CD28-independent, TRAF2-dependent Costimulation of Resting T Cells by 4-1BB Ligand (original) (raw)
Abstract
4-1BB ligand (4-1BBL) is a member of the tumor necrosis factor (TNF) family expressed on activated antigen-presenting cells. Its receptor, 4-1BB, is a member of the TNF receptor family expressed on activated CD4 and CD8 T cells. We have produced a soluble form of 4-1BBL using the baculovirus expression system. When coimmobilized on plastic with anti-CD3, soluble 4-1BBL induces interleukin (IL)-2 production by resting CD28+ or CD28− T cells, indicating that 4-1BBL can function independently of other cell surface molecules, including CD28, in costimulation of resting T cell activation. At low concentrations of anti-CD3, 4-1BBL is inferior to anti-CD28 in T cell activation. However, when 4-1BB ligand is provided together with strong TCR signals, then 4-1BBL and anti-CD28 are equally potent in stimulation of IL-2 production by resting T cells. We find that TNF receptor–associated factor (TRAF)1 or TRAF2 associate with a glutathione S-transferase–4-1BB cytoplasmic domain fusion protein in vitro_._ In T cells, we find that association of TRAF1 and TRAF2 with 4-1BB requires 4-1BB cross-linking. In support of a functional role for TRAF2 in 4-1BB signaling, we find that resting T cells isolated from TRAF2-deficient mice or from mice expressing a dominant negative form of TRAF2 fail to augment IL-2 production in response to soluble 4-1BBL. Thus 4-1BB, via the TRAF2 molecule, can provide CD28-independent costimulatory signals to resting T cells.
Keywords: T cells, costimulation, 4-1BB, TRAF2, signaling
Tcells require two signals for activation, an antigen-specific MHC-restricted signal through the TCR and a second costimulatory signal. CD28 on T cells is widely considered as the primary receptor for delivering costimulatory signals to resting T cells (1). However, CD28− T cells are not defective in all responses, suggesting the existence of other costimulatory pathways (2–4). One such alternate costimulatory pathway involves the TNFR family member 4-1BB (CDw137), a T cell activation antigen found on activated CD4 and CD8 T cells (5, 6). The ligand for 4-1BB is found on activated B cells, macrophages, and cultured dendritic cells, as well as on several B lymphomas and the thymoma, EL4 (7–11).
A number of studies have shown a role for 4-1BB in T cell activation using either transfected ligand (7), antibodies against the 4-1BB molecule (6, 12–14), or blocking studies with a soluble form of the 4-1BB receptor (10, 11, 15, 16). Anti–4-1BB antibodies are effective in the activation of T cells that have been preactivated via their TCR to induce high levels of 4-1BB receptor expression (12), but are poorer agonists for induction of IL-2 by resting T cells (6). More recently, Shuford et al. have shown that anti–4-1BB antibodies induce higher levels of proliferation of CD8 T cells compared with CD4 T cells, leading to the suggestion that 4-1BB is primarily a costimulatory molecule for CD8 T cells (14). In contrast, studies with APCs that express 4-1BB ligand (4-1BBL)1 have shown that 4-1BB interaction with its ligand can participate in induction of proliferation and IL-2 and IL-4 production by CD4 T cells. This role for 4-1BBL in the development of Th1 and Th2 cells is most apparent in the absence of a strong B7-CD28 interaction (11, 16).
Despite the accumulating body of evidence on the importance of 4-1BB as a costimulatory receptor on activated CD4 and CD8 T cells, there is controversy as to whether 4-1BBL alone can provide a costimulatory signal for resting T cells independently of other molecules present on the APC. In this report we describe the production of a soluble form of 4-1BBL (s4-1BBL) using the baculovirus expression system. When coimmobilized on plastic with anti-CD3 or when cross-linked in solution through its influenza hemagglutinin (HA) epitope tag, we find that s4-1BBL is a potent activator of IL-2 production by isolated high density CD28+ or CD28− T cells. When TCR signals are limiting, we find that immobilized anti-CD28 is more effective than 4-1BBL in inducing IL-2 production. However, at higher anti-CD3 concentrations, 4-1BBL and anti-CD28 are equally potent in inducing IL-2 production by resting T cells. Thus, isolated 4-1BBL can costimulate resting T cells via a CD28-independent pathway.
The observation that 4-1BB signaling can replace CD28 signaling in costimulation of T cell activation under some circumstances raises the question of how signals from the 4-1BB receptor synergize with signals from the TCR to induce high level IL-2 production. 4-1BB is a member of the TNFR superfamily. Other members of this family have been shown to signal via the TNFR-associated factor (TRAF) family of signaling molecules first identified by their ability to interact with the cytoplasmic domains of TNFR family members (17, 18). Six members of the TRAF family (TRAF1–6) have been identified to date (17–25). The TRAF proteins appear to function as adapter proteins that link TNFR family members to downstream signaling pathways. TRAF family members have in common a conserved TRAF-C domain involved in homotypic interactions or in heterotypic interactions with other TRAF molecules and with the cytoplasmic tails of TNFR family members. The TRAF2 molecule has been shown to interact directly with the cytoplasmic tails of CD40, CD30, and TNFR2 and indirectly with TNFR1 (17, 18, 26–31) and has been shown to induce both nuclear factor (NF)-κB and c-Jun NH2-terminal kinase (JNK) activation in response to TNF stimulation (26, 32–34).
Transgenic mice expressing a dominant negative (DN) form of TRAF2 (TRAF2 241-501) in their lymphoid cells (TRAF2 DN mice) have reduced JNK activity, but relatively normal NF-κB activation (35). Similar results have also been obtained using mice that have been rendered TRAF2 negative by gene targeting (36). Here we show that T lymphocytes isolated from traf2−/− or TRAF2 DN mice fail to augment IL-2 production in response to s4-1BBL. Thus the 4-1BB costimulatory signal leading to IL-2 production is dependent on the TRAF2 molecule.
Materials and Methods
Cell Lines, Mice, Antibodies and Reagents.
The T hybrid, C8.A3, was obtained from Dr. Laurie Glimcher (Harvard Medical School, Boston, MA). CD28− mice (2) were backcrossed on to the H-2b background (n = 10). C57BL/6 mice were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). CD28− or B6 mice were used at 8–10 wk of age.
TRAF2 DN mice have been described previously (35). These mice had been backcrossed on to the BALB/c background. TRAF2 DN mice express TRAF2 (241-501) under the control of the IgH enhancer to drive the transgene expression only in B and T cells. TRAF2 (241-501) lacks the RING finger domain and this form of TRAF2 has been previously shown to act as a dominant negative inhibitor of TNF and CD40 signaling (26). Spleen cells and lymph nodes were obtained from TRAF2 DN mice or their transgene-negative littermates at 8–10 wk of age.
traf2−/− mice were generated by gene targeting as described elsewhere (36). TRAF2 expression was ablated using a replacement vector that replaces the entire RING finger coding region and intervening intron of traf2 with the PGK-Neo gene in the reverse orientation to the endogenous traf2 gene. traf2−/− mice were generated by breeding heterozygous mice. Lymph nodes were obtained from surviving traf2−/− mice or their traf2+/+ littermates at ∼3 wk of age. Hereafter, we will refer to these mice as TRAF2− and TRAF2+, respectively.
Mice overexpressing TRAF1 under control of the H-2k promoter have been described previously (38). These mice express ∼10-fold more TRAF1 in their spleen and lymph nodes compared with wild-type mice, but have normal numbers of lymphocytes in their peripheral lymphoid organs. The anti-CD3-producing hybridoma 145-2C11 (39) was provided by Dr. Jeff Bluestone (University of Chicago, Chicago, IL). The 12CA5 producing hybridoma (40) was obtained from Dr. Bob Phillips (University of Toronto, Toronto, Canada). The hybridomas N418 (anti-CD11c), Y3-P (anti-Ab), RA3-6B2 (anti-B220), TIB-128 (anti-MAC-1), YN1/1.7.4 (anti–intercellular adhesion molecule [ICAM]-1), RG7/7.6H2 (anti–rat Ig κ chain), and M1/69 (anti–heat stable antigen) were obtained from the American Type Culture Collection (Rockville, MD). Antibodies were purified from hybridoma supernatants using protein G– or protein A–Sepharose (Pharmacia Biotech, Piscataway, NJ), according to the manufacturer's instructions. The anti–CD28-secreting hybridoma 37.51.1 (41) was provided by Dr. Jim Allison (University of California, Berkeley, CA). 3T3 cells secreting 4-1BB linked to alkaline phosphatase (AP) were provided by Dr. Byoung Kwon (Indiana University, Indianapolis, IN). 4-1BB–AP was purified on anti–AP-Sepharose as previously described (9). AP from human placenta was obtained from Sigma Chemical Co. (St. Louis, MO). Anti–4-1BB (1AH2) was purchased from Pharmacia Biotech.
