Murine Macrophages Secrete Interferon γ upon Combined Stimulation with Interleukin (IL)-12 and IL-18: A Novel Pathway of Autocrine Macrophage Activation (original) (raw)
Abstract
Interferon (IFN)-γ, a key immunoregulatory cytokine, has been thought to be produced solely by activated T cells and natural killer cells. In this study, we show that murine bone marrow– derived macrophages (BMMΦ) secrete large amounts of IFN-γ upon appropriate stimulation. Although interleukin (IL)-12 and IL-18 alone induce low levels of IFN-γ mRNA transcripts, the combined stimulation of BMMΦ with both cytokines leads to the efficient production of IFN-γ protein. The macrophage-derived IFN-γ is biologically active as shown by induction of inducible nitric oxide synthase as well as upregulation of CD40 in macrophages. Our findings uncover a novel pathway of autocrine macrophage activation by demonstrating that the macrophage is not only a key cell type responding to IFN-γ but also a potent IFN-γ–producing cell.
Keywords: macrophage, interferon γ, interleukin 12, interleukin 18, innate immunity
Interferon (IFN)-γ regulates a variety of important immunological programs. It is the predominant cytokine during Th1-dominated immune reactions, participates importantly during antigen presentation, and is the prototypical macrophage-activating cytokine. Consequently, a pivotal role of IFN-γ in the clearance of various intracellular pathogens has been amply demonstrated (1). One of the key events during innate immune reactions is the production of IL-12 mainly by macrophages (2). IL-12 induces NK cells to rapidly secrete IFN-γ, which then acts back to activate macrophages early in an immune response. Furthermore, IL-12 induces IFN-γ production by T cells and is the key cytokine driving Th1 cell differentiation.
A recently discovered novel cytokine, IL-18, shares many functional properties with IL-12. IL-18 was described originally as a Kupffer cell–derived costimulating factor essential for the production of IFN-γ in a murine LPS–induced shock model (3). IL-18 has been cloned recently (4), and was shown subsequently to induce IFN-γ in human (5) and murine (6) T cells. Moreover, strong synergistic effects between IL-12 and IL-18 in the induction of IFN-γ secretion of T cells (5, 6) or NK cells (7) were described.
A few reports described the secretion of low levels of IFN-γ by murine (8, 9) or human (10) macrophages stimulated with IFN-γ itself (8), IL-12 (9), or Mycobacterium tuberculosis (10). Because these findings are at variance with the widely accepted view that T cells and NK cells are the sole producers of IFN-γ, the general significance of these observations remained uncertain. A recent report demonstrated the secretion of IFN-γ even by B cells upon combined stimulation with IL-12 and IL-18 (11). In this study, we demonstrate that these two cytokines synergistically induce macrophages to secrete large amounts of IFN-γ which is biologically active in an autocrine fashion. The data suggest that macrophage activation may operate by an autocrine positive feedback loop involving multiple cytokines, including IFN-γ.
Materials and Methods
Medium and Reagents.
All cell culture was performed in DMEM supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 60 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1× nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO BRL, Paisley, UK).
Recombinant murine IFN-γ was obtained from Genentech Inc. (South San Francisco, CA), IL-12 was purchased from R&D Systems (Abingdon, UK), and IL-18 and TNF-α from PeproTech, Inc. (London, UK). Biotinylated anti-CD40 (clone 3/23) and rIgG2a isotype control antibodies were obtained from PharMingen (San Diego, CA). Streptavidin-PE was purchased from GIBCO BRL.
Animals and Generation of Bone Marrow–derived Macrophages.
Mice of strains AKR/N, C57BL/6, and 129Sv and mice homozygous for a targeted mutation of the IFN-γ receptor (IFN-γR−/−; 129Sv) (12) were obtained from the specific pathogen– free animal facilities of the Max-Planck-Institut and were used between 6 and 8 wk of age.
Macrophages were derived from bone marrow cells (BMMΦ) as described previously (12).
IFN-γ Assay, Nitric Oxide Measurement, and Analysis of CD40 Expression by FACS®.
IFN-γ was determined by a commercially available (PharMingen) sandwich ELISA test according to the manufacturer's protocol. The measuring range of the ELISA test was 0.5–100 ng/ml. Nitric oxide (NO) was measured as nitrite using the Griess reagent as described previously (13).
