Receptor-Mediated Uptake of Antigen/Heat Shock Protein Complexes Results in Major Histocompatibility Complex Class I Antigen Presentation via Two Distinct Processing Pathways (original) (raw)
Abstract
Heat shock proteins (HSPs) derived from tumors or virally infected cells can stimulate antigen-specific CD8+ T cell responses in vitro and in vivo. Although this antigenicity is known to arise from HSP-associated peptides presented to the immune system by major histocompatibility complex (MHC) class I molecules, the cell biology underlying this presentation process remains poorly understood. Here we show that HSP 70 binds to the surface of antigen presenting cells by a mechanism with the characteristics of a saturable receptor system. After this membrane interaction, processing and MHC class I presentation of the HSP-associated antigen can occur via either a cytosolic (transporter associated with antigen processing [TAP] and proteasome–dependent) or an endosomal (TAP and proteasome–independent) route, with the preferred pathway determined by the sequence context of the optimal antigenic peptide within the HSP-associated material. These findings not only characterize two highly efficient, specific pathways leading to the conversion of HSP-associated antigens into ligands for CD8+ T cells, they also imply the existence of a mechanism for receptor-facilitated transmembrane transport of HSP or HSP-associated ligands from the plasma membrane or lumen of endosomes into the cytosol.
Keywords: immunology, vaccines, macrophages, T cells, peptides
Introduction
Activation of the T cell limb of the adaptive immune system involves the recognition of small ligands bound to MHC or MHC-like proteins on plasma membranes. This antigen presentation paradigm is best understood for peptides associated with MHC class I or class II molecules, with a large body of work having defined two distinct pathways by which protein antigens are converted to shorter fragments occupying the polymorphic binding domains of these MHC glycoproteins 1. For MHC class I molecules, peptides are mainly created by the action of proteasomal enzymes on proteins present in the cytosol. These peptides are then transported by the transporter associated with antigen processing (TAP) dimer through the membrane of the endoplasmic reticulum (ER), after which they are bound to nascent MHC class I heavy chain–β2-microglobulin complexes within the ER lumen 2. The occupied MHC molecules then pass through the secretory pathway to the cell surface where they can interact with the receptors of CD8+ T cells. MHC class II molecules instead interact primarily with large polypeptides within various endosomal compartments 3 4 5, after which exopeptidases remove the unprotected segments of the antigen outside of the MHC molecule binding groove 6 7. The resulting MHC class II–peptide complexes are then exported to the plasma membrane for recognition by CD4+ T cells.
Heat shock proteins (HSPs) are highly conserved peptide-binding molecules that control the folding of proteins and prevent their aggregation 8. The same peptide-binding capacity appears to allow HSPs to acquire proteinaceous antigenic material within cells, and when administered exogenously, to induce priming of CD8+ T lymphocytes in vivo 9 10 11 12 13. This property suggests that there is efficient transfer of antigen from HSPs to MHC class I molecules under these conditions. Such transfer is surprising both because peptide loading of MHC class I molecules typically involves a cytosol to ER route, and because while we understand the role of several well-defined receptor systems in facilitating MHC class II–peptide presentation 14 15 16 17 18, little is known about any specific pathways for capture and delivery of exogenous antigens to (intracellular) MHC class I molecules. In circumstances other than those known to involve HSP 19 20 21 22, MHC class I presentation of peptides derived from exogenous protein sources has been reported and ascribed to either endosomal degradation of particulate antigens, followed by “regurgitation” of the derived peptides for loading of surface class I molecules 23, or to delivery of antigens from endosomes and/or phagosomes into the cytosol by an unknown route 24 25 26 27. The relative contribution of either of these proposed pathways to presentation of HSP-associated antigen has not been determined, nor is much known about the cell biology of such exogenous pathways for MHC class I–peptide acquisition.
Several groups have recently reported that various HSP molecules show cell surface binding and/or cellular uptake that in some 28 29 but not other 30 cases has the characteristics expected of a saturable receptor system. However, these studies did not link these binding events to functional antigen presentation, or address by what route the antigen in the bound HSP is converted into peptides associated with MHC class I molecules. Given other studies showing active signaling by HSP for induction of cytokine secretion by various cell types 31, it was possible that the binding reported in these earlier investigations was related to this functional stimulation and not necessarily to antigen presentation. Here, we analyze HSP delivery of antigenic material to MHC class I molecules using both functional and morphologic methods. Our data reveal that saturable uptake of the complexes, apparently through interaction with one or more surface receptors, is important for delivering HSP-associated antigen to the cell for processing via either an endosomal or a cytosolic route. Which processing pathway is preferred appears to depend on the sequence context of the antigenic peptide bound to the HSP. The evidence presented here for proteasome-dependent processing of HSP-derived material also implies the existence of an uncharacterized mechanism for transport of HSP complexes, or their cargo, across plasma or endosomal membranes into the cytosol.
