Stat5b Is Essential for Natural Killer Cell–mediated Proliferation and Cytolytic Activity (original) (raw)
Abstract
We have analyzed the immune system in Stat5-deficient mice. Although Stat5a−/− splenocytes have a partial defect in anti-CD3-induced proliferation that can be overcome by high dose interleukin (IL)-2, we now demonstrate that defective proliferation in Stat5b−/− splenocytes cannot be corrected by this treatment. Interestingly, this finding may be at least partially explained by diminished expression of the IL-2 receptor β chain (IL-2Rβ), which is a component of the receptors for both IL-2 and IL-15**,** although other defects may also exist. Similar to the defect in proliferation in activated splenocytes, freshly isolated splenocytes from Stat5b−/− mice exhibited greatly diminished proliferation in response to IL-2 and IL-15. This results from both a decrease in the number and responsiveness of natural killer (NK) cells. Corresponding to the diminished proliferation, basal as well as IL-2– and IL-15–mediated boosting of NK cytolytic activity was also greatly diminished. These data indicate an essential nonredundant role for Stat5b for potent NK cell–mediated proliferation and cytolytic activity.
Keywords: natural killer cells, Stat5b, Stat5a, interleukin 2, interleukin 15
Signal transducers and activators of transcription (STAT)1 proteins are cytosolic latent transcription factors that are rapidly activated after cellular exposure to interferons, cytokines, or growth factors (1–3). A considerable amount of investigation has centered on the specificity of the signals transduced by each of the seven mammalian STAT proteins. For three of these STATs (Stat1, Stat4, and Stat6), knockout mice reveal specific defects limited to the immune system consistent with defective signaling in response to interferons (4, 5), IL-12 (6, 7), or IL-4/IL-13 (8– 10), respectively. In contrast, Stat3 knockout mice exhibit fetal lethality (11), a finding consistent with the activation of Stat3 by many important cytokines, including but not limited to the IL-6 family of cytokines (IL-6, IL-11, ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, and cardiotrophin 1; reference 3).
Stat5 was discovered as a mammary gland factor induced by prolactin (12). Subsequently, a number of studies revealed the existence of two closely related Stat5-like proteins with >90% amino acid identity, now denoted as Stat5a and Stat5b (13–17). The genes encoding Stat5a and Stat5b are closely linked on human chromosome 17 (17) and mouse chromosome 11 (18). Both Stat5a and Stat5b are activated not only by prolactin but also by a very wide range of other cytokines, including growth hormone, erythropoietin, thrombopoietin, hematopoietic cytokines (IL-3, GM-CSF, and IL-5), and cytokines more specific for lymphocytes (IL-2, IL-7, IL-9, and IL-15; reference 3). Despite their similarities, Stat5a and Stat5b differ in their COOH-terminal transactivation domains (19), and relatively specific actions have been attributed to Stat5a and Stat5b. For example, mice lacking Stat5a exhibit defective lactation with impaired lobuloalveolar proliferation of the mammary epithelium during pregnancy, and reduced expression of whey acidic protein (20), indicative of defective prolactin signaling. Mice lacking Stat5b exhibit a loss of sexually dimorphic growth, which results from sex differences in the pattern of growth hormone secretion from the pituitary (21). A recent study analyzing mice in which the two Stat5 genes were deleted individually or together (by retargeting Stat5a+/− embryonic stem cells with a Stat5b targeting construct) confirmed these findings and additionally showed a greater defect in growth in mice lacking both genes (22). Thus, Stat5a and Stat5b have distinctive roles but appear also to have partially redundant roles (22). Interestingly, the double knockout mice also exhibited a significant decrease in expression of Stat3 (which results in fetal lethality when expression is totally eliminated; reference 11), raising the possibility that diminished Stat3 levels contributed to some of the abnormalities in these mice, such as the diminished response of bone marrow cells to G-CSF (22).
