Identification of a Human Enterocyte Lipoxin A4 Receptor That Is Regulated by Interleukin (IL)-13 and Interferon γ and Inhibits Tumor Necrosis Factor α–induced IL-8 Release (original) (raw)
Abstract
Epithelial cells of the alimentary tract play a central role in mucosal immunophysiology. Pathogens and/or agonists that interact with mucosal surfaces often elicit epithelial responses that upregulate inflammation. Therefore, it was of interest to explore potential epithelial targeted antiinflammatory signals. Here we identified and sequenced a human enterocyte lipoxin (LX) A4 [5(S),6(R),15(S)-trihydroxy-7,9,13-_trans_-11-cis eicosatetraenoic acid] receptor, and demonstrate that transcription of this receptor was controlled by cytokines, of which lymphocyte-derived interleukin (IL)-13 and interferon γ were the most potent. When lipoxins and LXA4 stable analogs were evaluated for enterocyte functional as well as immune responses, lipoxins sharply inhibited TNF-α–induced IL-8 release but did not alter either barrier function or agonist-stimulated chloride secretion. 15_R/S_-methyl-LXA4 and 16-phenoxy-LXA4 each attenuated (IC50 ∼10 nM) IL-8 release. Cyclooxygenase (COX) II is emerging as an important component in wound healing and proliferation in intestinal epithelia and when acetylated by acetylsalicylic acid (aspirin) initiates the biosynthesis of a LXA4 receptor ligand. We therefore determined whether colonic cell lines (HT-29 Cl.19A, Caco-2, or T84) express the COX II isozyme. Results for RT-PCR and Western blot analysis showed that COX I as well as an IL-1β– and TNF-α–inducible COX II are expressed in HT-29 Cl.19A. In addition, aspirin-treated enterocytes generated 15R-HETE, a precursor of 15-epi-LXA4 biosynthesis, whose potent bioactions were mimicked by the stable analog 15_R/S_-methyl-LXA4. Taken together, these results identify an endogenous pathway for downregulating mucosal inflammatory events and suggest a potential therapeutic benefit for LXA4 stable analogs.
Epithelial cells that line the alimentary tract serve as the barrier separating the lumen from underlying tissues. It is now apparent that enterocytes play a critical role in mucosal immunophysiology that, in part, consists of a paracrine network between enterocytes and the underlying immune and inflammatory cells (1–5). This view is supported by the observation that the release of lymphocyte-derived mediators alters enterocyte phenotype and function as evidenced by the impact of IFN-γ on human enterocytes in vitro. IFN-γ attenuates barrier function and agonist-stimulated chloride secretion as well as induces expression of MHC class II molecules for enterocyte immune accessory functions (1, 6). In addition, lymphocytes can convert enterocytes into M cells that transport antigen to underlying immune cells, an action constituting an important component of oral vaccination and immunization (4).
Enterocytes can contribute to the regulation of mucosal immune responses by releasing cytokines and chemokines that can in turn activate and recruit inflammatory as well as immune cells to the mucosa. For example, TNF-α or pathogens can induce release of a potent leukocyte chemoattractant, namely IL-8, by intestinal epithelial cell lines as well as freshly isolated human colon enterocytes (7, 8). Only pathways that upregulate the inflammatory/immune response are recognized to date in agonist- or pathogen-stimulated enterocytes (for review see reference 1).
It is therefore of interest to elucidate regulatory signals that could attenuate mucosal immune functions. In this regard, endogenously generated lipoxin A4 (LXA4: 5(S), 6(R),15(S)-trihydroxy-7,9,13-_trans_-11-cis eicosatetraenoic acid)1 is of interest because it inhibits the action of proinflammatory stimuli both in vitro and in vivo (for review see reference 9). LXA4 is a potent inhibitor of both neutrophil adhesion and transmigration across endothelia or epithelia. It is then of special interest that acetylsalicylic acid (aspirin) triggers the biosynthesis of a recently discovered novel pathway that leads to transcellular production of 15-epi-LXA4, since enterocytes are probably among the first cell types to encounter orally administered aspirin. This transcellular route involves acetylation of cyclooxygenase (COX) II by aspirin in both human endothelia and lung epithelia (10, 11) and formation of 15-epi-LXA4 by neighboring neutrophils. The C-15 alcohol of 15-epi-LXA4 is in the rectus (R) configuration, rather than sinister (S) as in native LXA4. This switch in chirality enhances its bioactivity as well as resistance to metabolic inactivation (9). The inhibitory actions of LXA4 and the aspirin-triggered 15-epi-LXA4 provided an intriguing opportunity to develop stable LXA4 and 15-epi-LXA4 analogs. These analogs were recently evaluated in a mouse in vivo inflammation model and proved to be topically active, and also more potent inhibitors of LTB4-initiated inflammation than was the well-known antiinflammatory steroid, namely dexamethasone (12).
The bioactions of LXA4, 15-epi LXA4, and LXA4 stable analogs are transduced by a high affinity myeloid G protein–coupled receptor that has been sequenced and cloned for both mouse (12) and human leukocytes (13, 14). In addition, LXA4 actions with vascular endothelial and mesangial cells are mediated via a distinct nonmyeloid receptor that remains to be cloned (9). In the gastrointestinal tract, COX II upregulation is associated with human colorectal adenocarcinomas (15, and see reference 16 for review), mucosal lesions (17), colitis (18), and acute pathogen invasion (19). This localization of the enzyme and its regulation can provide a strategic in vivo milieu “primed” for the biosynthesis of aspirin-triggered 15-epi-lipoxins.
Since enterocytes are in close proximity to cell types of the immune system and are among the first cell types to encounter orally administered aspirin, the actions of 15-epi-LXA4 on these cells are of potential interest. Also, in view of cytokine-mediated upregulation of epithelial immune function and its associated increase in IL-8 release in human ulcerative colitis (20) and Crohn's colitis (21), as well as increased levels of TNF-α (22), it was of interest to elucidate endogenous signals that might attenuate mucosal inflammatory responses. To this end, we identified the first nonmyeloid LXA4R in human enterocytes, and we demonstrate here that expression of this receptor is regulated by cytokines and that stable analogs of LXA4 and aspirin-triggered 15-epi-LXA4 are potent inhibitors of agonist-induced IL-8 secretion by enterocytes. These results provide the first evidence that LXA4R gene expression is associated with immune functions of human enterocytes.
Materials and Methods
Materials.
Synthetic LXA4, LXB4 [5(S),14(R),15(S)-trihydroxy-6,10,12-trans-8-cis-eicosatetraenoic acid], LTD4 [5(S)-hydroxy- (6R)-S-cysteinyl-glycyl-7,9-trans-11,14-eicosatetraenoic acid], 5-HETE (15-hydroxy-5,8,11-_cis_-13-_trans_-eicosatetraenoic acid), 12-HETE, and 15-HETE were purchased from Cascade Biochem Limited (Reading, Berkshire, UK). Arachidonic acid was obtained from Cayman Chemical Company (Ann Arbor, MI). Acetylsalicylic acid (aspirin) was purchased from Spectrum Chemical Mfg. Corp. (Gardena, CA). 16-phenoxy-LXA4-methyl ester and 15(R/ S)-methyl LXA4-methyl ester were prepared by total synthesis for the present experiments by Dr. Nicos Petasis and colleagues (University of Southern California, Los Angeles, CA) as in reference 23. LXA4 analogs used in these experiments were carboxy methyl esters. Recombinant human (r) IL-8, rIL-1β, rIL-4, rIL-6, rIL-13, and rIFN-γ were purchased from R&D Systems (Minneapolis, MN). Dulbecco's PBS (DPBS), DMEM, and Ham's F-12 were obtained from Bio Whittaker (Walkersville, MD) and HBSS was from GIBCO BRL (Gaithersburg, MD). First strand cDNA synthesis kit was purchased from Promega (Madison, WI), and all other molecular biology reagents were obtained from Boehringer Mannheim Corp. (Indianapolis, IN). Human colonic epithelial cell lines (Caco-2 and T84) and alveolar epithelial cells (A549) were purchased from the American Type Culture Collection (Rockville, MD), and HT-29 Cl.19A were generously provided by Dr. Laboisse (Universite de Nantes, Nantes, France). Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). Sep-Pak C18 cartridges were obtained from Alltech (Deerfield, IL). Methyl formate was purchased from Eastman Kodak Co. (Rochester, NY). Hexane and all HPLC solvents were purchased from J.T. Baker (Phillipsburg, NJ). Calcium ionophore (A23187) and all other reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Epithelial Cell Lines and Culture.