To assess CD40 expression by FACS®, 2 ×105 BMMΦ were preincubated with FcBlockTM (PharMingen) and stained with biotinylated anti-CD40 mAb or isotype control (biotin rIgG2a) for 30 min on ice. After another wash step, cells were stained with Streptavidin-PE for 30 min on ice and subsequently analyzed on a FACScan® (Becton Dickinson, Mountain View, CA), gating on viable cells by propidium iodide counterstaining. All washing and staining steps were performed in PBS/2% FCS.
Reverse Transcription PCR.
5 × 106 BMMΦ were seeded and stimulated in 55-mm Petriperm® hydrophob Petri dishes (Heraeus GmbH, Hanau, Germany) in a final volume of 5 ml. At the indicated time points, cells were harvested with a rubber policeman, and total cellular RNA was prepared by the method of Chomczynski and Sacchi (14). Reverse transcription was performed by standard procedures using Moloney murine leukemia virus reverse transcriptase (Pharmacia GmbH, Freiburg, Germany).
0.01–1 μl of the resulting cDNA (adjusted to a concentration of 50 ng/μl input RNA) was then amplified by PCR (annealing temperature 58°C, 1.5 mM MgCl2) for 35 cycles. The sequences for the primers used are as follows: β-actin sense primer, 5′ TGGAATCCTGTGGCATCCAT GAAAC 3′, and β-actin antisense primer, 5′ TAAAACGCAGCTCAGTAACAGTCCG 3′, generating a 348-bp PCR product; and IFN-γ sense primer, 5′ GCTCTGAGACAATGAACGCT 3′, and IFN-γ antisense primer, 5′ AAAGAGATAATCTGGCTCTGC 3′, generating a 227-bp PCR product. The PCR products were run on a 1.5% agarose gel and visualized by ethidium bromide staining.
RNA In Situ Hybridization.
In situ hybridization was performed using a digoxigenin-labeled riboprobe corresponding to the region between nucleotides 371 and 1075 of the mouse _IFN_-γ cDNA, using a protocol modified from Vignaud et al. (15). In brief, BMMΦ were cultured on glass chamber slides (Nunc, Inc., Naperville, IL), washed in PBS, fixed in 4% paraformaldehyde for 3 min, permeabilized in 100 mM glycine for 20 min, followed by a short wash in water, and postfixed in 4% paraformaldehyde for 5 min. The cells were then hybridized overnight at 55°C with 1 μg/ml of the riboprobe in 40% formamide, 5× SSC, 1× Denhardt's, 100 μg/ml denatured herring sperm DNA, and 100 μg/ml tRNA. After extensive washing in 2× SSC, 50% formamide at 50°C, unspecific antibody binding was blocked by incubation at room temperature for 1 h in TBS (25 mM Tris-HCl, pH 7.5, 140 mM NaCl, 2.7 mM KCl) containing 10% sheep serum. Alkaline phosphatase–conjugated antidigoxigenin antibodies (Boehringer Mannheim, Mannheim, Germany) were then applied at 4°C for 5 h in TBS containing 1% sheep serum. The cells were then washed extensively in TBS, equilibrated with 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2, and the alkaline phosphatase activity was developed with BCIP/NBT (Boehringer Mannheim) for 6 h. The preparations were mounted, and the cells were photographed under Nomarski optics.
Results
IL-12 and IL-18 Stimulate BMMΦ to Secrete Large Amounts of IFN-γ.