Materials and Methods
Cells.
Macrophages were collected by peritoneal lavage 6 d after injection of 1 ml of thioglycollate into C57BL/6 or (C3H × C57BL/6)F1 mice (The Jackson Laboratory). B3Z is a CD8+ T cell hybridoma specific for OVA 257–264 (SIINFEKL) bound to H-2Kb MHC class I molecules 32. 3A9 is a CD4+ T cell hybridoma specific for the hen egg lysozyme determinant corresponding to residues 46–61 bound to the MHC class II molecule I-Ak 33.
HSP.
Recombinant HSP 70 protein was expressed from the pMS236 plasmid constructed by cloning the 1.96-kb NcoI-XbaI fragment of the genomic mouse clone hsp70.1 34 into the pTrc99 expression vector (Amersham Pharmacia Biotech). Expression in DH5α cells was induced by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), harvested cells were disrupted, and clarified supernatants were loaded onto a DEAE Sephacel column (Amersham Pharmacia Biotech). Elution of HSP 70 was achieved by applying a 25–500 mM NaCl gradient. Fractions containing HSP 70 were loaded onto an ATP-agarose column (Sigma-Aldrich), and elution was achieved by applying a 0–1 mM ATP gradient. Fractions containing HSP 70 were precipitated with 80% ammonium sulfate, redissolved in low salt buffer containing magnesium acetate, dialyzed against the same buffer, and aliquots were stored at −80°C. Bovine HSP 70 was purchased from Sigma-Aldrich.
HSP/Antigen Complexes.
The loading of mouse HSP 70 was performed as described previously 11 with minor variations. The HSP and the hybrid peptide (synthesized by Bio-Synthesis, Inc.) were incubated together in PBS containing 1 mM KCl, 2 mM MgCl2, and 100 μM ATP for 45 min at room temperature. 1 mM ADP was added, and the incubation was extended for an additional 30 min. The free hybrid peptide was removed by extensive washing using a Centricon 30K (Amicon), until the residual free hybrid peptide was calculated to be in the picomolar range. The efficiency of the loading was determined with iodine-labeled hybrid peptide to be ∼20% in most preparations.
Bioassays.
105 thioglycollate-induced macrophages were cultured overnight in each well of a flat-bottomed 96-well plate in the absence or presence of the indicated antigens and 5 × 104 B3Z or 3A9 cells in a final volume of 200 μl. As an indication of the T cell activation, IL-2 accumulation in the supernatant at 16 h was measured by ELISA (BD PharMingen) 26. For the competition experiments, macrophages were preincubated with the indicated amount of unloaded bovine HSP 70 (Sigma-Aldrich) for 30 min at 37°C in a final volume of 100 μl. Without washing, loaded HSP 70 or antigenic peptide was then added along with 5 × 104 B3Z cells, and incubation continued for an additional 16 h before assay of IL-2 accumulation. Error bars show SEM; where no bars are visible, the errors were too small to plot.
Lactacystin Treatment.
(C57BL/6 × C3H)F1 macrophages were cultured for 8 h with the indicated antigens in the presence or absence of 25 μM lactacystin (Calbiochem). The APCs were then fixed with 1% paraformaldehyde for 10 min at room temperature, treated for 10 min with 0.1 M glycine, washed, and cultured overnight with either B3Z or 3A9 T cell hybridoma cells. IL-2 accumulation in the supernatant at 16 h was measured as an indication of T cell activation.
Immunofluorescence Staining.
Bovine HSP 70 (Sigma-Aldrich), BSA (Sigma-Aldrich), and 25-D1.16 (an mAb specific for the SIINFEL/Kb complex [see reference 41]) were biotinylated using _N_-hydroxy succinimide–biotin (Pierce Chemical Co.) according to the manufacturer's directions. FITC-OVA (Molecular Probes) was used at a concentration of 200 μg/ml. Rabbit anticalnexin (StressGen Biotechnologies) and FITC-conjugated mouse anti–rabbit antibodies (Jackson ImmunoResearch Laboratories) were used at 5 μg/ml. Steptavidin–Texas red (Southern Biotechnology Associates) was used at 2 μg/ml. Biotinylated 25-D1.16 was used at 10 μg/ml. Details of intracellular staining analyzed by immunofluorescence confocal microscopy are given in the legend to Fig. 3. Details of the surface staining and internalization assay, analyzed by immunofluorescence confocal microscopy, are given in the legend to Fig. 4. In brief, 5 × 105 thioglycollate-induced macrophages were grown overnight in 24-well plates on poly-l-lysine (Sigma-Aldrich) coated coverslips. The next day, the coverslips were washed to remove the nonadherent cells and incubated with the indicated antigens in 250 μl of complete medium. Cells were fixed with 1% paraformaldehyde (see Fig. 3) or with 3% paraformaldehyde (see Fig. 4) followed by quenching with 0.1 M glycine. Unless otherwise indicated, cells were permeabilized with 0.1% Brij. In Fig. 4 A, cells were stained with Streptavidin–Texas red without permeabilization. Coverslips were mounted with Fluorosave (Calbiochem). Digital images were acquired using a Leica LSCM, composed in Adobe Photoshop®, and printed after formatting in ClarisDraw.
