Expression of Interleukin 9 in the Lungs of Transgenic Mice Causes Airway Inflammation, Mast Cell Hyperplasia, and Bronchial Hyperresponsiveness (original) (raw)
Abstract
Interleukin (IL)-9, a pleiotropic cytokine produced by the Th2 subset of T lymphocytes has been proposed as product of a candidate gene responsible for asthma. Its wide range of biological functions on many cell types involved in the allergic immune response suggests a potentially important role in the complex pathogenesis of asthma. To investigate the contributions of IL-9 to airway inflammation and airway hyperresponsiveness in vivo, we created transgenic mice in which expression of the murine IL-9 cDNA was regulated by the rat Clara cell 10 protein promoter. Lung selective expression of IL-9 caused massive airway inflammation with eosinophils and lymphocytes as predominant infiltrating cell types. A striking finding was the presence of increased numbers of mast cells within the airway epithelium of IL-9–expressing mice. Other impressive pathologic changes in the airways were epithelial cell hypertrophy associated with accumulation of mucus-like material within nonciliated cells and increased subepithelial deposition of collagen. Physiologic evaluation of IL-9–expressing mice demonstrated normal baseline airway resistance and markedly increased airway hyperresponsiveness to inhaled methacholine. These findings strongly support an important role for IL-9 in the pathogenesis of asthma.
Keywords: interleukin 9, transgenic mice, asthma, mast cells, eosinophilia
topic asthma is a chronic inflammatory disorder of the airways associated with reversible airway obstruction and bronchial hyperresponsiveness (1). The response to antigen in the airways involves various types of inflammatory cells, including eosinophils, B cells, mast cells, and activated T cells, in particular CD4+ T cells of the Th2 subset (2, 3). Although the precise mechanisms of this response are still unknown, there is evidence that complex interaction between these cells leads to the production of cytokines and other inflammatory proteins with important impact in the pathogenesis of asthma (4, 5).
Genetic susceptibility to atopic asthma is thought to be multigenic (6, 7). Although the gene(s) predisposing to asthma, atopy, and bronchial hyperresponsiveness have not yet been identified, various genetic studies in humans have linked these characteristics to chromosome 5q31-q33 (8– 10). This chromosomal region contains several genes that are implicated in bronchial inflammation associated with asthma (11–13). Recently, one of them, IL-9, has been suggested as a candidate gene for asthma (10, 14).
IL-9 is a T cell–derived cytokine with pleiotropic effects on various cell types (15). IL-9 seems to be produced in vitro and in vivo by CD4+ T cells, preferentially of the Th2 subset (16–22). The in vitro biological functions of IL-9 include its ability to stimulate proliferation of activated T cells (23–25), to enhance production of immunoglobulin in B cells (26, 27), and to promote proliferation and differentiation of mast cells (28, 29) and hematopoietic progenitors (30, 31). The involvement of IL-9 in lymphomagenesis has been suggested by in vivo studies with transgenic mice expressing IL-9 constitutively in which a higher susceptibility to develop thymic lymphoma was observed (32).
The pleiotropic functions of IL-9 suggest a major role for this cytokine in the complex immune response associated with allergic forms of asthma. To study in vivo potential contributions of IL-9 to the pathogenesis of asthma, we created transgenic mice that overexpress IL-9 selectively within the lungs. We show that expression of IL-9 in the airway epithelium of transgenic mice results in airway inflammation, mast cell hyperplasia, and dramatically increased airway hyperresponsiveness.
Materials and Methods
Production of Transgenic Mice.
The 443-bp murine IL-9 cDNA (Genetics Institute, Cambridge, MA) was cloned as a XhoI– EcoRI fragment into the NdeI–BglII sites of plasmid pCC10-SV40 (33) using SalI–NdeI and EcoRI–BglII oligonucleotide adapters. The resulting plasmid pCC10-IL9-SV40 was purified from large scale cultures using the Plasmid Maxi Kit (QIAGEN, Inc., Chatsworth, CA). After digestion of the plasmid with XhoI and SacII, the CC10-IL9-SV40 transgene was separated by electrophoresis through a 1% agarose gel (SeaKem GTG BioProducts, FMC; Rockland, ME), isolated by electroelution, and then purified as previously described (33) before injection into (C3H × C57BL/6) F2 eggs (34). Positive founder animals were identified by Southern blot analysis of tail DNA using a 32P-labeled full-length fragment of the murine IL-9 cDNA. Founder animals were backcrossed onto strain B10.D2 (The Jackson Laboratory, Bar Harbor, ME) and transgene-positive animals from subsequent generations were identified by PCR analysis of tail DNA. PCR was performed using the following primers to identify the IL-9 construct: 5′ CAT CCT TGC CTC TGT TTT GC 3′ (sense); and 5′ CGT CCC CAG GAG ACT CTT C 3′ (antisense). Amplification of the inserted IL-9 cDNA was performed by 25 cycles at 95°C, 60°C, 72°C for 1 min each to yield a IL-9 DNA product of 379 bp that was detected by agarose gel electrophoresis. Transgene-positive animals (heterozygous for the transgene) and their transgene-negative littermates were analyzed between 6 and 12 wk of age.
Lung Lavage, Tissue Fixation, and Staining.
Mice were anesthetized by methoxyflurane inhalation and killed by exsanguination or carbon dioxide inhalation. Blood was allowed to clot at room temperature for 30 min and serum was recovered by centrifugation (10,000 rpm, 5 min at 4°C), frozen on dry ice, and stored at −70°C until use. Lung lavage was performed by inserting a cannula into the trachea and lavaging with three successive aliquots of 1 ml PBS as previously described (33). Aliquots of lavage fluid were centrifuged and the supernatants were harvested and stored individually at −70°C until use. All measurements were made on the first aliquot of lavage fluid (85–90% of the 1-ml input volume was retrieved). Lavage cells were resuspended in PBS and counted using a hemocytometer. Differential cell counts were performed on cytospin cell preparations stained with Diff-Quik (VWR Scientific Products, Bridgeport, NJ). Lungs were then excised completely from the chest, inflated with 10% formalin, and immersed in 10% formalin. Paraffin embedding, various histological stainings, and immunostaining for α-smooth muscle actin of lung sections were performed by the Yale Medical School Research Pathology or Dermatopathology Laboratories.
Cytokine Assays.
Quantitation of IL-4, IL-5, or IL-9 in lavage fluid or serum was performed by ELISA using monoclonal antibodies (PharMingen, San Diego, CA) according to the manufacturer's recommendation. Assays were standardized with recombinant murine IL-4 (DNAX, Palo Alto, CA), IL-5 (PharMingen), or IL-9 (PharMingen). The minimum detectable level of cytokine in each of the ELISAs was 16 pg/ml (IL-4), 40 pg/ml (IL-5), and 0.31 ng/ml (IL-9) in lung lavage fluid and 0.62 ng/ml (IL-9) in serum. For the detection of IL-9 in serum, recombinant murine IL-9 diluted in mouse serum was used as a positive control.
Transmission Electron Microscopy.
Lung tissue was prepared for electron microscopic analysis as previously described (35). In brief, lung tissue was fixed in 3% glutaraldehyde for a minimum of 1 h, postfixed in 1% osmium, dehydrated, and embedded in Epox (Ernest F. Fullam, Inc., Latham, NY). 1-μm-thick sections were stained with toluidine blue and screened by light microscopy for representative areas containing airways. These areas were then selected for electron microscopy. Thin sections (80 nm) were stained with uranyl acetate and lead citrate, examined, and photographed with a Philips 300 electron microscope (Eindhoven, The Netherlands).
Histamine Assay.
Histamine levels in lavage fluid were determined using a Histamine-Enzyme Immunoassay Kit (Immunotech, Westbrook, ME) according to the manufacturer's instructions. The minimum detectable level of histamine was 0.2 nM for this assay.
Immunohistochemistry.