Generation of s4-1BBL.
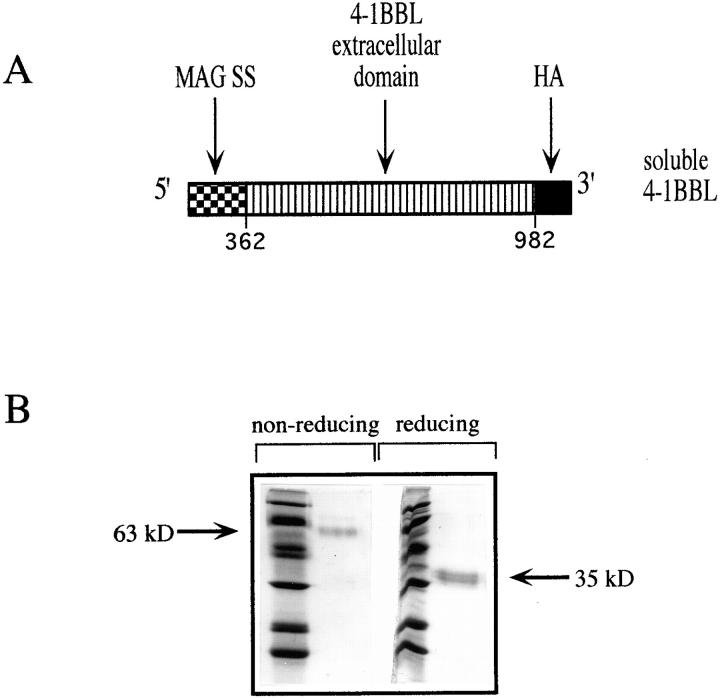
4-1BBL is a type II glycoprotein. To generate s4-1BBL in insect cells, we linked cDNA encoding the 4-1BBL extracellular COOH-terminal domain to the signal sequence from myelin-associated glycoprotein, a signal sequence previously shown to be effective in directing the secretion of heterologous proteins in the baculovirus expression system (reference 42 and see Fig. 1 A). An HA epitope tag was linked to the COOH terminus of the 4-1BBL molecule to facilitate isolation of the secreted molecule.
Figure 1.
Generation of a soluble form of 4-1BBL. (A) Schematic representation of the construct used to generate s4-1BBL. MAG SS, MAG signal sequence. (B) Coomassie blue–stained SDS-PAGE of s4-1BBL produced in baculovirus and isolated from insect cell supernatants using 12CA5 anti-HA affinity chromatograpy as described in Materials and Methods.
cDNA encoding the extracellular domain of 4-1BBL was obtained by reverse transcriptase (RT) PCR amplification of RNA isolated from the BALB/c B lymphoma K46J, since this cell line has been shown to express functional 4-1BBL. RNA was extracted from K46J cells using Promega RNAgents Total RNA Isolation System (Promega Corp., Madison, WI). Single-stranded cDNA was synthesized from 2 μg of total RNA using the First-Strand cDNA Synthesis Kit (Pharmacia Biotech). PCR was performed with the primers 5′-GCT TCT CGA GGG GGA CAC CGC ACC GAG CCT CGG CCA GC-3′ and 5′-ATT GCC GGC CCT TCC CAT GGG TTG TCG GGT T-3′ (Oligonucleotide Synthesis Laboratory, University of Toronto, Toronto, Canada) and 1 μg of template cDNA, using an initial 2-min denaturation at 94°C, followed by 30 cycles of each of the following: 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and a final 6-min extension at 72°C to produce a 645-bp product (including 4-1BBL 104-309). The PCR product was inserted into the pCR II vector using the TA Cloning Kit (Invitrogen Corp., Carlsbad, CA) and transformed into One Shot Cells (Invitrogen Corp.) for sequencing using the T7 Sequencing Kit (Pharmacia Biotech). Four independently derived RT-PCR products, derived from the K46J cell line, were sequenced. Sequencing of these independently derived RT-PCR products revealed a difference from the published sequence of C to A at NTD 476, resulting in a change from Lys to Gln at amino acid 142. Goodwin et al. originally isolated 4-1BBL from the EL4 thymoma, which originates from C57Bl/6 mice, whereas the 4-1BBL cDNA isolated here was from a BALB/c lymphoma (7). Therefore, this difference in sequence may represent a polymorphism between mouse strains.
The myelin-associated glycoprotein (MAG) signal sequence was added 5′ to the 4-1BBL 104-309 gene by subcloning into the XhoI–AvaI sites of pShoNEX 1.1 (obtained from Rob Dunn, McGill University, Montreal, Canada). The HA tag was added 3′ to the MAG–4-1BBL 104-309 gene by NheI insertion into the baculovirus vector pETL-HA (obtained from C. Richardson, Amgen Institute, Toronto, Canada; reference 43). The recombinant gene was inserted by homologous recombination into the wild-type AcMNPV baculovirus in SF9 cells by cotransfection using the Linear Transfection Module (Invitrogen Corp.). Recombinant baculovirus clones were identified by plaque assay, and their purity was confirmed by PCR analysis using the primers 5′-TTT ACT GTT TTC GTA ACA GTT TTG-3′ and 5′-CAA CAA CGC ACA GAA TCT AG-3′.
s4-1BBL in SF9 cell supernatants was detected by capture ELISA using antibodies to the HA tag (12CA5) for capture and s4-1BB receptor, 4-1BB–AP, for detection. Wells were coated at 37°C for 1 h with 20 μg/ml (100 μl/well) of 12CA5 in PBS. Wells were then blocked with PBS/5% skim milk/0.1% Tween 20 two times for 30 min at 37°C. After blocking, the wells were coated with various dilutions of supernatants from uninfected cultures or cultures infected with recombinant or wild-type virus (100 μl). After 90 min at room temperature, wells were washed three times with PBS/0.1% Tween 20. 5 μg/ml of 4-1BB–AP (100 μl/well) diluted in PBS/5% skim milk/0.1% Tween 20 was added to each well for 45 min at room temperature, and plates were washed three times with PBS/0.1% Tween 20. Finally, 1 mg/ml of _p_-nitrophenylphosphate (Sigma Chemical Co.) in 10% diethanolamine was added to the wells, and OD405 was measured using a Titertek Plus reader (Flow Laboratories, McLean, VA).
The recombinant virus was amplified in SF9 cells grown in Grace's insect media supplemented with 2% lactalbumin hydrolysate (GIBCO BRL, Gaithersburg, MD), 2% yeastolate (GIBCO BRL), plus 10% fetal bovine serum (GIBCO BRL), and titered by plaque assay before use for protein production.
Purification of s4-1BBL.
s4-1BBL was produced and purified as follows. A 3-liter suspension culture of 1–2 × 106 SF9 cells/ml grown in SF900II (GIBCO BRL) at room temperature was infected with recombinant virus at a multiplicity of infection of 10– 20 and harvested 6 d after infection. s4-1BBL molecules were purified from the supernatant by affinity chromatography on 12CA5 Sepharose. 12CA5 Sepharose 4B was prepared using cyanogen bromide–activated Sepharose (Pharmacia Biotech) according to the manufacturer's instructions. To ensure that effects of s4-1BBL were not influenced by the presence of endotoxin, the level of endotoxin in the purified s4-1BBL was measured using the Limulus amebocytes lysate QCL 1000 kit (BioWhittaker Inc., Walkersville, MD) and found to be less than 0.1 U/ml.
Generation of 4-1BB Cytoplasmic Tail (CT)–GST Fusion and Measurement of 4-1BB CT–TRAF Association.
The entire cytoplasmic tail of 4-1BB was obtained by PCR from the 4-1BB full length cDNA using primers 5′-GGGAATTCAAATGGATCAGGAAAAAATTCCC-3′ and 5′-GGGGATCCTCACAGCTCATAGCCTCCTCCTC-3′. Amplified PCR fragments were sequenced and fused in-frame to GST by cloning into EcoRI and BamHI sites of pEBG, a mammalian GST expression vector, to generate pEBG–4-1BBCT. The plasmids, pEBG, pEBG-4-1BB, and pEBG-CD30CT (28), were transfected into 293 cells. 48 h after transfection, GST and GST fusion proteins were purified with glutathione beads. For in vitro association experiments, the full-length murine TRAF1 and TRAF2 cDNA in pBluescript (Stratagene Inc., La Jolla, CA) were transcribed and translated in vitro using the TNT coupled reticulocytes system (Promega) with 35S-labeled methionine. Equal amounts of in vitro translated TRAF1 or TRAF2 were incubated in binding buffer (PBS containing 0.1% NP-40, 0.5 mM DTT, 10% glycerol, 1 mM PMSF, and 2 μg/ml aprotinin) with ∼5 μg of fusion protein bound to glutathione beads for 45 min at 4°C. After washing five times with binding buffer, the proteins were eluted by boiling in SDS sample buffer for 5 min and subsequently analyzed by SDS-PAGE.
