CD4+ T Cell Tolerance to Parenchymal Self-Antigens Requires Presentation by Bone Marrow–derived Antigen-presenting Cells (original) (raw)
Abstract
T cell tolerance to parenchymal self-antigens is thought to be induced by encounter of the T cell with its cognate peptide–major histocompatibility complex (MHC) ligand expressed on the parenchymal cell, which lacks appropriate costimulatory function. We have used a model system in which naive T cell receptor (TCR) transgenic hemagglutinin (HA)-specific CD4+ T cells are adoptively transferred into mice expressing HA as a self-antigen on parenchymal cells. After transfer, HA-specific T cells develop a phenotype indicative of TCR engagement and are rendered functionally tolerant. However, T cell tolerance is not induced by peptide–MHC complexes expressed on parenchymal cells. Rather, tolerance induction requires that HA is presented by bone marrow (BM)–derived cells. These results indicate that tolerance induction to parenchymal self-antigens requires transfer to a BM-derived antigen-presenting cell that presents it to T cells in a tolerogenic fashion.
Keywords: peripheral tolerance, CD4+ T cells, antigen-presenting cells, adoptive T cell transfer, transgenic mice
The immune system has the ability to recognize and neutralize pathogens. However, it must remain tolerant of self-antigens so as not to destroy what it has evolved to protect. In the case of T lymphocytes, the majority of cells with specificities for self-antigens are eliminated during development in the thymus (1, 2). T cells whose TCRs recognize self determinants not presented in the thymus will mature and migrate to the peripheral lymphoid organs. Therefore, additional mechanisms of peripheral tolerance are needed to prevent the development of autoimmunity (3).
There appear to be several potential mechanisms for peripheral T cell tolerance. In the simplest case, naive T cells ignore parenchymal tissues expressing their cognate antigen (4, 5). The lack of expression of T cell costimulatory ligands such as B7 on the antigen-expressing parenchyma may play a role in preventing these T cells from becoming activated (6, 7). The most profound form of tolerance is physical deletion of potentially autoreactive T cells (8–10), a process that may proceed via an initial activation phase before ultimate elimination (11–13). T cell inhibition by expression of Fas ligand by cells in certain organs such as the eye (14) and testis (15), as well as downregulation of the TCR (16) or coreceptor molecules (i.e., CD8) (17), represent additional mechanisms for self-tolerance generation observed in certain model systems.
Another form of peripheral tolerance that is commonly observed is the induction of anergy (18). Anergic T cells are neither deleted nor altered with regard to expression of their TCR and coreceptor molecules, but are refractory to antigenic stimuli that would activate naive T cells (19–21). The two-signal model predicts that a T cell will become activated when its TCR recognizes the cognate MHC– peptide ligand on the surface of a professional (i.e., bone marrow [BM]1–derived) APC that also expresses a second costimulatory signal, whereas TCR engagement in the absence of costimulation results in anergy (22). Consistent with this model is the observation that transgenic expression of an allo-class II MHC molecule on pancreatic islet β cells, which do not express costimulatory activity, induces anergy in the cognate CD4+ T cell population (20), whereas coexpression of B7 together with allo-class II MHC on β cells diminishes their capacity for tolerance induction (23). The more recent observation that anergy induction by injection of soluble antigen can be blocked in vivo by preventing B7–CTLA4 interaction (24) suggests that counterregulatory pathways also influence the ultimate outcome of TCR engagement.
In contrast to parenchymal cells, antigen presentation by BM-derived cells is generally considered to be stimulatory rather than tolerogenic. Nonetheless, antigen presentation by some subsets of BM-derived cells, such as B cells, have been shown to induce tolerance in vivo (25, 26). Therefore, it is possible that tolerance induction to peripheral self-antigens could involve transfer to a subset of BM-derived APCs specialized to present them to T cells in a tolerogenic fashion. To distinguish whether tolerance induction to parenchymal self-antigens involves direct presentation versus transfer to a tolerizing BM-derived APC, we have developed a model in which naive TCR transgenic CD4 cells specific for the hemagglutinin (HA) antigen are rendered anergic after adoptive transfer into mice expressing HA as a parenchymal self-antigen. Analysis of tolerance induction in parent → F1 chimeras demonstrates the absolute requirement of transfer to and presentation by BM-derived APCs in tolerance induction.
Materials and Methods
Generation of Transgenic Mice.
The 7.1 kb C3(1) genomic clone (27) was provided by Elizabeth Wilson (University of North Carolina, Chapel Hill, NC). The initiation codon (ATG) located in the first exon at +55 bp as well as a potential cryptic initiation codon (ATG) at +104 bp were altered to ATT and TTG, respectively, by subcloning the 5′ 4.4-kb HindIII–PstI fragment into the pALTER-1 plasmid (Altered Sites In Vitro Mutagenesis System; Promega, Madison, WI) and following the manufacturer's protocol for site-directed mutagenesis using the mutagenic oligonucleotide 5′-CAA CAT TAA GCT GGT GTT TCT ATT CTT GTT GGT CAC CAT CCC CAT TTG CTG CTT TGC CAG. After DNA sequencing was performed to confirm the correct alterations, the HindIII–PstI fragment was ligated into the pGem4 vector (Promega). The 1.7-kb HA gene coding sequence derived from the influenza virus A/PR/8/34 (Mount Sinai strain) (28) was ligated downstream of the C3(1) promoter as a SalI–BamHI fragment. The 2.4 kb 3′ fragment of C3(1) was PCR amplified with Pfu polymerase (Stratagene, La Jolla, CA) using the primers 5′-GGT TCT GGA TCC AGT ATT CTA and 5′-CGT AGA TGA TCA GTG GGT GTG GG to add BamHI (5′) and BclI (3′) restriction sites, respectively, and then ligated into the BamHI site downstream of the HA gene.
The C3-HA expression cassette was isolated by digesting with HindIII and KpnI followed by gel purification using Elutip DNA purification columns (Schliecher & Schuell, Inc., Keene, NH) before microinjection into fertilized B10.D2 embryos (performed at the University of Michigan Biomedical Research Core Facilities, Ann Arbor, MI). Transgenic progeny were identified by PCR analysis of DNA extracted from tail biopsies using the primers 5′-GTG TTC TTT GGC TCT TCT TCG and 5′-GCA GAG AAA TCT GAT CAT AAC. Southern blot analysis indicated that the C3-HA transgenic founder line No. 142 contained approximately three intact copies of the transgene (data not shown).
The TCR transgenic mouse line 6.5 (reference 29; provided by Harald von Boehmer, Institut Necker, Paris, France) that expresses a TCR recognizing an I-Ed restricted HA epitope (110SFERFEIFPKE120) was backcrossed 8 generations onto the B10.D2 genetic background. Transgenic progeny were typed either by flow cytometry using the 6.5 mAb (see below) or by PCR analysis using the primers 5′-AGT CGT GCC CTG GTC CG and 5′-GCT GCA GTC ACC CAA AGC.
Reverse Transcriptase PCR Analysis of Tissue Expression of Transgene.