In Stat5a−/− mice, bone marrow–derived macrophages exhibit defective GM-CSF–induced proliferation and gene expression (23); moreover, these mice also exhibit several immunological defects, including reduced expansion of T cells in vivo (24). This latter defect is associated with diminished IL-2–mediated induction of the IL-2Rα chain, although TCR-mediated IL-2Rα induction appears to be relatively normal; thus, impaired IL-2 signaling is believed to at least partially explain the defects in these mice.
We have now analyzed the immunological properties in Stat5b−/− mice and shown that they have a modest decrease in both thymic and splenic cellularity. Moreover, Stat5b−/− splenocytes exhibit a marked decrease in IL-2– mediated proliferation and a more moderate decrease in the number of NK cells. Although Stat5a−/− mice also exhibit a partial decrease in the number of NK cells, only the Stat5b−/− mice have a dramatic defect in NK cell function. The implications of these findings are discussed below.
Materials and Methods
Mice.
The Stat5a−/− (20) and Stat5b−/− mice (21) have been described. These mice were backcrossed with C57BL/6 once, and then littermates were intercrossed for this study. All experiments were performed under protocols approved by National Institutes of Health Animal Use and Care Committee and followed the National Institutes of Health guidelines “Using Animals in Intramural Research.”
Flow Cytometric Analysis.
Single-cell suspensions from the thymus and spleen were stained and analyzed using a FACSort® (Becton Dickinson, San Jose, CA) with CELLQuest software (Becton Dickinson). The following antibodies were purchased from PharMingen (San Diego, CA): PE-conjugated anti–TCR-α/β (anti-TCR-α/β–PE; H57-597 to TCR-β); anti-TCR-γ/δ–FITC (GL3); anti-CD3ε–allophycocyanin (145-2C11); anti-CD4–FITC, –PE, and –Cy-Chrome (RM4-5); anti-CD8–PE, –Cy-Chrome, and –allophycocyanin (53-6.7); anti-IL-2Rα (CD25)-FITC (7D4); anti-CD44–Cy-Chrome (IM7); anti-CD45R (B220)–PE (RA3-6B2); anti-IL-2Rβ–FITC and –PE (TM-β1); anti-Pan-NK cells– FITC (DX5); and anti-γc–PE (4G3). In some experiments, dead cells were excluded by staining with propidium iodide.
RNA Analysis.
To evaluate IL-2–induced IL-2Rα and IL-2Rβ mRNA expression in activated lymphocytes, fresh splenocytes were stimulated in Falcon 3003 plates (Becton Dickinson) coated with 10 μg/ml anti-CD3ε mAb (145-2C11) for 48 h, washed twice with PBS, and then cultured with or without 2 nM recombinant human IL-2 (Hoffmann-La Roche, Nutley, NJ) for 10 h. RNA was extracted using Trizol (GIBCO BRL, Gaithersburg, MD), fractionated on a 0.8% formaldehyde/agarose gel, and transferred to nylon membranes. Blots were hybridized with 1.2 kb IL-2Rα, 1.7 kb IL-2Rβ (24), or control probe (0.3 kb pHe7 cDNA; reference 25) labeled with 32P using the Prime-It Rmt Kit (Stratagene, La Jolla, CA). To evaluate perforin mRNA expression, RNA was extracted from fresh splenocytes or splenocytes cultured for 90 h at 2 × 106/ml in RPMI 1640 medium containing 10% fetal bovine serum, 50 μM 2-ME, and either IL-2 (10 nM) or IL-15 (15.5 nM). The blot was sequentially hybridized with murine perforin (26) and pHe7 probes.
Western Blotting.
Whole cell extracts (15 μg/sample) were fractionated on 8% SDS polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA). Blots were processed as previously described (24). In brief, after blocking, blots were incubated with antisera specific for Stat5a or Stat5b (17), followed by the incubation with anti–rabbit IgG conjugated with horseradish peroxidase (Nycomed Amersham, Little Chalfont, Buckinghamshire, UK). Blots were washed and developed with an enhanced chemiluminescent substrate (Pierce Chemical Co., Rockford, IL). To examine Stat6 expression, anti-Stat6 antibody (Transduction Laboratories, Lexington, KY) was used.
Proliferation Assay.