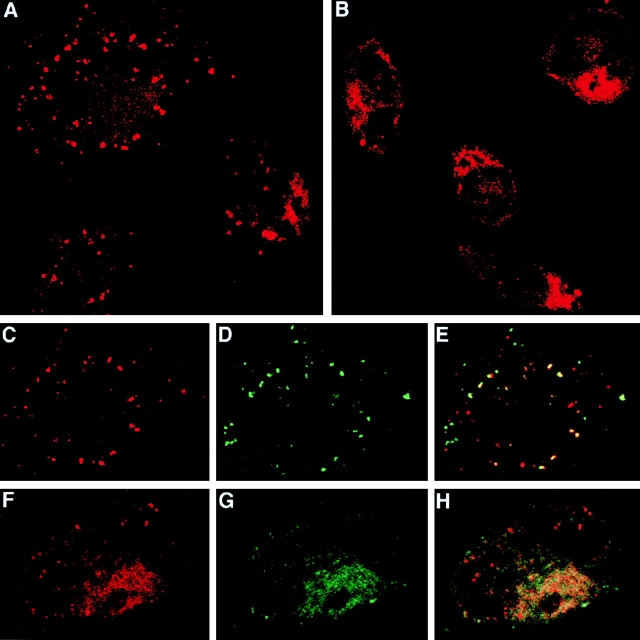
Figure 3.
Direct visualization of two distinct intracellular sites of SIINFEKL association with MHC class I molecules after antigen delivery via HSP 70. C57BL/6 macrophages were incubated for 6 h at 37°C with either 100 μg/ml of HSP/BiP-OVA (A, C–E) or HSP/OVA-BiP (B, F–H). During the last 30 min of incubation, FITC-OVA was added to some of the samples. A, B, C, and F show the red channel fluorescence of cells stained with biotinylated 25-D1.16 followed by Streptavidin–Texas red. C shows the red channel (SIINFEKL/Kb complexes) for one cell, demonstrating the predominant vesicular location of these complexes; D shows the green channel (FITC-OVA) for the same high-power field; and E shows the colocalization of the two signals (yellow), indicating the endosomal nature of at least a fraction of the SIINFEKL/Kb-containing structures. In F–H, cells were double stained with biotinylated 25-D1.16 followed by Streptavidin–Texas red as well as with rabbit anticalnexin followed by FITC-conjugated anti–rabbit Ig. F shows the red channel (SIINFEKL/Kb complexes), revealing primarily reticular cytoplasmic staining along with a few stained vesicles. G shows the green channel (calnexin) for the same cell. Colocalization of the two signals (yellow) is seen in H, consistent with an ER location for the bulk of the SIINFEKL/Kb complexes.
Figure 4.

A subset of normal macrophages expresses a surface receptor for HSP 70 that mediates internalization into endosomes. (A) C57BL/6 macrophages were incubated with 100 μg/ml of biotinylated HSP 70 for 30 min at 4°C; (B–D) after incubation as in A, the excess HSP was washed away and the macrophages were incubated for an additional 30 min at 37°C in the presence of FITC-OVA. All the samples were washed and then stained with Streptavidin–Texas red either without permeabilization (A), or after permeabilization with 0.1% Brij (B–D). B shows the red channel (HSP 70), and C shows the green channel (FITC-OVA). D shows the partial colocalization of the two signals (yellow). (E) 3 × 105 macrophages were incubated at 4°C with 50 μg/ml of biotinylated BSA (gray histogram), with 50 μg/ml of biotinylated HSP 70 (thick solid line), or with 500 μg/ml of unlabeled HSP 70 for 30 min, followed without washing by addition of 50 μg/ml of biotinylated HSP 70 and incubation for an additional 30 min (thin solid line), or with 500 μg/ml of unlabeled BSA for 30 min, followed without washing by addition of 50 μg/ml of biotinylated HSP 70 and incubation for an additional 30 min (dotted line). Cells were stained with FITC-anti-CD11b and streptavidin-PE. Only propidium iodide–negative CD11b+ cells are shown. (F) 5 × 105 macrophages were incubated at 4°C with increasing concentrations of biotinylated HSP 70 or biotinylated BSA. Cells were washed and stained with FITC–anti-CD11b and Streptavidin-PE. Only propidium iodide–negative CD11b+ cells are included in the analysis. The mean fluorescence of the CD11bbright subpopulation of macrophages that shows high binding to HSP 70 is plotted. (G) Macrophages were cultured overnight in the presence or absence of the indicated antigens and 5 × 104 B3Z cells. Where indicated “competitor,” macrophages were preincubated for 30 min with 2 or 20 μg of unloaded HSP 70 in 100 μl of medium, followed without washing by addition of 2 μg of antigen-loaded HSP or SIINFEKL and B3Z cells. In four independent experiments, the inhibition of the presentation of either HSP70/OVA-BiP or HSP 70/BiP-OVA by 20 μg of competitor averaged 50%.