Tissues were homogenized in Trizol buffer (GIBCO BRL, Gaithersberg, MD) and RNA purified as recommended by the manufacturer. 1 μg of DNAsed RNA was reverse transcribed by standard methods and PCR amplified for 50 cycles using primers to the ubiquitously expressed β-2–microglobulin (β2M) gene, 5′-GAT GCT GAT CAC ATG TCT CG and 5′-CAA ATT CAA GTA TAC TCA CGC, as well as the C3-HA transgene transcript, 5′-CAT TTG CTG CTT TGC CAG TG and 5′-GGT TTC CCA AGA GCC ATC. Both primer sets spanned introns so that the resulting PCR products of 280 (β2M) and 310 bp (C3-HA) could only be amplified from cDNA and not from contaminating genomic DNA. PCR reactions were electrophoresed on 4% polyacrylamide gels and stained with ethidium bromide.
Flow Cytometry.
106 cells were preincubated with the Fc-γ receptor blocking antibody 2.4G2 (HB-197; American Type Culture Collection, Rockville, MD). mAbs used for staining were: biotinylated anti-clonotypic TCR (6.5, provided by H. von Boehmer); avidin-PE; and FITC-conjugated anti-CD4 (CT-CD4; Caltag, Burlingame, CA). Cy-chrome–conjugated anti-CD4 (RM4-5), FITC-conjugated anti-CD44 (IM7), and FITC-conjugated anti-CD45RB (16A) were purchased from PharMingen (San Diego, CA). 100,000 lymphocyte-gated events were collected for double staining, and 200,000 CD4+ gated events were collected for triple staining on a FACScan® (Becton Dickinson, San Jose, CA).
Adoptive Transfer.
Pooled axillary, inguinal, cervical, and mesenteric LNs from the 6.5 transgenic mice were dissociated in RPMI media (GIBCO BRL), passed over nylon mesh, and washed in RPMI. After FACS® staining to determine the proportion of clonotypic CD4 cells, cell preparations containing 2.5 × 106 clonotypic cells were injected intraperitoneally in 0.5 ml sterile HANKS buffer (GIBCO BRL) into unirradiated male recipients. T cell transfers using intraperitoneal versus intravenous delivery yielded equivalent results (data not shown). For control transfers, pooled LN cells from nontransgenic (NT) mice were injected into recipients with the total number of transferred cells equaling that given to mice receiving 6.5 clonotypic T cells. At the indicated time points after transfer, pooled LN and spleen were extracted from the recipients and dissociated in CTL media (RPMI with 10% FBS [HyClone, Logan, UT], 0.1 mM 2-ME, 2 mM l-glutamine,1 mM sodium pyruvate, and 1× nonessential amino acids and penicillin/streptomycin solutions (Sigma Chemical Co., St. Louis, MO)) for further analysis. Time course studies indicated that the responses of the clonotypic CD4 cells after transfer into the respective recipients were manifested by 9 d and persisted through 21 d after transfer (data not shown). Although data presented is exclusively from LN, T cells recovered from either the LN or spleen of the same recipient always exhibited a similar phenotype (data not shown). For transfers into parent → F1 chimeras, the 6.5 mice were made H-2bxd by mating with NT C57BL/6 mice. Before adoptive transfer, LN T cells from 6.5 bxd progeny were depleted of endogenous class II–expressing cells using streptavidin-conjugated dynabeads (DYNAL, Lake Success, NY) and the biotinylated anti-I-Ed mAb 14.4.4s as per the manufacturer's instructions.
Proliferation and IL-2 Assays.
1.5 × 105 LN cells or splenocytes extracted from transfer recipients were incubated in round-bottomed 96-well tissue culture plates with the indicated concentration of synthetic HA peptide. In parent → F1 chimera experiments, 5 × 104 cells prepared from the transfer recipients were mixed with 105 splenocytes from an unmanipulated NT mouse (F1, bxd) and HA peptide. For proliferation assays, 72-h cultures were pulsed with 1 μCi [3H]thymidine and incubated an additional 24 h before harvest and determination of the amount of incorporated radioactive counts. LN and spleen APCs extracted from C3-HA mice did not express endogenous HA peptide on their surface in sufficient quantities to either stimulate or suppress the response of naive clonotypic cells in this assay (data not shown). To measure IL-2 levels, media was removed from 72-h cultures and analyzed using a murine IL-2 ELISA kit (Endogen, Woburn, MA) as per the manufacturer's instructions. Individual data points in both proliferation and IL-2 assays represent the average of triplicate wells.
Generation of BM Chimeras.
2-mo-old mice were treated with 1,000 rads of ionizing radiation and then given 4 × 106 BM cells depleted of T cells using complement and the mAbs J1j (anti-Thy1), C3PO (anti-CD2), RL172 (anti-CD4), and 3.155 (anti-CD8). Animals were given a 2–3-mo recovery period before performance of T cell transfer experiments. For parent → F1 chimeras, H-2bxd BM recipients (generated by mating C3-HA transgenic and NT mice [on a B10.D2 background, H-2d] with C57BL/6 mice [H-2b]) received BM grafts from NT B10.D2 or C57BL/6 mice. After reconstitution, blood samples were stained with the mAbs 14.4.4s (anti-I-Ed) and Y-3P (anti-I-Ab) to confirm the BM haplotypes before initiation of T cell transfer experiments.
Results
Generation of C3-HA Transgenic Mice.
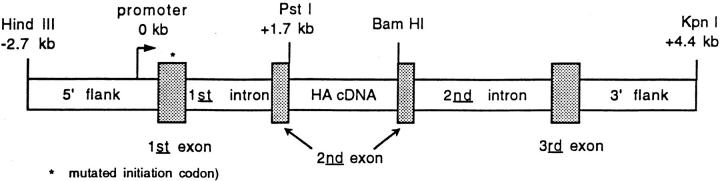
The rat C3(1) gene encodes a polypeptide that comprises one of the three subunits of the oligomeric prostatic steroid-binding protein, which is the predominant protein present in rat prostatic fluid (30). A large genomic fragment containing the three coding exons, two introns and several kb of both 5′ and 3′ flanking sequences was previously shown in transgenic mice to direct the expression of C3(1) mRNA in several organs. In addition to male reproductive organs such as the prostate and testes, the C3(1) transgene was also expressed in nonreproductive tissues such as the heart, salivary gland, skeletal muscle, and lung (31). A 7.1-kb C3(1) genomic fragment (27) was modified by ligating the coding sequence for the influenza HA gene (PR8 Mount Sinai strain) into the C3(1) second exon. The C3(1) initiation codon, located within the first exon, was altered by site-directed mutagenesis so that translation of the chimeric transgene mRNA would begin and terminate within the HA coding sequence (Fig. 1). The C3-HA expression cassette was microinjected into fertilized B10.D2 embryos. A transgene line No. 142 was characterized that contained approximately three intact copies of the transgene (data not shown).
Figure 1.
C3-HA transgene expression vector. The C3-HA transgene expression cassette consists of a 7.1-kb C3(1) HindIII–KpnI genomic fragment in which the 1.7-kb HA cDNA has been ligated into the second exon of C3(1). The natural initiation codon within the first exon of C3(1) was altered (*) so that translation would initiate and terminate within the HA cDNA sequence.
Using reverse transcriptase (RT)-PCR analysis, HA transcript–derived PCR products were consistently generated from RNA prepared from several peripheral organs including male sex accessory tissues such as the prostate, testis, vas deferens, and seminal vesicles, as well as a number of nonreproductive organs including the salivary gland, kidney, heart, and lung. HA expression was detected inconsistently in other tissues such as the thymus, BM, and LNs, suggesting that HA mRNA expression levels were near the threshold detection limit for the RT-PCR assay (Table 1). Anti-HA antibody staining of RT-PCR–positive tissues did not reveal any signal above background (data not shown), suggesting that HA expression in the No. 142 line was relatively low.