Fresh splenocytes (2 × 105 cells/well) were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics with or without 2 nM IL-2 or 100 ng/ml (7.75 nM) IL-15 (Peprotech, Rocky Hill, NJ) for 48 h and pulsed with 1 μCi of [3H]thymidine (6.7 Ci/mmol, NEN, Boston, MA) for the last 12 h of culture. For proliferation of anti-CD3-stimulated splenocytes, cells (105/well) were cultured for 24 h in the presence or absence of 1 nM IL-2 with a 1 μCi [3H]thymidine pulse for the last 10 h. Because IL-4 alone cannot potently stimulate splenocytes, we stimulated splenocytes with a combination of PMA (1 ng/ml) and murine IL-4 (1,000 U/ml) for 48 h and pulsed them with 1 μCi of [3H]thymidine for the last 12 h of culture in order to evaluate the ability of IL-4 to induce proliferation in Stat5-deficient mice.
Cytotoxicity Assay.
51Cr-release assays were performed by standard methods in final volumes of 200 μl. YAC-1 (H-2A), an NK-sensitive cell, was used to assess NK activity with effector/ target ratios of 100, 50, 25, and 12.5:1 and 40, 10, 2.5, and 0.6:1, respectively. 10 nM IL-2 or 15.5 nM IL-15 were added to wells. Data are expressed as lytic units (LU) in 107 cells (1 LU corresponds to the number of effector cells lysing 20% of the targets) and obtained by fitting the titration curves to scale families of curves, as previously described (27).
Results and Discussion
The majority of mice lacking Stat5a have normal levels of Stat5b in thymus and spleen (24). Correspondingly, Stat5b−/− mice express relatively normal levels of Stat5a. Thus, in both Stat5a−/− and Stat5b−/− mice, the loss of expression of one Stat5 gene did not typically affect the expression of the other Stat5 gene in either thymus or spleen, although occasional variations were seen (Fig. 1 A, reference 24, and data not shown). Furthermore, both Stat5a−/− (24) and Stat5b−/− mice had normal levels of Stat3 (data not shown).
Figure 1.
(A) Extracts from thymuses (lanes 1–3) or spleens (lanes 4–6) were isolated from wild-type mice (lanes 1, 4), Stat5a−/− mice (lanes 2, 5), or Stat5b−/− mice (lanes 3, 6), and proteins were then separated by SDS-PAGE and Western blotted with antisera specific for Stat5a (top) or Stat5b (bottom). (B) Diminished thymocyte numbers in Stat5b−/−mice (7–10-wk-old mice were analyzed). (C and D) Flow cytometric analysis of thymocytes from 8-wk-old mice. (C) CD4 versus CD8 flow cytometric profiles. (D) CD25 versus CD44 flow cytometric profiles of double negative thymocytes. (E) Diminished numbers of splenocytes in Stat5b−/− mice (7–10-wk-old mice were analyzed). (F–H) Flow cytometric profiles (8-wk-old mice) show a decrease in the ratio of CD3+ to B220+ cells (F) and a decrease in the percentage of CD8+ single positive T cells (G) in Stat5b−/− mice. The percentage of TCR-α/β and TCR-γ/δ cells was normal in Stat5b−/− mice (H).
We first analyzed lymphoid development in Stat5b−/− mice. In these mice, thymocyte numbers were modestly diminished (P < 0.01; Fig. 1 B). Flow cytometric analysis of thymocytes from these mice revealed that all populations were represented, with almost normal numbers of CD4 and CD8 single positive and CD4+CD8+ double positive thymocytes (Fig. 1 C). However, among the double negative cells there was a moderate decrease in the percentage of CD44+CD25− cells and increase in the percentage of CD44−CD25+ cells (Fig. 1 D).
In the periphery, Stat5b−/− mice tended to have decreased numbers of splenocytes (P < 0.001; Fig. 1 E), as is observed in Stat5a−/− mice (24). The T/B cell ratio was slightly decreased (Fig. 1 F). Flow cytometric analysis revealed diminished numbers of single positive splenocytes; the number of CD4−CD8+ splenocytes was substantially reduced, whereas the number of CD4+CD8− cells was at most only slightly affected (Fig. 1 G). Interestingly, whereas Stat5a−/− mice have decreased numbers of TCR-γ/δ cells in the spleen (24), this was not the case for Stat5b−/− mice (Fig. 1 H).