Saturation and Competition Binding Assays.
For saturation binding analysis, 5 × 105 thioglycollate-induced macrophages were incubated with increasing concentrations of either biotinylated HSP 70 or biotinylated BSA for 45 min on ice, then washed. For the binding competition assay, macrophages were incubated first with the indicated amount of unlabeled HSP 70 or BSA for 45 min on ice, and followed without washing by incubation with biotinylated HSP 70 for an additional 45 min. Cells were washed two times and incubated with Streptavidin-PE (Molecular Probes) and FITC-CD11b (BD PharMingen), then washed again. Data were collected using a FACScan™ flow cytometer (Becton Dickinson) after addition of propidium iodide and analyzed using CELLQuest™ software (Becton Dickinson). Gates were set for CD11b+, propidium iodide–negative cells, and either flow histograms of HSP staining of CD11b+ cells staining were generated (competition experiment), or mean fluorescence values of HSP staining for this cell subpopulation were calculated (saturation analysis).
Results
Differential Roles of the Proteasome and TAP in MHC Class I Presentation of SIINFEKL Present in Two Distinct HSP Complexes.
To gain a better understanding of HSP antigenicity, with its implications for both the basic biology of antigen presentation and the development of HSP-based vaccines, we first addressed whether the proposed “regurgitation” route or the cytosol to ER route, or both, contributes to the presentation of HSP-associated antigen. This was accomplished by examining the effects of inhibiting proteasome activity or eliminating TAP function on T cell stimulation by HSP/antigen complexes. Mouse HSP 70 was loaded in vitro with synthetic antigens containing the amino acid sequence SIINFEKL (residues 257–264 of OVA) that is presented by H-2Kb. The binding motif of HSP 70 consists of a hydrophobic core of four to five amino acids and two flanking regions enriched in basic residues 8 35. Because SIINFEKL does not contain this motif, we synthesized hybrid peptides containing a sequence derived from BiP that binds avidly to HSP 70 and that is connected to SIINFEKL via a GSG linker at the antigenic peptide's NH2 terminus (HWDFAWPWGSGSI INFEKL, referred to as BiP-OVA) or the converse (SIIN FEKLGSGHWDFAWPW, referred to as OVA-BiP). The two different orientations of SIINFEKL and BiP motif were tested because the sequence context of a MHC class I–binding determinant within a protein has been shown to affect its efficiency of presentation 36 37. With both of these hybrid peptides, the loading efficiency of HSP 70 was typically 20%, versus only 1% occupancy using antigenic peptides not optimized for HSP binding (11; and data not shown). These HSP/hybrid peptide complexes are highly effective at priming CTL responses in vivo 38.
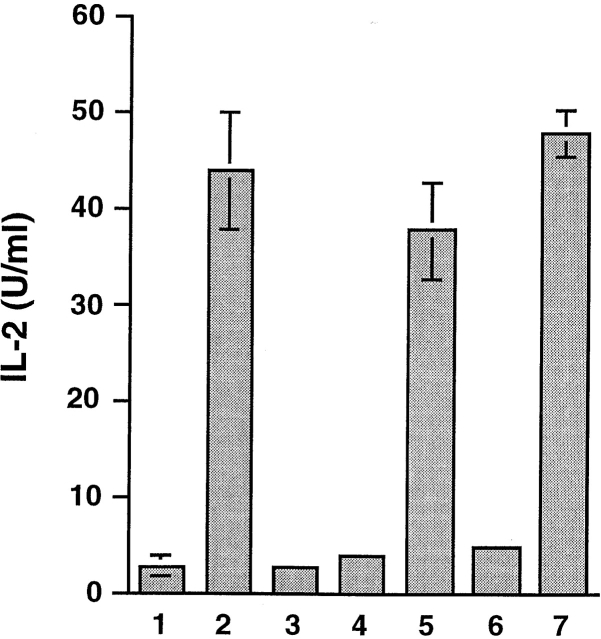
In the presence of macrophages as APCs, HSP 70 loaded with either BiP-OVA (HSP/BiP-OVA) or OVA-BiP (HSP/OVA-BiP) stimulated IL-2 secretion from the SIINFEKL/Kb-specific T cell hybridoma B3Z, whereas neither hybrid peptide added alone to macrophages was effective (Fig. 1). This excludes the possibility that activation achieved using HSP/hybrid peptide complexes is mediated by residual free peptide and suggests that an active cellular process is necessary for generation of the SIINFEKL/Kb ligand. HSP 70 itself, or HSP 70 mixed with hybrid peptides under conditions that do not support stable complex formation, was not immunogenic. Taking into account the 20% loading, the HSP/antigen complexes were as efficient on a molar basis as the potent optimal-length free SIINFEKL peptide, despite their requirement for additional processing.