Table 1.
RT-PCR Analysis of Transgene mRNA Distribution
| Organ | HA mRNA expression |
|---|---|
| Male reproductive | |
| Ventral prostate | +≳ |
| Dorsal lateral prostate | + |
| Seminal vesicles | + |
| Testis | + |
| Vas deferens | + |
| Bulbourethral gland | + |
| Penis | + |
| Epididymis | +/−‡ |
| Coagulating gland | −§ |
| Lymphoid tissues | |
| Bone marrow | +/− |
| Thymus | +/− |
| Lymph node | +/− |
| Spleen | − |
| Others | |
| Lung | + |
| Heart | + |
| Salivary gland | + |
| Kidney | + |
| Skeletal muscle | + |
| Intestine | +/− |
| Bladder | − |
| Liver | − |
| Pancreas | − |
| Brain | − |
Naive HA-specific CD4+ T Cells Become Tolerant when Transferred into HA-expressing Transgenic Mice.
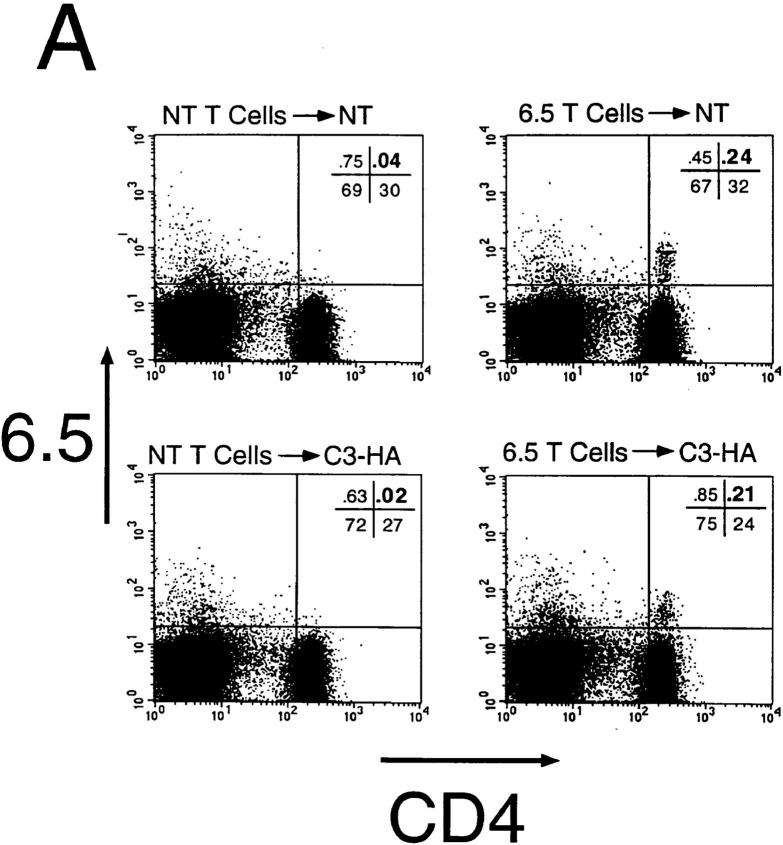
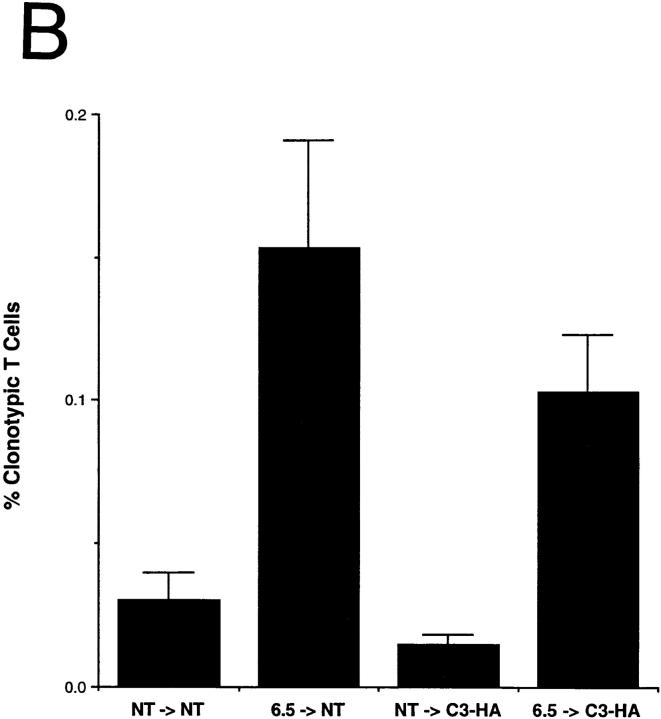
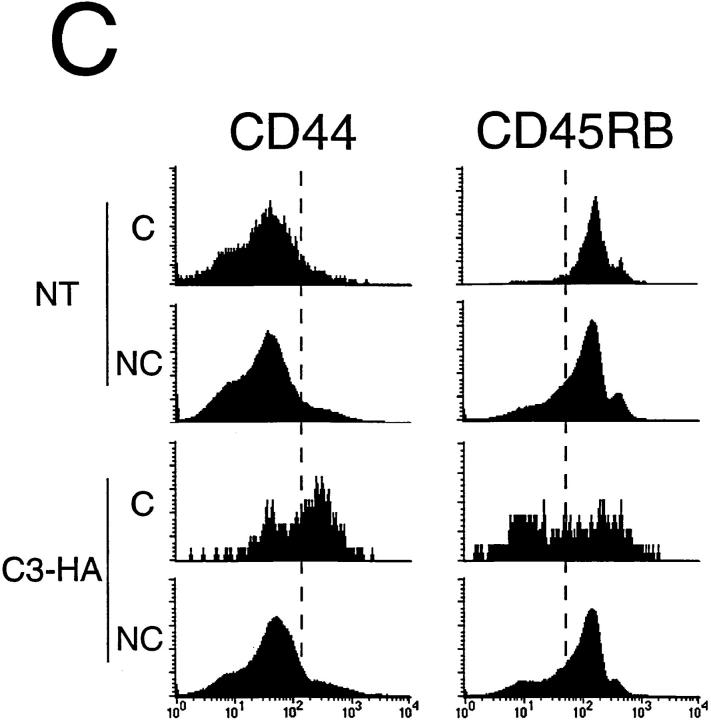
To study how HA-specific CD4+ T cells respond when they encounter HA antigen expressed in the C3-HA transgenic mice, naive clonotypic cells from 6.5 TCR transgenic mice (expressing a TCR specific for an I-Ed–restricted HA epitope) were adoptively transferred into unirradiated C3-HA and NT animals. 9 d after transfer into NT animals, the clonotypic CD4 cells were retrieved from pooled LN and their frequency was determined by FACS® (Fig. 2 A). In a representative NT transfer recipient, the clonotypic cells (which stain positively for both CD4 and the 6.5 TCR) comprised 0.24% of the total lymphocytes. The clonotypic CD4 cells were also present in a C3-HA recipient in approximately the same proportion (0.21%). The background staining, determined by analyzing control NT and C3-HA mice that had received T cell preparations from NT mice, was 0.04 and 0.02%, respectively. Comparing the average proportion of clonotypic T cells present in the two groups of recipients, there might have been slightly fewer clonotypic cells in the C3-HA mice (Fig. 2 B). Although the double staining did not reveal a significant difference between the number of clonotypic T cells that were present in the NT and those present in C3-HA recipients, triple staining using the cell surface markers CD44 and CD45RB suggested that the HA-specific CD4 cells had in fact encountered and responded to their cognate antigen after transfer into the C3-HA recipients (Fig. 2 C). Clonotypic T cells transferred into NT recipients exhibited CD44 and CD45RB expression profiles which were similar to those observed on gated non-clonotypic CD4 cells from the same animals, suggesting that these clonotypic cells remained in a naive state. In contrast, the clonotypic T cells retrieved from the C3-HA recipients had upregulated CD44 expression and downregulated CD45RB expression relative to the nonclonotypic CD4 cells, a phenotype consistent with antigen recognition. The average mean fluorescence values for each marker on the clonotypic cells were altered approximately twofold in the C3-HA recipients (data not shown). That the clonotypic, but not the nonclonotypic, T cells in the C3-HA and NT transfer recipients exhibited different expression profiles for CD44 and CD45RB consistent with the presence or absence of HA, respectively, provides confirmatory evidence that the cell population in the upper right quadrant of the FACS® plots (Fig. 2 A) are in fact clonotypic CD4 cells.