Human colonic adenocarcinoma cell lines were grown and passaged in culture conditions as previously described for T84 (24), Caco-2 (25), and HT-29 Cl.19A (26) at 37°C in an atmosphere of 5% CO2. In brief, T84 cells were propagated in 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 14 mM NaHCO3, 40 mg/liter penicillin, 9 mg/liter streptomycin, 8 mg/liter ampicillin, 5% newborn calf serum, and 15 mM Na+ Hepes buffer, pH 7.5. HT-29 Cl.19A and Caco-2 were propagated in DMEM containing a standard (4.5 gram/liter) glucose concentration and supplemented with 14 mM NaHCO3, 40 mg/liter penicillin, 9 mg/liter streptomycin, 8 mg/liter ampicillin, 10% fetal bovine serum, and 15 mM Hepes buffer, pH 7.5. The human lung adenocarcinoma cell line A549 was grown and passaged as in reference 11.
Polarized colonic epithelial cells were split near confluency by incubating cells with 0.1% trypsin and 0.9 mM EDTA in Ca2+- and Mg2+-free DPBS for 5–20 min. Cells were diluted in media alone or in media containing IL-1β (1 ng/ml), IL-4 (10 ng/ml), IL-6 (1,000 U/ml), IL-13 (10 ng/ml), IFN-γ (1,000 U/ml), and LPS (500 ng/ml), and then were incubated for 24 and/or 48 h. Cytokine selection and concentrations selected for evaluation in our experiments were based on results from published studies using epithelial cells (6, 11, 27). In experiments analyzing enterocyte barrier function, chloride secretion, and IL-8 release, polarized monolayers of T84 were grown on collagen-coated permeable supports and maintained until steady-state transepithelial resistance (i.e., >600 Ω × cm2) was achieved, as previously detailed in reference 24.
Reverse Transcription and PCR.
Total RNA from colonic (T84, Caco-2, HT-29 Cl.19A) or alveolar epithelial cells (A549), which were exposed to either IL-1β (1 ng/ml) or media alone for 24 h, was extracted with TriZol reagent (GIBCO BRL) according to the manufacturer's instructions. RNA was further purified by chloroform/phenol/isoamyl (24:25:1) extraction and cDNA was produced by reverse transcription. Oligonucleotide sense and antisense primers were constructed from published sequences of COX II (28), myeloid 15-lipoxygenase (LO; reference 29), 12-LO (30), and 5-LO (31). Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as internal controls, as described in reference 11. Amplification protocols for COX II and GAPDH consisted of 25 repetitive cycles of denaturing at 94°C (1 min), annealing at 58°C (2 min), and extension at 72°C (3 min). 5-, 12- and 15-LO were amplified by 35 repetitive cycles of denaturing at 94°C (1 min), annealing at 55°C (2 min), and extension at 72°C (2.5 min). Amplified cDNA was separated by agarose gel electrophoresis and visualized with ethidium bromide.
Identification and cDNA Cloning of the Intestinal Epithelial LXA4R.
Total RNA was isolated from T84, HT-29 Cl.19A, Caco-2, or A549 as described above, and 0.5 μg of RNA was reverse transcribed. Oligonucleotide primers, sense primer 5′-CACCAGGTGCTGCTGGCAAG-3′ and antisense primer 5′-AATATCCCTGACCCCATCCTCA-3′, were designed to amplify the published human myeloid LXA4R coding region (∼1.1 kb; reference 12). Amplification protocols, using the high fidelity polymerase PCR system Expand (Boehringer Mannheim Corp.), consisted of 35 repetitive cycles of denaturing at 94°C (30 s), annealing at 64°C (45 s), and extension at 72°C (80 s). In parallel analysis, oligonucleotide primers designed for the recently sequenced myeloid LTB4 receptor (32), sense primer 5′-GGCAAGCTTATGAACACT ACATCTTCT-3′ and antisense primer 5′-GGCAGGACCTCTAGTTCAGTTCGTTTA-3′, were used to amplify the LTB4 receptor coding region (∼1.1 kb). The amplification protocol, using vent (New England Biolabs, Beverly, MA) as the polymerase, consisted of 35 repetitive cycles of denaturing at 98°C (60 s), annealing at 60°C (60 s), and extension at 72°C (70 s). PCR analysis of LXA4R demonstrated a single PCR product from T84 cDNA with an apparent size of ∼1.1 kb, which was subcloned into pBluescript KS(+), and three independent clones were isolated and selected for analysis at the Children's Hospital sequencing facility (Boston, MA). The following oligonucleotide primers were used to sequence the 1,053-bp coding region of the human enterocyte LXA4R: nucleotides 0–264 [pBluescript KS (+) reverse primer] and 884–1053 (M13-20 sense primer); nucleotides 210–631 (5′-ATCTGTTACCTGAACCTGGC-3′) and 514–914 (5′-GTCTCTCTCGGAAGTCTTGG-3′); and nucleotides 477– 907 (5′-TTGCTCTAGTCCTTACCTTGC-3′) and 461–818 (5′-GATGTCAATGATTTTGTACTTGC-3′).
Northern Blot Analysis of Enterocyte LXA4R Messenger RNA.
Confluent T84 cells (resistance >600 Ω × cm2) were exposed to media alone or selected cytokines (IL-1β, IL-4, IL-6, IL-13, IFN-γ, or LPS) for 24 or 48 h. PolyA+ RNA was directly isolated from cells using the FastTrack® 2.0 messenger (m)RNA isolation system (Invitrogen, San Diego, CA) according to the manufacturer's instructions. mRNA (3 μg) was purified by gel electrophoresis in 1% agarose gel containing 1.9% formaldehyde and was then blotted to nylon membrane. The cDNA of the human leukocyte LXA4R coding region was labeled with α-[32P]dCTP using Oligolabeling kit (Pharmacia Biotech AB, Piscataway, NJ) and hybridization was performed in 1% BSA, 7% SDS, 0.5 M phosphate buffer (pH 6.8), and 1 mM EDTA as in reference 33. Filters were washed in buffer A (0.5% BSA, 5% SDS, 40 mM phosphate buffer [pH 6.8], and 1 mM EDTA) twice for 20 min at 65°C, and then in buffer B (1% SDS, 40 mM phosphate buffer [pH 6.8], and 1 mM EDTA) four times for 20 min at 65°C. After quantitation using a PhosphorImager (Molecular Dynamics, San Lorenzo, CA), the filter was washed in 50% formamide to remove the LXA4R probe and was reprobed with α-32P-labeled actin cDNA (Clontech, Palo Alto, CA). Northern hybridization was quantitated by PhosphorImager using Image-Quant programming (Molecular Dynamics), and LXA4R mRNA was normalized to actin mRNA levels in each treatment condition.
Barrier Function and Chloride Secretion in Human Enterocytes.