Figure 1.
Antigenic peptides derived from material bound to HSP 70 in vitro can be presented by MHC class I molecules of normal macrophages. C57BL/6 macrophages were cultured overnight in 1 the absence of any antigen, 2 the presence of 1 nM SIINFEKL peptide, 3 2 μg of unloaded HSP 70, 4 20 nM OVA-BiP, 5 2 μg HSP70/OVA-BiP, 6 20 nM BiP-OVA, or 7 2 μg HSP 70/BiP-OVA complexes and 5 × 104 B3Z. IL-2 accumulation in the supernatant at 16 h was measured as an indication of T cell activation. Comparable results were obtained in >10 similar experiments.
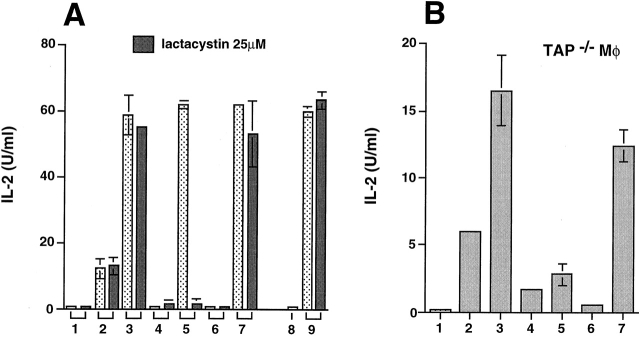
The role of proteasomal enzymes in the presentation of SIINFEKL from the HSP 70/antigen complexes was examined by incubating peritoneal macrophages with either of the HSP/hybrid peptide complexes in the presence of the potent proteasomal inhibitor lactacystin 39. Only stimulation by HSP/OVA-BiP, but not by HSP/BiP-OVA, was substantially inhibited by lactacystin used at 25 μM, a concentration that did not influence presentation of free SIINFEKL peptide or of an endosomally processed determinant of hen egg lysozyme that binds to MHC class II molecules (Fig. 2 A). Such selective inhibition argues against either a general toxic effect or a nonspecific inhibition of cellular proteases, and suggests that HSP can use two distinct intracellular processing pathways to deliver antigenic peptides to MHC class I molecules, only one of which involves proteasome activity. To further define these pathways, we tested the role of TAP. TAP-deficient macrophages express lower levels of MHC class I molecules at the cell surface than wild-type cells, but this limitation can be overcome by growing the cells at reduced temperature in β2-microglobulin 40. TAP−/− macrophages were therefore grown at 26°C for 24 h and transferred to 37°C when incubation with the antigens and the T cells was started. In agreement with the data obtained using lactacystin, TAP-deficient macrophages showed greatly impaired presentation of SIINFEKL from HSP/OVA-BiP but not from HSP/BiP-OVA (Fig. 2 B).
Figure 2.
Presentation of SIINFEKL from HSP/OVA-BiP but not HSP/BiP-OVA is dependent on proteasomal proteases and TAP. (A) The effect of 25 μM lactacystin on the presentation of the indicated antigens to either the B3Z or the 3A9 T cell hybridoma cells was assayed. In bars 1–7, the presentation to B3Z hybridoma cells was analyzed in 1 the absence of any antigen, 2 the presence of 1 nM SIINFEKL peptide, 3 10 nM SIINFEKL peptide, 4 20 nM OVA-BiP, 5 2 μg HSP70/OVA-BiP, 6 20 nM BiP-OVA, or 7 2 μg HSP 70/BiP-OVA complexes. In four independent experiments, the presentation of HSP 70/BiP-OVA was not affected by lactacystin, whereas the inhibition of the presentation of HSP70/OVA-BiP ranged between 70 and 90%. The effect of lactacystin on the presentation of HEL-46-61 to 3A9 hybridoma cells was tested 8 in the absence of any antigen, or 9 in the presence of 1 mg/ml hen egg lysosyme. (B) 105 TAP-1−/− macrophages (C57BL/6 × 129) were grown at 26°C for 24 h and transferred to 37°C when incubation with the indicated antigens and the T cells was started. The presentation to B3Z hybridoma cells was analyzed in 1 the absence of any antigen, 2 the presence of 1 nM SIINFEKL peptide, 3 10 nM SIINFEKL peptide, 4 20 nM OVA-BiP, 5 2 μg HSP70/OVA-BiP, 6 20 nM BiP-OVA, or 7 2 μg HSP 70/BiP-OVA complexes. Similar results were obtained in three independent experiments.