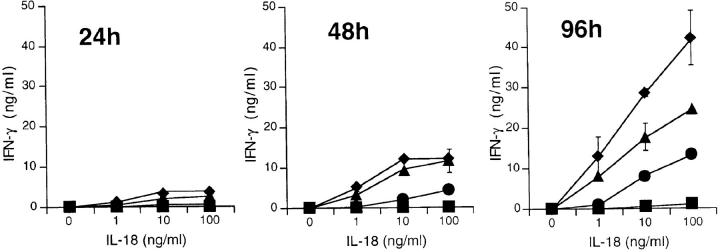
AKR/N-BMMΦ were stimulated with various concentrations of IL-12 or IL-18 alone or in combination (Fig. 1). At different time points, the IFN-γ concentrations in the supernatants were determined by ELISA. The stimulation of the macrophages with IL-12 or IL-18 alone did not result in detectable IFN-γ secretion. In sharp contrast, both cytokines synergized strongly to induce readily detectable levels of IFN-γ in the supernatants at all time points tested. The cytokine accumulated from 3.7 ng/ml per 105 cells at 24 h to 42 ng/ml per 105 cells IFN-γ at 96 h. Stimulation of the macrophages with IL-12 and IL-18 at 96 h was still suboptimal, as a plateau of IFN-γ secretion has not been reached. The induced levels of IFN-γ are comparable to those reached upon anti-CD3 stimulation of the prototypical Th1 T cell clone AE7 (81 ng/ml per 105 cells, data not shown). The ability of the macrophages to secrete IFN-γ is not restricted to the AKR/N strain of mice. We also tested BMMΦ of C57BL/6 (see also Fig. 3 A) and BALB/c (data not shown) mice with similar results. To demonstrate the purity of our BMMΦ population and to exclude the possibility of contaminating NK, T, or B cells in our assay, we analyzed the BMMΦ by FACS®. The population was consistently found to be homogeneously positive for F4/80, MAC-1, I-Ak, B7.1, and CD16/32. T cell antigens (CD3, CD4, and CD8), NK cell marker (DX5, and also NK1.1 for C57BL/6 mice) or B cell antigens (B220, CD19, and CD5) were uniformly negative (data not shown).
Figure 1.
IL-12/IL-18–induced IFN-γ synthesis of BMMΦ. 105 AKR/N-BMMΦ were stimulated in a final volume of 200 μl in 96-well flat-bottomed plates with increasing concentrations of IL-12 alone (squares, no IL-12; circles, 0.001 ng/ml; triangles, 0.1 ng/ml; diamonds, 10 ng/ml), IL-18 alone, or both cytokines together in a final volume of 200 μl in 96-well flat-bottomed plates. At the indicated time points, culture supernatants were harvested and analyzed for IFN-γ concentration by ELISA. Data represent means of triplicate with SD indicated. Similar results were obtained in a total of three independent experiments.
Figure 3.
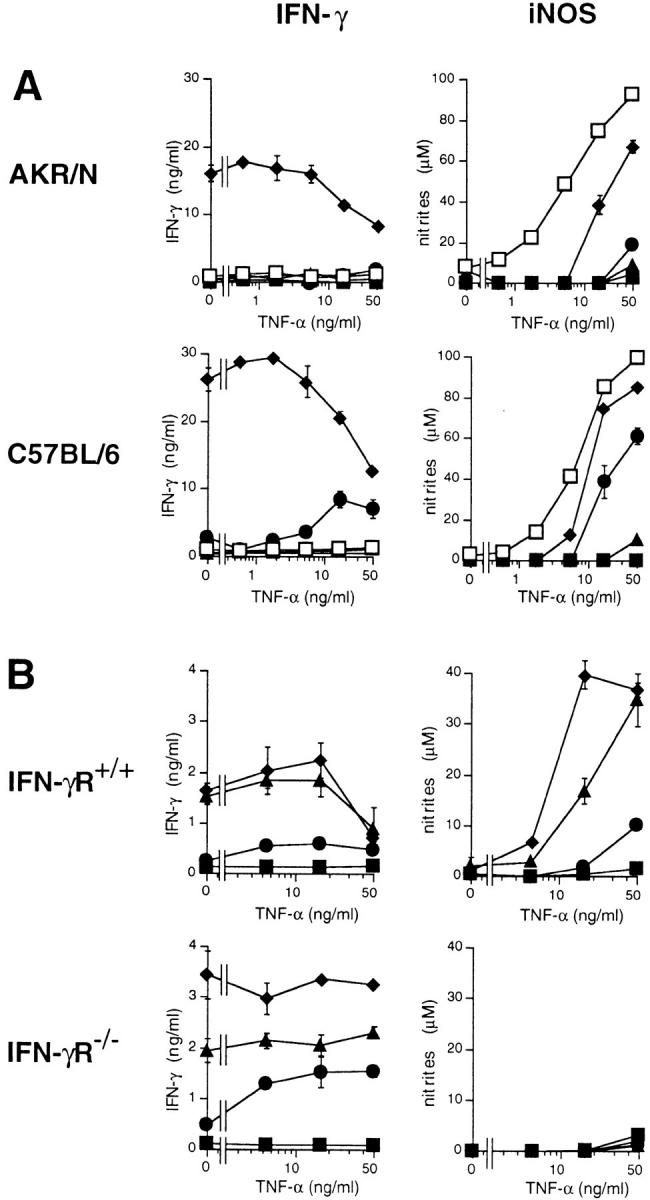
Endogenously produced IFN-γ induces autocrine macrophage stimulation: iNOS induction. (A) 105 AKR/N-BMMΦ were cultivated in a final volume of 200 μl in 96-well flat-bottomed plates with medium alone (filled squares) or stimulated with 10 ng/ml IL-12 alone (circles), 50 ng/ml IL-18 alone (triangles), with both cytokines together (diamonds), or with 10 ng/ml IFN-γ (open squares) in a final volume of 200 μl in 96-well flat-bottomed plates. To allow for synergistic iNOS induction, increasing concentrations of TNF-α were titrated into each culture. After 96 