Lungs, completely excised from the chest, were inflated with a 1:3 dilution of O.C.T. Tissue-Tek compound (Miles Labs., Inc., Elkhart, IN) in PBS and frozen in O.C.T. by submersion in 2-methylbutane (Aldrich Chemical Co., Milwaukee, WI) cooled with dry ice. Tissue sections were cut, transferred onto silane-treated glass slides, and allowed to dry at room temperature for 1 h. Sections were fixed in acetone for 10 min and then stored at −20°C. For staining, sections were rehydrated in wash buffer (0.1 M Tris-Cl, pH 7.4, and 0.01% Triton X-100) for 10 min. Sections were blocked with blocking buffer (3% BSA, 0.1 M Tris-Cl, pH 7.4, and 0.01% Triton X-100) for 30 min and with Avidin/Biotin Blocking Kit (Vector Labs., Burlingame, CA) for 15 min each before incubation with diluted biotinylated antibody for 1 h. The sections were then washed three times for 5 min in wash buffer and incubated with prediluted streptavidin-alkaline phosphatase solution (Kirkegaard and Perry Labs., Inc., Gaithersburg, MD) for 40 min. The sections were washed and developed using HistoMark Red Staining System (Kirkegaard and Perry Labs., Inc.) according to the manufacturer's instructions. The slides were counterstained with Meyer's hematoxylin and then mounted using Permount (Fisher Scientific Co., Fairlawn, NJ).
For staining of lung sections, monoclonal antibodies, biotinylated anti–mouse CD4 and CD8a (PharMingen), biotin-conjugated anti–mouse B220 (Caltag Labs., San Francisco, CA), and biotin-conjugated anti–mouse Mac-1 (Serotec, Inc., Raleigh, NC) were used at appropriate dilutions in 1% BSA/wash buffer.
Flow Cytometric Analysis.
To detect cytokine production at a single cell level, intracellular staining for IL-4 and INF-γ was performed according to a modified protocol described elsewhere (36). In brief, cells retrieved by lung lavage as described above were washed and cultured at a concentration of 5 × 106 in the presence of 0.05 μg/ml PMA (Sigma Chemical Co., St. Louis, MO) and 0.5 μg/ml ionomycin (Calbiochem Corp., La Jolla, CA) for 6 h. After 2 h of incubation, monensin (PharMingen) was added to a final concentration of 2 μM. Cells were washed once in staining buffer (1× PBS, 1% FBS, and 0.1% sodium azide, pH 7.4) and incubated with monoclonal antibodies for the detection of cell surface markers diluted in staining buffer containing Fc-Block (PharMingen) for 30 min at 4°C. Cells were washed once in staining buffer and then fixed in PBS containing 4% paraformaldehyde for 30 min at 4°C. Cells were washed twice in staining buffer and incubated with monoclonal antibodies for the detection of intracellular cytokines diluted in permeabilization buffer (0.1% saponin/staining buffer) containing Fc-Block for 30 min at 4°C. Cells were washed twice in staining buffer and kept at 4°C in the dark until analysis by FACScan® using CellQuest (Becton Dickinson, San Jose, CA). Murine Th1 and Th2 cell clones, producing either INF-γ or IL-4 (37), were used as a positive control for intracellular cytokine production. Negative controls consisted of isotype-matched, directly conjugated, nonspecific antibodies (PharMingen).
For the detection of cell surface markers, Cy-Chrome–conjugated monoclonal antibodies (anti–mouse CD4, anti–mouse CD8a; PharMingen) were used, and for intracellular cytokine staining, FITC-conjugated anti–mouse INF-γ and PE-conjugated anti–mouse IL4 (PharMingen) were used.
Physiologic Assessment.
Mice were assessed physiologically by an investigator blinded to the genotype of the mice, using a method for the determination of airway baseline resistance and hyperresponsiveness to inhaled methacholine described elsewhere (34). Mice were anesthetized by intraperitoneal injection of pentobarbital (90 mg/kg) and tracheostomized with an 18-gauge angiocatheter (Baxter Scientific, McGraw Park, IL). Airway resistance was then measured using the modifications of the techniques of Martin et al. (38). In these assessments, changes in the lung volume of anesthetized and tracheostomized mice were measured plethysmographically by determining pressure in a plexiglass chamber using an in-line microswitch pressure transducer. Flow was measured by differentiation of the volume signal, and transpulmonary pressure was determined by a second microswitch pressure transducer placed in line with the plethysmograph and an animal ventilator. Resistance was then calculated using the method of Amdur and Mead (39). Resistance of the tracheostomy catheter was eliminated and baseline measurements of pulmonary resistance were obtained by ventilating the mouse in the plethysmograph at volumes of 0.4 ml at a rate of 150 breaths/min, which has been shown previously to produce normal arterial blood gases (38). Each mouse was studied at baseline. Bronchial hyperresponsiveness was then determined by methacholine challenge as previously described (34). Increasing concentrations of methacholine in PBS were administered by nebulization (20 1-ml breaths), and pulmonary resistance was calculated precisely 60 s later. Stepwise increases in methacholine dose were then given until the pulmonary resistance had at least doubled in comparison with the baseline level. The data are expressed as the provocative challenge 100 (PC100), the dose of methacholine at which pulmonary resistance was 100% above baseline level.
Statistical Analysis.
Values are expressed as means ± SD. The data were normally distributed, and group means were compared with Student's two tailed, unpaired t test using Excel 5 for Apple Macintosh (Microsoft Corporation, Redmond, WA). P < 0.05 was considered significant.
Results
Generation of Transgenic Mice.
We constructed several independent lines of transgenic mice in which the expression of the murine IL-9 cDNA was under the control of the rat Clara cell protein, CC10, promoter. Previous studies in mice had demonstrated by chloramphenicol acetyltransferase expression assay (40), RNase protection assay (33), and Northern blot analysis (41) that expression regulated by the CC10 promoter is selective to lung tissue. Of 29 original progeny screened by Southern blot analysis and confirmed by PCR analysis of tail DNA, 9 animals (31%) were positive for the CC10 transgene. All founder animals were backcrossed with B10.D2 mice. Based upon the range of IL-9 expression levels, four independent lines, 9, 16, 20, and 25, were chosen for a more detailed analysis.
Assessment of IL-9 Levels within Lung and Serum.
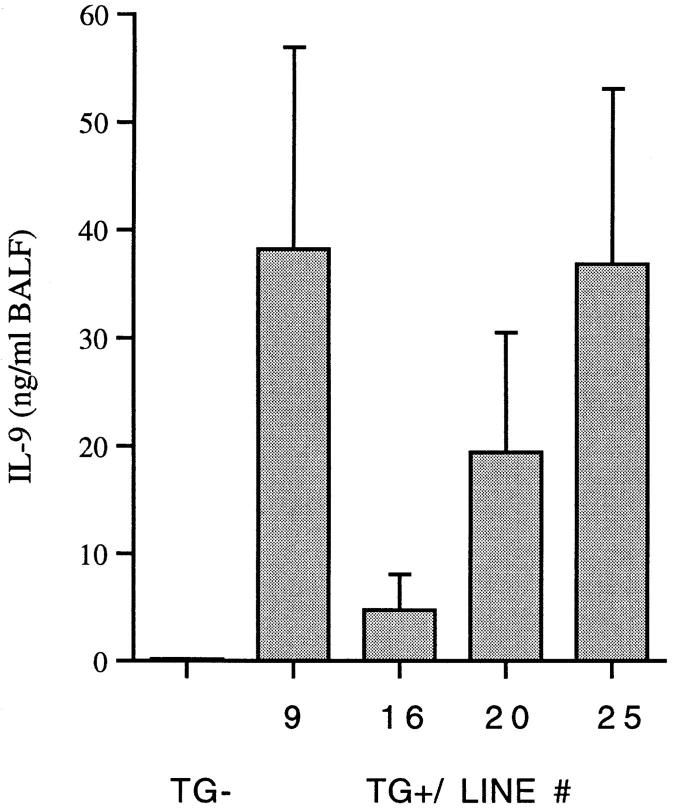
The expression of IL-9 in the lung was assessed by mIL-9 levels in lung lavage fluid by ELISA (Fig. 1). Lower levels of IL-9 were detected in lavage fluid from transgene-positive mice of lines 16 (4.7 ± 3.3 ng/ml) and 20 (19.4 ± 11.1 ng/ml), and higher levels in transgene-positive mice of lines 9 (38.2 ± 18.7 ng/ml) and 25 (36.8 ± 16.3 ng/ml). The levels of IL-9 were below the detection limit (<0.31 ng/ml) in lung lavage fluid from transgene-negative mice of all four independent lines. IL-9 was not detectable in serum of transgene-positive or -negative animals (<0.62 ng/ml). Lung lavage fluid from mice expressing IL-9 did not contain detectable levels of IL-4 (<16 pg/ml) or IL-5 (<40 pg/ml).