Measurement of TRAF1 and TRAF2 Association with 4-1BB in C8.A3 T Cells.
For measurement of TRAF1 and TRAF2 association in C8.A3 T hybrids, 2 × 107 C8.A3 cells were incubated on ice with 5 μg/ml of anti–4-1BB (1AH2) or with rat anti– ICAM-1 (YN1) control antibody for 5 min. The antibody was cross-linked with 20 μg/ml of RG7 (anti–rat κ chain) for 15 min at 37°C. After stimulation the cells were lysed with 10 mM Tris, 1 mM MgCl2, 50 mM NaCl, 0.5% NP-40, 2 μg/ml aprotinin, and 1 mM PMSF. Lysates were immunoprecipitated for 4 h at 4°C with 40 μl of protein G–Sepharose (Sigma Chemical Co.). For unstimulated controls, antibody and second step were added on ice and the samples were carried through the same immunoprecipitation protocol. Precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose. TRAF proteins were detected by polyclonal rabbit anti–murine TRAF1 (S-19) and anti–murine TRAF2 (C-20) (_Santa Cruz Biotech_nologies, Santa Cruz, CA). Bound antibodies were detected with goat anti–rabbit Ig–horseradish peroxidase (Molecular Probes, Eugene, OR) and detected by chemiluminescence according to the manufacturer's protocol (ECL system; Amersham Life Science, Arlington Heights, IL).
T Cell Isolation.
APCs were depleted from spleen or lymph node cell suspensions in HBSS (GIBCO BRL)/2.5% FCS/50 μM 2-ME, with a cocktail of antibodies, anti–class II (Y3P), anti-B220, anti–heat stable antigen (M1/69), anti–MAC-1, and anti-CD11c (N418) each at a final concentration of 10 μg/ml at 4°C for 30 min. A 1:10 dilution of baby rabbit complement (Cedarlane Labs. Ltd., Hornby, Ontario, Canada) was added, and the cultures were further incubated at 37°C for 40 min. To remove adherent cells, the cell suspensions were passaged through a Sephadex G10/nylon wool column, and then centrifuged through Percoll gradients consisting of 60, 70, and 80% Percoll layers. Small (high density) T cells were isolated from the 70/80% interface and used in all subsequent experiments.
T Cell Activation Assays.
Monoclonal anti-CD3 (145-2C11) was immobilized on the surface of 96-well plates (Nunc, Gaithersburg, MD) by incubating for 5 h at 37°C. Where indicated, s4-1BBL or anti-CD28 antibodies were added to the wells at the same time. 0.5–1 × 105 small high density T cells, from either C57BL/6, CD28− mice, TRAF2 DN mice, TRAF2−, or littermate controls were cocultured with either immobilized anti-CD3 antibody alone, or in the presence of immobilized s4-1BBL, immobilized anti-CD28, or control antibody as indicated in the figure legends. After 2 d the culture supernatants were collected, and assayed for their ability to induce proliferation of the IL-2– dependent cell line CTLLs as previously described (10).
Results
Generation of s4-1BBL Using the Baculovirus Expression System.
At the time we began these studies, Hurtado et al. had shown that antibodies to 4-1BB could activate preactivated T cells but were rather poor in the activation of resting T cells (12). In contrast, we had found that B lymphomas expressing 4-1BBL could induce high levels of IL-2 and IL-4 production by resting T cells (10, 16). Therefore, it was possible either that 4-1BBL was more potent in activating resting T cells than were available antibodies or that other molecules on the APC contributed to T cell activation by 4-1BBL. To distinguish these possibilities, we generated a soluble form of 4-1BBL using the baculovirus expression system.The entire extracellular domain of 4-1BBL was expressed in isolation with the addition of an influenza HA epitope tag at the COOH terminus of the protein to facilitate purification (Fig. 1 A). Fig. 1 B shows a Coomassie blue–stained gel of purified s4-1BBL isolated from the insect cells using 12CA5 (anti-HA) affinity chromatography. It can be seen that s4-1BBL forms a disulfide-linked dimer of ∼63 kD, consistent with the expected molecular weight for the glycosylated form of the extracellular domain. The band is slightly heterogeneous, possibly due to variable glycosylation or due to limited proteolysis.
Activation of CD28+ and CD28− T Cells by 4-1BBL.
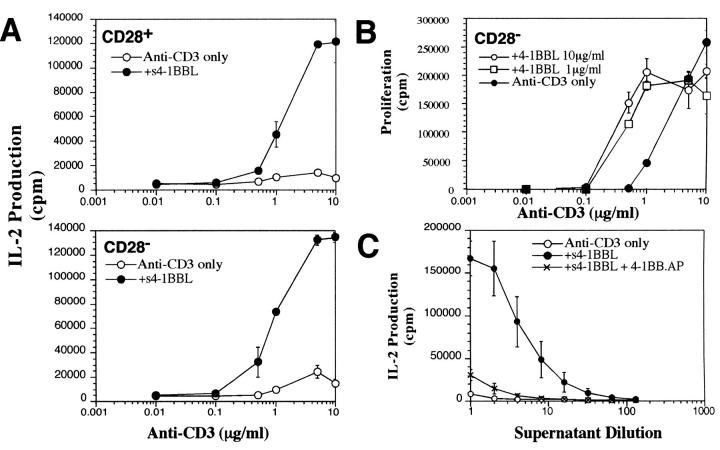
To assess the activity of s4-1BBL in the activation of resting T cells, high density resting T cells from the spleens of CD28+ or CD28− mice were isolated by complement and Sephadex G10 depletion of APCs followed by Percoll gradient fractionation as described in Materials and Methods. CD28− T cells were used to eliminate the possibility of any contribution from CD28 signaling. Fig. 2 A shows IL-2 production by purified T cells responding to immobilized anti-CD3 in the presence or absence of immobilized s4-1BBL, added at 1 μg/ml. It can be seen that for both CD28+ and CD28− T cells, no IL-2 is generated in response to immobilized anti-CD3 alone, but that coimmobilization of s4-1BBL leads to significant IL-2 production. s4-1BBL added in solution did not stimulate a response, but s4-1BBL added in solution together with anti-HA antibody to cross-link the molecule via the HA tag was also effective in costimulation (data not shown). Fig. 2 B shows the proliferation of CD28− T cells in response to immobilized anti-CD3 alone or with 1 or 10 μg/ml immobilized s4-1BBL. It can be seen that at suboptimal anti-CD3 concentrations, s4-1BBL enhances T cell proliferation. Fig. 2 C shows that the effect of s4-1BBL can be fully blocked by coculture with a soluble form of the receptor, 4-1BB–AP, whereas AP alone had no such effect. Thus, the enhancement of IL-2 production in response to 4-1BBL is specific to the soluble ligand.
Figure 2.
Immobilized s4-1BBL can costimulate proliferation and IL-2 production by CD28+ or CD28− T cells. (A) High density resting T cells isolated from the spleens of CD28+ or CD28− mice were incubated with various concentrations of anti-CD3 alone or with anti-CD3 plus immobilized s4-1BBL added to the plastic wells at 1 μg/ml as indicated in the figures. After 48 h, culture supernatants were removed and serial dilutions of supernatant were incubated with IL-2–dependent CTLL cells for 18–24 h. [3H]thymidine was added for the last 6 h of the CTLL culture. Data are shown for the first dilution of culture supernatant and are representative of four independent experiments. (B) CD28− T cells were cultured with immobilized anti-CD3 alone or with immobilized anti-CD3 plus s4-1BBL at 1 or 10 μg/ml as indicated in the figure. After 48 h, [3H]thymidine was added to the wells and thymidine uptake was determined after a further 6 h of culture. Similar results were obtained in three separate experiments. (C) CD28− cells were cultured with anti-CD3 (1 μg/ml) in the presence or absence of s4-1BBL (1 μg/ml) or in the presence of s4-1BBL (1 μg/ml) plus 4-1BB-AP (10 μg/ml). After 48-h incubation, culture supernatants were analyzed for induction of thymidine incorporation by CTLL. 4-1BB–AP alone had no effect on IL-2 production. Results are shown for serial dilutions of the culture supernatants and are representative of four independent experiments.