h, supernatants were harvested, and IFN-γ as well as nitrites were determined as described in Materials and Methods. Data represent means of triplicate with SD indicated. Similar results were obtained in a total of four independent experiments. (B) 105 BMMΦ of IFN-γR+/+ (129Sv) or IFN-γR−/− strains of mice were stimulated with increasing concentrations of IL-12 plus IL-18 (squares, no cytokines; circles, 0.1/0.5 ng/ml; triangles, 1/ 5 ng/ml; diamonds, 10/50 ng/ml, respectively) in a final volume of 200 μl in 96-well flat-bottomed plates. Increasing concentrations of TNF-α were titrated into each culture. After 96 h, supernatants were harvested, and IFN-γ as well as nitrites were determined as described in Materials and Methods. Data represent means of triplicate with SD indicated. One of two independent experiments with similar results is shown.
We also assayed the commonly used mouse macrophage cell lines RAW 264.7, J774, RAW 309Cr, P388D1, and IC 21. In contrast to BMMΦ, none of the five cell lines produced IFN-γ upon stimulation with IL-12 and IL-18 (data not shown).
IL-12 and IL-18 Induce IFN-γ mRNA in BMMΦ.
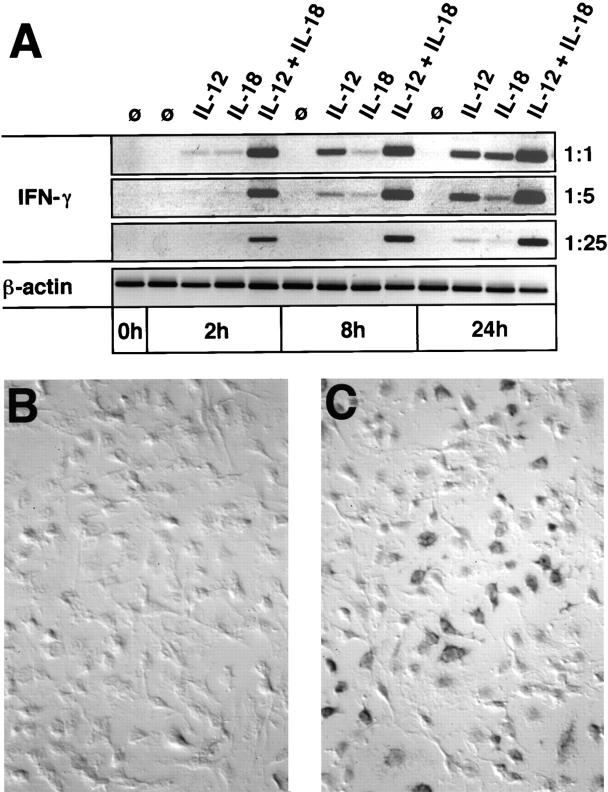
T o address the level of regulation of IFN-γ synthesis, we stimulated BMMΦ with IL-12 (10 ng/ml) or IL-18 (50 ng/ ml), alone or in combination, and prepared mRNA after 2, 8, and 24 h of stimulation. IFN-γ mRNA expression was then evaluated by reverse transcription (RT)-PCR (Fig. 2 A). In the case of BMMΦ stimulated with IL-12 plus IL-18, a prominent band of IFN-γ mRNA appeared as early as 2 h after addition of the cytokines, and increased further at later time points after stimulation. The PCR product was proven to result from IFN-γ mRNA by sequencing (data not shown). Surprisingly, although no secreted IFN-γ was detectable in the supernatant of BMMΦ stimulated with IL-12 alone or IL-18 alone, IFN-γ mRNA was clearly induced. This induction appears to be more prominent in the case of stimulation with IL-12 than with IL-18 and increases over time during the culture period. Nevertheless, as shown semiquantitatively by titrating the input cDNA of the PCR reaction, the induction of IFN-γ mRNA by combined stimulation with IL-12 and IL-18 is clearly far more efficient, consistent with the synergism observed at the protein level. To estimate the frequency of IFN-γ–producing macrophages within the whole population, we performed RNA in situ hybridization experiments (Fig. 2, B and C). After 18 h of stimulation with IL-12 plus IL-18, ∼25–30% of the BMMΦ stained strongly positive for IFN-γ mRNA (Fig. 2 C), whereas unstimulated control BMMΦ were homogeneously negative (Fig. 2 B). Control stainings with the riboprobe in sense orientation also yielded completely negative results (data not shown).