Direct Visualization of the Sites of SIINFEKL-Kb Binding in Cells Exposed to HSP/Antigen Complexes.
These functional experiments suggest that HSP/OVA-BiP is preferentially processed via the classical class I pathway and imply that the HSP/ligand complex, or the ligand alone, is translocated into the cytosol after which proteasomal cleavage and TAP transport contribute to MHC class I loading of released peptide in the ER. In contrast, HSP/BiP-OVA appears to be predominantly processed by a distinct pathway. To visualize directly the site(s) of SIINFEKL-MHC class I association after exposure of cells to HSP/antigen complexes, macrophages were incubated with each of the HSP complexes, and the cells were stained with 25-D1.16, an mAb to SIINFEKL/Kb complexes 41. Compared with cells exposed to HSP only (not shown), we could detect SIINFEKL/Kb complexes with 25-D1.16 when macrophages were incubated with either of the HSP/antigen preparations. In agreement with the implications of the antigen presentation studies, the intracellular distribution of these MHC class I/peptide complexes varied depending on the antigen employed. With HSP/BiP-OVA, whose presentation is insensitive to proteasomal inhibition and is TAP independent, a vesicular staining predominates (Fig. 3 A). 25-D1.16 staining (Fig. 3 C) partially colocalizes with OVA internalized by fluid phase endocytosis (Fig. 3D–E), indicating that with this HSP/antigen combination, SIINFEKL-loaded MHC class I molecules are found preferentially in endosomes. In contrast, when macrophages are pulsed with HSP/OVA-BiP, the staining of vesicular structures is infrequent (Fig. 3 B), and the 25-D1.16 staining (Fig. 3 F) largely colocalizes with calnexin used to identify the ER (Fig. 3G and Fig. H). Together with the above data on proteasome and TAP dependence of HSP/OVA-BiP presentation, these staining data support the existence of an HSP-mediated pathway for the translocation of polypeptides into the cytosol, in agreement with a recent study involving a lymphoma cell line 30.
These functional and morphologic results thus reveal that two distinct routes of processing can lead to MHC class I presentation of HSP-associated antigenic material, and also suggest that which pathway is preferentially used depends on the relationship of the class I binding sequence to the surrounding peptide structure. Having a free COOH-terminal anchor residue (BiP-OVA) seems to be associated with effective processing by an endosomal route. In contrast, having this key binding residue within an extended polypeptide chain (OVA-BiP) appears to require proteasomal action for efficiently placing this amino acid at the COOH terminus of the processed material 42 43. Additional peptide sequences will need to be tested to determine if this is truly a general rule, which would have important implications for the design of HSP-based vaccines.
Possible Role of Receptor-mediated Uptake in HSP-dependent Antigen Presentation.
The efficiency of presentation using HSP/hybrid peptide complexes suggests the existence of a specific mechanism that can maximize the available pool of antigenic substrate within the cell. Several recent studies have provided evidence for receptor-mediated binding of various HSPs to cell surfaces 28 and/or receptor-mediated uptake into endosomes 28 29. To look for evidence of a receptor uptake system under the conditions employed here, we examined the interaction of biotinylated HSP with peritoneal macrophages. Incubation with biotinylated HSP 70 at 4°C resulted in binding to the cell surface (Fig. 4 A). The bound HSP was internalized into endocytic vesicles when the cells were warmed to 37°C (Fig. 4 B), where it partially colocalized with OVA provided as a fluid phase marker (Fig. 4C and Fig. D). This binding and rapid endocytic uptake were specific to HSP 70, as biotinylated BSA gave no surface staining at 4°C and little concentration in vesicles after a longer time at 37°C under these conditions (not shown). These data are in agreement with and extend recent results showing that glucose-regulated protein (Grp) 96 and heat shock cognate protein (HSC) 70 can bind to the plasma membrane and internalize into endosomal-like structures 28 29.