Fig. 3 compares the sensitivity of CD28+ and CD28− T cells to s4-1BBL. It can be seen that the IL-2 response of CD28+ and CD28− T cells saturates at a concentration of s4-1BBL of between 2 and 5 μg/ml. This titration experiment was repeated using a different batch of purified s4-1BBL and showed a similar saturation point (data not shown). The response of CD28− T cells to s4-1BBL is somewhat reduced compared with that of CD28+ T cells. Higher responses of CD28+ versus CD28− T cells to s4-1BBL might be due to low level expression of B7.2 on the T cells or due to the presence of B7 on contaminating APCs. Differences between the magnitude of the response of CD28+ and CD28− T cells to s4-1BBL were not apparent in all experiments (for example, see Fig. 2) but tended to vary with the particular T cell preparation. Similar differences between CD28+ and CD28− T cell responses to 4-1BBL were previously observed using 4-1BBL on APCs (11). Flow cytometry analysis of CD28+ and CD28− T cells after overnight treatment with anti-CD3 showed similar levels of induction of 4-1BB on the surface (data not shown). Thus the difference in response to s4-1BBL on CD28+ or CD28− T cells does not appear to be due to differential expression of 4-1BB in response to anti-CD3 treatment of CD28+ or CD28− T cells.
Figure 3.
Effect of 4-1BBL titration on CD28+ and CD28− T cell responses. High density resting T cells isolated from the spleens of CD28+ or CD28− mice were incubated with anti-CD3 alone (1 μg/ml) or with anti-CD3 plus immobilized s4-1BBL added to the plastic wells at the concentration indicated in the figures. After 2 d, serial dilutions of the culture supernatant were incubated with IL-2– dependent CTLL cells overnight. [3H]thymidine was added to the CTLL cultures for the last 6 h of culture. Similar results were obtained in three independent experiments.
Kinetics of IL-2 Production in Response to Anti-CD3 Plus s4-1BBL.
4-1BB is an activation antigen on T cells. 4-1BB mRNA appears within 3 h of anti-CD3 stimulation (45), but surface expression of 4-1BB is not apparent until several hours later (6). Therefore, it was important to establish the kinetics of response to s4-1BBL. Fig. 4 shows IL-2 production by resting CD28− T cells at various times after exposure to immobilized anti-CD3 plus immobilized s4-1BBL. It can be seen that IL-2 production by resting T cells responding to anti-CD3 plus immobilized s4-1BBL is detectable by 24 h with further increases in IL-2 production by 48 h. The kinetics of response to s4-1BBL is similar to the response of T cells to 4-1BBL expressed on B lymphomas, where we detected some IL-2 production by 12 h with increasing amounts at 24–48 h (11). Thus the kinetics of the response to s4-1BBL appears to be rapid enough to explain the ability of 4-1BBL to activate resting T cells and suggests that quite low levels of 4-1BB on the T cell surface may be sufficient to provide T cell costimulation.
Figure 4.
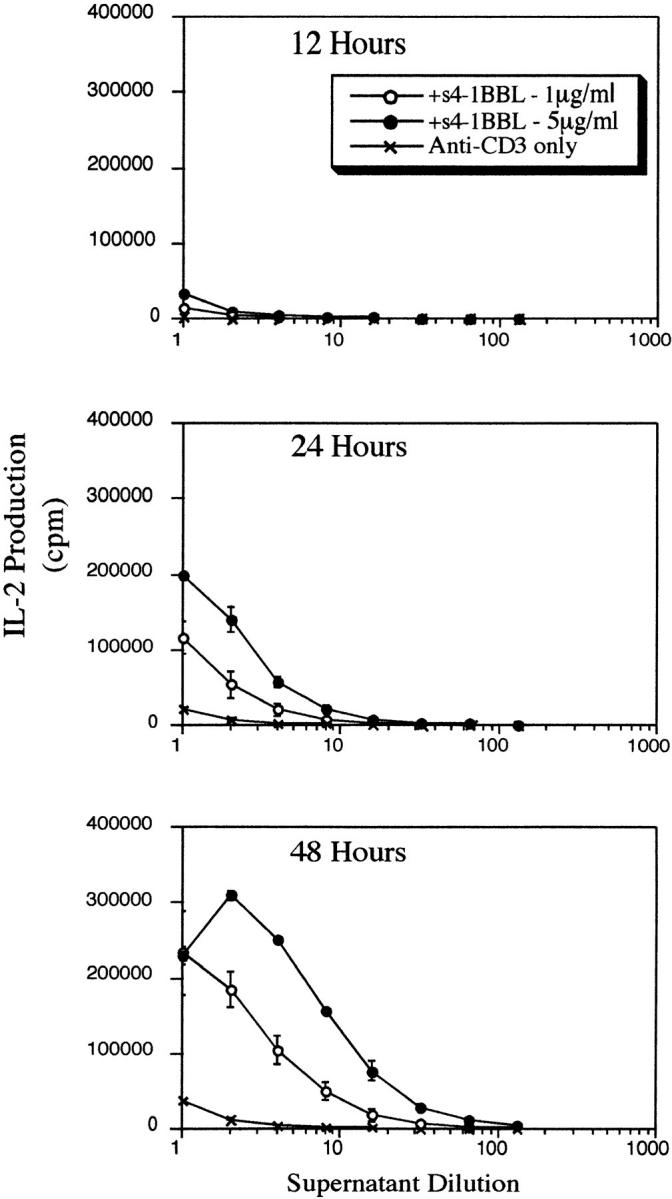
Kinetics of IL-2 production in response to 4-1BBL. Resting T cells isolated from the spleens of CD28− mice were incubated with anti-CD3 alone (1 μg/ml) or with anti-CD3 plus s4-1BBL immobilized at the concentrations indicated on the figure. After 12, 24, or 48 h, culture supernatants were removed and serial dilutions were analyzed for induction of proliferation of IL-2–dependent CTLL cells. Results are representative of two separate experiments.
Comparison of Costimulatory Activity of 4-1BBL with Anti-CD28 Antibodies.
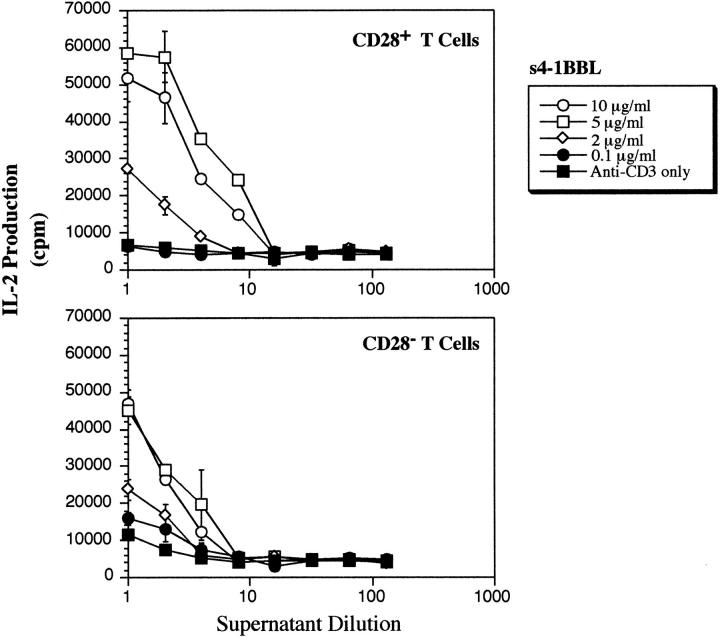
To compare the efficacy of the 4-1BB versus CD28 costimulatory pathways for resting T cell activation, we compared T cell responses to immobilized anti-CD28 and immobilized s4-1BBL in the presence of various doses of anti-CD3 as shown in Fig. 5. Anti-CD28 was titrated in a separate experiment (data not shown) and the concentration which gives maximum stimulation when immobilized with 1 μg/ml of anti-CD3 (10 μg/ml anti-CD28) was compared with s4-1BBL at 5 μg/ml. It can be seen that at low doses of anti-CD3, anti-CD28 is more effective than s4-1BBL in stimulating IL-2 production by resting CD28+ T cells. However, if one increases signals through the TCR, via increasing the density of immobilized anti-CD3, then 4-1BBL and anti-CD28 induce similarly high levels of IL-2 production by resting CD28+ T cells. As expected, only s4-1BBL but not anti-CD28 enhanced IL-2 production by CD28− T cells. Thus, 4-1BBL can provide a similar level of costimulation as anti-CD28 when provided with a strong TCR stimulus, but is less effective when signals through the TCR are limiting.
Figure 5.