Figure 2.
IL-12/IL-18–induced IFN-γ mRNA in BMMΦ. (A) 5 × 106 AKR/N-BMMΦ were seeded in 55-mm Petriperm® hydrophob plates and either cultivated in medium alone (control, ø) or stimulated with 10 ng/ml IL-12 alone, 50 ng/ml IL-18 alone, or with both cytokines together. At the indicated time points, cells were harvested, mRNA was extracted, and cDNA was prepared as described in Materials and Methods. 1, 0.2, or 0.04 μl of input cDNA was amplified by PCR with IFN-γ–specific primers. As a control, β-actin mRNA was also amplified with 0.01 μl input cDNA. One of three independent experiments, yielding identical results, is shown. (B and C) 106 AKR/N-BMMΦ were cultivated on glass chamber slides with medium alone (B) or with 10 ng/ml IL-12 plus 50 ng/ml IL-18 (C). After 18 h, RNA in situ hybridization for IFN-γ mRNA was performed as described in Materials and Methods; ×200.
Autocrine Macrophage Stimulation by Macrophage-derived IFN-γ.
To test the biological activity of IFN-γ produced endogenously by BMMΦ stimulated with IL-12 plus IL-18, we tested two different indicators of IFN-γ–mediated macrophage activation. First, IFN-γ is known to synergize with TNF-α in the induction of inducible NO synthase (iNOS) with concomitant production of nitric oxide (NO) in macrophages (16). Therefore, we stimulated BMMΦ of AKR/N and C57BL/6 mice with optimal concentrations of IL-12, IL-18, or IL-12 plus IL-18 and titrated increasing concentrations of TNF-α into these cultures. Control cultures received IFN-γ. As a readout for iNOS induction, we determined nitrite, the stable end product of NO, in the supernatants after 96 h of stimulation (Fig. 3 A). IFN-γ was measured simultaneously in the supernatants. In macrophages exposed to IL-12 plus IL-18, increasing concentrations of TNF-α induced iNOS very efficiently, although somewhat less than upon optimal stimulation with exogenously added IFN-γ. Experiments with mice lacking the IFN-γ receptor (IFN-γR−/−) proved that endogenously produced IFN-γ is responsible for the induction of iNOS: although IFN-γR−/−-BMMΦ stimulated with IL-12 plus IL-18 plus TNF-α secreted IFN-γ comparable to control IFN-γR+/+-BMMΦ (129Sv), no nitrites were detectable in the supernatant of the IFN-γR−/−-BMMΦ, reflecting a failure of iNOS induction when IFN-γ is unable to signal via its receptor (Fig. 3 B). Indeed, nitrite accumulation reflected iNOS induction, as demonstrated by the addition of the iNOS inhibitor l-monomethyl-l-arginine, which completely abolished detectable nitrites without influencing IFN-γ induction (data not shown).