Polarized monolayers of T84 (resistance >600 Ω × cm2) were grown on matrix-coated permeable supports that divided the monolayer into an upper (apical) and lower (basolateral) compartment. Apical and basolateral epithelial compartments were exposed to media alone, LXA4, or LXB4 at indicated concentrations for 24 or 48 h. After 24 h, complete media was replaced and eicosanoid treatment was repeated for both apical and basolateral compartments. The impact of LXA4 analogs that resist metabolic inactivation was evaluated by exposing apical and basolateral compartments to 15_R/S_-methyl-LXA4 or 16-phenoxy-LXA4 (1 nM–1 μM) for 30 min to 24 h. After exposure to lipoxin, LXA4 analog, or vehicle, cells were washed with HBSS and all subsequent measurements were made in a 37°C environment. Enterocytes were stimulated with forskolin (10 μM) for 10 min to stimulate chloride secretion. Short circuit currents, transepithelial potentials, and resistances were measured with a voltage clamp (Iowa Dual Voltage Clamps, Bioengineering, University of Iowa, Iowa City, IA) interfaced with an equilibrated pair of calomel electrodes and a pair of Ag–AgCl electrodes as in reference 24.
TNF-α–induced IL-8 Release by Human Enterocytes.
Polarized T84 grown on matrix-coated permeable supports were washed three times with HBSS and the basolateral (300 μl) and apical (100 μl) compartments were exposed to HBSS containing either LXA4, 15_R/S_-methyl-LXA4, or 16-phenoxy-LXA4 at indicated concentrations, or vehicle alone (0.1% EtOH). After incubation (30 min, 37°C), human recombinant TNF-α (Endogen, Inc., Woburn, MA) was added at a 1:100 (vol/vol) ratio to both apical and basolateral compartments. TNF-α was tested in the concentration range of 0.02–0.5 nM to evaluate the impact of LXA4 and LXA4 analogs on the distinct signal transduction pathways of the TNF receptors CD120a (K d 0.5 nM) and CD120b (K d 0.1 nM) (34–36). After a 5-h exposure, IL-8 release was measured in the basolateral compartment by ELISA, which has been previously identified as the site of chemokine release in polarized human enterocytes (7, 8). IL-8 release was measured by ELISA as in reference 8. In brief, 96-well plates (Libro/Titretek, ICN Biomedicals, Aurora, OH) were coated overnight with goat α-human IL-8 antibody (R&D Systems). Rabbit α-human IL-8 (Endogen, Inc.) was used as the detection antibody and peroxidase conjugated rabbit α-goat IgG was used as the reporting antibody. After addition of the substrate, the plates were read at a wavelength of 650 nm.
Western Blot Analysis of COX I and II Proteins in Human Enterocytes.
Subconfluent HT-29 Cl.19A were lifted with trypsin/ EDTA and resuspended in media containing IL-1β (1 ng/ml) and/or TNF-α (50–100 ng/ml) or media alone and incubated for 24 or 48 h. Enterocytes were washed twice in DPBS, resuspended, and incubated in lysis buffer for 10 min (50 mM Tris-HCl, pH 7.5; 10 mM EDTA; 1% Triton X-100; 1 mM PMSF; 200 μM leupeptin; and 50 μM pepstatin A) and sonicated (three 10-s bursts) with a Sonic Dismembrator (Model 60; Fisher Scientific Co., Pittsburgh, PA). Protein concentrations in the cell extract were determined using a Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Cell extracts were boiled for 5 min in gel-loading buffer and equal amounts of protein were separated by SDS-PAGE according to Laemmli's method. Proteins were transferred to a polyvinylidene difluoride microporous membrane by electroblotting. The membrane was blocked in 1% blocking reagent (Boehringer Mannheim Corp.) in TBST (Tris-buffered saline containing 0.1% vol/vol Tween 20 [0.9% NaCl and 0.1% Tween 20 in 20 mM Tris-HCl, pH 7.4]) and probed (1 h, room temperature) with either an anti-human COX II polyclonal antibody or polyclonal anti-COX I peptide antibody (Oxford Biomedical Research, Oxford, MI). Membranes were washed three times in TBST followed by an incubation with horseradish peroxidase–linked goat anti–rabbit IgG (1:10,000 dilution) for 30 min. Immunoreactive bands were developed with chemiluminescence substrates (Boehringer Mannheim) and visualized by exposure to x ray film. COX protein levels were quantitated by densitometry.
Analysis of Mono-HETE Generation by Human Enterocytes.
Confluent HT-29 Cl.19A cells that were exposed for 24 h to either media alone or to both IL-1β (1 ng/ml) and TNF-α (50 ng/ml) were washed twice and suspended in DPBS. Enterocytes were incubated with either vehicle alone or aspirin (300 μM). After a 20 min incubation, cells were permeabilized by two rapid freeze– thaw cycles and stimulated with 20 μM of arachidonic acid for 30 min as in reference 11. Incubations were terminated with the addition of 2 vol of cold MeOH and 200 ng of PGB2 were added as an internal standard.
Eicosanoids were extracted using Sep-Pak C18 cartridges as in reference 37. Materials eluting in the methyl formate fraction were taken to dryness using a stream of nitrogen and suspended in HPLC mobile phase. The reverse phase (RP)-HPLC system consisted of a dual pump gradient (LKB, Bromma, Sweden), a diode array detector (Hewlett-Packard 1040M series II; Hewlett Packard, San Fernando, CA), and HPLC3D ChemStation software. Collected UV data were recalled at 234 nm for detecting the presence of conjugated dienes (mono-HETEs). All spectra were acquired using step = 4 nm and bandwidth = 10 nm, with a sampling interval of 0.96 s. Monohydroxy eicosanoids (i.e., 5-, 12-, and 15-HETE) from HT-29 Cl.19A cells were chromatographed using an Ultrasphere-ODS (5 μm, 4.6 mm × 25 cm) column (Beckman Instruments, Fullerton, CA) with MeOH/ H2O/acetic acid (65:35:0.01; vol/vol/vol) as phase 1 (0–20 min), and a linear gradient with MeOH/acetic acid (99.99:0.01; vol/ vol) as phase 2 (20–45 min) at a flow rate of 1.0 ml/min. The area beneath the peaks of eluting compounds was corrected for recovery of PGB2 (used as internal standard), integrated using ChemStation software and quantitated by direct comparison to peak areas of their corresponding authentic standards in this system.
Statistical Analysis.
Unless otherwise indicated, all values are represented as mean values ± SEM. Results were analyzed using Student's t test. Differences were considered significant at the P <0.05 level.
Results
Cloning of the Human Enterocyte LXA4R and Its Regulation by Cytokines.
To elucidate potential bioactions of LXA4 and 15-epi-LXA4 with human enterocytes, we first sought to determine whether LXA4Rs are present in human colonic epithelia using established model cell lines. Reverse transcription (RT)-PCR analysis of enterocyte-derived cDNA demonstrated a single band with the expected size of ∼0.7 kb present in T84, HT-29 Cl.19A, and Caco-2. It is of interest to note that another epithelial cell line from the airway, A549, did not show appreciable levels of the LXA4R cDNA (data not shown). To determine the sequence homology of the human enterocyte LXA4R and those of the human and mouse myeloid receptors (9, 12), specific LXA4 primers were designed to amplify the complete coding region (∼1.1 kb; see Materials and Methods). T84 cells gave an ∼1.1 kb PCR product that was isolated, cloned, and sequenced. Three independent clones contained a 1,053-bp cDNA that was identical to the human myeloid LXA4R. Of interest, RT-PCR analysis did not provide evidence for expression of the recently identified human myeloid LTB4 receptor in these particular epithelial cell lines. These findings clearly demonstrated that the LXA4R is not only expressed in enterocytes but that it is conserved in both human leukocytes and enterocytes. The cDNA sequence of the human enterocyte LXA4R is available from EMBL/ GenBank/DDBJ under accession number AF054013.