Figure 1.
IL-9 levels in bronchoalveolar lavage fluid (BALF ). Transgene-positive animals (TG+) from four independent lines (9, 16, 20, and 25) expressed different amounts of IL-9. Each column represents the mean ± SD of three representative animals. No IL-9 was detectable in lung lavage fluid from transgene-negative animals (_TG_−).
Cellular Constituents of Lung Lavage Fluid.
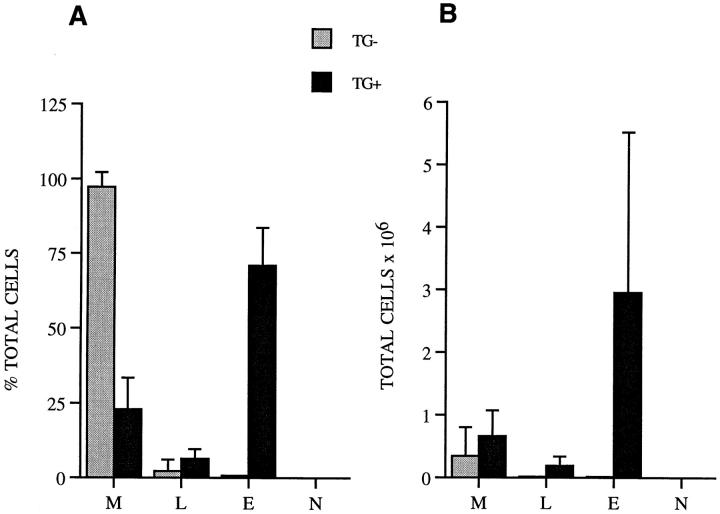
Analysis of lung lavage fluid from IL-9–expressing mice revealed the presence of inflammatory cells. The total number of cells retrieved was significantly increased in mice that expressed IL-9 (3.79 ± 2.98 × 106, n = 13), compared with the number in transgene-negative mice that did not express IL-9 (0.35 ± 0.46 × 106, n = 4; P < 0.04). Differential counts on lung lavage cells revealed a great increase in the number of eosinophils along with substantial accumulations of lymphocytes (Fig. 2, A and B). In contrast, in transgene-negative animals the majority of cells were macrophages (Fig. 2, A and B). Neutrophils were not identified in lung lavage fluid from transgene-positive and -negative animals (Fig. 2, A and B, respectively).
Figure 2.
Inflammatory cells in lung lavage fluid. Differential cell counts were derived from at least 500 cell counts and are presented as percentage of each cell type from total cells (A) and as total cell numbers (B). Data are the mean ± SD of counts from 13 IL-9–expressing mice (black bars) and four transgene-negative control mice (gray bars) from all four transgenic lines. The number of eosinophils (E) and lymphocytes (L) were significantly increased in IL-9–expressing mice compared with transgene-negative mice (P ≤ 0.04 and P < 0.03; B), whereas the number of macrophages (M) was only slightly but not significantly increased (B). N, neutrophils.
Histologic Assessment of Lung Tissue.
Examination of lung tissue stained with hematoxylin and eosin by light microscopy revealed the same impressive histologic changes in the lungs of all IL-9–expressing mice from four independent lines (Fig. 3, B–D), which were not observed in lung tissue from transgene-negative control mice (Fig. 3 A).
Figure 3.
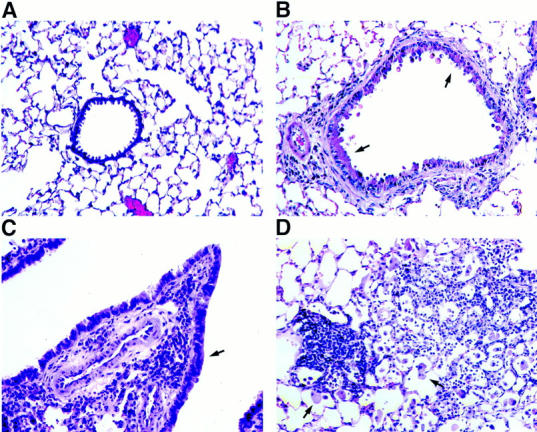
Lung histology of conducting airways and parenchyma. Sections of formalin-fixed lung tissue from a transgene-negative control mouse (A) and an IL-9–expressing mouse of line 9 (B–D) were stained with hematoxylin and eosin before examination by light microscopy. Lung sections from IL-9–expressing mice revealed the presence of inflammatory cells in the subepithelium of conducting airways of all different sizes, smaller airways (B) and larger airways (C), and around blood vessels, which was not seen in sections from transgene-negative mice (A). The airway epithelium was hypertrophic (B and C, arrows) compared with that in transgene-negative animals (A). Cellular infiltrations in the lung parenchyma from IL-9–expressing mice were often associated with nodule-like accumulations of mononuclear cells and large macrophages (D, arrows). Original magnification, A–D: ×250.
Infiltration of lung tissue with mononucleated as well as multinucleated inflammatory cells occurred around airways and blood vessels of different sizes (Fig. 3, B and C). A higher cellularity was also noted within the lung parenchyma with the appearance of large macrophages and accumulations of mononuclear cells in areas of high infiltration (Fig. 3 D) Histologic analysis of the airways from IL-9– expressing mice revealed that the epithelium from most conducting airways was hypertrophic (Fig. 3, B and C). Many epithelial cells seemed to be enlarged due to the accumulation of material that appeared homogenous within the cytoplasm that stained positively for mucin with alcian blue/periodic acid–Schiff (Fig. 4 B). Higher magnification of the stained epithelium showed that accumulation of mucin-like material appeared in nonciliated but not ciliated epithelial cells (Fig. 4 C). In contrast, airway epithelia were not hypertrophic and did not stain positively for mucin with alcian blue/periodic acid–Schiff in lung sections from transgene-negative mice (Fig. 4 A).
Figure 4.
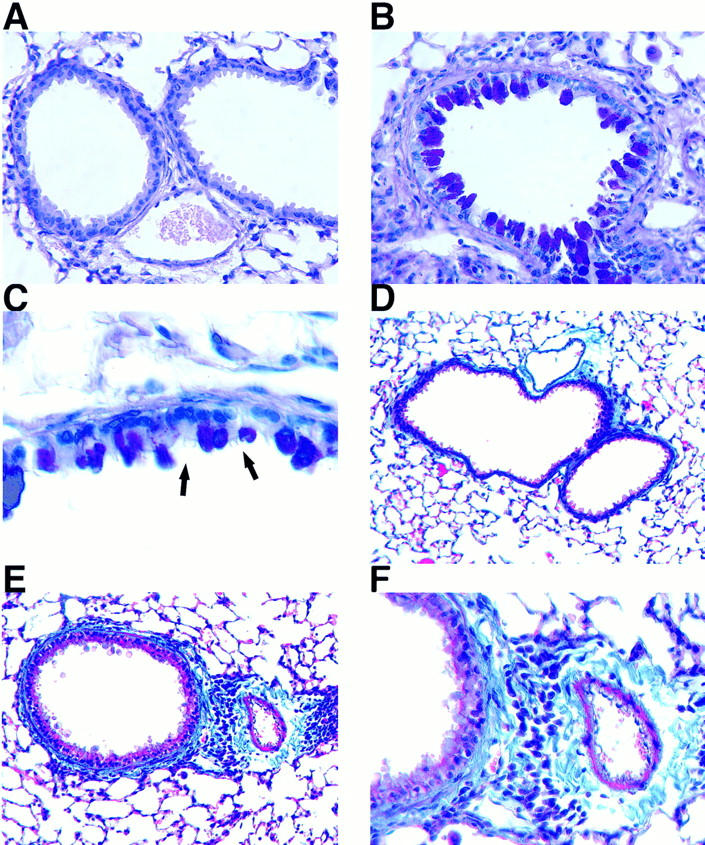
Histologic staining for mucin and collagen in lung sections. Light micrographs of formalin-fixed tissue from a transgene-negative control mouse (A and D) and an IL-9–expressing mouse (B, C, E, and F ) stained with alcian blue/periodic acid-Schiff (A–C) or Masson's trichrome (D–F ). The hypertrophic airway epithelium in sections from IL-9–expressing mice stained positively (magenta) for mucins with alcian blue/periodic acid–Schiff (B). Higher magnification of the epithelium revealed that only nonciliated epithelial cells but not ciliated cells (arrows) were hypertrophic and stained positive for mucin (C). The airway epithelium in sections from transgene–negative mice did not stain for mucin (A). Lung sections stained with Masson's trichrome demonstrated that thickening of the airway wall in IL-9–expressing mice is associated with increased deposition of material staining positively (blue) for collagen (E), which was not present in transgene-negative mice (D). A higher magnification of the same area demonstrates extracellular, intensely blue staining collagen adjacent to inflammatory cells around airway and blood vessel (F ). Original magnifications: A, B, and F, ×500; C, ×1,000; D and E, ×250.