Two additional interesting observations were consistently made. First, with increasing concentrations of TNF-α, IFN-γ levels stimulated with IL-12 plus IL-18 decrease (Fig. 3, A and B). IFN-γR−/−-BMMΦ are an exception to this rule (Fig. 3 B), pointing to a possible role of enhanced consumption of IFN-γ upon increasing concentrations of TNF-α. The phenomenon is unrelated to the simultaneously produced NO, known to be potentially autotoxic to the secreting macrophage itself (16), because l-monomethyl-l-arginine had no influence on the observed decrease of IFN-γ (data not shown). Second, in the case of the C57BL/6-BMMΦ (Fig. 3 A), IL-12 alone synergizes with TNF-α to induce IFN-γ as well as iNOS. In AKR/N mice, a different and/or less efficient synergism seems to operate: in five independent experiments, a slight induction of nitrites was noted consistently upon costimulation with 50 ng/ml TNF-α, whereas IFN-γ in the supernatant was either undetectable or only marginally elevated (e.g., 1.5 ng/ml in Fig. 3 A).
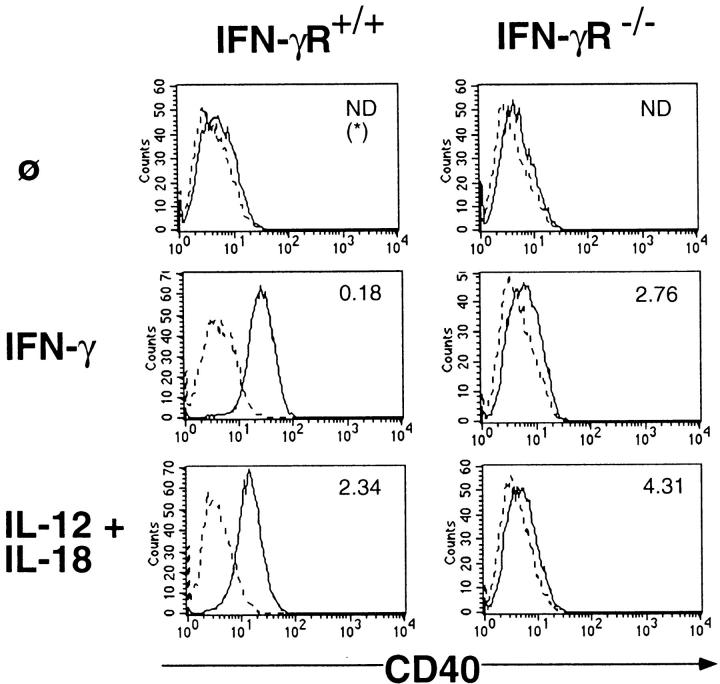
As a second system to demonstrate the functional potential of the BMMΦ-derived IFN-γ, we assayed the IFN-γ–mediated upregulation of CD40. Resting BMMΦ are negative or only slightly positive for CD40, whereas stimulation with IFN-γ for 48 h leads to an upregulation of this costimulatory molecule (M. Munder, unpublished observation). The same induction was noted on IFN-γR+/+-BMMΦ (129Sv) stimulated with IL-12 plus IL-18. Again, endogenously produced IFN-γ was responsible for this effect, as demonstrated by the lack of CD40 induction on IFN-γR−/−-BMMΦ stimulated with IL-12 plus IL-18 (Fig. 4). BMMΦ of mice with 129 background are definitively less efficient in IFN-γ production upon IL-12/IL-18 stimulation (Fig. 3 B, and Fig. 4) than BMMΦ of strains AKR/N or C57BL/6 (Fig. 1, and Fig. 3 A). Nevertheless, even these lower amounts of induced IFN-γ clearly autoactivate the macrophages to upregulate iNOS and CD40.
Figure 4.
Macrophage-derived IFN-γ is biologically active: CD40 upregulation. 1.5 × 106 BMMΦ of IFN-γR+/+ (129Sv) or IFN-γR−/− strains of mice were seeded in 35-mm Petriperm® hydrophob plates in a final volume of 1.5 ml. They were either not stimulated (ø, medium only) or stimulated with 10 ng/ml IFN-γ (positive control) or with the combination of 10 ng/ml IL-12 plus 50 ng/ml IL-18. After 48 h, BMMΦ were harvested with a rubber policeman and analyzed by FACS® for CD40 upregulation as described in Materials and Methods. (*) Simultaneous determination of IFN-γ concentration (ng/ml) in the 48-h supernatant. ND, Not determined. One of two independent experiments with similar results is shown.