Figure 2.
FACS® analysis of clonotypic HA-specific CD4 cells after adoptive transfer into C3-HA transgenic mice. HA-specific CD4 cells were retrieved from the LN of C3-HA and NT recipients 9 d after transfer of 2.5 × 106 clonotypic cells. Two-color staining from representative transfer recipients (A) was performed using FITC-labeled anti-CD4 and biotinylated 6.5 followed by PE-labeled streptavidin. Clonotypic cells (upper right quadrant) stain positively for both CD4 and 6.5 surface markers. As controls, LN preparation taken from NT and C3-HA recipients of NT T cells were also stained. The relative percentage of cells in each quadrant is shown in the upper right corner of each plot with the percentage of cells in the upper right quadrant in larger print. Bar graph showing the average frequency of clonotypic CD4 cells in the different transfer recipient groups (mean ± SEM, n = 4) (B) Three color staining was performed using cy-chrome–labeled anti-CD4, FITC-labeled CD44 or CD45RB, and biotinylated 6.5 followed by PE-labeled streptavidin. (C) Histogram plots of representative clonotypic transfer recipients for CD44 and CD45RB expression on gated clonotypic (C) and nonclonotypic (NC) CD4 cells.
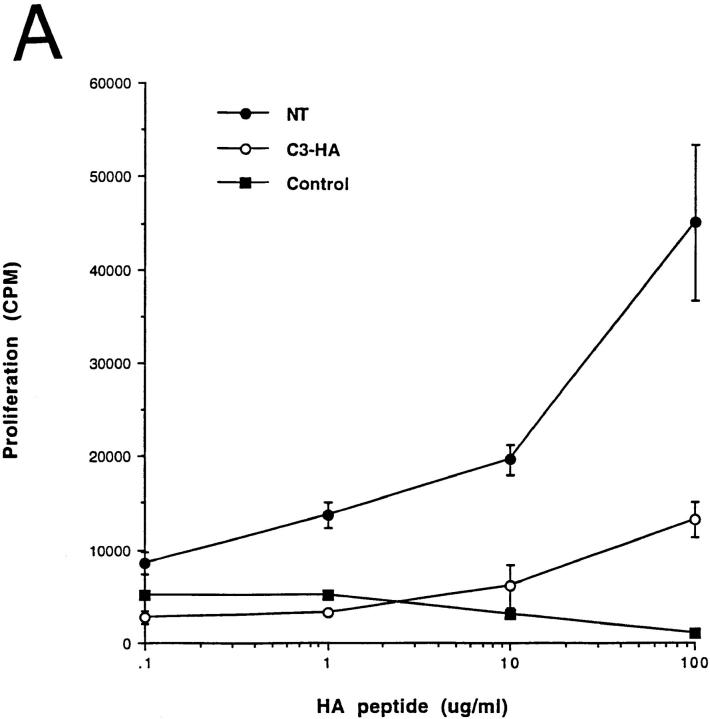
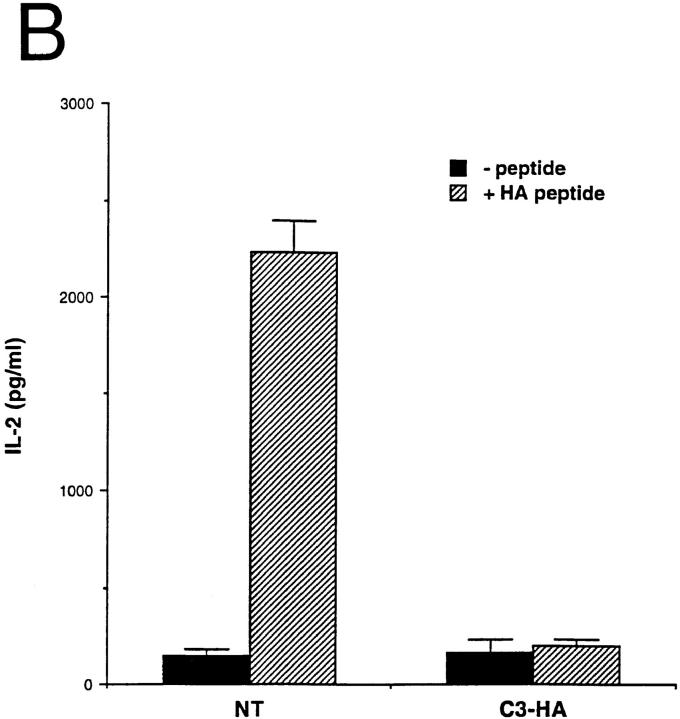
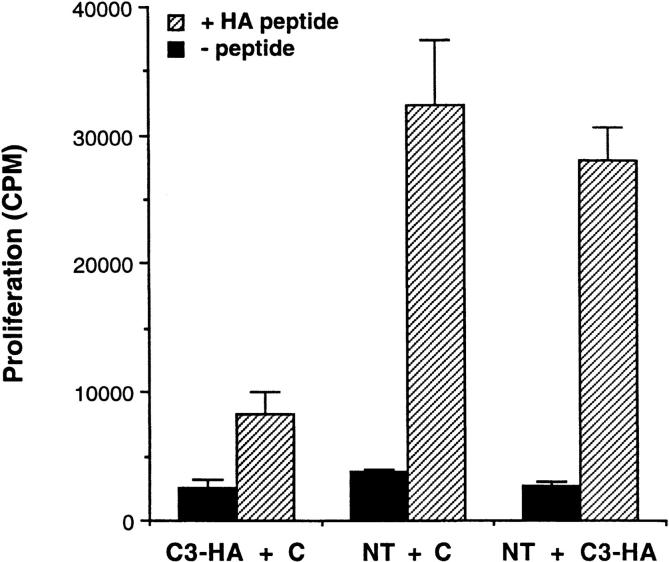
Functionally, the HA-specific CD4 cells retrieved from the C3-HA recipients were refractory to antigenic stimulation by their cognate peptide epitope. In comparison to clonotypic cells retrieved from NT recipients, they were impaired in their abilities to proliferate (Fig. 3 A) and secrete IL-2 (Fig. 3 B) when cultured with APCs pulsed with HA peptide. IL-2 secretion by clonotypic CD4 cells retrieved from C3-HA recipients was consistently greater than sevenfold lower than cells retrieved from NT recipients even at shorter (48-h) in vitro culture times (data not shown). These clonotypic T cells did not appear to have acquired suppressor activity or to mediate cytolytic destruction of APCs presenting HA peptide in culture, since clonotypic cells retrieved from NT recipients proliferated equivalently when mixed with either the clonotypic T cells retrieved from C3-HA recipients or control cell preparations (Fig. 4). Furthermore, HA-expressing organs did not exhibit any gross or histological evidence of autoimmunity after transfer of clonotypic T cells (data not shown). Taken together, these results indicate that the HA-specific CD4 cells had been rendered tolerant upon transfer into HA-expressing transgenic mice.