We next evaluated immune function in the periphery in Stat5b−/− mice and found a defect in the responsiveness of splenocytes to mitogenic stimulation. Previously, we had found that Stat5a−/− splenocytes exhibited a defect in IL-2– induced proliferation of splenocytes that had been preactivated for 48 h with anti-CD3. However, the diminished proliferation in Stat5a−/− splenocytes could be overcome with high concentrations of IL-2 (24), suggesting that this defect primarily related to impaired IL-2–mediated induction of IL-2Rα (24), a protein that is required for formation of high-affinity IL-2 receptors (28). In Stat5b−/− splenocytes, we observed a similar defect in IL-2–induced IL-2Rα expression, both at the protein (Fig. 2 A) and mRNA (Fig. 2, B and C) levels. This defect in IL-2– induced IL-2Rα expression seen in both Stat5a−/− and Stat5b−/− mice can be explained by the presence of an IL-2 response element in the IL-2Rα gene that is dependent on Stat5 proteins (29–31). However, in contrast to the ability of high doses of IL-2 (sufficient to titrate intermediate affinity receptors) to normalize proliferation in Stat5a−/− mice (reference 24 and data not shown), this was not the case for Stat5b−/− mice (Fig. 2 D). The diminished expression of IL-2Rβ (Fig. 2, B and C) presumably contributed to this effect. Thus, although diminished proliferation in Stat5a−/− mice is primarily related to diminished IL-2Rα expression (24), the Stat5b−/− mice appeared to have a more defective IL-2-signaling pathway involving but not necessarily limited to defective IL-2Rα and IL-2Rβ expression. Indeed, additional defects are indicated given that the Stat5b−/− splenocytes also exhibited defective proliferation to a combination of PMA and IL-4 (Fig. 2 E), even though IL-4 activates Stat6 rather than Stat5 proteins. Because Stat6−/− mice exhibit diminished IL-4–dependent proliferation (8–10), we confirmed that Stat6 expression was normal in Stat5b−/− splenocytes (Fig. 2 F). These data are consistent with a more general defect or perhaps multiple defects that together diminish but not abrogate T cell proliferation.
Figure 2.
(A) Diminished IL-2–induced IL-2Rα expression in both CD4+ and CD8+ subpopulations of T cells after their preactivation with anti-CD3. Fresh splenocytes were stimulated in Falcon 3003 plates coated with 10 μg/ml anti-CD3ε mAb for 48 h, washed twice with PBS, and then cultured with or without 2 nM IL-2 for 48 h. Cells were stained with anti-CD25–FITC, anti-CD4–PE, and anti-CD8–Cy-Chrome. Shown are IL-2Rα expression and mean fluorescent intensity on CD4+ or CD8+ T cells from one representative mouse out of eight mice analyzed. (B) Northern blotting for IL-2Rα, IL-2Rβ, or control pHe7 using RNA from preactivated splenocytes not treated or treated with IL-2. Shown are representative data from three mice. (C) The relative levels of IL-2Rα and IL-2Rβ mRNA expression in B were normalized for pHe7 expression. (D) High dose IL-2 (1 nM) could not restore proliferation of Stat5b−/− splenocytes preactivated with anti-CD3. Shown are the mean ± SEM from a compilation of two independent experiments. (E) Proliferation to PMA plus IL-4 was done in Stat5b−/− splenocytes. Shown are the mean ± SD from a compilation of two experiments. In one experiment, we confirmed that treatment of cells with PMA alone or IL-4 alone yielded minimal proliferation even in wild type mice. (F) Equivalent expression of Stat6 in splenocytes from wild-type, Stat5a−/− and Stat5b−/− mice.