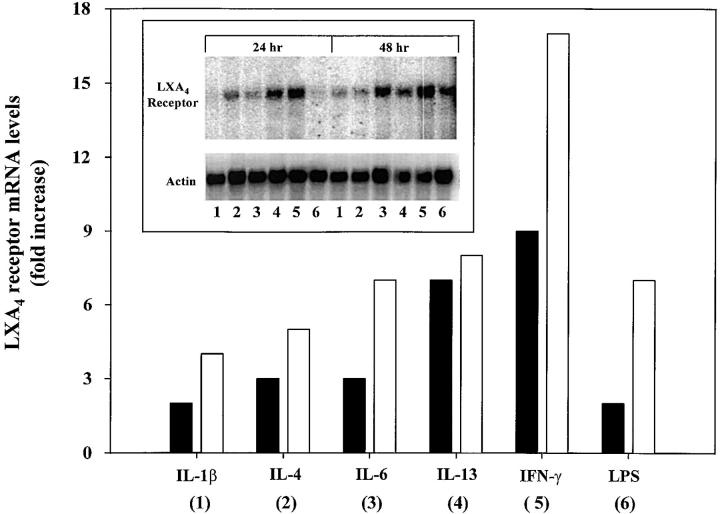
Selected cytokines were recently found to be modulators of phenotype and enterocyte function (6, 27). To evaluate whether LXA4R expression was altered by these cytokines, Northern blot analysis of poly+ RNA was used to monitor LXA4R transcription in T84 cells. Cytokines for which enterocytes bear functional receptors were chosen for evaluation (i.e., see Fig. 1) (6, 27). After 24 h, exposure to these cytokines increased LXA4R mRNA levels compared with those of cells that were exposed to media alone (Fig. 1). Among the panel of cytokines evaluated, IL-1β and LPS were the least potent cytokines, inducing a 1.8-fold increase in LXA4R mRNA, whereas exposure to either IL-4 or IL-6 increased receptor message levels by ∼3-fold. The most potent cytokines proved to be IFN-γ and IL-13, which increased LXA4R mRNA by ∼6.8- and 8.6-fold, respectively. This pattern of cytokine selective upregulation of LXA4R transcription was also observed for the 48-h interval. LXA4R mRNA levels increased in each experimental group from 24 to 48 h compared with those of the control group in which message levels remained unchanged. After 48 h of exposure, IL-1β and IL-4 increased LXA4R message levels by a total of ∼4.0- to 4.5-fold, whereas IL-6 gave an ∼6.6-fold and LPS an ∼7.3-fold increase. The most potent of the cytokines examined proved to be IL-13 and IFN-γ, as they increased LXA4R mRNA by 8.4- and 17.3-fold in 48 h when compared with the parallel controls. Similar patterns of selective regulation of the receptor message levels by these cytokines were also observed using RT-PCR analysis of total RNA from T84 (data not shown). Together these results indicate that IL-13 and IFN-γ each selectively increase LXA4R mRNA levels.
Figure 1.
Cytokines regulate epithelial LXA4R expression. Northern blot analysis of LXA4R mRNA levels in human enterocytes (T84) exposed to cytokines or buffer alone (inset). T84 were exposed to either IL-1β (1 ng/ml), IL-4 (10 ng/ml), IL-6 (1,000 U/ml), IL-13 (10 ng/ml), IFN-γ (1,000 U/ml), LPS (500 ng/ml), or buffer alone for 24 (▪) or 48 h (□). RNA was isolated and 3 μg of poly+RNA was separated by gel electrophoresis, and then filters were washed and hybridized as described in Materials and Methods. LXA4R mRNA was quantitated by a PhosphorImager and normalized to corresponding actin mRNA for each treatment protocol. Changes in receptor mRNA levels are expressed as fold increase compared with T84 cells that were exposed to media alone.
Lipoxins Do Not Modulate Epithelial Barrier Function or Forskolin-induced Chloride Secretion.
Epithelial cells that line the intestine perform a primary function as a selective barrier and secretory cell (1, 26). We next examined whether LXA4 or LXB4 could have an impact on epithelial barrier function and/or agonist-induced chloride secretion when compared with the established effect of IFN-γ on enterocyte function (6). Neither LXA4 nor LXB4 altered T84 barrier function after 24 or 48 h of exposure when compared with vehicle alone (data not shown; n = 3). In addition, exposure to either LXA4 or LXB4 for 48 h did not stimulate chloride secretion or alter agonist-activated (forskolin, 10 μM) chloride secretion in enterocytes when compared with the vehicle control (data not shown; n = 3). Essentially similar findings were observed after 24 h of exposure to either LXA4 or LXB4. Furthermore, results obtained with 15_R/S_-methyl-LXA4 and 16-phenoxy-LXA4 confirm these findings, since neither analog in a concentration range of 1 nM to 1 μM and an exposure of 30 min to 24 h altered these enterocyte functional responses (data not shown; n = 3). Together these results indicate that LXA4R-mediated actions include neither modulation of epithelial barrier function nor chloride secretion.
LXA4 Stable Analogs Are Potent Inhibitors of TNF-α– induced IL-8 Secretion in Human Enterocytes.
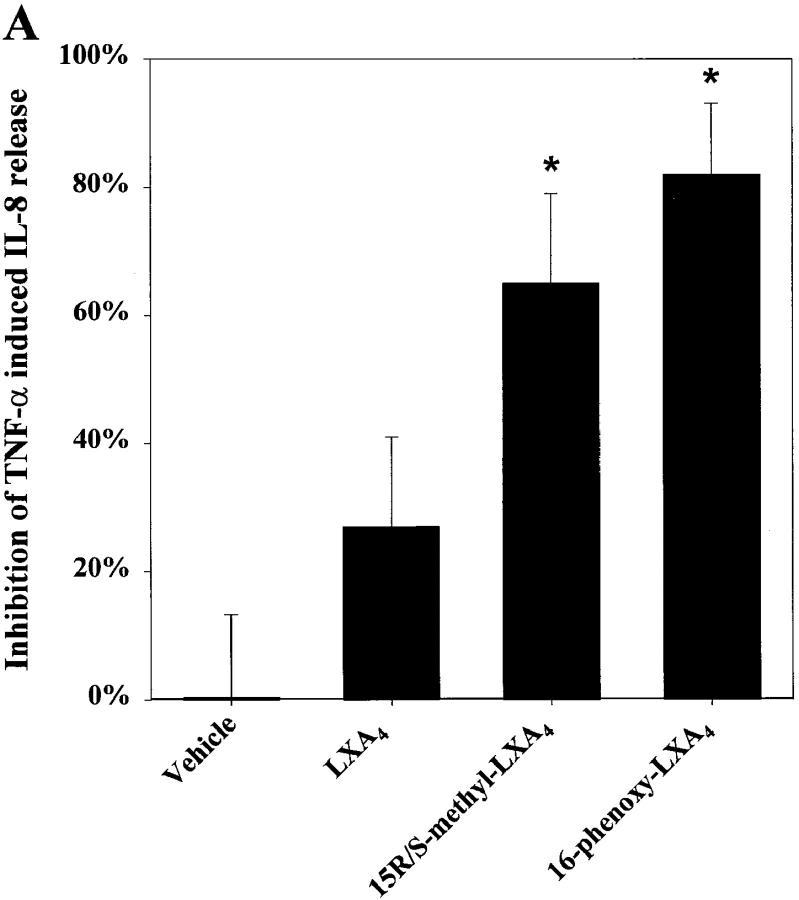
Intestinal epithelia constitutively express low levels of IL-8 that can be markedly upregulated by pathogens and proinflammatory cytokines such as TNF-α. This increase in basolateral IL-8 secretion is held to be important in PMN recruitment to the epithelium (7, 8). To evaluate whether LXA4 and LXA4 stable analogs modulate IL-8 release, T84 monolayers were stimulated with TNF-α, and IL-8 release was determined after epithelial exposure to LXA4 and/or stable analogs. IL-8 secretion in untreated T84 cells ranged from less than the ELISA detection limit to ∼20 pg/epithelial cell monolayer. TNF-α, in the concentration range expected to use the high affinity receptor (K d ∼0.1 nM) (34), induced epithelial IL-8 secretion that was significantly inhibited (P <0.006) by both 15_R/S_-methyl-LXA4 and 16-phenoxy-LXA4 (Fig. 2 A).