Masson's trichrome staining of lung tissue demonstrated that infiltration around the airways in IL-9–expressing mice was often accompanied by an increased deposition of collagen in the subepithelial region (Fig. 4, E and F ), which was not found in airways of transgene-negative animals (Fig. 4 D).
Blood vessels of all different sizes in lungs from IL-9– expressing mice showed thickening of their walls due to mild medial hypertrophy as demonstrated in van Gieson–stained sections (Fig. 5 B). This was not observed in lung sections of transgene-negative animals (Fig. 5 A). Immunostaining of lung sections from IL-9–expressing mice for α-smooth muscle actin demonstrated that hypertrophy was due at least in part to marked muscle cell proliferation in the media with migration into the intima (Fig. 5 D). Staining for smooth muscle actin was much less intensive in lung sections from transgene-negative mice (Fig. 5 C).
Figure 5.
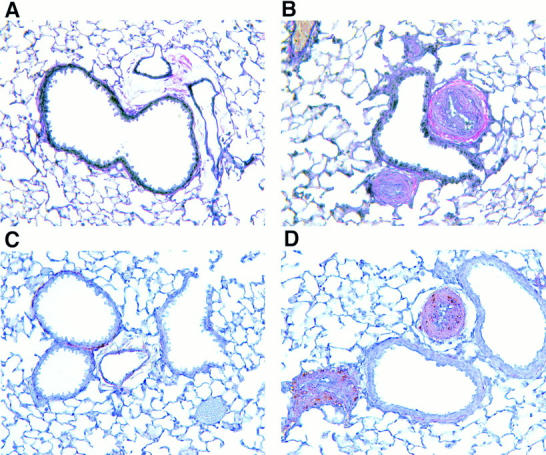
Histologic analysis of blood vessels. Blood vessels in van Gieson–stained sections from formalin-fixed lung tissue of an IL-9–expressing mouse (B) showed medial hypertrophy compared with vessels from a transgene-negative control mouse (A). Immunostaining for α-smooth muscle actin was strongly positive (brown) in wall and lumen of blood vessels found in sections from an IL-9–expressing mouse (D), which was not observed in blood vessels from transgene-negative mice (C). Original magnification, A–D, ×500.
One of the most striking histologic findings in the airways of IL-9–expressing mice was the presence of mast cells within the airway epithelium. Mast cells were detected in epithelial and subepithelial regions of all conducting airways in lung tissue stained with toluidine blue (Fig. 6, B and C) or using Leder's chloracetate esterase reaction (Fig. 6 D). As expected, mast cells were not present in airway epithelia from transgene-negative animals (Fig. 6 A).
Figure 6.
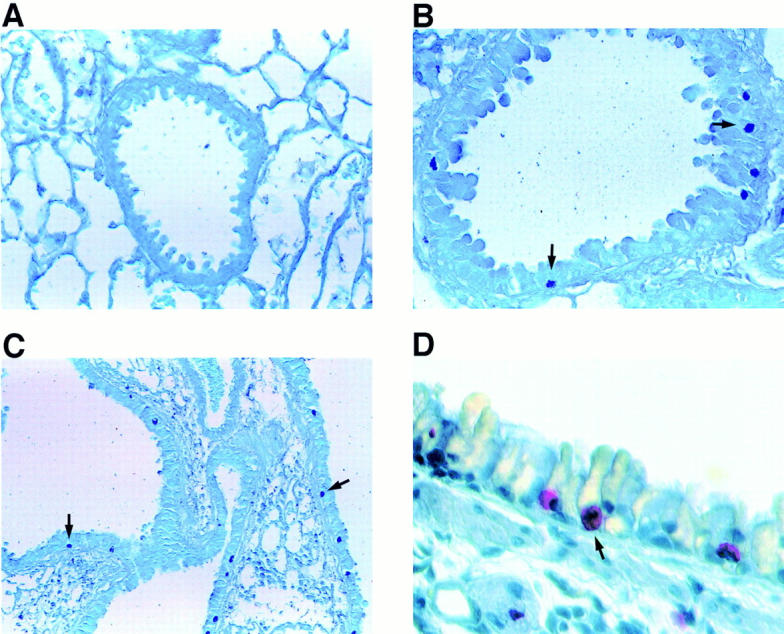
Histologic staining for mast cells. Toluidine blue staining of sections from formalin-fixed lung tissue revealed mast cells in the airway epithelium from an IL-9–expressing mouse (B and C) but not in the epithelium from transgene-negative mice (A). Dark blue–stained mast cell granules (arrows) were present in the epithelium of smaller (B) and larger (C) airways. Higher magnification shows red-stained mast cells (arrow) in the airway epithelium using Leder's chloracetate esterase reaction (D). Original magnifications: A and B, ×500; C, ×250; D, ×1,000.
Electron Microscopic Examination of Airway Epithelium.
Electron microscopic analysis of the epithelium of conducting airways of the lower respiratory tract from IL-9– expressing mice confirmed the presence of mast cells (Fig. 7 A) that appeared to be fully granulated (Fig. 7, A and B). Mast cells were not present in the airway epithelium from transgene-negative control mice (Fig. 7 C).
Figure 7.
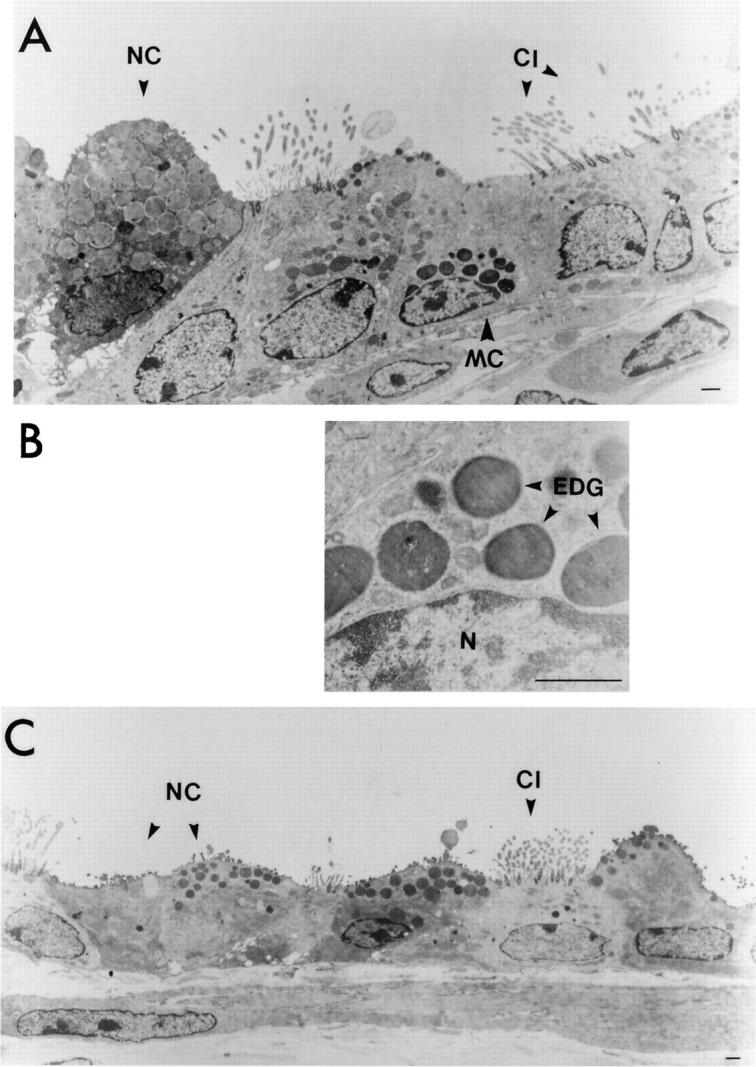
Electron micrographs of epithelium from conducting airways of the lower respiratory tract. Representative area of the airway epithelium from an IL-9–expressing mouse shows that fully granulated mast cells (MC) were present (A). A higher magnification of mast cell granules from the same cell as shown in A did not reveal any signs of degranulation (B). N, nucleus, EDG, electron-dense granules. Mast cells were not present in airway epithelium from transgene-negative control mice (C). Nonciliated epithelial cells (NC) in IL-9–expressing mice were hypertrophic and showed accumulations of low-density vacuoles, whereas ciliated cells (CI ) appeared normal (A). Epithelium from a transgene-negative mouse shows normal nonciliated (NC) and ciliated (CI ) epithelial cells (C). Bars in A, B, and C represent 1 μm. Original magnifications: A, ×5,000; B, ×22,400; C, ×3,300.