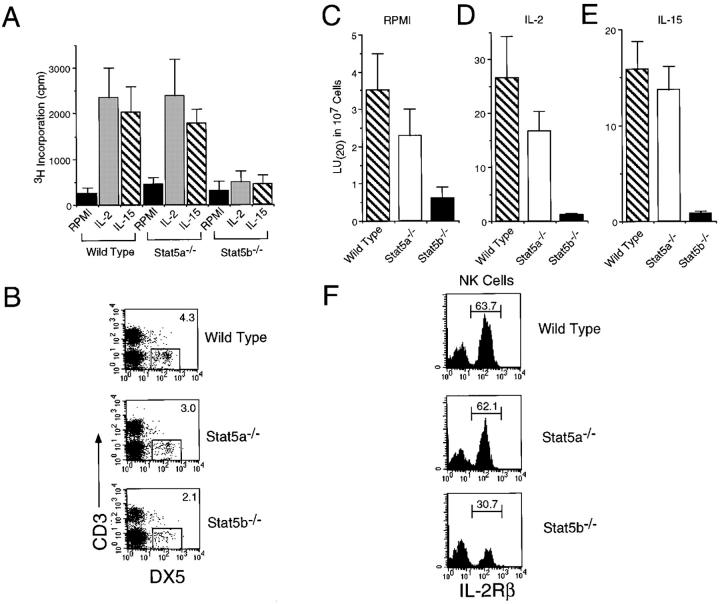
Considering the above partial defect in proliferation of preactivated T cells, we next tested the ability of freshly isolated splenocytes to proliferate in response to IL-2 and IL-15. Proliferation of Stat5a−/− splenocytes was at most slightly diminished, whereas IL-2– and IL-15–induced proliferation of Stat5b−/− splenocytes was greatly diminished (Fig. 3 A). We speculated that this defect in proliferation might result from a decrease in the number and/or function of NK cells, given that NK cells represent a prominent IL-2–responsive cell population in murine spleens and that IL-2−/− mice exhibit essentially normal T and B cell responses but compromised NK cell responses (32). Indeed, flow cytometric analysis revealed that the percentage of NK (CD3−DX5+) cells was uniformly diminished in Stat5b−/− mice (∼55% of the percentage seen in wild-type mice; Fig. 3 B). Interestingly, the percentage of NK cells in Stat5a−/− mice was also diminished, although to a lesser extent (∼70% of the percentage seen in wild-type mice). However, the much greater defect in proliferation in Stat5b−/− compared with Stat5a−/− mice indicated that the defect was not proportional to the number of NK cells but rather reflected a greater functional defect in Stat5b−/− mice.
Figure 3.
(A) Diminished proliferation of freshly isolated splenocytes from Stat5b−/− mice to 2 nM IL-2 or 7.75 nM IL-15. Shown are the mean ± SD from a compilation of two independent experiments. (B) Flow cytometric analysis of CD3−DX5+ NK cells in splenocytes from wild type, Stat5a−/−, and Stat5b−/− mice. (C–E) Decreased NK cytolytic activity in Stat5b−/− mice in the presence of no cytokine (C), IL-2 (D), or IL-15 (E). Shown are the mean ± SEM from combining two or three independent experiments, each of which had three mice of each genotype. (F) Defective IL-2Rβ expression in NK cells in Stat5b−/− mice, as compared with levels in wild type or Stat5a−/− mice. A total of 14 Stat5a−/− and 17 Stat5b−/− mice were examined.
Corresponding to the greater defect in proliferation in freshly isolated splenocytes from Stat5b−/− versus Stat5a−/− mice (Fig. 3 A), NK cytolytic activity was much more impaired in Stat5b−/− than in Stat5a−/− splenocytes (Fig. 3 C). The defect in Stat5b−/− mice was even more evident when either IL-2 (Fig. 3 D) or IL-15 (Fig. 3 E) was added during the NK assay (note the difference in the scale on the ordinate in Fig. 3, C–E). Thus, Stat5b−/− mice exhibited substantial defects in both proliferative and cytolytic activities.