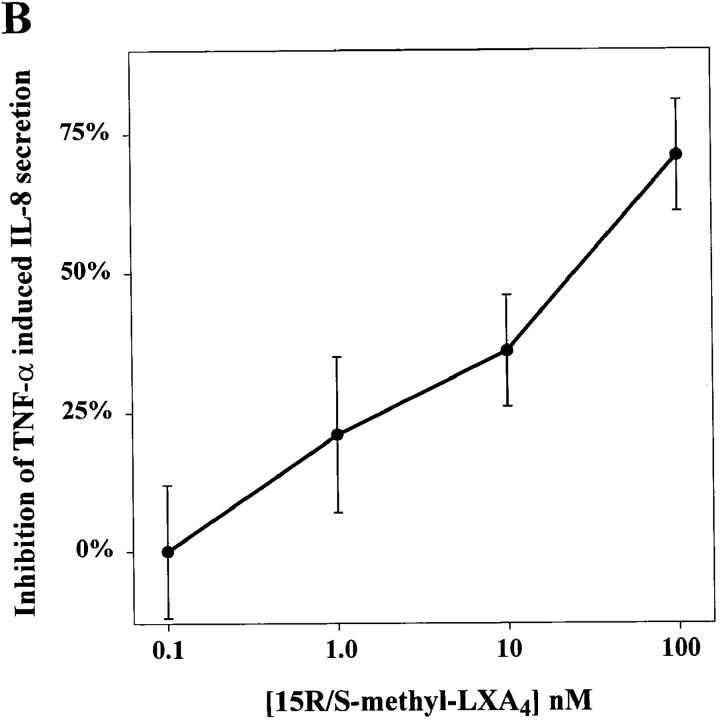
Figure 2.
LXA4 and aspirin-triggered 15-epi-LXA4 stable analogs are potent inhibitors of TNF-α–induced IL-8 secretion in human enterocytes_._ Monolayers of T84 were grown on matrix-coated permeable supports. (A) Monolayers were treated with either LXA4 (100 nM), 15_R/S_-methyl-LXA4 (100 nM), 16-phenoxy-LXA4 (100 nM), or vehicle (0.1% EtOH) alone. (B) Monolayers were exposed to indicated concentrations of 15_R/S_-methyl-LXA4. After 30 min of lipoxin exposure, enterocytes were stimulated with TNF-α (0.1 nM). After 5 h the basolateral supernatants were removed and IL-8 concentration was measured by ELISA. Results are presented as percentage of inhibition of TNF-α–induced IL-8 release (∼60 pg/monolayer of T84); each panel shows results from a representative experiment consisting of three independent monolayers for each experimental parameter. Essentially identical patterns were observed in at least three separate experiments.
15_R/S_-methyl-LXA4 inhibition of TNF-α–induced IL-8 secretion was concentration dependent (Fig. 2 B) and maximal inhibition (65 ± 14%) was observed at the highest concentration tested, namely, 100 nM. 16-phenoxy-LXA4 at 100 nM proved to be even more potent, inhibiting agonist-induced IL-8 secretion by 82 ± 11% (Fig. 2 A). Furthermore, IL-8 secretion induced by 0.02 and 0.2 nM TNF-α was also significantly inhibited (∼50%) by 15_R/S_-methyl-LXA4 (data not shown; n = 3). Native LXA4 did not exhibit a statistically significant effect on TNF-α–induced IL-8 (Fig. 2 A), although a trend toward suppression of IL-8 secretion was consistently noted. It is likely that a decrease in LXA4 concentration during the incubation (5 h) brought about by metabolic inactivation may explain its diminished action compared with its stable LXA4 analogs. Stimulation of IL-8 secretion with higher concentrations of TNF-α that were in the range of the low affinity TNF-α receptor (∼0.5 nM) (34) led to greater IL-8 secretion that was not subject to inhibition by 100 nM 15_R/S_-methyl-LXA4 (data not shown). These results indicate that LXA4 stable analogs are potent inhibitors of TNF-α–induced IL-8 release by intestinal epithelial cells and appear to selectively inhibit signals transduced via the CD120b TNF receptor (35, 36).
COX II Is Expressed in Human Colonic Cell Lines.
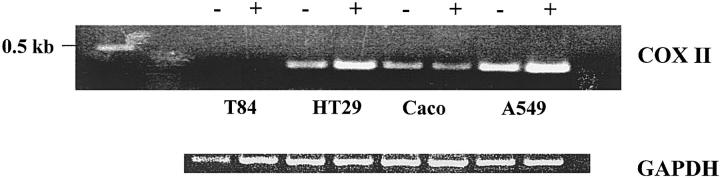
Upregulation of COX II in enterocytes, an enzymatic target for nonsteroidal antiinflammatory drug action, is associated with intestinal inflammation and cell proliferation (16). To determine whether human enterocytes have the enzymatic capacity to serve as a site or donor for aspirin-triggered 15-epi-lipoxin production, cDNA from untreated as well as IL-1β–primed enterocyte cell lines was examined for the presence of COX II. In addition, we evaluated the presence of the lipoxygenases 5-LO, 12-LO, and myeloid 15-LO, which are other components of the transcellular pathways for LXA4 and 15-epi-LXA4 biosynthesis (for review see reference 9). In the absence of cytokine stimulation, HT-29 Cl.19A and Caco-2, but not T84, expressed low levels of COX II. Exposure to IL-1β enhanced COX II expression in HT-29 Cl.19A cells, but no significant change in COX II RNA levels was observed for Caco-2 cells. T84 cells did not express appreciable amounts of COX II (Fig. 3). Indeed, the level of enterocyte basal and cytokine-induced COX II mRNA was less than that observed with the airway epithelial cell line A549 (Fig. 3). It is of interest to note that each of these colonic epithelial cell lines (HT-29 Cl.19A, Caco-2, and T84) expressed 5-LO mRNA to some extent. In contrast, these enterocyte cell lines expressed neither 12-LO nor 15-LO RNA (data not shown). These findings indicate that human enterocytes (HT-29 Cl.19A and Caco-2) exposed to cytokines have the enzymatic capacity to generate COX II–derived eicosanoids.
Figure 3.
Expression of COX II in human colonic cell lines_._ Total RNA was isolated from colonic (T84, Caco-2, and HT-29 Cl.19A) or airway (A549) epithelial cell lines treated with either IL-1β (1 ng/ml) or media alone for 24 h. cDNA was analyzed by RT-PCR using specific primers designed for human COX II or GAPDH. PCR products were separated on a 2% agarose gel stained with ethidium bromide. Similar patterns were observed in three separate experiments.
Transient and Cytokine-specific Induction of COX II in Human Enterocytes.