Electron micrographs of airway epithelium from IL-9– expressing mice also demonstrated that only nonciliated epithelial cells were hypertrophic due to massive accumulations of vacuoles containing a homogenous, low-density material (Fig. 7 A), confirming the results obtained from alcian blue/periodic acid–Schiff–stained epithelium (Fig. 4 C). These vacuoles were not observed in ciliated cells that were not hypertrophic (Fig. 7 A), nor in any cells of the airway epithelium from transgene-negative animals (Fig. 7 C).
Histamine Levels within Lung Lavage Fluid.
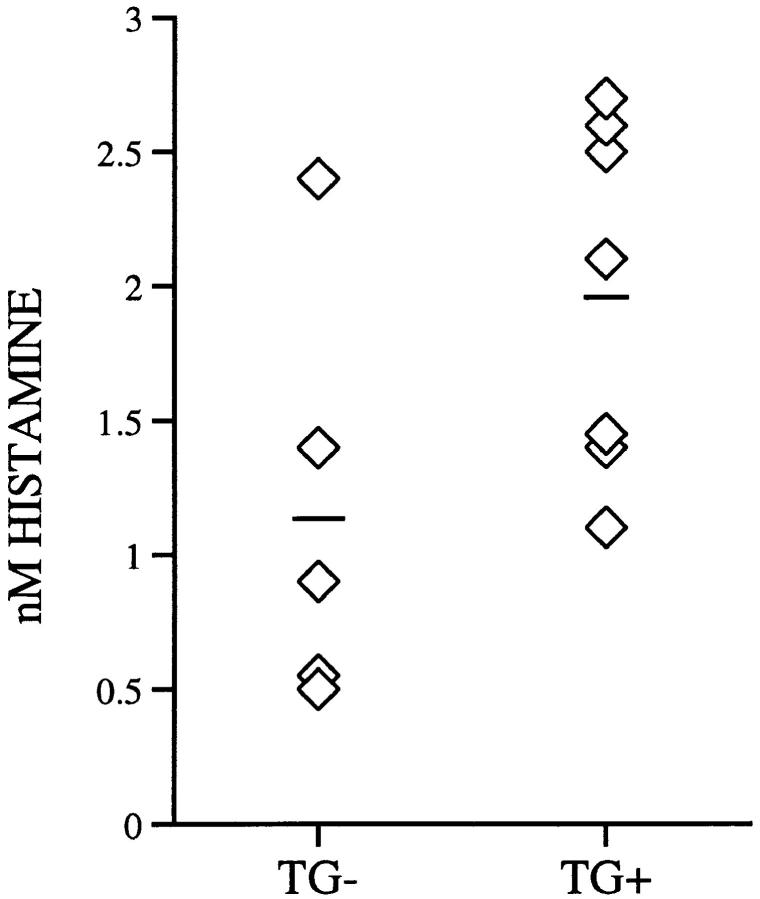
To assess the potential release of mediators from mast cells within the airway epithelium from IL-9–expressing mice, lung lavage fluid was analyzed for histamine. Low levels of histamine were detected in lung lavage fluid of both IL-9–expressing mice (1.98 ± 0.65 nM) and transgene–negative mice (1.14 ± 0.79 nM) of all four independent lines (Fig. 8). The slightly but not significantly increased histamine levels found in lavage fluid of IL-9–expressing mice might be explained by increased numbers of mast cells in the airway epithelia of these mice.
Figure 8.
Histamine levels in lung lavage fluid. IL-9–expressing mice (TG+) and transgene negative mice (_TG_−) had similar histamine levels in lung lavage fluid as determined by enzyme immunoassay. Levels are expressed as individual data points for each mouse (diamonds) and as means (lines).
Characterization of Infiltrating Cells by Histology and Flow Cytometry.
New vital red staining of lung sections from IL-9–expressing mice demonstrated that high numbers of eosinophils were among the infiltrating cells around airways and blood vessels (Fig. 9 A) and within the parenchyma. Mononuclear cells were identified and further characterized by immunostaining of frozen lung sections. Both CD4+ and CD8+ lymphocytes were detected among infiltrating cells (Fig. 9, B and C), whereas B220+ cells were less present and appeared in clusters (Fig. 9 D). Furthermore, a substantial number of infiltrating cells were probably macrophages that stained positive for Mac-1 (Fig. 9 E). No cellular infiltrations were detected by either new vital red staining or immunostaining in lung sections from transgene-negative animals.
Figure 9.
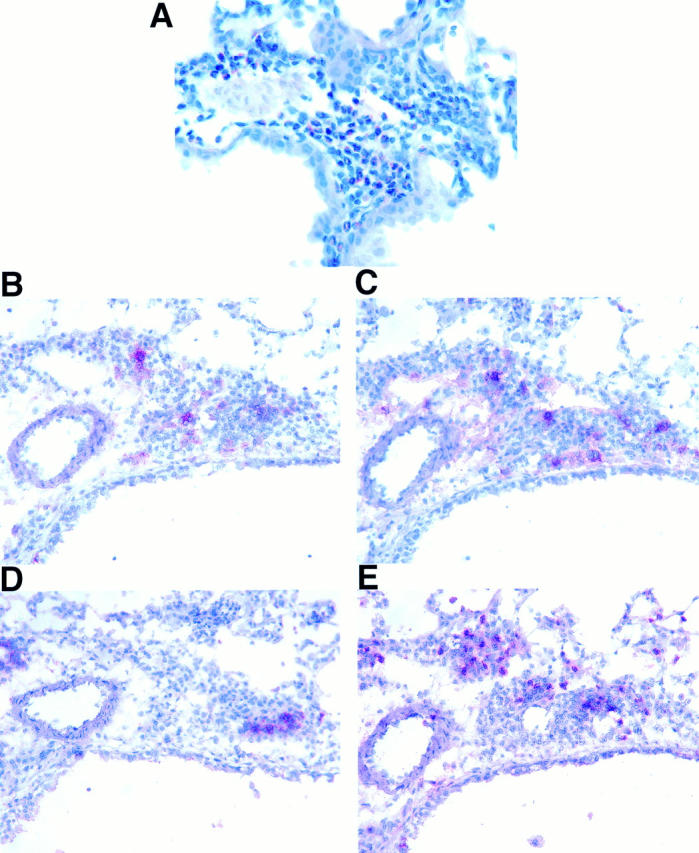
Characterization of inflammatory cells in lung sections from an IL-9–expressing mouse. Formalin-fixed tissue was stained with new vital red to demonstrate that eosinophils were present in high numbers in lung tissue from IL-9–expressing mice (A). Immunostaining of frozen lung sections (B–E) revealed that cell infiltrates also contained CD4+ (B), CD8+ (C), B220+ (D), and Mac-1+ (E) mononuclear cells. Original magnifications: A, ×600; B–E, ×500.