Because of these IL-2 and IL-15 signaling defects, and because the IL-2 and IL-15 receptors both contain IL-2Rβ and γc as critical receptor components (33, 34), we evaluated expression of these proteins in NK cells. No defect in γc expression was observed in either Stat5a−/− or Stat5b−/− mice (data not shown). Interestingly, however, the number of NK cells expressing IL-2Rβ expression was uniformly decreased in all Stat5b−/− mice examined (n = 17; Fig. 3 F); in contrast, in Stat5a−/− NK cells, IL-2Rβ expression was typically normal, although 2 out of 14 mice examined had a significantly diminished percentage of IL-2Rβ–expressing NK cells. Overall, the decreased number of IL-2Rβ+ NK cells in Stat5b−/− mice generally correlated with the decreased proliferative response to IL-2 and IL-15 (Fig. 3 A) as well as the marked decrease in IL-2– and IL-15–mediated boosting of NK cytolytic activity (Fig. 3, D and E). Again, however, the defects in IL-2– and IL-15–induced proliferation and cytolytic activity were more severe than would have been predicted based solely on the decreased number of CD3−DX5+IL-2Rβ+ cells, suggesting that Stat5b plays an additional important and specific role(s) in IL-2– and IL-15–induced signaling in NK cells.
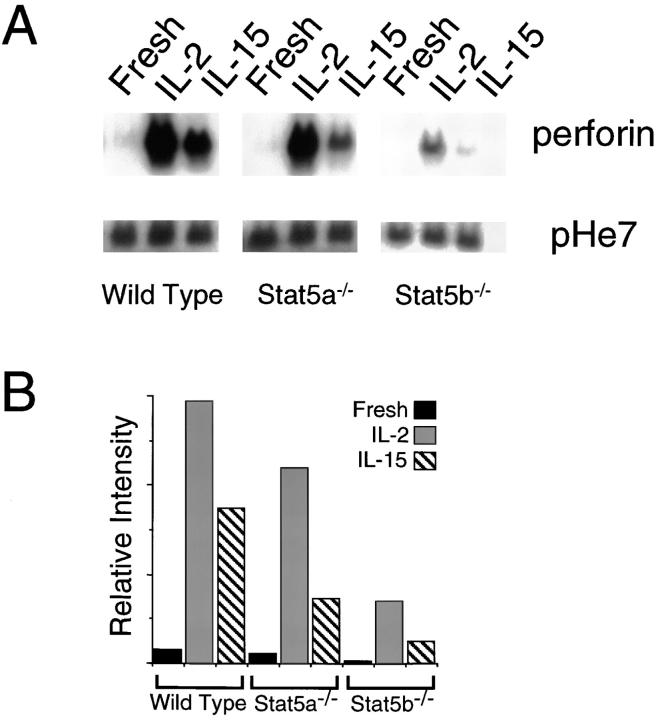
To further investigate the basis for defective NK function, we examined β2-integrin (CD18) and perforin expression. CD18 is known to be important for recognition of target cells by NK cells (35), and perforin is known to be one of the key molecules for NK cell–mediated cytolysis (36). Interestingly, although cell surface CD18 expression was not diminished (data not shown), induction of perforin mRNA by IL-2 and IL-15 was markedly decreased in Stat5b−/− splenocytes (Fig. 4).
Figure 4.
(A) Northern blotting for perforin or control pHe7 using RNA from fresh splenocytes or from splenocytes treated with IL-2 or IL-15 for 90 h. (B) For each condition, the relative level of perforin mRNA expression in A was normalized for pHe7 expression.
The mechanism of NK cell development has been an area of active investigation. NK cells do not develop in several different knockout mice, including those lacking IL-2Rβ (37), γc (38, 39), or Jak3 (40–42), which are receptor or signaling components for both IL-2 and IL-15. Interestingly, although IL-2 potently augments NK cytolytic activity, the fact that IL-2−/− mice have NK cells indicates that IL-2 is not absolutely required for NK cell development. Instead, IL-15 has been implicated as playing an important role in NK cell development (43–46). Supporting this concept, the absence of NK cells in IRF1−/− mice has been correlated with defective IL-15 production (47). Our data now reveal a partial decrease in NK cells in both Stat5a−/− and Stat5b−/− mice, indicating that Stat5 activation is vital for normal NK cell development.