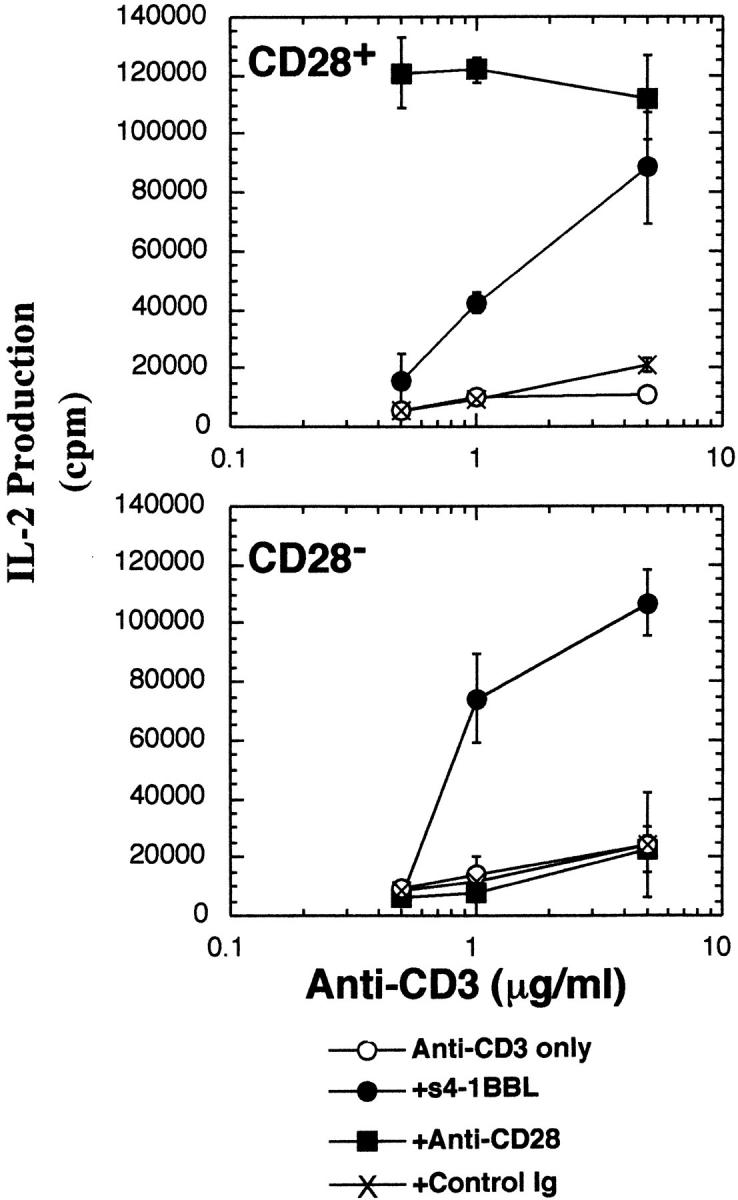
Comparison of immobilized s4-1BBL with immobilized anti-CD28 for costimulation of IL-2 production by CD28+ and CD28− T cells_._ Resting T cells isolated from CD28+ or CD28− T cells were stimulated by immobilized anti-CD3 at the indicated concentrations plus or minus 4-1BBL immobilized at 5 μg/ml or anti-CD28 at 10 μg/ml or control Ig at 10 μg/ml. After 48 h, supernatants were removed and analyzed for the presence of IL-2 using CTLL cells. Results are representative of two independent experiments.
Association of 4-1BB with TRAF1 and TRAF2.
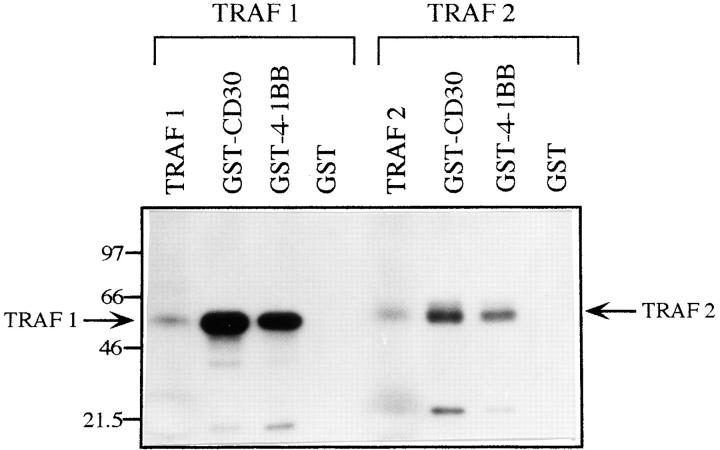
The mechanism by which 4-1BB signals T cells to costimulate IL-2 production is not known. 4-1BB is a member of the TNFR family of signaling molecules. As discussed above, the TRAF family of signaling molecules appear to function as adapter proteins in the signal transduction cascades leading to NF-κB and JNK activation by members of this family. Since TRAF2 has been shown to interact directly with the cytoplasmic tails of several TNFR family molecules, we chose to focus on a possible role for TRAF2 in 4-1BB signaling. Therefore, the 4-1BB cytoplasmic domain was expressed as a GST fusion protein in 293 cells. Purified 4-1BB– CT-GST bound to glutathione Sepharose was used to probe the association of the 4-1BB cytoplasmic domain with in vitro translated 35S-methionine labeled TRAF1 and TRAF2 proteins (Fig. 6). TRAF1 and TRAF2 were found to associate with 4-1BB–CT-GST as well as CD30-GST (included as a positive control). No association was observed between TRAF1 and TRAF2 and GST alone.
Figure 6.
Association between the 4-1BB cytoplasmic tail-GST fusion protein and TRAF1 and TRAF2 proteins. As described in Materials and Methods, purified GST, GST-CD30CT, and GST–4-1BBCT proteins bound to glutathione beads were incubated with 35S-labeled in vitro– translated TRAF1 or TRAF2. After washing, samples were eluted and analyzed by SDS-PAGE followed by autoradiography. In vitro–translated TRAF1 and TRAF2 were also run directly on the gel (lanes marked TRAF1 and TRAF2) for comparison.
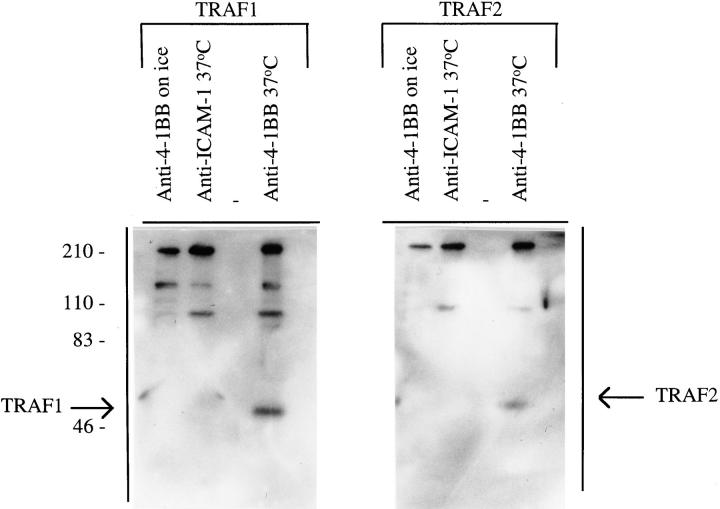
We also attempted to detect TRAF association with the 4-1BB–CT-GST protein in 293 cells, but were not successful (data not shown), which suggests that in cells, aggregation of 4-1BB might be required to induce TRAF association as has been shown for CD40/TRAF association (44). To test this hypothesis, we analyzed the association of TRAF1 and TRAF2 with full-length 4-1BB in the T cell hybridoma C8.A3. This T cell hybridoma has been shown previously to respond to 4-1BB signaling and expresses low levels of 4-1BB in the resting state, with further upregulation of 4-1BB after overnight treatment with anti-CD3 (10). C8.A3 cells were incubated with anti–4-1BB and goat anti–rat Ig for 15 min at 37°C or on ice, after which time the cells were lysed and protein G–Sepharose added to immunoprecipitate the complex. When 4-1BB was cross-linked at 0°C little or no TRAF1 or TRAF2 could be detected by Western blot analysis of the immunoprecipitated material (Fig. 7). In contrast, aggregation of 4-1BB at 37°C using anti–4-1BB followed by anti–rat Ig induced the association of TRAF1 and TRAF2 with the 4-1BB precipitates. Cross-linking of ICAM-1 on the T cells using rat anti–ICAM-1 and anti–rat Ig at 37°C did not result in significant levels of TRAF1 or TRAF2 in the ICAM-1 immunoprecipitates (Fig. 7). Thus, the presence of TRAF1 and TRAF2 in the immunoprecipitates is specific to the 4-1BB molecule. These results show that cross-linking of 4-1BB on T cells results in its enhanced association with TRAF1 and TRAF2 and that there is little or no TRAF1 and TRAF2 associated with 4-1BB in the resting state.
Figure 7.