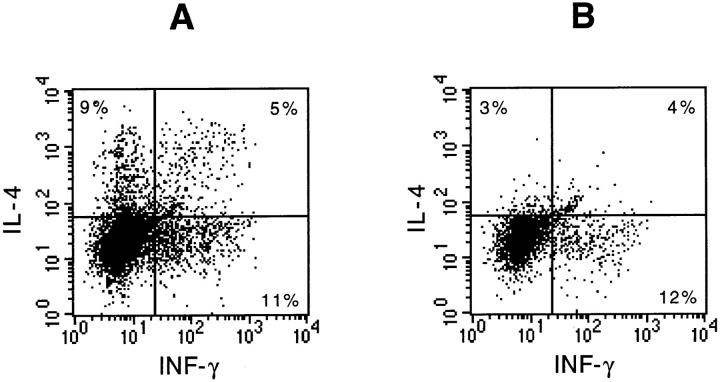
To investigate if the expression of IL-9 leads to selective infiltration of the lungs with lymphocytes producing either Th1- or Th2-type cytokines, lung lavage cells from IL-9– expressing mice were analyzed individually by flow cytometry for the presence of intracellular IL-4 and INF-γ. Fig. 10 shows typical dot-plots of IL-4 and INF-γ staining in CD4+ (Fig. 10 A) and CD8+ (Fig. 10 B) lymphocytes retrieved by lung lavage that were present at a 2:1 ratio (data not shown). The percentage of INF-γ–producing cells among CD4+ as well as CD8+ lymphocytes was slightly higher compared with IL-4–producing cells or cells staining positively for both cytokines (Fig. 10, A and B). Nonetheless, there was no selective accumulation of either IL-4 or INF-γ–producing CD4+ lymphocytes detectable in lung lavage fluid from IL-9–expressing mice.
Figure 10.
Intracellular detection of IL-4 and INF-γ in lymphocytes from lung lavage fluid of IL-9–expressing mice. Data shown are generated by three-color flow cytometric analysis of total lung lavage cells and pooled from four IL-9–expressing littermates after short-term culture in the presence of PMA, ionomycin, and monensin. Dot-blots were gated on CD4+ lymphocytes (A) or CD8+ lymphocytes (B). Number of cells staining for each cytokine are expressed as a percentage of CD4+ or CD8+ cells.
Assessment of Lung Physiology.
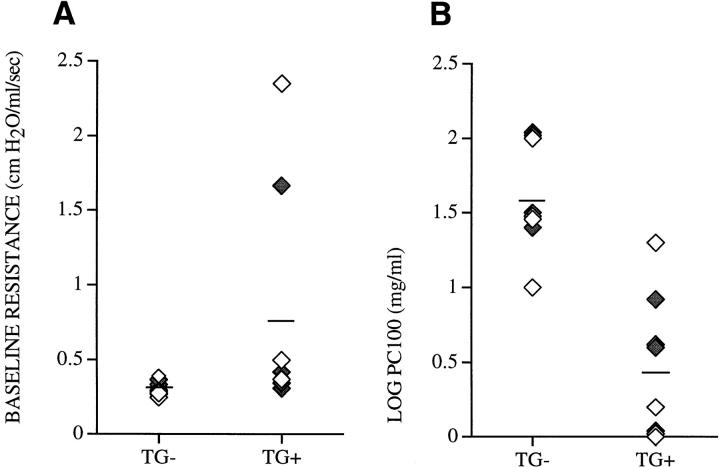
To assess the physiologic changes associated with lung selective expression of IL-9 in the airways of transgenic mice, both baseline airway resistance and the bronchial responsiveness to inhaled methacholine was determined. Several IL-9–expressing mice had increased airway resistance at baseline compared with transgene-negative control mice. However, when analyzed as a group, IL-9–expressing mice exhibited no significant difference in baseline airway resistance, 0.79 ± 0.77 cm H2O/ml/s (n = 8), compared with transgene-negative littermates, 0.31 ± 0.05 cm H2O/ml/s (n = 8, P > 0.09) (Fig. 11 A). Challenge with methacholine showed that IL-9–expressing mice required a significantly lower dose of methacholine to achieve a 100% increase in respiratory resistance (PC100), 0.452 ± 0.487 (n = 8), than did transgene-negative littermates, 1.606 ± 0.362 (n = 8, P < 0.0001) (Fig. 11 B). This difference reflects a 30-fold increase in airway hyperresponsiveness compared with transgene-negative littermates. Thus, airway-selective overexpression of IL-9 in the lungs of transgenic mice resulted in a nonsignificant trend toward increased baseline airway resistance as well as markedly increased airway hyperresponsiveness.
Figure 11.
Assessment of lung physiology. Two independent experiments were performed to compare airway baseline resistance (A) and airway hyperresponsiveness to inhaled methacholine, LOG PC100 (B), in IL-9–expressing mice (TG+) and in transgene-negative littermates (_TG_−). Mice derived from two independent lines, 9 (open diamonds) and 25 ( filled diamonds), are expressed as individual data points and as means (lines).
Discussion
Our results strongly support a potential and major role for IL-9 in the pathogenesis of allergic asthma. Expression of IL-9 selectively within the lungs of our transgenic mice caused hypertrophy of airway epithelium, submucosal accumulation of collagen, cellular infiltrates with eosinophils and lymphocytes, and mast cell hyperplasia. These mice also demonstrated the critical physiological response to inhaled methacholine that is characteristic of patients with asthma, namely hyperresponsiveness.
Genetic studies have linked asthma, atopy, and bronchial hyperresponsiveness to human chromosome 5q31-q33 (10), which contains several genes involved in the allergic immune response. The gene for IL-9 is located within that region (12), and allelic association between IL-9 and total serum IgE levels has been demonstrated (10). Recently, studies with different inbred strains of mice linked bronchial hyperresponsiveness to mouse chromosome 13, which shares homology with human chromosomal region 5q31-q33 and on which the murine homologue of the IL-9 gene is located (14). Correlation between strain-specific mRNA and protein levels of IL-9 and hyperresponsiveness has been demonstrated. In particular, these studies have shown that reduced expression levels of IL-9 were associated with hyporesponsiveness (14). Based on these data and on its pleiotropic functions, IL-9 was proposed as a critical factor involved in the pathogenesis of asthma (14). Expression of IL-9 in vivo in the lungs of our transgenic mice resulted in increased native airway hyperresponsiveness. Thus, the overexpression of one cytokine, selectively within the lungs, even in the absence of exposure of these mice to known antigens, resulted in marked increase in sensitivity to the bronchoconstrictor agonist. This new finding is in accord with recently published data (14) suggesting a role for IL-9 in the development of airway hyperresponsiveness.
There appears to be a clear association between airway hyperresponsiveness that defines asthma and airway inflammation (1). However, the exact mechanism(s) leading to this altered airway physiology is still unknown. Eosinophilic and lymphocytic infiltration of the airways is a common feature of human asthma (42, 43). A similar inflammatory response was seen in the lungs of IL-9–expressing mice that demonstrated airway hyperresponsiveness to methacholine. Eosinophils and lymphocytes were found surrounding airways and blood vessels and were present in lung lavage fluid. These findings mimic important histologic characteristics of human asthma. Several studies have demonstrated a correlation between the degree of inflammation, in particular eosinophilia, and airway hyperresponsiveness in asthmatic patients (44–46). The application of animal models to the study of experimental atopic asthma has not yet confirmed the requirement of eosinophils and their products in the development of airway hyperresponsiveness, which remains controversial (47–49). For example, studies in mice have demonstrated that hyperresponsiveness might occur in the absence of eosinophilic airway inflammation (48, 49). Therefore, how eosinophils and their products may contribute to the altered human airway physiology typical of asthma remains to be determined.