Figure 3.
Clonotypic CD4 cells exhibit impaired response to antigenic stimulation after adoptive transfer into C3-HA transgenic recipients. (A) In vitro proliferative response of clonotypic cells, retrieved 9 d after transfer, to peptide stimulation. LN cells prepared from NT (closed circles; n = 3), C3-HA transgenic (open circles; n = 3), and control (closed squares; n = 1) recipients were cultured with the indicated concentration of synthetic HA peptide. (B) T cell proliferation was measured by [3H]thymidine incorporation, and data were expressed as the total number of incorporated radioactive counts (cpm, mean ± SEM). IL-2 secretion by clonotypic cells stimulated in vitro. Media were collected from 72-h cultures with (hatched bars) and without (solid bars) 100 μg/ml HA peptide and tested by ELISA. Data is expressed as pg/ ml IL-2 (mean ± SEM, n = 3).
Figure 4.
Tolerized clonotypic T cells do not inhibit the proliferative response of naive clonotypic T cells in vitro. Equal parts of the indicated LN preparations taken from day 9 transfer recipients were combined; tolerized T cells with control cells (C3-HA + C), naive T cells with control cells (NT + C), and naive with tolerized T cells (NT + C3-HA). 1.5 × 105 total cells were cultured in vitro with (hatched bars) or without (solid bars) 100 μg/ml HA peptide and proliferation was determined by [3H]thymidine incorporation (cpm, mean ± SEM, n = 3).
HA Expression on BM-derived Cells Is Not Required for Tolerance Induction.
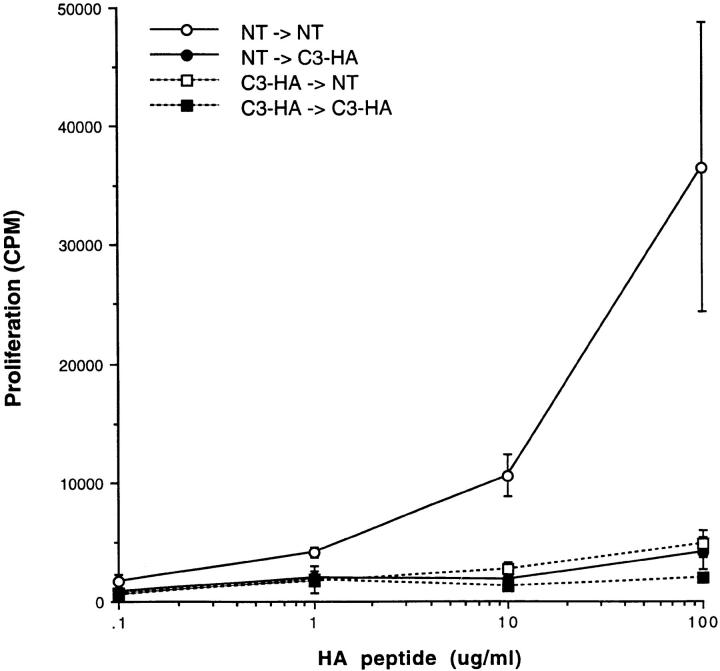
RT-PCR analysis (Table 1) indicated that HA mRNA was being expressed in several peripheral tissues and perhaps in the BM as well. To ascertain whether T cells were being tolerized as a result of HA expression by BM-derived cells, parenchymal cells, or both, a set of BM chimeras were generated in which BM from NT animals was used to reconstitute lethally irradiated C3-HA transgenic animals (NT → C3-HA), and conversely C3-HA BM was used to reconstitute irradiated NT mice (C3-HA → NT). As positive and negative controls for tolerance induction, C3-HA BM–reconstituted C3-HA recipients (C3-HA → C3-HA) and NT BM–reconstituted NT recipients (NT → NT) were also analyzed. HA-specific 6.5 clonotypic T cells retrieved from the C3-HA → NT chimeric transfer recipients did in fact exhibit an impaired proliferative response to HA peptide (Fig. 5). This tolerization might have resulted from low levels of HA expression in the BM, although we cannot rule out the possibility that contaminating APCs in the BM preparation that had acquired HA antigen from the periphery of the donor were mediating this effect. Nonetheless, tolerance induction in the NT → C3-HA chimeras was also apparent, indicating that HA need not be expressed in BM-derived cells for tolerance induction to take place.
Figure 5.
HA expression on BM-derived cells is not required for tolerance induction. C3-HA transgenic and NT mice were lethally irradiated and reconstituted with BM prepared from either NT or C3-HA donors. Clonotypic cells were retrieved 21 d after adoptive transfer from chimeric recipients: open circles, NT → NT (BM donor → irradiated recipient); closed circles, NT → C3-HA; open squares, C3-HA → NT; and closed squares, 3-HA → C3-HA. Proliferation assays were established as in Fig. 3 A. Two-color FACS® analysis indicated that equivalent numbers of clonotypic cells were present in the various chimeras (data not shown). Each data point (mean ± SD) is derived from two animals. This experiment was representative of two independent trials (data not shown).
Tolerance Induction Is Exclusively Mediated by an Antigen Transfer Pathway Involving BM-derived APCs.
Because tolerance induction did not appear to require that HA be expressed in BM-derived cells, either the HA expressing parenchymal cells were able to directly induce tolerance, or the acquired BM-derived APCs shed antigen and presented the I-Ed–restricted HA epitope in a tolerogenic manner. To distinguish between these possibilities, parent → F1 chimeras were generated in which the parenchymal cells expressing HA expressed the appropriate MHC haplotype for HA epitope presentation (H-2d), whereas the BM expressed either the restricting (H-2d) or a nonrestricting (H-2b) MHC haplotype for the transgenic TCR. C3-HA transgenic mice (F1, bxd) were reconstituted with either NT H-2d BM (H-2d → H-2bxd/HA) or NT H-2b BM (H-2b → H-2bxd/HA). Clonotypic CD4 cells prepared from H-2bxd F1 6.5 transgenic mice were depleted of endogenous APCs before adoptive transfer.