Association of TRAF1 and TRAF2 with 4-1BB in a T cell hybrid is induced by receptor aggregation at 37°C. C8.A3 T cells (2 × 107 cells/ lane) were stimulated with anti– 4-1BB or with anti–ICAM-1 on ice for 5 min and then cross-linked with anti–rat Ig for 15 min on ice or at 37°C. Lysates were immunoprecipitated with protein G–Sepharose. Immunoprecipitates were separated by SDS-PAGE and analyzed by Western blotting with either anti-TRAF1 or anti-TRAF2. This experiment is representative of three separate experiments.
Role of the TRAF Family of Signaling Molecules in Costimulation of IL-2 Production by s4-1BBL.
Based on the association of the 4-1BB cytoplasmic domain with TRAF2 (Figs. 6 and 7) and the importance of TRAF2 in JNK activation in response to TNF, we explored the functional role of TRAF2 using T cells isolated from TRAF2− mice, as well as using T cells isolated from TRAF2 DN mice.
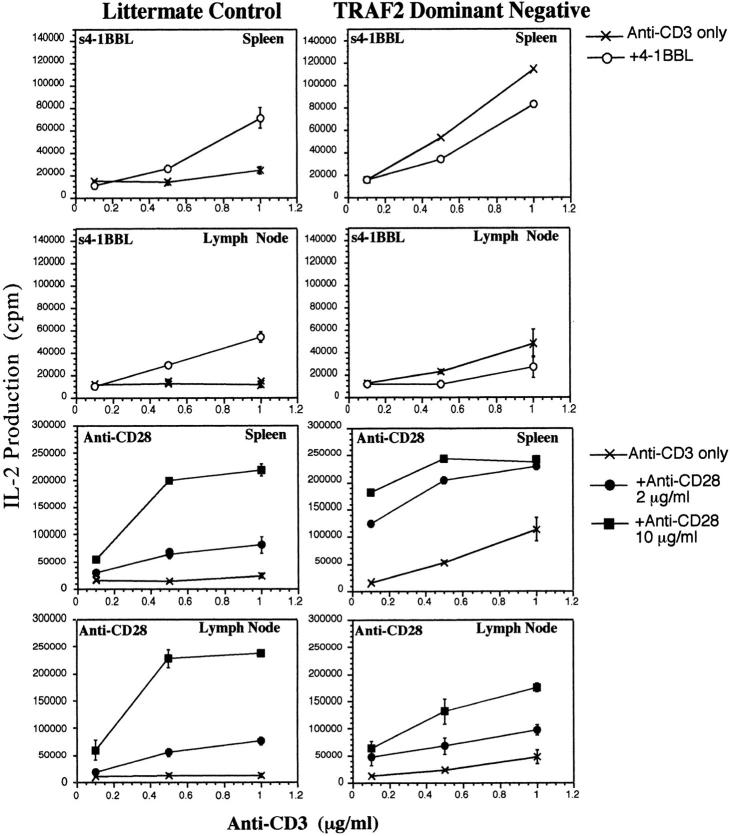
To test the effect of the TRAF2 DN mutant on 4-1BB signaling leading to IL-2 production, we isolated high density resting T cells from TRAF2 DN mice and their transgene negative littermates. Fig. 8 compares IL-2 production by the T cells in response to anti-CD3 alone, anti-CD3 plus immobilized s4-1BBL or anti-CD3 plus immobilized anti-CD28 antibody. For the wild-type T cells, immobilized s4-1BBL clearly augments the response of resting spleen or lymph node T cells to immobilized anti-CD3. As was observed in Fig. 5, the effects of s4-1BBL on IL-2 production are smaller than those of anti-CD28. The TRAF2 DN T cells showed a higher background response to anti-CD3 alone and addition of s4-1BBL actually led to a decrease in response of the TRAF2 DN T cells. In contrast, anti-CD28 augmented IL-2 production in response to anti-CD3 in both the wild-type mice and TRAF2 DN mice. Although responses to anti-CD28 were retained in all instances, with lymph node T cells we sometimes observed a lower response to anti-CD28 in transgenic T cells compared with the nontransgenic controls (e.g., anti-CD28 at 10 μg/ml in Fig. 8). In contrast, splenic T cells sometimes showed hyperresponsiveness to anti-CD28 (e.g., anti-CD28 2 μg/ml, Fig. 8). In spite of the variable response to anti-CD28, the observation that the TRAF2 DN mice largely retain responses to anti-CD28 but fail to show augmentation of IL-2 production in response to immobilized s4-1BBL suggests that the TRAF2 signaling molecule is required for 4-1BB signaling.
Figure 8.
Decreased response to 4-1BBL by T cells isolated from mice expressing a dominant negative form of TRAF2. Resting T cells were isolated from spleen and lymph nodes TRAF2 DN mice or their transgene-negative littermates, as described in Materials and Methods. T cells were incubated with anti-CD3 immobilized on plastic at the concentrations indicated in the figures in the presence or absence of immobilized s4-1BBL (5 μg/ml) or immobilized anti-CD28 at 2 or 10 μg/ml as indicated in the figure. At 48 h of culture, supernatants were analyzed for IL-2 production using CTLL cells. Results shown are for a single representative mouse. Similar results were obtained in three independent experiments with two control and two mutant mice analyzed separately in each experiment.
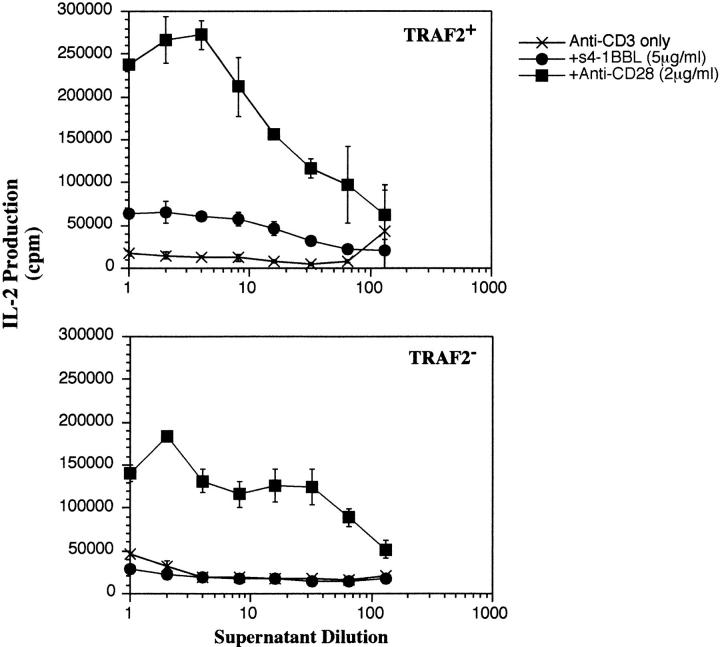
Fig. 9 shows a similar experiment carried out using T cells isolated from the lymph nodes of TRAF2− mice. As described elsewhere, TRAF2− mice have small lymphoid organs and die on average at day 10–14 after birth (36). However, some mice survived to 3 wk and were used for these experiments. The spleens of these mice are atrophied and could not be used for T cell isolation, but a small number of resting T cells could be isolated from the lymph nodes of these animals. It can be seen that the T cells from the TRAF2− mice retain the ability to respond to anti-CD28, but show no enhancement of anti-CD3 signaling by immobilized 4-1BBL. Again, the response to anti-CD3 alone was higher in the TRAF2-deficient animals than the littermate controls and the addition of 4-1BBL actually reduces the response to anti-CD3 alone. Similarly to the results with lymph node T cells from the TRAF2 DN mice (Fig. 8), the response of lymph node T cells to anti-CD3 plus anti-CD28 was also somewhat reduced.
Figure 9.
Decreased response to 4-1BBL by T cells isolated from TRAF2− mice. Resting T cells were isolated from the lymph nodes of TRAF2− mice or their wild-type TRAF2+ littermates. T cells were incubated with anti-CD3 alone (immobilized at 1 μg/ml) or with anti-CD3 plus immobilized s4-1BBL (5 μg/ml) or immobilized anti-CD28 (2 μg/ml). Results are shown from one mouse and are representative of two independent experiments with two mice per experiment.
In addition to TRAF2, the 4-1BB cytoplasmic domain also interacts with TRAF1 (Figs. 6 and 7). T cells from transgenic mice that overexpress TRAF1 have been shown to proliferate normally in response to TCR engagement, but are resistant to antigen-induced apoptosis (38). Consistent with the observations that TRAF1 overexpression does not influence T cell proliferative responses (38), we find that T cells from mice overexpressing TRAF1 showed no differences in IL-2 production in response to anti-CD3 plus immobilized s4-1BBL compared with wild-type controls (data not shown).