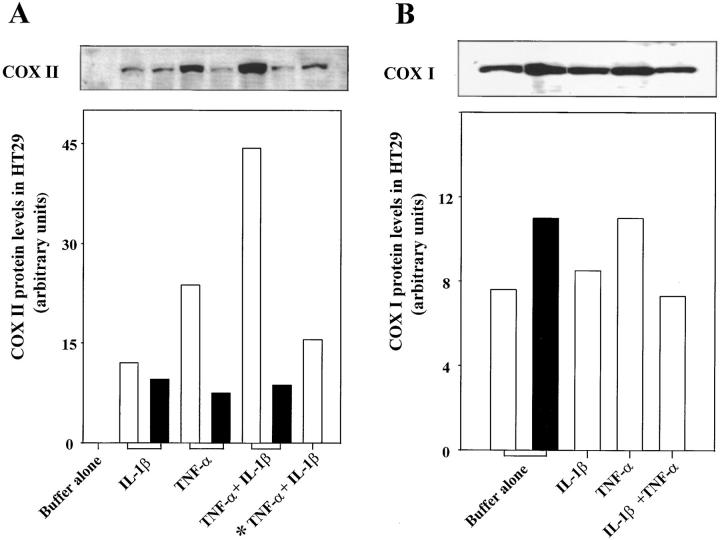
Do changes in COX II mRNA translate to protein levels and active enzyme in cytokine-primed enterocytes? To achieve high levels of COX II expression, HT-29 Cl.19A were treated with TNF-α, a cytokine that also induces COX II in a variety of cell types (1), or were treated in combination with IL-1β. COX II protein levels were quantitated and compared with COX I by Western blot. COX II protein was not observed with the untreated cells but was induced by both IL-1β and TNF-α after 24 h (Fig. 4 A). TNF-α–induced COX II levels were approximately twofold higher than IL-1β–induced protein levels. Treating cells with both cytokines gave an approximately fourfold greater increase in COX II protein as compared with IL-1β treatment alone. Extending exposure to these cytokines from 24 to 48 h decreased COX II protein levels. These findings indicate that upregulated COX II expression was transient. In addition, increasing TNF-α concentration from 50 to 100 ng/ml (TNF-α + IL-1β exposed for 24 h) induced COX II protein at levels that were higher than those observed with IL-1β alone but less than levels observed with 50 ng/ml of TNF-α alone or in combination with IL-1β (24 h of exposure). These findings are consistent with the well-characterized cytotoxic effect of TNF-α with tumor cells such as HT-29 (38, 39).
Figure 4.
IL-1β and TNF-α induce COX II in human enterocytes_._ (A) Monolayers of HT-29 Cl.19A were exposed to IL-1β (1 ng/ml) and/or TNF-α (50 ng/ml) for 24 (□) or 48 h (▪). In select experiments, cells were exposed to TNF-α (100 ng/ml) and IL-1β (1 ng/ml) for 24 h (indicated by *). Cells were homogenized and proteins (30 μg/lane) were separated on 10% polyacrylamide SDS gel, transferred to polyvinylidene difluoride microporous membrane, and probed with a COX II–specific antibody. (B) Protein (3 μg/lane) from HT-29 Cl.19A treated with IL-1β and/or TNF-α (50 ng/ml) for 24 h or buffer alone for 24 (□) or 48 h (▪) was probed with a COX I–specific antibody. Immunoreactive bands were quantitated by densitometry. Identical patterns were observed in three separate experiments.
For the purpose of direct comparison, COX I protein levels were analyzed by Western blot. Results in Fig. 4 indicate that COX I was a highly abundant protein in HT-29 Cl.19A (note that protein concentrations were reduced by 10-fold in comparison to the COX II immunoblot to achieve visualization of immunoreactive bands). COX I was detected in untreated cells as well as in cytokine-primed enterocytes (Fig. 4 B). However, protein levels for COX I were not significantly altered when these cells were exposed to either IL-1β and/or TNF-α. Thus, in enterocytes, COX II levels were most effectively increased by combined exposure to TNF-α and IL-1β. This “upregulation” of COX II was transient and did not extend to COX I, whose protein levels were not significantly altered by exposure to these cytokines.
Aspirin-dependent 15-HETE Generation by Enterocytes.
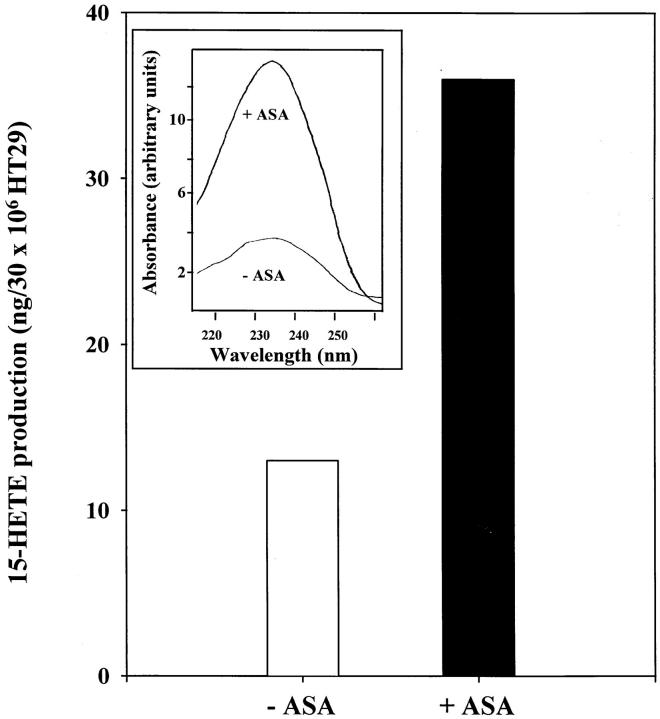
Do cytokine-primed enterocytes generate 15R-HETE when treated with aspirin? It was of interest to determine if enterocytes (HT-29 Cl.19A) that demonstrated cytokine-regulated COX II at both RNA and protein level (Figs. 3 and 4) also had the capacity to generate 15R-HETE when exposed to aspirin. To this end cytokine-primed enterocytes were incubated with aspirin, and lipid extracts were subjected to analysis using RP-HPLC. Aspirin-treated enterocytes gave a product that coeluted with authentic 15-HETE standard and displayed a characteristic conjugated diene UV chromophore with a maximum absorbance at 234 nm (Fig. 5, chromophore inset). 15-HETE production by cytokine-primed enterocytes exposed to aspirin increased by approximately threefold (Fig. 5), a finding that is consistent with the notion of aspirin-dependent 15R-HETE generation demonstrated in both endothelial cells (10) and airway epithelial cells (11). These findings are also consistent with results obtained with isolated recombinant COX II and 15R-HETE biosynthesis (40). Of interest, RP-HPLC profiles from permeabilized cytokine-treated enterocytes did not show 5-HETE generation, although we found message for this enzyme to be present. These results are consistent with previous studies that detected no 5-LO activity in intact HT-29, and only after sonication were small amounts of 5-LO products observed (41). Together, these findings indicate that cytokine-primed enterocytes in the presence of aspirin can generate 15R-HETE.
Figure 5.
Aspirin-dependent 15-HETE generation by IL-1β– and TNF-α–treated enterocytes_._ Monolayers of human enterocytes (30 × 106 HT-29 Cl.19A) were exposed to TNF-α (50 ng/ml) and IL-1β (1 ng/ml) for 24 h. After cytokine treatment, cells were treated with aspirin (300 μM) or vehicle alone for 20 min. Enterocytes were freeze–thaw permeabilized and incubated with 20 μM arachidonic acid for 30 min. After extraction, products that eluted in the methyl formate fraction were analyzed by reverse phase HPLC (see Materials and Methods). Inset shows the characteristic mono-HETE UV chromophore of the aspirin-dependent enterocyte product 15-HETE. Results are representative of two separate experiments that gave similar results.