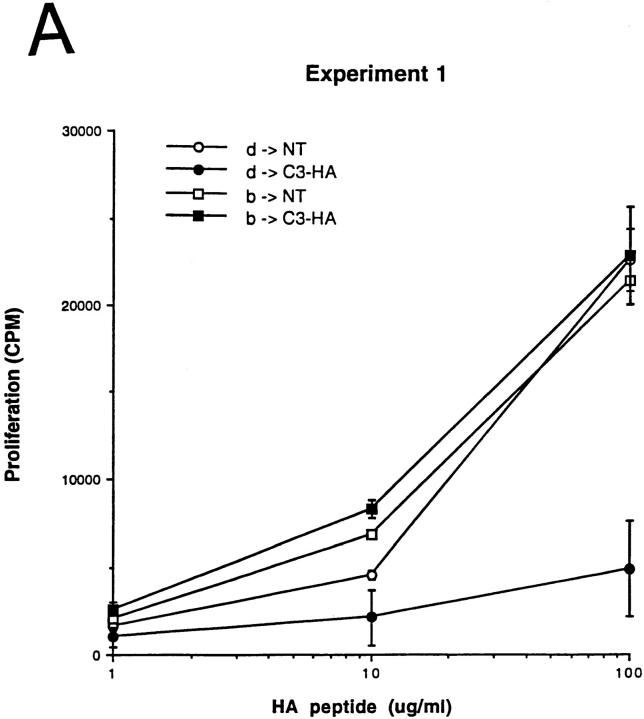
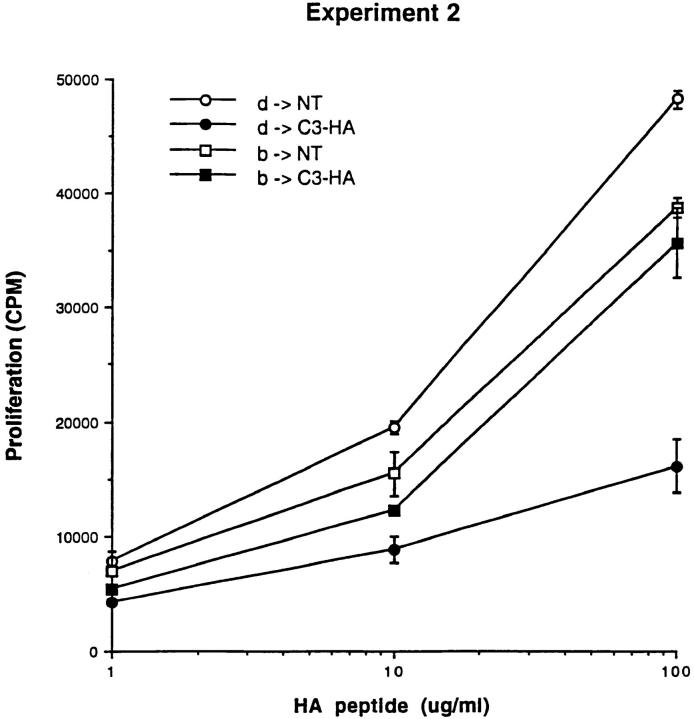
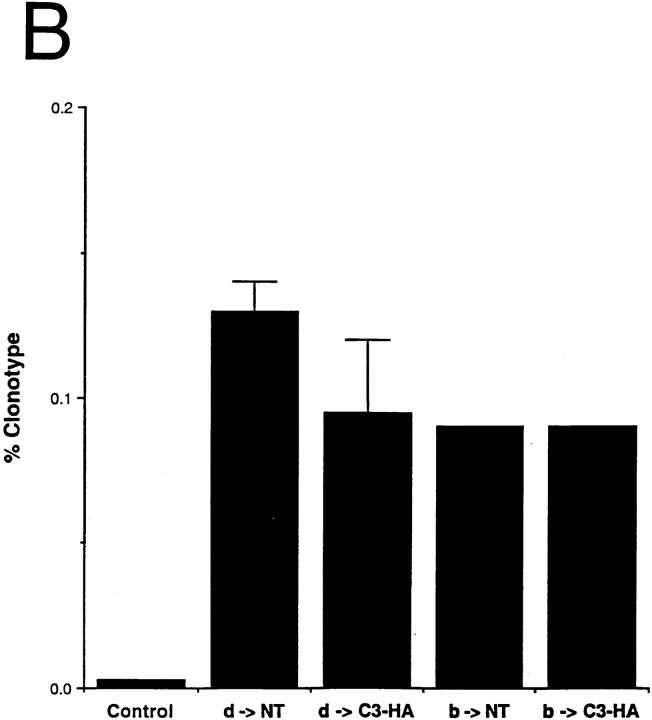
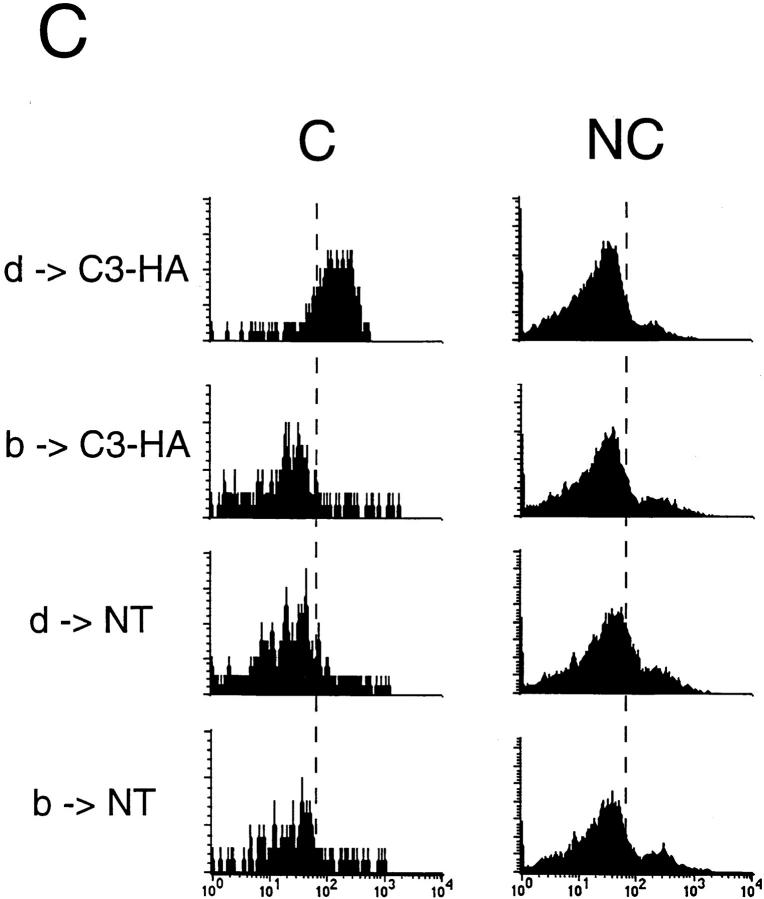
As expected, in two independent experiments, clonotypic cells transferred into H-2d → H-2bxd/HA chimeras exhibited impaired proliferative responses to HA peptide in vitro relative to NT chimeric controls. In contrast, clonotypic cells retrieved from H-2b → H-2bxd/HA chimeras were not tolerized, proliferating as vigorously as did the NT control chimeras (Fig. 6 A). As an increase in expression of CD44 on the surface of clonotypic T cells correlates with antigen recognition and the induction of tolerance (see Fig. 2), the clonotypic CD4 cells from experiment 1 were analyzed by FACS® to quantitate both their frequencies as well as their level of surface CD44 expression. Although similar numbers of clonotypic T cells were measured in each group (Fig. 6 B), only clonotypic cells in the positive control group (H-2d → H-2bxd/HA) exhibited increased CD44 expression levels relative to the nonclonotypic CD4 cells (Fig. 6). The finding that the clonotypic CD4 cells retrieved from the H-2b → H-2b×d/HA experimental group expressed low levels of CD44 indicated that they had not come into contact with the cognate I-Ed-HA peptide complex in vivo. Taken together, these results are inconsistent with a mechanism by which T cells are directly tolerized by HA-expressing parenchyma. Furthermore, the data also argues against the presence of radio-resistant host APCs (H-2bxd) that were capable of inducing tolerance. That tolerance induction required BM-derived APCs to express the restricting MHC haplotype clearly indicates that tolerance was induced by APCs that had acquired HA by means of an antigen transfer pathway.