Discussion
The delivery of a signal through the CD28 molecule has been shown to be critical for activation of T cells and for prevention of induction of cell death or an anergic state. For a costimulatory receptor to prevent the induction of anergy, it is thought that the costimulatory signal must be delivered simultaneously or within a few hours of signaling through the TCR (46). Previous studies using antibodies to 4-1BB to stimulate T cells have found that unprimed T cells require 4–5 d to respond to anti–4-1BB, but that T cells that have been preactivated for 2–3 d to induce a high level 4-1BB expression, respond within 48 h (6, 12). Our data using 4-1BBL on APCs (11) or using s4-1BBL (this report) show that T cells can produce IL-2 in response to 4-1BBL within 24 h in the absence of CD28 costimulation. Other studies in which antibodies to 4-1BB have been used to costimulate with anti-CD3 either used preactivated T cells (12) or did not separate resting from activated T cells (14). Because B7 family members are expressed on activated T cells, one cannot rule out that low levels of B7 were also contributing to the response in these studies (12, 14). Although we have previously shown that CD28− T cells can respond to 4-1BBL expressed on APCs (16), in those studies it was possible that other molecules present on the cell surface contributed to this signal. Our study therefore used purified resting T cells from CD28− mice and soluble 4-1BBL to test the role of 4-1BBL in isolation. The data presented here clearly show that 4-1BBL alone can provide an effective costimulatory signal for resting T cell activation, independent of other cell surface molecules including the CD28 molecule.
The ability of s4-1BBL to induce IL-2 in T cells more rapidly than anti–4-1BB antibody may be due to the natural ligand for 4-1BB providing a more potent signal than that delivered by antibody ligation. Although surface expression of 4-1BB is not obvious until at least 24 h after anti-CD3 treatment of resting T cells (6), 4-1BB mRNA is readily detectable within 3 h of anti-CD3 treatment (45). Our data showing that functional responses to isolated 4-1BBL occur within 24 h of T cell activation suggests that it may take only a few 4-1BB molecules on the T cell surface to allow costimulatory signals to be transduced. Thus, our data show that 4-1BBL can, under appropriate conditions, function in a similar way to and independently of the CD28 molecule to provide a costimulatory signal for induction of IL-2 production by resting T cells.
A comparison of the costimulatory activity of immobilized anti-CD28 and immobilized s4-1BBL (Fig. 5) showed that anti-CD28 is more effective than immobilized s4-1BBL in inducing IL-2 production at low doses of anti-CD3. However, at higher concentrations of anti-CD3, one can achieve similar levels of IL-2 production in response to CD28 or 4-1BB engagement. CD28 is expressed on resting T cells, whereas 4-1BB is expressed only after signaling through the TCR. When anti-CD3 is bound to the plates at low density, the level of 4-1BB receptor induced may be limiting, thus explaining the poorer response to s4-1BBL at low anti-CD3 concentrations. Alternatively or additionally, the lower response to s4-1BBL at low anti-CD3 doses might be due to 4-1BBL providing a weaker costimulatory signal than anti-CD28. This weaker signal from 4-1BBL– 4-1BB interaction may be compensated for by increasing signals through the TCR. Nevertheless, when signals through the TCR are optimized, 4-1BBL can function as well as anti-CD28 in induction of IL-2 production by resting T cells (Fig. 5).
Shahinian et al. have shown that CD28− T cells are unimpaired in their immune response to LCMV infection (2). Interestingly, there is evidence that it is the duration of TCR signaling that allows CD28-independent immune responses to LCMV (47). In light of our data, one could interpret the data of Kündig et al. (47) as suggesting that antigens that induce strong signals through the TCR may allow other CD28-independent costimulatory pathways, such as the 4-1BB pathway, to take over. The kinetics of the response to s4-1BBL (Fig. 4) suggest that if signal 1 is strong enough, then 4-1BB can be induced and receive sufficient signals to induce IL-2 production within 24 h.
A critical question is how the various cell surface molecules involved in T cell activation integrate the signals from distinct signal transduction pathways to induce high level IL-2 gene expression. T cell receptor signaling and CD28 signaling have been shown to synergize at the level of JNK (48). TRAF2 has been implicated in the ability of both TNFR1 and CD40 to activate JNK (32–36). Therefore, an attractive hypothesis is that 4-1BB signaling can replace CD28 signaling by synergizing with the TCR at the level of JNK activation. As a first step toward testing this model, we have analyzed the role of TRAF2 in T cell activation by 4-1BB.
Using a GST fusion protein, we showed that the cytoplasmic domain of 4-1BB could associate with TRAF1 and TRAF2 in vitro (Fig. 6). However, attempts to demonstrate an association between the 4-1BB CT and TRAF1 and 2 in 293 cells using the same GST fusion protein were not successful. This suggested that aggregation of 4-1BB might be required for TRAF association in cells. In support of this, we found that TRAF1 and TRAF2 association with 4-1BB in a T cell hybrid required cross-linking of 4-1BB at 37°C. During the revision of this manuscript, two groups have also reported an association of TRAF1 and TRAF2 with the 4-1BB cytoplasmic domain using yeast 2 hybrid analysis (49, 50). Arch and Thompson (49) demonstrated TRAF1 and TRAF2 association with the murine 4-1BB cytoplasmic domain. In addition, using a fusion protein between the murine 4-1BB cytoplasmic domain and the transmembrane/extracellular domains of CD28, Arch and Thompson found constitutive association of 4-1BB with TRAF 2 in HEK293 cells (49). Jang et al. have reported that TRAF1 and TRAF2 associate with human 4-1BB and they find that when full-length 4-1BB and the TRAF proteins are overexpressed in 293 these associations are observed in the absence of 4-1BB receptor cross-linking (50). The difference between those studies and our results may reflect the higher level of proteins expressed in the 293 cells versus our use of endogenously expressed molecules in T cells.
In this report we have also shown that resting T cells isolated from mice that lack TRAF2 or express a dominant negative form of TRAF2 in their lymphoid subset fail to respond to 4-1BB signaling. TRAF2− mice have very small lymphoid organs and die at 2–3 wk of age, whereas mice expressing a TRAF2 DN transgene in their lymphoid cells are viable and have enlarged lymphoid organs due to the presence of an abnormal number of B cells. In spite of these marked phenotypic differences, both types of TRAF2-deficient mice have demonstrated an essential role for TRAF2 in JNK activation in response to TNF (35, 36). Similarly, in this report we have demonstrated that the two different types of TRAF-deficient mice have very similar outcomes for the T cell response to 4-1BBL. That is, responses to anti-CD28 were retained, whereas responses to 4-1BBL actually went from being stimulatory in wild-type mice, to being inhibitory to anti-CD3–induced IL-2 production in TRAF2-deficient mice (Figs. 8 and 9). The observation that signaling via 4-1BB is actually slightly inhibitory to anti-CD3–stimulated T cells is unexplained. It is possible that this result is due to an inhibitory signal from 4-1BB that is normally masked by the positive signaling effects of TRAF2.
For both TRAF2 DN mice and TRAF2− mice, we also observed an increase in background response to anti-CD3 alone. The reason for this increase in background is not clear. The purity of T cells from TRAF2 DN mice or their transgene-negative littermates was indistinguishable and addition of CTLA-4 Ig to block effects of B7 family molecules had no effect on the response (data not shown). Thus, the increased response of isolated resting T cells to immobilized anti-CD3 does not appear to be due to the presence of contaminating B7-expressing APCs. Lee et al. have shown that the response of TRAF2 DN lymph node T cells to anti-CD3 presented by accessory cells is decreased compared with transgene-negative littermates (35). The data in our report, showing a slight decrease in the response of lymph node TRAF2 DN T cells to anti-CD3 and anti-CD28, agrees with that finding. However, the increased response to anti-CD3 alone (Figs. 8 and 9) is puzzling. Yeh et al. have shown that serum levels of TNF are increased in TRAF2-deficient animals and have suggested that lack of TRAF2 leads to dysregulation of TNF synthesis (36). It is possible that the dysregulation of TRAF2 prevents feedback inhibition of the expression of other molecules produced by or expressed on the purified T cells, and that these in turn allow enhancement of the response to anti-CD3 alone.