Discussion
Our results demonstrate that human intestinal epithelial cells express LXA4Rs and that the stable analogs of LXA4 and aspirin-triggered 15-epi-LXA4 attenuated TNF-α– induced IL-8 release in human enterocytes. Also, lymphocyte-derived cytokines exhibiting potent actions on enterocyte function and phenotype (6, 27) were shown here for the first time to upregulate gene expression of this receptor. RT-PCR analysis demonstrated that the LXA4R is expressed in established model cell lines of colonic epithelial cells. These cells form functional columnar epithelia that resemble natural in vivo crypt and brush border epithelia (6, 25, 26, 42). Sequence analysis revealed that the enterocyte LXA4R is identical to the human myeloid receptor (13, 14) and 76% homologous to the mouse leukocyte receptor (12). It is therefore of interest to note that RT-PCR analysis of RNA from other epithelial tissues including human cornea also proved positive for the LXA4R (data not shown), yet epithelial cells from the airway were negative. The finding of LXA4R expression in human epithelial cells suggests conserved receptor function and provides support for the immunoregulatory role of enterocytes. This conclusion is further supported by results from experiments with human neutrophils indicating that the LXA4R transduces signals that counterregulate proinflammatory mediators such as LTB4 and FMLP both in vitro (9) and in vivo (12).
The intestinal mucosa forms the most extensive barrier separating the external environment from the internal milieu (for review see reference 1). Enterocytes are in communication with the interdispersed intraepithelial lymphocytes and submucosal inflammatory cells to fulfill their function as an absorptive/secretory and immune accessory cell. In this respect, enterocytes are considered a primary lymphoid organ and a component of the mucosal immune system (for review see references 1 and 3). It is thus of particular interest that lymphocyte-derived cytokines upregulated gene expression of the epithelial LXA4R (Fig. 1). These cytokines initiate enterocyte immune functions that include antigen presentation, expression of secretory components, and transport of immunoglobulin A into the intestinal lumen. Cytokines associated with these phenotypic changes, namely IL-13 (27) and IFN-γ (6), were the most potent in inducing LXA4R transcription. Of interest, IL-13 also induces 15-LO, an important enzyme in native lipoxin biosynthesis (43).
Our results also suggest that signals transduced by the LXA4R are associated with the immune function of human enterocytes. Evidence to support this hypothesis is twofold. First, LXA4, LXB4, and stable LXA4 analogs did not alter enterocyte barrier function or cAMP-mediated chloride secretion, a finding consistent with previous results showing that exposure to LXA4 for 2 h did not impact monolayer integrity (44). This further distinguishes lipoxin A4 from the bioaction of prostaglandin E2, whose receptors and action have been characterized in human enterocytes and include stimulation of cAMP-mediated chloride secretion (45). Also, our results indicate that lipoxins do not activate secondary mediators such as prostaglandin or arachidonic acid release in enterocytes, since these compounds are known to stimulate chloride secretion (45, 46). Second, unlike the lack of impact on human enterocyte secretory and barrier function, LXA4 and stable LXA4 analogs attenuated epithelial immune function, namely the inhibition of IL-8 release.
In the mucosal milieu, enterocytes play an important role in leukocyte recruitment by releasing basolaterally the potent chemoattractant IL-8 (8), which is associated with the pathogenesis of diseases such as Crohn's (21) and ulcerative colitis (20). The stable LXA4 analogs 15_R/S_-methyl-LXA4 and 16-phenoxy-LXA4 proved to be potent inhibitors of IL-8 release induced by the high affinity (CD120b) TNF-α receptor (Fig. 2), attenuating IL-8 release by as much as 62 ± 14% and 82 ± 11%, respectively. Therefore, these are the first results demonstrating that a lipid mediator inhibits IL-8 release. These findings suggest a potentially important new role for LXA4 as an endogenous “stop-signal” in the progression of an enterocyte-initiated inflammatory response.
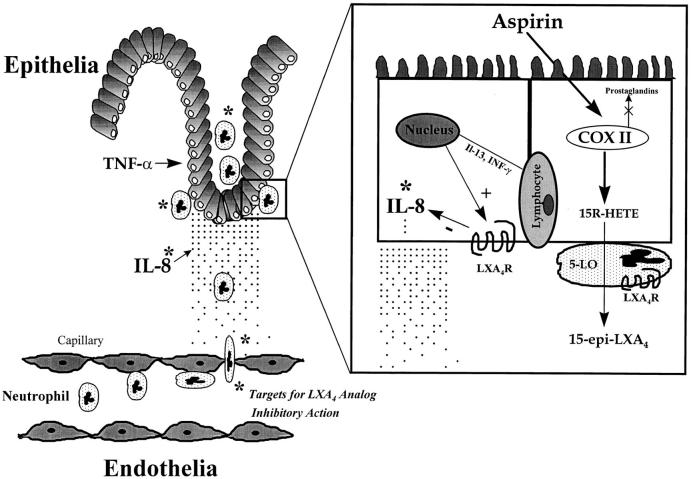
COX II is expressed in human colonic tumors and adenocarcinoma epithelial cell lines (for review see references 1 and 16) as well as in normal epithelial tissue in acute bacterial infections (19), mucosal lesions (17), and colitis (18). Activation of this early response gene (e.g., COX II) is associated with both epithelial cell proliferation (for review see reference 16) and wound healing, and selective inhibition of COX II exacerbates colon injury in several in vivo models (1, 17, 18). It is of interest to point out that the analog 15_R/S_-methyl-LXA4, which inhibited enterocyte immune function (Fig. 2), is a structurally related mimetic of aspirin-triggered 15-epi-LXA4 (9). The observations that cytokines upregulated enterocyte COX II and that these cells (HT-29 Cl.19A) generate 15-HETE in the presence of aspirin are in agreement with results reported with both human vascular endothelial and lung epithelial cells (10, 11). Together, these in vitro findings suggest that, in scenarios where COX II is induced, enterocytes have the potential in vivo to augment the biosynthesis of 15-epi-LXA4, which may serve to inhibit further PMN accumulation during mucosal inflammation (Fig. 6).
Figure 6.
Hypothetical scheme of the antiinflammatory actions of 15-epi-LXA4 and LXA4 stable analogs in the alimentary tract. Multistep recruitment of neutrophils to a site of mucosal inflammation and enterocyte LXA4R gene regulation by intraepithelial lymphocyte-derived cytokines is depicted. Activation of receptors, present on both neutrophils and enterocytes, inhibits PMN migration to sites of inflammation. Targets or sites of action are indicated by * for LXA4, aspirin-triggered LXA4 (15-epi-LXA4), and their stable analogs, which include inhibition of PMN adhesion to and transmigration across both endothelia and epithelia as well as inhibition of the generation of a gradient of IL-8. The biosynthesis of aspirin-triggered 15-epi-LXA4 is illustrated during PMN and enterocyte interactions.
In summary, this is the first evidence demonstrating that a cytokine regulates LXA4R expression in intestinal epithelial cells, suggesting that the LXA4R is associated with enterocyte immune functions. In addition, our findings indicate that LXA4 directly modulates the initiation of inflammatory events by inhibiting the release of the potent chemokine IL-8 at the initial site of pathogen exposure, namely at the epithelial barrier. These results indicate that the potential for antiinflammatory actions of LXA4 in the gastrointestinal tract are multifaceted by acting on both myeloid cells to counterregulate proinflammatory mediators (Fig. 6 and for review see reference 9) and directly attenuating the initiation of inflammation by modulating IL-8 release from epithelia (Fig. 2). Thus, these findings with enterocytes and lipoxins expand the bioactivity of these endogenous lipid mediators and provide potential new uses for LXA4 stable analogs in mucosal immunobiology.