Figure 6.
Tolerance induction is mediated by BM-derived cells which acquire HA via an antigen transfer pathway. C3-HA transgenic and NT mice (F1, bxd) were lethally irradiated and reconstituted with BM from NT donors of either the H-2d (HA-restricting) or H-2b (nonrestricting) haplotype. (A) Naive HA-specific CD4 cells (prepared from 6.5 TCR transgenic mice [F1, bxd]) were depleted of endogenous class II positive cells before adoptive transfer into parent → F1 chimeric recipients. LN cells prepared from day 14 adoptive transfer were tested for in vitro proliferative response to HA peptide; H-2d → H-2bxd/ HA (d → HA, closed circles), H-2b → H-2bxd/HA (b → HA, closed squares), H-2d → H-2bxd/NT (d → NT, open circles) and H-2b → H-2bxd/NT (b → NT, open squares). Experiments 1 and 2 are two independent trials in which each data point (mean ± SD) is derived from two separate mice, except for the b → NT group in experiment 1, which represents a single mouse. (B) Bar graph showing average clonotype frequencies from experiment 1 (mean ± SD). (C) Representative histogram plots of CD44 expression on the surface of clonotypic (C) and nonclonotypic (NC) T cells in experiment 1.
Discussion
Several previous studies have described in vivo model systems to study peripheral T cell tolerance. Although some systems have examined heterogenous T cell populations, analysis at the level of a single T cell specificity has been achieved by breeding double transgenic mice that express both a model antigen in the periphery and the cognate TCR on a large fraction of their mature T cells (32). The limitation of this approach is that T cells might not behave normally when the T cell repertoire is so heavily dominated by the clonotypic specificity. This problem can be circumvented by adoptively transferring a relatively small number of clonotypic T cells, derived from TCR transgenic mice, into mice with a normal T cell repertoire (33). In this study we have used such an adoptive transfer system to study peripheral tolerance mechanisms for a clonotypic CD4+ T cell population to a peripherally expressed self-antigen.
Naive HA-specific CD4 cells adoptively transferred into transgenic mice that express HA in several tissues become functionally tolerized as evidenced by their inability to proliferate and secrete IL-2 when reextracted and cultured with peptide-pulsed APCs. Although we cannot rule out the possibility that a portion of these tolerized T cells were deleted or had downregulated expression of their CD4 or clonotypic TCR molecules, our data do indicate that a significant number of these T cells persist that express normal levels of CD4 and TCR but are refractory to subsequent antigenic stimulation in vitro. These results are compatible with anergy as the form of tolerance that is induced in this system. Given that some of the transgenic T cells probably express both endogenous and transgenic TCRs, the observed nonresponsiveness could still reflect deletion of transgenic cells bearing single transgenic TCRs, leaving CD44+ T cells bearing two TCRs. However, Figs. 2 and 6 demonstrate that the proportion of clonotype-positive cells expressing CD44 goes from ∼5% in control animals to >60% in the HA transgenic recipients. Given that there is no difference in total number of clonotype-positive cells, the putative CD44+ double TCR cells would have to expand quite a bit and then become functionally nonresponsive in the in vitro assay. It is also possible that tolerized cells may be dying off in subsequent weeks after the time period that was analyzed. In fact, in other models of CD4+ T cell tolerance induction to peripheral self-antigens, most of the CD4 cells were found to be deleted at later time points (although there was also evidence of residual anergic cells) (34).
That BM-derived APCs expressing low levels of HA might have been able to directly induce T cell tolerance is consistent with previous studies indicating that the same clonotypic T cells become tolerized when transferred into transgenic mice expressing HA directly on hemopoietic cells (35). Even more significantly, when BM-derived APCs do not express HA themselves, they are still able to induce T cell tolerance by means of a transfer pathway in which they acquire the HA epitope from parenchymal cells. The elucidation of this cross-tolerization pathway resolves several issues regarding tolerance induction in CD4 cells to peripherally expressed self-antigens. For example, tolerance could be efficiently induced even if the parenchymal cell type expressing the cognate antigen expresses little or no class II MHC. Secondly, naive T cells that do not normally traffic through nonlymphoid tissues (36) could interact with tolerizing APCs in secondary lymphoid organs. Although it has previously been shown in several systems that BM-derived APCs can induce T cell tolerance in vivo to either exogenous antigens (25, 37) or antigens expressed directly on the APCs themselves (26, 35, 38), our study firmly establishes that APCs can play a central role in maintaining tolerance to parenchymally expressed self-antigens.
The class II–restricted HA epitope might enter the cross-tolerance pathway as a function of its location within the extracellular domain of a plasma membrane protein. Fragments of the HA protein containing the class II epitope might be released into the circulation through the action of serum proteases, thus allowing APCs located in peripheral lymphoid organs to acquire, process, and present the epitope in a tolerogenic manner. Previously, it has been shown that class II epitopes derived from HA expressed on pancreatic islet β cells can be presented on BM-derived APCs (39). Furthermore, tolerance induction in a population of clonotypic CD4 cells specific for the SV40 T antigen appears to be initially manifested in the LNs draining the pancreas in transgenic mice expressing T antigen under the control of the rat insulin promoter (34). Although mechanisms other than cross-tolerance might be operating in this system, it is possible that T antigen (normally localized in the nucleus) might be released from the β cells due to an increased cell turnover resulting from the oncogenic transformation initiated by T antigen itself. Interestingly, cross-presentation of a class I–restricted self-epitope, derived from a membrane-bound form of ovalbumin by APCs, appears to play a role in inducing deletion of the cognate CD8 cell population (40).
It is curious that the immune system would use a tolerance induction pathway for an epitope that is not normally presented by the cells that express it. One possibility is that it is a fail-safe mechanism that would prevent autoimmunity if inflammation induces expression of class II MHC molecules on antigen-expressing parenchymal cells. Arguing against this possibility, parenchyma expressing class II MHC in the absence of costimulatory molecules might induce tolerance rather than autoimmunity (23). Alternatively, anergized CD4 cells might play a role in regulating the responses of CD8 or B cells to the same antigen whose respective epitopes might be recognizable on the antigen-expressing parenchyma.