In addition to associating with TRAF2 (Figs. 6 and 7), the 4-1BB cytoplasmic tail can also associate with TRAF1 in vitro. The biological significance of this finding remains to be tested. In contrast to the results with TRAF2-negative mice or with mice expressing a dominant negative form of TRAF2, T cells from mice that overexpress wild-type TRAF1 showed no perturbation of responses to anti-CD3 plus anti-CD28 or s4-1BBL (data not shown). These results are not surprising in view of the data from Speiser et al., which showed that T cells from the TRAF1 overexpressing mice have normal proliferative responses but are resistant to antigen-induced apoptosis (38). This lack of perturbation of 4-1BB signaling by overexpression of TRAF1 could be due to the dispensability of TRAF1 for 4-1BB costimulatory signals or could be because the amount of TRAF1 recruited during 4-1BB signaling in wild-type mice is not limiting. It was also possible that overexpression of TRAF1 would interfere with signaling via TRAF2 by forming heterodimers that prevent other interactions of the TRAF2 molecule, but this did not seem to occur, as 4-1BB signaling was neither impaired nor enhanced by overexpression of TRAF1 (data not shown).
In summary, the results presented here show that 4-1BBL can function in isolation to costimulate T cell responses that are independent of the CD28 molecule. When signaling through the TCR is strong, 4-1BB signaling is as effective as anti-CD28 signaling in stimulating IL-2 production by T cells. In contrast, at low anti-CD3 doses, the CD28 costimulatory pathway seems to be more effective than the 4-1BB costimulatory pathway. The induction of IL-2 in response to the 4-1BB costimulatory signal is dependent on the presence of a functional TRAF2 molecule. Given the importance of TRAF2 in JNK activation in response to CD40L or TNF, a plausible model is that synergistic activation of JNK by TCR plus 4-1BB via TRAF2 leads to high level IL-2 gene transcription and in this way the 4-1BB molecule can replace the CD28 molecule in resting T cell activation. Thus, our data add to the accumulating body of evidence for a pivotal role for the TRAF2 molecule in signaling by TNFR family members.
Acknowledgments
We thank Chris Richardson for advice on the baculovirus expression system and for providing the pETL-HA vector, Sf 9, and Hi5 insect cell lines. We thank Rob Dunn for providing the MAG signal sequence-containing vector, and Pam Ohashi for helpful suggestions.
This work was funded by a grant from the Medical Research Council of Canada (MRC) to T.H. Watts, by a grant from the National Cancer Institute of Canada (NCIC) to T.W. Mak, and by a grant from the Howard Hughes Medical Institute to Y. Choi; K. Saoulli and M.D. Goldstein are funded by MRC studentships; W.-C. Yeh is funded by an MRC postdoctoral fellowship; and T.H. Watts is a Senior Scientist of the NCIC with funds from the Canadian Cancer Society.
Abbreviations used in this paper
4-1BBL
4-1BB ligand
AP
alkaline phosphatase
CT
cytoplasmic tail
DN
dominant negative
GST
glutathione S-transferase
HA
hemagglutinin
ICAM-1
intercellular adhesion molecule 1
JNK
c-Jun NH2-terminal kinase
NF-κB
nuclear factor–κB
RT
reverse transcriptase
s4-1BBL
soluble form of 4-1BBL
TRAF
TNF receptor–associated factor
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 3.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Shahinian A, Mak TW, Ohashi PS. Skin allograft rejection in CD28-deficient mice. Transplantation. 1996;61:352–355. doi: 10.1097/00007890-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollok KE, Kim Y-J, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4-1BB: analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 7.Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 8.Alderson MR, Smith CA, Tough TW, Davis-Smith T, Armitage RJ, Falk B, Roux E, Baker E, Sutherland GR, Din WS, Goodwin RG. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 9.Pollok KE, Kim Y-J, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T cell antigen binds to mature B cells and macrophages, and costimulates anti-μ-primed splenic B cells. Eur J Immunol. 1994;24:367–374. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- 10.DeBenedette MA, Chu NR, Pollok KE, Hurtado J, Wade WF, Kwon BS, Watts TH. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by cAMP. J Exp Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28−T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 12.Hurtado JC, Kim Y-J, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 13.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 14.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, et al. 4-1BB costimulatory signals preferentially induce CD8+T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtado JC, Kim SH, Pollok KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation: comparison with the costimulatory molecule CD28. J Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- 16.Chu NR, DeBenedette MA, Stiernholm BJN, Barber BH, Watts TH. Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28−T cells. J Immunol. 1997;158:3081–3089. [PubMed] [Google Scholar]
- 17.Hu HM, O'Rourke K, Boguski MS, Dixit VM. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- 18.Rothe M, Wong SC, Henzel WJ, Goeddel DG. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Cleary AM, Ye Z-S, Hong DI, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 20.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 21.Régnier CH, Tomasetto C, Moog-Lutz C, Chenard M-P, Wendling C, Basset P, Rio M-C. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem. 1995;270:25715–25721. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- 22.Song HY, Donner DB. Association of a RING finger protein with the cytoplasmic domain of the human type-2 tumour necrosis factor receptor. Biochem J. 1995;309:825–829. doi: 10.1042/bj3090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Irie S, Reed JC. A novel member of the TRAF family of putative signal transducing proteins binds to the cytosolic domain of CD40. FEBS Lett. 1995;358:113–118. doi: 10.1016/0014-5793(94)01406-q. [DOI] [PubMed] [Google Scholar]
- 24.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware CF, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 25.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Science. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 26.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Rothe M, Goeddel DV. Anatomy of TRAF2: distinct domains for nuclear factor-κB activation and association with tumor necrosis factor signaling proteins. J Biol Chem. 1996;271:19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Park CG, Choi Y. T cell receptor–dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor–associated factors. J Exp Med. 1996;183:669–674. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansieau S, Scheffrahn I, Mosialos G, Brand H, Duyster J, Kaye K, Harada J, Dougall B, Hubinger G, Kieff E, et al. Tumor necrosis factor receptor–associated factor (TRAF)-1, TRAF-2, and TRAF-3 interact in vivo with CD30 cytoplasmic domain; TRAF-2 mediates CD30-induced nuclear factor kappa B activation. Proc Natl Acad Sci USA. 1996;93:14053–14058. doi: 10.1073/pnas.93.24.14053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Boucher L-M, Marengere LEM, Lu Y, Thukral S, Mak TW. Binding sites of cytoplasmic effectors TRAF1, 2 and 3 on CD30 and other members of the TNF receptor superfamily. Biochem Biophys Res Commun. 1997;233:592–600. doi: 10.1006/bbrc.1997.6509. [DOI] [PubMed] [Google Scholar]
- 31.Gedrich RW, Gilfillan MC, Duckett CS, Van Dongen JL, Thompson CB. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor–associated factor family of signal transducing proteins. J Biol Chem. 1996;271:12852–12858. doi: 10.1074/jbc.271.22.12852. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z-g, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 33.Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 34.Reinhard C, Shamoon B, Shyamala V, Williams LT. Tumor necrosis factor α–induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO (Eur Mol Biol Organ) J. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 36.Yeh W-C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, et al. Early lethality, functional NF-κB activation and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 37.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky RA. Establishment and characterization of Balb/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 38.Speiser DE, Lee SY, Wong B, Arron J, Santana A, Kong Y-Y, Ohashi PS, Choi Y. A regulatory role for TRAF1 in antigen-induced apoptosis of T cells. J Exp Med. 1997;185:1777–1783. doi: 10.1084/jem.185.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field J, Nikawa J-I, Broek D, MacDonald B, Rodgers L, Wilson IA, Lerner RA, Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiaeby use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 42.Attia, J., S. Gupta, and R.J. Dunn. 1995. Expression and secretion of a soluble form of myelin-associated glycoprotein (MAG). In Methods in Molecular Biology, Vol. 39: Baculovirus Expression Protocols. C.D. Richardson, editor. Humana Press Inc., Totowa, NJ. 363–384. [DOI] [PubMed]
- 43.Lalumière, M., and C.D. Richardson. 1995. Production of recombinant baculoviruses using rapid screening vectors that contain the gene for β-galactosidase. In Methods in Molecular Biology, Vol. 39: Baculovirus Expression Protocols. C.D. Richardson, editor. Humana Press Inc., Totowa, NJ. 161–177. [DOI] [PubMed]
- 44.Kuhne MR, Hambor JE, Mackey MF, Kosaka Y, Nishimura T, Gigley JP, Noelle RJ, Calderhead DM. Assembly and regulation of the CD40 receptor complex in human B cells. J Exp Med. 1997;186:377–342. doi: 10.1084/jem.186.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon BS, Kim GS, Prystowsky MB, Lancki DW, Sabath DE, Pan JL, Weissman SM. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci USA. 1987;84:2896–2900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? . J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kündig TM, Shahinian A, Kawai K, Mittrücker H-W, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 48.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 49.Arch RH, Thompson CB. 4-1BB and OX40 are members of a tumor necrosis factor (TNF)–nerve growth factor receptor subfamily that bind TNF receptor–associated factors and activate nuclear factor κB. Mol Cell Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-κB. Biochem Biophys Res Commun. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]