Interestingly, although diminished NK cells were seen in both Stat5a−/− and Stat5b−/− mice, it was only the NK cells from Stat5b−/− mice that exhibited a dramatic defect in their ability to respond to IL-2 and IL-15 (Fig. 3, D and E), and only Stat5b−/− mice that had a lower percentage of NK cells expressing IL-2Rβ (Fig. 3 F). The basis for this decrease in IL-2Rβ+ NK cells remains unclear; although a number of transcription factors including early growth response (Egr)-1, Ets-1, and GABP have been shown to be important for IL-2Rβ promoter activity (48, 49), a Stat5b-dependent response element for this gene has not been reported. The decrease in perforin mRNA in IL-2– and IL-15–treated Stat5b−/− splenocytes is consistent with perforin being a Stat5b-regulated gene.
In conclusion, just as Stat4 and Stat6, respectively, are important for normal Th1 and Th2 cell development and function (6–10), our results reveal that both Stat5a and Stat5b contribute to normal NK cell development with a greater defect being seen particularly for NK function in Stat5b−/− mice. Although Stat5a and Stat5b have similar (but not identical) binding activities in vitro and can both drive expression of similar reporter constructs in transfected cells, the data presented here are consistent with published studies suggesting differential roles for these proteins in nonlymphoid settings in vivo (20–22). It will be vital to further clarify the range of target genes that are controlled by Stat5a and Stat5b. We have shown that IL-2Rα is defective in the absence of either Stat5 protein, whereas IL-2Rβ and perforin appear more dependent on Stat5b. Moreover, by demonstrating that Stat5b plays a particularly critical role for proliferation and cytolytic activity of NK cells, we now provide a key example of different biological roles for these two Stat5 proteins within the immune system.
Acknowledgments
We thank Drs. Lothar Hennighausen, Xiuwen Liu, and Anthony Wynshaw-Boris (National Institutes of Health [NIH]) for Stat5a−/− mice, and Dr. Alex Grinberg (NIH) for thawing and implanting Stat5b−/− frozen embryos. We thank Dr. Thomas R. Malek (University of Miami, Miami, FL) for the murine IL-2Rβ cDNA; Drs. Grace Ju and John Hakimi (Hoffmann-La Roche) for recombinant IL-2; Dr. James E. Darnell, Jr. (The Rockefeller University, New York, NY) for pHe7 cDNA; Dr. William E. Paul (NIH) for murine IL-4; and Drs. Dana P. Ascherman and Robert D. Schreiber for valuable discussions/critical comments.
Abbreviation used in this paper
STAT
signal transducers and activators of transcription
Footnotes
K. Imada was supported in part by a Japanese Society for the Promotion of Science research fellowship for Japanese biomedical and behavioral researchers at NIH and by the Sankyo Foundation of Life Science.
References
- 1.Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 2.Horvath CM, Darnell JE., Jr The state of STATs: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–239. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 3.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 4.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 5.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 6.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 9.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO (Eur Mol Biol Organ) J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mui AL, Wakao H, O'Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologues. EMBO (Eur Mol Biol Organ) J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azam M, Erdjument-Bromage H, Kreider BL, Xia M, Quelle F, Basu R, Saris C, Tempst P, Ihle JN, Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO (Eur Mol Biol Organ) J. 1995;14:1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA. 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J-X, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–10744. [PubMed] [Google Scholar]
- 18.Copeland NG, Gilbert DJ, Schindler C, Zhong Z, Wen Z, Darnell JE, Jr, Mui AL, Miyajima A, Quelle FW, Ihle JN, Jenkins NA. Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics. 1995;29:225–228. doi: 10.1006/geno.1995.1235. [DOI] [PubMed] [Google Scholar]
