230. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Kaji M, Takahashi T, van den Berg TK, Dijkstra CD. Host origin of follicular dendritic cells induced in the spleen of SCID mice after transfer of allogeneic lymphocytes. Immunology. 1995;84:117–126. [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-β–specific receptor. Science. 1994;264:707–710. [PubMed] [Google Scholar]
- 25.Kosco MH, Pflugfelder E, Gray D. Follicular dendritic cell–dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 26.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosco-Vilbois MH, Scheidegger D. Follicular dendritic cells: antigen retention, B cell activation, and cytokine production. Curr Top Microbiol Immunol. 1995;201:69–82. doi: 10.1007/978-3-642-79603-6_4. [DOI] [PubMed] [Google Scholar]
- 28.Imazeki N, Takeuchi A, Senoo A, Fuse Y. New monoclonal antibodies directed against mouse follicular dendritic cells. J Histochem Cytochem. 1994;42:329–335. doi: 10.1177/42.3.8308249. [DOI] [PubMed] [Google Scholar]
- 29.Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147:763–771. [PMC free article] [PubMed] [Google Scholar]
- 30.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 31.Ni J, Porter AG, Hollander D. β7 integrins and other cell adhesion molecules are differentially expressed and modulated by TNFβ in different lymphocyte populations. Cell Immunol. 1995;161:166–172. doi: 10.1006/cimm.1995.1023. [DOI] [PubMed] [Google Scholar]
- 32.Melani C, Silvani A, Parmiani G, Colombo MP. Lymphotoxin gene expression by melanocytes and melanoma cell lines and persistence of unspliced mRNA. FEBS Lett. 1993;335:114–118. doi: 10.1016/0014-5793(93)80451-y. [DOI] [PubMed] [Google Scholar]
- 33.Ware CF, Crowe PD, Grayson MH, Androlewicz MJ, Browning JL. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 34.Millet I, Ruddle NH. Differential regulation of lymphotoxin (LT), lymphotoxin-β (LT-β), and TNF-α in murine T cell clones activated through the TCR. J Immunol. 1994;152:4336–4346. [PubMed] [Google Scholar]
- 35.Worm M, Geha RS. CD40 ligation induces lymphotoxin α gene expression in human B cells. Int Immunol. 1994;6:1883–1890. doi: 10.1093/intimm/6.12.1883. [DOI] [PubMed] [Google Scholar]
- 36.Cerny A, Zinkernagel RM, Groscurth P. Development of follicular dendritic cells in lymph nodes of B-cell–depleted mice. Cell Tissue Res. 1988;254:449–454. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 37.Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin α (LTα) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]