The surface binding observed microscopically could also be detected using flow cytometry. Only 25–30% of isolated peritoneal macrophages, a population composed largely of the CD11b-brightest cells (data not shown), exhibited substantial cell surface binding of biotinylated HSP 70 (Fig. 4 E). Interestingly, this matches the proportion of HSP-pulsed macrophages that can be lysed by CTLs specific for HSP-associated antigen 12. Thus, the level of surface receptor expression correlates with and may determine effective presentation of HSP-associated ligand. Consistent with the existence of a saturable, specific receptor system, surface binding of biotinylated HSP to the CD11bbright cells could be partially but substantially inhibited by a 10-fold excess of unlabeled HSP, but not by BSA (Fig. 4 E), and exposure to increasing concentrations of HSP 70 resulted in staining approaching a plateau at 200 μg/ml among the CD11bbright cells (Fig. 4 F). A 10-fold excess of unloaded HSP (100 μg/ml) also substantially inhibited B3Z responses to either of the HSP/hybrid peptide complexes (Fig. 4 G), in accord with the idea that the receptor-mediated binding observed by staining contributes to antigen presentation by both processing pathways detailed above.
Discussion
The evidence provided here for both a cytosolic and endocytic route for MHC class I presentation of HSP-associated antigen provides new mechanistic insight into a well-recognized functional property of HSP. The two pathways we have directly visualized correspond to those previously proposed to underlie presentation of material associated with artificial particulate antigens or with bacteria 23 24 25 26 27. In our own previous study of latex bead–associated protein, the efficiency of MHC class I–dependent antigen presentation was low, which we considered inconsistent with the operation of a highly evolved specific pathway 26. In contrast, HSP-mediated antigen presentation is quite efficient and our congruent microscopic and functional evidence support the existence of a specific receptor uptake system for HSP/antigen complexes, extending both morphological data and extensive quantitative uptake data from other recent studies 28 29 by showing a functional relationship between such receptor-dependent surface binding and HSP-dependent MHC class I antigen presentation.
Receptor-mediated uptake improves MHC class II antigen presentation by up to four orders of magnitude 14 15 16 17 18. The presumably similar enhancing effects of such uptake on the HSP-dependent presentation pathway, together with the expression of these putative receptors on subsets of professional APCs involved in initiating immune responses (including splenic dendritic cells [Castellino, F., unpublished results]), strongly imply that class I, and possibly also class II, MHC molecule presentation of HSP-associated antigens is a physiologically relevant pathway. The heterogeneity seen for HSP 70 binding to peritoneal mononuclear cells suggests that expression of the HSP binding structures is regulated during cellular differentiation, whereas the continued small increment in staining observed at very high HSP concentrations (>200 μg/ml; our unpublished observations) raises the possibility that more than one affinity class of receptor may exist. The staining at very high HSP concentrations could reflect weak cross-binding among the different receptors proposed by Arnold-Schild et al. to mediate Grp 96 versus HSP 70 binding 28.
Antigen cross-presentation plays a key role in responses to tumors and possibly to virally infected nonhematopoietic cells, as well as in the establishment of peripheral tolerance 44. The mechanism of antigen acquisition and the cells involved in in vivo presentation in these circumstances are poorly understood. Uptake of apoptotic cells by phagocytic dendritic cells has been suggested as a key event 22, but recent data favor the activation of dendritic cells by material from necrotic cells 45, followed by efficient antigen presentation of released material by MHC class I molecules 22 46. The loss of HSP/protein complexes from cells dying a necrotic death could be readily imagined to lead to antigen delivery via the processing pathways described here, in agreement with the proposal of Melcher et al. 47. Some antigen-associated HSP might also be released from viable infected or transformed cells due to saturation of the KDEL-retrieval pathway 48 after the HSP upregulation that results from stress due to microbial invasion or the poor oxygenation of growing tumors. In these cases, the complexes could contribute to an “early warning” system that promotes T cell activation by professional APCs that have efficiently bound them through surface receptors and converted them into peptide/MHC molecule ligands suitable for T cell recognition.
Finally, from a cell biology point of view, we are left with trying to understand how HSP/antigen complexes or the antigenic cargo of HSP molecules move across endosomal or plasma membranes into the cytosol. The only described pathway across such membranes for normal cellular proteins is in the opposite direction and involves lysosomes 49. Whether the same pathway can operate in reverse or whether another transport pathway remains to be discovered poses an intriguing question.
Acknowledgments
The authors wish to thank Dr. Jon Yewdell for his very helpful suggestions during this study and the preparation of this manuscript, as well as Dr. Owen Schwartz of the National Institute of Allergy and Infectious Diseases Confocal Microscope Facility for his help in obtaining the images used in this report.
A.N. Houghton was supported in part by a grant from Swim-Across-America and from the National Cancer Institute.
Footnotes
Abbreviations used in this paper: ER, endoplasmic reticulum; HSP, heat shock protein; TAP, transporter associated with antigen processing.