Previous in vitro studies have suggested that anergy induction results from TCR recognition of peptide–MHC ligand in the absence of costimulation (22). The recent finding that anergy can be prevented by blocking the B7-CTLA4 pathway suggest that the induction of anergy in vivo to exogenous antigens can be induced by cells expressing costimulatory signals (24). Although we currently do not know whether costimulation plays a role in the cross-tolerance pathway, our data do suggest that tolerance induction in CD4 cells to peripheral self-antigens in vivo is more complex than originally thought since T cells are not directly tolerized by parenchyma expressing the cognate epitope but rather by an intermediary APC. However, we cannot rule out the existence of a second tolerization pathway mediated by parenchymal cells expressing antigen and class II MHC, but not costimulatory molecules. This pathway might require either a higher T cell precursor frequency or a longer period of time to develop than was used in our current study. In fact, this type of tolerization pathway has been observed in double transgenic mice expressing an allo-class II MHC on parenchymal cells (lacking costimulatory activity) as well as the cognate clonotypic TCR on a large proportion of its mature CD4 cells (23). In the event that multiple pathways are able to induce tolerance in vivo, cross-tolerance would probably be the dominant pathway as parenchymal cells only express class II MHC under conditions of inflammation, whereas cross-tolerance would be operative constitutively.
Dissection of the cross-tolerance pathway will require identification of the APC involved in mediating this phenomenon. All three APC subtypes (B cells [25, 26], macrophages [38], and dendritic cells [37, 41]) have been shown in various systems to be capable of inducing T cell tolerance. It will be particularly interesting to determine whether the APC that mediates cross-tolerance is the same as the APC that can activate T cells in the cross-priming pathway used by certain tumor vaccines (42). One possibility is that one specific APC subtype is dedicated to inducing tolerance while another activates T cells. Alternatively, the same APC could induce either tolerance or activation depending upon whether an appropriate secondary signal is provided simultaneously. For tumor vaccines, expression of antigen in conjunction with cytokine (43) would program an APC to activate T cells. In the case of peripheral tolerance to self-antigens, the APC would acquire antigen from parenchymal tissues in the absence of a second signal, thus resulting in a tolerizing APC.
Acknowledgments
We would like to thank Harald von Boehmer and Elizabeth Wilson for the generous gifts of the 6.5 TCR transgenic mice and the C3(1) genomic clone, respectively. Eduardo Sotomayor and Hy Levitsky provided invaluable advice concerning T cell adoptive transfer experiments. Thom Saunders and Diane Robins provided helpful advice regarding the generation of transgenic mice. We would also like to thank Ephram Fuchs for critically reviewing the manuscript.
Abbreviations used in this paper
BM
bone marrow
HA
hemagglutinin
NT
nontransgenic
RT
reverse transcriptase
Footnotes
A.J. Adler is the recipient of an American Cancer Society Postdoctoral Fellowship and is an American Foundation For Urological Disease Research Scholar. This work was supported by a National Cancer Institute Prostate SPORE grant (CA-58236) and gifts by the Topercer family and the Telinde fund.
Gregory S. Yochum's current address is Molecular Biology Program, University of Utah, Salt Lake City, UT 84132.
References
- 1.Kappler J, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.von Boehmer H, Kisielow P. Self-nonself discrimination by T cells. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- 3.Lo D, Burkly LC, Flavell RA, Palmiter RD, Brinster RL. Tolerance in transgenic mice expressing class II major histocompatibility complex on pancreatic acinar cells. J Exp Med. 1989;170:87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 5.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 6.Harlan DM, Hengartner H, Huang ML, Kang YH, Abe R, Moreadith RW, Pircher H, Gray GS, Ohashi PS, Freeman GJ, et al. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci USA. 1994;91:3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Herrath MG, Guerder S, Lewicki H, Flavell RA, Oldstone MB. Coexpression of B7-1 and viral (“self”) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity. 1995;3:727–738. doi: 10.1016/1074-7613(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 8.Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science. 1990;250:1726–1739. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- 9.Carlow DA, Teh SJ, van Oers NS, Miller RG, Teh HS. Peripheral tolerance through clonal deletion of mature CD4−CD8+T cells. Int Immunol. 1992;4:599–610. doi: 10.1093/intimm/4.5.599. [DOI] [PubMed] [Google Scholar]
- 10.Zhang LI, Martin DR, Fung-Leung WP, Teh HS, Miller RG. Peripheral deletion of mature CD8+antigen-specific T cells after in vivo exposure to male antigen. J Immunol. 1992;148:3740–3745. [PubMed] [Google Scholar]
- 11.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 12.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 13.Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JF. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kbin the liver. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 14.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand–induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 15.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 16.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A.M. Schmitt-Verhulst, B. Malissen, G.J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 65293–65304. [DOI] [PubMed]
- 17.Zhang L, Fung-Leung W, Miller RG. Down-regulation of CD8 on mature antigen-reactive T cells as a mechanism of peripheral tolerance. J Immunol. 1995;155:3464–3471. [PubMed] [Google Scholar]
- 18.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 19.Ramsdell F, Lantz T, Fowlkes BJ. A nondeletional mechanism of thymic self tolerance. Science. 1989;246:1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- 20.Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989;342:564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- 21.Burkly LC, Lo D, Flavell RA. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science. 1990;248:1364–1368. doi: 10.1126/science.1694042. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 23.Guerder S, Meyerhoff J, Flavell R. The role of the T cell costimulator B7-1 in autoimmunity and the induction and maintenance of tolerance to peripheral antigen. Immunity. 1994;1:155–166. doi: 10.1016/1074-7613(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 24.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 25.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 27.Tan J, Marschke KB, Ho KC, Perry ST, Wilson EM, French FS. Response elements of the androgen-regulated C3 gene. J Biol Chem. 1992;267:7958. [PubMed] [Google Scholar]
- 28.Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 29.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+single positive cells with a class II major histocompatibility complex–restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker M, White R, Hurst H, Needham M, Tilly R. Prostatic steroid-binding protein. Isolation and characterization of C3 genes. J Biol Chem. 1983;258:12–15. [PubMed] [Google Scholar]
- 31.Allison J, Zhang Y, Parker M. Tissue-specific and hormonal regulation of the gene for rat prostatic steroid-binding protein in transgenic mice. Mol Cell Biol. 1989;9:2254–2257. doi: 10.1128/mcb.9.5.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JF, Flavell RA. T-cell tolerance and autoimmunity in transgenic models of central and peripheral tolerance. Curr Opin Immunol. 1994;6:892–899. doi: 10.1016/0952-7915(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 33.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 34.Forster I, Lieberam I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur J Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- 35.Lanoue A, Bona C, von Boehmer H, Sarukhan A. Conditions that induce tolerance in mature CD4+T cells. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 37.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 38.Miyazaki T, Suzuki G, Yamamura K. The role of macrophages in antigen presentation and T cell tolerance. Int Immunol. 1993;5:1023–1033. doi: 10.1093/intimm/5.9.1023. [DOI] [PubMed] [Google Scholar]
- 39.Lo D, Reilly CR, Scott B, Liblau R, McDevitt HO, Burkly LC. Antigen-presenting cells in adoptively transferred and spontaneous autoimmune diabetes. Eur J Immunol. 1993;23:1693–1698. doi: 10.1002/eji.1830230744. [DOI] [PubMed] [Google Scholar]
- 40.Kurts C, Kosaka H, Carbone FR, Miller JFAP, Heath WR. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steptoe RJ, Thomson AW. Dendritic cells and tolerance induction. Clin Exp Immunol. 1996;105:397–402. doi: 10.1046/j.1365-2249.1996.d01-779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow–derived cells in presenting MHC class I–restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 43.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]