Acknowledgments
We thank Mary Halm Small for assistance in manuscript preparation.
These studies were supported in part by National Institutes of Health (NIH) grant GM-38765, a research grant from Schering AG (to C.N. Serhan), and NIH grants DK-47662 and DK-35392 (to J.L. Madara). K. Gronert is the recipient of a postdoctoral fellowship from the National Arthritis Foundation and A. Gewirtz is a recipient of an individual National Research Service Award.
Footnotes
1 Abbreviations used in this paper: 15-epi-LXA4, 5(S),6(R),15(R)-trihydroxy-7,9,13-_trans_-11-cis eicosatetraenoic acid; 15 (R/S)-methyl LXA4, 5(S),6(R), 15(R/S)-trihydroxy-15-methyl-7,9,13-_trans_-11-_cis_-eicosatetraenoic acid; 16-phenoxy-LXA4, 16-phenoxy-17,18,19,20-tetranor-LXA4; aspirin, acetylsalicylic acid; COX, cyclooxygenase (PGHS); 15-HETE, 15-hydroxy-5,8,11-_cis_-13-_trans_-eicosatetraenoic acid; LO, lipoxygenase; LXA4, 5(S),6(R),15(S)-trihydroxy-7,9,13-_trans_-11-cis eicosatetraenoic acid; LXA4R, lipoxin A4 receptor; RP-HPLC, reverse phase-HPLC.
The present address of Drs. Gewirtz and Madara is Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30322.
References
- 1.Stenson WF, Alpers DH. A parable on the dangers of overclassification: can an enterocyte assume immune functions? . Curr Opin Gastroenterology. 1994;10:121–124. [Google Scholar]
- 2.Madara JL. The chameleon within: improving antigen delivery. Science. 1997;277:910–911. doi: 10.1126/science.277.5328.910. [DOI] [PubMed] [Google Scholar]
- 3.Shanahan F. A gut reaction: lymphoepithelial communication in the intestine. Science. 1997;275:1897–1898. doi: 10.1126/science.275.5308.1897. [DOI] [PubMed] [Google Scholar]
- 4.Kernéis S, Bogdanova A, Kraehenbuhl J-P, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Whetsell M, Klein JR. Local hormone networks and intestinal T cell homeostasis. Science. 1997;275:1937–1939. doi: 10.1126/science.275.5308.1937. [DOI] [PubMed] [Google Scholar]
- 6.Colgan SP, Parkos CA, Matthews JB, D'Andrea L, Awtrey CS, Lichtman AH, Delp-Archer C, Madara JL. Interferon-γ induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994;267:C402–C410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann L, Jung HC, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 8.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimuriumto intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell–cell interactions or a therapeutic opportunity? . Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- 10.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell– leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)–neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 12.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4stable analogs are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4–lipoxin A4receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 14.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 15.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 16.Levy GN. Prostaglandin H synthases, nonsteroidal antiinflammatory drugs, and colon cancer. FASEB (Fed Am Soc Exp Biol) J. 1997;11:234–247. [PubMed] [Google Scholar]
- 17.Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 18.Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckmann L, Stenson WF, Savidge TC, Lowe DC, Barrett KE, Fierer J, Smith JR, Kagnoff MF. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. J Clin Invest. 1997;100:296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C. Interleukin-8 and neutrophil markers in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol. 1992;87:1447–1452. [PubMed] [Google Scholar]
- 21.Sher M, D'Angelo A, Stein T, Bailey B, Burns G, Wise L. Cytokines in Crohn's colitis. Am J Surg. 1995;169:133–136. doi: 10.1016/s0002-9610(99)80121-4. [DOI] [PubMed] [Google Scholar]
- 22.Raab Y, Gerdin B, Ahlstedt S, Hällgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993;34:1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 24.Dharmsathaphorn K, Madara JL. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- 25.Grasset E, Bernabeu J, Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol. 1985;248:C410–C418. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JB, Smith JA, Tally KJ, Awtrey CS, Nguyen H, Rich J, Madara JL. Na-K-2Cl cotransport in intestinal epithelial cells. J Biol Chem. 1994;269:15703–15709. [PubMed] [Google Scholar]
- 27.Zünd G, Madara JL, Dzus AL, Awtrey CS, Colgan SP. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem. 1996;271:7460–7464. doi: 10.1074/jbc.271.13.7460. [DOI] [PubMed] [Google Scholar]
- 28.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigal E, Craik CS, Highland E, Grunberger D, Costello LL, Dixon RAF, Nadel JA. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem Biophys Res Commun. 1988;157:457–464. doi: 10.1016/s0006-291x(88)80271-7. [DOI] [PubMed] [Google Scholar]
- 30.Funk CD, FitzGerald GA. Eicosanoid forming enzyme mRNA in human tissues. J Biol Chem. 1991;266:12508–12513. [PubMed] [Google Scholar]
- 31.Dixon RAF, Jones RE, Diehl RE, Bennett CD, Kargman S, Rouzer CA. Cloning of the cDNA for human 5-lipoxygenase. Proc Natl Acad Sci USA. 1988;85:416–420. doi: 10.1073/pnas.85.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokomizo T, Izumi T, Chang K, Takuwa T, Shimizu T. A G-protein–coupled receptor for leukotriene B4that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 33.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dembic Z, Loetscher H, Gubler U, Pan Y-CE, Lahm H-W, Gentz R, Brockhaus M, Lesslauer W. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine. 1990;2:231–237. doi: 10.1016/1043-4666(90)90022-l. [DOI] [PubMed] [Google Scholar]
- 35.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 36.Rink L, Kirchner H. Recent progress in the tumor necrosis factor–α field. Int Arch Allergy Immunol. 1996;111:199–209. doi: 10.1159/000237369. [DOI] [PubMed] [Google Scholar]
- 37.Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85:772–780. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbara JA, Van Ostade X, Lopez A. Tumour necrosis factor–alpha (TNF-α): the good, the bad and potentially very effective. Immunol Cell Biol. 1996;74:434–443. doi: 10.1038/icb.1996.73. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiero V, Latham K, Baglioni C. Cytostatic and cytotoxic activity of tumor necrosis factor on human cancer cells. J Immunol. 1987;138:2711–2717. [PubMed] [Google Scholar]
- 40.Xiao G, Tsai A-L, Palmer G, Boyar WC, Marshall PJ, Kulmacz RJ. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 1997;36:1836–1845. doi: 10.1021/bi962476u. [DOI] [PubMed] [Google Scholar]
- 41.Cortese JF, Spannhake EW, Eisinger W, Potter JJ, Yang VW. The 5-lipoxygenase pathway in cultured human intestinal epithelial cells. Prostaglandins. 1995;49:155–166. doi: 10.1016/0090-6980(95)00003-s. [DOI] [PubMed] [Google Scholar]
- 42.Lammers KM, Jansen J, Bijlsma PB, Ceska M, Tytgat GNJ, Laboisse CL, van Deventer SJH. Polarised interleukin secretion by HT 29/19A cells. Gut. 1994;35:338–342. doi: 10.1136/gut.35.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nassar GM, Morrow JD, Roberts LJ, II, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- 44.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers KV, Goldman PS, Frizzell RA, McKnight GS. Regulation of Cl−transport in T84 cell clones expressing a mutant regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1990;87:8975–8979. doi: 10.1073/pnas.87.22.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett KE, Bigby TD. Involvement of arachidonic acid in the chloride secretory response of intestinal epithelial cells. Am J Physiol. 1993;264:C446–C452. doi: 10.1152/ajpcell.1993.264.2.C446. [DOI] [PubMed] [Google Scholar]