M. Mayhew's present address is Mojave Therapeutics, Inc., Tarrytown, NY 10591.
References
- Germain R.N. MHC-dependent antigen processing and peptide presentationproviding ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Pamer E., Cresswell P. Mechanism of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Castellino F., Germain R.N. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- Castellino F., Zappacosta F., Coligan J.E., Germain R.N. Large protein fragments as substrates for endocytic antigen capture by MHC class II molecules. J. Immunol. 1998;161:4048–4057. [PubMed] [Google Scholar]
- Rudensky A., Preston-Hurlburt P., Hong S.C., Barlow A., Janeway C.J., Jr. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Lindner R., Unanue E.R. Distinct antigen MHC class II complexes generated by separate processing pathways. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6910–6920. [PMC free article] [PubMed] [Google Scholar]
- Hartl U.F. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Srivastava P.K., Menoret A., Basu S., Binder R.J., McQuade K.L. Heat shock proteins come of ageprimitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- Ciupitu A.M., Petersson M., O'Donnell C.L., Williams K., Jindal S., Kiessling R., Welsh R.M. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 1998;187:685–691. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachere N.E., Li Z., Chandawarkar R.Y., Suto R., Jaikaria N.S., Basu S., Udono H., Srivastava P.K. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto R., Srivastava P.K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Chandawarkar R.Y., Wagh M.S., Srivastava P.K. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J. Exp. Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K.L., Benacerraf B., Abbas A.K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med. 1984;160:1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Snider D.P., Segal D.M. Targeted antigen presentation using crosslinked antibody heteroaggregates. J. Immunol. 1987;139:1609–1616. [PubMed] [Google Scholar]
- Casten L.A., Kaumaya P., Pierce S.K. Enhanced T cell responses to antigenic peptides targeted to B cell surface Ig, Ia, or class I molecules. J. Exp. Med. 1988;168:171–180. doi: 10.1084/jem.168.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Swiggard W.J., Heufler C., Peng M., Mirza A., Steinman R.M., Nussenzweig M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- Bevan M.J. Class discrimination in the world of immunology. Nature. 1987;325:192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- Carbone F.R., Bevan M.J. Class I–restricted processing and presentation of exogenous cell-associated antigen in vivo. J. Exp. Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Benvenuto R., Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I–restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258–260. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.D., Wick M.J., Roberts R.L., Findlay K., Normark S.J., Harding C.V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Norbury C.C., Hewlett L.J., Prescott A.R., Shastri N., Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;6:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C., Germain R.N. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J. Exp. Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A., Lankar D., Lacabanne V., Rodriguez A., Thery C., Rescigno M., Saito T., Verbeek S., Bonnerot C., Ricciardi-Castagnoli P. Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Schild D., Hanau D., Spehner D., Schmid C., Rammensee H.-G., de la Salle H., Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- Wassenberg J.J., Dezfulian C., Nicchitta C.V. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J. Cell Sci. 1999;112:2167–2175. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- Fujihara S.M., Nadler S.G. Intranuclear targeted delivery of functional NF-kappaB by 70 kDa heat shock protein. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:411–419. doi: 10.1093/emboj/18.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A., Lichtman A.H., Finberg R.W., Libby P., Kurt-Jones E.A. Heat shock protein (HSP) 60 activates the innate immune responseCD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Karttunen J., Sanderson S., Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P.M., Strydom D.J., Unanue E.R. Processing of lysozyme by macrophagesidentification of the determinant recognized by two T-cell hybridomas. Proc. Natl. Acad. Sci. USA. 1984;81:2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Flynn G.C., Pohl J., Flocco M.T., Rothman J.E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Del Val M., Schlicht H.J., Ruppert T., Reddehase M.J., Koszinowski U.H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- Niedermann G., Butz S., Ihlenfeldt H.G., Grimm R., Lucchiari M., Hoschutzky H., Jung G., Maier B., Eichmann K. Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Mayhew M., Trcka J., Hoe M., Takechi Y., Hartl F.U., Rothman J.E., Houghton A.N. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc. Natl. Acad. Sci. USA. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K.L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Ljunggren H.G., Stam N.J., Öhlén C., Neefjes J.J., Höglund P., Heemels M.T., Bastin J., Schumacher T.N., Townsend A., Kärre K., Ploegh H.L. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Porgador A., Yewdell J.W., Deng Y., Bennink J.R., Germain R.N. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D.H., Peterson P.A., Wilson I.A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992;257:927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Stern L.J., Wiley D.C. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure. 1994;2:245–251. doi: 10.1016/s0969-2126(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Carbone F.R., Kurts C., Bennett S., Miller J.F., Heath W.R. Cross-presentationa general mechanism for CTL immunity and tolerance. Immunol. Today. 1998;8:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Lolkema M., Matzinger P. Natural adjuvantsendogenous activators of dendritic cells. Nat. Med. 1999;11:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell deathexposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher A., Todryk S., Hardwick N., Ford M., Jacobson M., Vile R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- Dorner A.J., Wasley L.C., Kaufman R.J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J. Biol. Chem. 1989;264:20602–20607. [PubMed] [Google Scholar]
- Cuervo A.M., Dice J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]