Recently, interest has focused on the association between activated CD4+ T lymphocytes, in particular the Th2 subset, and the pathogenesis of asthma (50–52). It has been shown that CD4+ Th2 cells are present and activated in the bronchial wall and lavage fluid of atopic asthmatics (53, 54). IL-4 and IL-5, produced by Th2 cells, have been postulated to have central roles in the initiation and maintenance of allergic inflammation because IL-4 is essential for sustaining a Th2-type immune response (55), acts on mast cells and eosinophils (56), and is a critical factor for IgE isotype switching in B cells (57). IL-5 regulates growth, differentiation, and activation of eosinophils (58, 59). Expression of either IL-4 or IL-5 in the lungs of transgenic mice promoted an impressive inflammatory response (33, 60). Despite evidence for an important role of these Th2 cell–derived cytokines in the inflammatory response in the lungs of asthmatics, proof of direct contributions of these factors to pathologic and physiologic changes in the airways of asthmatics has remained elusive. Expression of IL-9, another Th2 cell–derived cytokine (15), in the lungs of our transgenic mice also resulted in an impressive inflammatory response that showed differences from that observed in IL-4 or IL-5 transgenic mice. Accumulations of B220+ lymphocytes as found in the lungs of IL-4 transgenic mice (Temann, U.-A., unpublished observation) or expansion of bronchus-associated lymphoid tissue, mostly consisting of B cells, as in IL-5 transgenic mice (60), were not seen in the lungs of IL-9–expressing mice. Only a minor population of the infiltrating cells were B220+. Instead, cell infiltrates seen in the lungs of IL-9–expressing mice, predominantly eosinophils but also CD4+ and CD8+ lymphocytes, were very similar to those seen in asthmatic lungs. In human asthma, a predominance of Th2-like lymphocytes has been demonstrated in several studies (53, 54). We show that IL-4 as well as INF-γ–producing lymphocytes were identified among infiltrating cells in lung lavage fluid after short-term stimulation in vitro, which suggests that local expression of IL-9 alone does not lead to a Th2 specificity of the infiltrating CD4+ lymphocytes in the lungs. Interestingly, similar results have recently been published on lung lavage cells from allergic asthmatics (61), demonstrating that only a smaller portion of T cells are producing IL-4 while the majority produces INF-γ. Therefore, the postulated predominantly Th2-regulated immune response in allergic asthma remains controversial.
Overexpression of IL-9 in the lungs of our transgenic mice resulted in certain, important pathologic and physiologic changes characteristic of human asthma. These in vivo findings support a new and important central role for IL-9 in the pathogenesis of asthma. Also of interest is that other typical features of human asthma, such as airway epithelial shedding, mucus plugs, or airway smooth muscle hypertrophy, were not observed in IL-9–expressing mice used in this study. Furthermore, the vascular changes seen in the lungs of IL-9–expressing mice are not typical of human asthma. The observed medial hypertrophy in vessel walls that are due, at least in part, to smooth muscle cell proliferation are histologic changes seen more commonly in primary pulmonary hypertension (62).
An important new finding that distinguishes IL-9 transgenic mice from those expressing other Th2 cytokines selectively within the lungs was the presence of mast cells in the airway epithelium of all conducting airways. Mast cells are important in immediate allergic responses and acute bronchoconstriction. Cross-linking of allergen-specific IgE bound to high affinity receptors FcεRI on the surface of mast cells by antigen leads to activation and secretion of a wide variety of mediators causing direct bronchoconstriction and enhancement of airway inflammation (63, 64). In the human lung, mast cells are located in the trachea, mucosa, and submucosa of all airways and within the parenchyma (65). Their number is increased in the lungs of asthmatics compared with nonasthmatics, and they often exhibit signs of degranulation (66). In contrast, mast cells are sparse in the murine lung and only limited numbers are present in the tracheal tissue. Mast cells were detected in great numbers in the airway epithelium of our IL-9–expressing mice. The presence of fully granulated mast cells in the airway epithelium demonstrated by electron microscopic analysis as well as similar histamine levels in lung lavage fluid from IL-9– expressing mice and transgene-negative control mice suggest no significant mast cell degranulation with release of mediators. However, whether or not these mast cells play a role in the development of the observed phenotype in IL-9–expressing mice is still not completely elucidated. A further characterization of these mast cells, especially for their protease phenotype, may allow a more specific assessment of the potential contributions these cells may make to pathologic and physiologic changes in the lungs of IL-9– expressing mice. IL-9 is known to promote growth and differentiation of mast cells in vitro (28, 29) and in vivo (67). Therefore, the presence of mast cells in the airway epithelium from IL-9–expressing mice seems to be a direct effect of IL-9, since mast cell hyperplasia in the airway epithelium has not been described in transgenic mice expressing other Th2 cytokines, IL-4, IL-5, or IL-6 selectively within their lungs (33, 60, 34). Our observations in vivo support the function of IL-9 as a primary growth and differentiation factor of mast cells. They further suggest that mast cells are recruited to, or proliferate and differentiate in, the airways of asthmatics as a consequence of IL-9 produced locally within the lungs by activated Th2 lymphocytes. Our data provide functional evidence for a potential important role of IL-9 in the pathogenesis of asthma.
We propose that IL-9 derived from CD4+ Th2 cells may play an important role in inflammatory responses in the lung and potentially contributes to both pathologic and physiologic changes characteristic of the asthmatic airway.
Acknowledgments
We would like to thank J.A. Elias and R.J. Homer for helpful discussion and Cindy Hughes and Debbie Butkus for generating transgenic mice.
This work was supported in part by grant HL-56389 from the National Institutes of Health (NIH). G.P. Geba was supported by NIH grants HL-03229 and HL-56389. J.A. Rankin was supported by NIH grant HL-54450 and by the Veterans Administration. R.A. Flavell is an investigator of the Howard Hughes Medical Institute.
References
- 1.Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- 2.Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. doi: 10.1165/ajrcmb.13.1.7598934. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistant airway inflammation. Am J Respir Crit Care Med. 1995;152:388–393. doi: 10.1164/ajrccm.152.1.7599853. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Cytokines as mediators of chronic asthma. Am J Respir Crit Care Med. 1994;150:S42–S49. doi: 10.1164/ajrccm/150.5_Pt_2.S42. [DOI] [PubMed] [Google Scholar]
- 5.Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Activation and regulation of chemokines in allergic airway inflammation. J Leukocyte Biol. 1996;59:13–17. doi: 10.1002/jlb.59.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Morton NE. Major loci for atopy? . Clin Exp Allergy. 1992;22:1041–1043. doi: 10.1111/j.1365-2222.1992.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 7.The Collaborative Study on the Genetics of Asthma (CSGA) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 8.Marsh DG, Neely JD, Breazeale DR, Gosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty TH. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 9.Meyers DA, Postma DS, Panhuysen CIM, Xu J, Amelung PJ, Levitt RC, Bleeker ER. Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics. 1994;23:464–470. doi: 10.1006/geno.1994.1524. [DOI] [PubMed] [Google Scholar]
- 10.Doull IJM, Lawrence S, Watson M, Besishuili T, Beasley RW, Lampe F, Holgate ST, Morton NE. Allelic association of gene markers on chromosomes 5q and 11q with atopy and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 1996;153:1280–1284. doi: 10.1164/ajrccm.153.4.8616554. [DOI] [PubMed] [Google Scholar]
- 11.Van Leeuwen BH, Martinson ME, Webb GC, Young IG. Molecular organization of the cytokine gene cluster, involving human IL-3, IL-4, IL-5, and GM-CSF genes, on chromosome 5. Blood. 1989;73:1142–1148. [PubMed] [Google Scholar]
- 12.Kelleher K, Bean K, Clark SC, Leung W-Y, Yang TL. Human interleukin-9: genomic sequence, chromosomal location, and sequences essential for its expression in human T cell leukemia virus (HTLV)-I–transformed human T cells. Blood. 1991;77:1436–1441. [PubMed] [Google Scholar]