Discussion
These experiments were stimulated by the results of several authors showing synergism between IL-12 and IL-18 in the induction of IFN-γ by human (5) and murine (6) T cells and murine NK cells (7). Remarkably, both cytokines were shown recently to cooperatively induce IFN-γ secretion of murine B cells (11). We now extend these observations by demonstrating that macrophages become efficient IFN-γ–producing cells upon combined stimulation with IL-12 and IL-18. The levels of IFN-γ were of a similar magnitude as that produced by T cells, and we observe efficient autocrine macrophage activation by endogenously generated IFN-γ in vitro.
We found that a considerable proportion but not all BMMΦ express IFN-γ mRNA under our conditions of in vitro stimulation. Moreover, five established macrophage cell lines failed to produce IFN-γ under these conditions. A detailed comparison (17) of various IFN-γ–induced activation parameters between immortalized macrophage cell lines and ex vivo–derived peritoneal macrophages demonstrated that each immortalized cell line exhibited only a part of the spectrum of the determined activation markers, whereas all were detected in normal macrophages. It is not clear whether these and our findings reflect a genuine quantitative or qualitative macrophage heterogeneity in the response to cytokines, and/or a loss of functional potency associated with prolonged tissue culture maintenance.
Although the combined stimulation of the BMMΦ with IL-12 and IL-18 is required to stimulate IFN-γ secretion, both cytokines alone suffice in inducing detectable IFN-γ mRNA. Whether this difference reflects merely a limitation of the ELISA sensitivity or hints towards unknown posttranscriptional regulatory mechanisms remains to be elucidated. Furthermore, Fultz et al. described the detection of IFN-γ mRNA without secreted protein upon LPS stimulation of various types of murine macrophages, including BMMΦ (18), a finding we were unable to reproduce in the present study (not shown).
An interesting side-aspect of our work is the finding that IL-12 and TNF-α, similar to their synergistic effects in the induction of IFN-γ by NK cells (19), also cooperate in the induction of IFN-γ secretion by BMMΦ. Among the three strains tested, this effect is seen only in C57BL/6-BMMΦ, possibly reflecting a genetic polymorphism related to the known genetic differences between inbred mice in the ability to generate Th1- or Th2-dominated immune responses. Thus, a detailed comparison of this phenomenon between different mouse strains might prove fruitful.
Our work demonstrates for the first time that macrophages secrete high levels of IFN-γ upon combined stimulation with IL-12 and IL-18. Upon appropriate activation by pathogens or LPS, macrophages are known to be important producers of IL-12 (2) or IL-18 (3). Thus, our findings unravel a novel potential pathway of autocrine macrophage activation involving endogenously produced IFN-γ. This pathway might play a pivotal role during early innate immune reactions, i.e., before the development of adaptive immunity, in infectious diseases (7, 10), in septic shock (3), as well as during autoimmune reactions (20).
References
- 1.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 3.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S, Kurimoto M. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 5.Micallef M, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, et al. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 6.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive TH1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang TT, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformansthrough production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marzio P, Puddu P, Conti L, Belardelli F, Gessani S. Interferon-γ upregulates its own gene expression in mouse peritoneal macrophages. J Exp Med. 1994;179:1731–1736. doi: 10.1084/jem.179.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf SF, Belardelli F, Gessani S. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 10.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. . Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimoto T, Okamura H, Tagawa Y, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 13.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of nitric oxide synthase/ arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi L. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Vignaud JM, Martinet N, Martinet Y, Plenat F. In situ hybridization for localization of mRNAs in mononuclear phagocytes in cell culture and tissue sections. J Immunol Methods. 1994;174:281–296. doi: 10.1016/0022-1759(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 16.MacMicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Lambert LE, Paulnock DM. Differential induction of activation markers in macrophage cell lines by interferon-γ. Cell Immunol. 1989;120:401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- 18.Fultz MJ, Barber SA, Dieffenbach CW, Vogel SN. Induction of IFN-γ in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 19.Tripp C, Wolf S, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon-γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothe H, Jenkins NA, Copeland NG, Kolb H. Active stage of autoimmune diabetes is associated with the expression of a novel cytokine, IGIF, which is located near Idd2. J Clin Invest. 1997;99:469–474. doi: 10.1172/JCI119181. [DOI] [PMC free article] [PubMed] [Google Scholar]