- 19.Moriggl R, Gouilleux-Gruart V, Jahne R, Berchtold S, Gartmann C, Liu X, Hennighausen L, Sotiropoulos A, Groner B, Gouilleux F. Deletion of the carboxyl-terminal transactivation domain of MGF-Stat5 results in sustained DNA binding and a dominant negative phenotype. Mol Cell Biol. 1996;16:5691–5700. doi: 10.1128/mcb.16.10.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Robinson GW, Wagner K-U, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Gen Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 21.Udy, G.B., R.P. Towers, R.G. Snell, R.J. Wilkins, S.-H. Park, P.A. Ram, D.J. Waxman, and H.W. Davey. 1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. USA. 94:7239–7244**.** [DOI] [PMC free article] [PubMed]
- 22.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 23.Feldman GM, Rosenthal LA, Liu X, Hayes MP, Wynshaw-Boris A, Leonard WJ, Hennighausen L, Finbloom DS. STAT5A-deficient mice demonstrate a defect in granulocyte-macrophage colony-stimulating factor- induced proliferation and gene expression. Blood. 1997;90:1768–1776. [PubMed] [Google Scholar]
- 24.Nakajima H, Liu XW, Wynshaw-Boris A, Rosenthal LA, Imada K, Finbloom DS, Hennighausen L, Leonard WJ. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor α chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 25.Kaczmarek L, Calabretta B, Kao H-T, Heintz N, Nevins J, Baserga R. Control of hsp70 RNA levels in human lymphocytes. J Cell Biol. 1987;104:183–187. doi: 10.1083/jcb.104.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinkai Y, Takio K, Okumura K. Homology of perforin to the ninth component of complement (C9) Nature. 1988;334:525–527. doi: 10.1038/334525a0. [DOI] [PubMed] [Google Scholar]
- 27.Bloom ET, Korn EL. Quantification of natural cytotoxicity by humans lymphocyte subpopulations isolated by density: heterogeneity of the effect cells. J Immunol Methods. 1983;58:323–335. doi: 10.1016/0022-1759(83)90360-5. [DOI] [PubMed] [Google Scholar]
- 28.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 29.Sperisen P, Wang SM, Soldaini E, Pla M, Rusterholz C, Bucher P, Corthesy P, Reichenbach P, Nabholz M. Mouse interleukin-2 receptor α gene expression: interleukin-1 and interleukin-2 control transcription via distinct cis-acting elements. J Biol Chem. 1995;270:10743–10753. doi: 10.1074/jbc.270.18.10743. [DOI] [PubMed] [Google Scholar]
- 30.John S, Robbins CM, Leonard WJ. An IL-2 response element in the human IL-2 receptor α chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO (Eur Mol Biol Organ) J. 1996;15:5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 31.Lecine P, Algarte M, Rameil P, Beadling C, Bucher P, Nabholz M, Imbert J. Elf-1 and Stat5 bind to a critical element in a new enhancer of the human interleukin-2 receptor α gene. Mol Cell Biol. 1996;16:6829–6840. doi: 10.1128/mcb.16.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 33.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur Mol Biol Organ) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. The interleukin (IL)-2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T cell proliferation and the induction of lymphokine activated killer cells. Proc Natl Acad Sci USA. 1994;92:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helander TS, Carpén O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 36.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin-2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 40.Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 41.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 42.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 43.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavazzana-Calvo M, Hacein-Bey S, de Saint G, Basile, de Coene C, Selz F, Le Deist F, Fischer A. Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from γc transduced severe combined immunodeficiency X1 bone marrow cells. Blood. 1996;88:3901–3909. [PubMed] [Google Scholar]
- 45.Leclercq G, Debacker V, de Smedt M, Plum J. Differential effects of interleukin-15 and interleukin-2 on the differentiation of bipotential T/natural killer progenitor cells. J Exp Med. 1996;184:325–336. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 47.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 48.Lin J-X, Bhat N, John S, Queale WS, Leonard WJ. Characterization of the human interleukin-2 receptor β chain gene promoter: regulation of promoter activity by etsgene products. Mol Cell Biol. 1993;13:6201–6210. doi: 10.1128/mcb.13.10.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin JX, Leonard WJ. The immediate-early gene product Egr-1 regulates the human interleukin-2 receptor β-chain promoter through non-canonical Egr and Sp1 binding sites. Mol Cell Biol. 1997;17:3714–3722. doi: 10.1128/mcb.17.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]