- 13.Holgate ST, Church MK, Howorth PH, Morton NE, Frew AJ, Ratko D. Genetic and environmental influences on airway inflammation in asthma. Int Arch Allergy Immunol. 1995;107:29–33. doi: 10.1159/000236921. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaides NC, Holroyed KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang L-Y, Messler CJ, et al. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci USA. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renauld J-C, Kermouni A, Vink A, Louahed J, van Snick J. Interleukin-9 and its receptor: involvement in mast cell differentiation and T cell oncogenesis. J Leukocyte Biol. 1995;57:353–360. doi: 10.1002/jlb.57.3.353. [DOI] [PubMed] [Google Scholar]
- 16.Renault J-C, Goethals A, Houssiau F, Merz H, van Roost E, van Snick J. Human P40/IL-9: expression in activated CD4+T cells, genomic organization, and comparison with the mouse gene. J Immunol. 1990;144:4235–4241. [PubMed] [Google Scholar]
- 17.Grencis RK, Hültner L, Else KJ. Host protective immunity to Trichinells spiralisin mice: activation of TH2 cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991;74:329–332. [PMC free article] [PubMed] [Google Scholar]
- 18.Else KJ, Hültner L, Grencis RK. Cellular immune response to the murine nematode parasite Trichuris muris. II. Differential induction of TH-subsets in resistant versus susceptible mice. Immunology. 1992;75:232–237. [PMC free article] [PubMed] [Google Scholar]
- 19.Gessner A, Blum H, Röllinghoff M. Differential regulation of IL-9-expression after infection with Leishmania majorin susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 20.Svetic A, Madden KB, Zhou XD, Lu P, Katona IM, Finkelman FD, Urban JF, Jr, Gause WC. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 21.Houssiau FA, Schandene L, Stevens M, Cambiaso C, Goldman M, van Snick J, Renauld J-C. A cascade of cytokines is responsible for IL-9 expression in human T cells. J Immunol. 1995;154:2624–2630. [PubMed] [Google Scholar]
- 22.Monteyne P, Renauld J-C, van Broeck J, Dunne DW, Brombacher F, Coutelier J-P. IL-4–independent regulation of in vivoIL-9 expression. J Immunol. 1997;159:2616–2623. [PubMed] [Google Scholar]
- 23.Uyttenhove C, Simpson RJ, van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T cell growth factor activity. Proc Natl Acad Sci USA. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houssiau FA, Renauld J-C, Stevens M, Lehman F, Lethe B, Coulie PG, van Snick J. Human T cell lines and clones respond to IL-9. J Immunol. 1993;150:2634–2640. [PubMed] [Google Scholar]
- 25.Schmitt E, Van Brandwijk R, van Snick J, Siebold B, Rüde E. TCGF III/P40 is produced by naive murine CD4+T cells but is not a general T cell growth factor. Eur J Immunol. 1989;19:2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- 26.Dugas B, Renauld J-C, Pène J, Bonnefoy JY, Petit-Frère C, Braquet P, Bousquet J, van Snick J, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4–induced immunoglobulin (IgG, IgM, and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 27.Petit-Frère C, Dugas B, Braquet P, Mencia-Huerta M. Interleukin-9 potentiates the interleukin-4– induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- 28.Hültner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rüde E, Dörmer P, van Snick J. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGF III (interleukin-9) Eur J Immunol. 1990;20:1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 29.Eklund KK, Ghildyal N, Austen KF, Stevens RL. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14–derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol. 1993;151:4266–4273. [PubMed] [Google Scholar]
- 30.Donahue RE, Yang Y-C, Clark SC. Human P40 T cell growth factor (interleukin-9) supports erythroid colony formation. Blood. 1990;75:2271–2275. [PubMed] [Google Scholar]
- 31.Williams DE, Morrissey PJ, Mochizuki DY, de Vries P, Anderson D, Cosman D, Boswell HS, Cooper S, Grabstein KH, Broxmeyer HE. T-cell growth factor P40 promotes the proliferation of myeloid cell lines and enhances erythroid burst formation by normal murine bone marrow cells in vitro. . Blood. 1990;76:906–911. [PubMed] [Google Scholar]
- 32.Renauld J-C, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- 33.Rankin JA, Picarella DE, Geba GP, Temann U-A, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett JA, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiCosmo B, Geba GP, Picarella D, Elias JA, Rankin JA, Stripp BR, Whitsett JA, Flavell RA. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94:2028–2035. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temann U-A, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 36.Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4 producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- 37.Dittel BN, Sant'Angelo DB, Janeway CA. Peptide antagonists inhibit proliferation and the production of IL-4 and/or INF-gamma in T helper 1, T helper 2, and T helper 0 clones bearing the same TCR. J Immunol. 1997;158:4065–4073. [PubMed] [Google Scholar]
- 38.Martin T, Gerard N, Galli S, Drazen J. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 39.Amdur MO, Mead J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958;192:364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- 40.Stripp RB, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10gene. J Biol Chem. 1992;267:14703–14712. [PubMed] [Google Scholar]
- 41.Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C, III, Flavell RA, Elias JA. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest. 1996;98:2845–2853. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wardlaw AJ, Dunette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 43.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 44.Bentley AM, Menz G, Storz C, Robinson DS, Bradley B, Jeffrey PK, Durham SR, Kay AB. Identification of T lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am Rev Respir Dis. 1992;146:500–506. doi: 10.1164/ajrccm/146.2.500. [DOI] [PubMed] [Google Scholar]
- 45.Taylor KJ, Luksza AR. Peripheral blood eosinophil counts and bronchial responsiveness. Thorax. 1987;42:452–456. doi: 10.1136/thx.42.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani A-MA, Schwartz LB, Durham SR, Jeffery PK, Kay AB. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 47.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airway hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hessel EM, van Oosterhout AJM, van Ark I, van Esch B, Hofman G, van Loveren H, Savelkoul HFJ, Nijkamp FP. Development of airway hyperresponsiveness is dependent on interferon-γ and independent of eosinophil infiltration. Am J Respir Cell Mol Biol. 1997;16:325–334. doi: 10.1165/ajrcmb.16.3.9070618. [DOI] [PubMed] [Google Scholar]
- 50.Garsson J, Nykamp FP, van der Vliet H, van Loveren H. T-cell–mediated induction of airway hyperreactivity in mice. Am Rev Respir Dis. 1991;144:931–938. doi: 10.1164/ajrccm/144.4.931. [DOI] [PubMed] [Google Scholar]
- 51.Gavett SH, Olen X, Finkelman FD, Wills-Karp M. Depletion of murine CD4+T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 52.Hogan SP, Koskinen A, Matthaie KI, Young JG, Foster PS. Interleukin-5 producing CD4+T cells play a pivotal role in aeroallergen-induced eosinophilia, bronchial hyperreactivity, and lung damage in mice. Am J Respir Crit Care Med. 1998;157:210–218. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- 53.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 54.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow J-C., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 55.Swain SL, Weinberg AD, English M, Hutson G. IL-4 directs the development of TH2 effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 56.Favre C, Saeland S, Caux C, Duvert V, De Vries JE. Interleukin-4 has basophilic and eosinophilic cell growth–promoting activity on cord blood cells. Blood. 1990;75:67–73. [PubMed] [Google Scholar]
- 57.Finkelman FD, Katona IM, Urban JF, Holmes J, Ohara J, Tung AS, Sample JG, Paul WE. IL-4 is required to generate and sustain in vivoIgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 58.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 62.Burke AP, Farb A, Virmani R. The pathology of primary pulmonary hypertension. Mod Pathol. 1991;4:269–282. [PubMed] [Google Scholar]
- 63.Schleimer RP, MacGlashan DW, Peters SP, Pinckard RN, Adkinson NF, Lichtenstein LM. Characterisation of inflammatory mediator release from purified human lung mast cells. Am Rev Respir Dis. 1986;133:614–617. doi: 10.1164/arrd.1986.133.4.614. [DOI] [PubMed] [Google Scholar]
- 64.Broide DH, Gleich GJ, Cuomo AJ, Coburn DA, Federman EC, Schwarz LB, Wasserman SI. Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol. 1991;88:637–648. doi: 10.1016/0091-6749(91)90158-k. [DOI] [PubMed] [Google Scholar]
- 65.Van Overveld FJ, Houben AMJ, Schmitz du Moulin FEM, Bruijnzeel PLB, Raaijmakers JAM, Terpstra GK. Mast cell heterogeneity in human lung tissue. Clin Sci. 1989;77:297–304. doi: 10.1042/cs0770297. [DOI] [PubMed] [Google Scholar]
- 66.Pesci A, Foresi A, Bertorelli G, Chetta A, Oliveri D. Histochemical characteristics and degranulation of mast cells in epithelium and lamina propria of bronchial biopsies from asthmatics and normal subjects. Am Rev Respir Dis. 1993;147:684–689. doi: 10.1164/ajrccm/147.3.684. [DOI] [PubMed] [Google Scholar]
- 67.Faulkner H, Humphreys N, Renauld JC, van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]