Unlinking chromosome catenanes in vivo by site-specific recombination (original) (raw)
Abstract
A challenge for chromosome segregation in all domains of life is the formation of catenated progeny chromosomes, which arise during replication as a consequence of the interwound strands of the DNA double helix. Topoisomerases play a key role in DNA unlinking both during and at the completion of replication. Here we report that chromosome unlinking can instead be accomplished by multiple rounds of site-specific recombination. We show that step-wise, site-specific recombination by XerCD-dif or Cre-loxP can unlink bacterial chromosomes in vivo, in reactions that require KOPS-guided DNA translocation by FtsK. Furthermore, we show that overexpression of a cytoplasmic FtsK derivative is sufficient to allow chromosome unlinking by XerCD-dif recombination when either subunit of TopoIV is inactivated. We conclude that FtsK acts in vivo to simplify chromosomal topology as Xer recombination interconverts monomeric and dimeric chromosomes.
Keywords: chromosome segregation, decatenation, FtsK, tangles, XerCD
Introduction
In the first descriptions of DNA structure, Watson and Crick (1953) identified the potential consequences of the interwound strands of the DNA double helix. The two strands of the duplex must be separated in order to be copied, but they are wrapped around each other once per helical turn, an average of ∼420 000 times in the supercoiled 4.6 Mbp Escherichia coli chromosome. Every one of these links must be removed in order to separate the two replicated chromosomes. Strand separation during replication pushes the helical twists into the region ahead of the replication fork, leading to an accumulation of positive supercoiling, which must be removed if DNA synthesis is to progress. This task is accomplished primarily by the essential type II topoisomerase, DNA gyrase. If any positive supercoiling diffuses behind the replication fork, interwinding of the two newly replicated sister duplexes in a partially replicated chromosome will occur, forming precatenanes (which become catenanes when replication is complete). In E. coli, a second type II topoisomerase, TopoIV, a heterotetramer of ParC and ParE, normally plays an essential role in chromosome segregation by acting preferentially on precatenanes or catenanes, particularly in the late stages of replication, when DNA gyrase may not be able to access the small region of unreplicated DNA between the converging replication forks (reviewed in (Espeli and Marians, 2004; Schvartzman and Stasiak, 2004)).
Both linear and circular chromosomes are locally catenated at the end of replication. However, in the case of circular chromosomes, crossing over by homologous recombination at stalled or broken replication forks generates dimeric circular chromosomes (Sherratt et al, 1995) in which any pre-catenation crossings would be converted to crossings in the knotted dimer. Dimeric chromosomes are converted to monomers by XerCD site-specific recombination acting at the recombination site, dif, located in the replication termination region (reviewed in Barre and Sherratt, 2005). FtsK, a multifunctional protein that coordinates chromosome segregation and cell division, plays an essential role in dimer resolution. FtsK localises to the septum during the late stages of chromosome replication through its N-terminal domain (FtsKN) which functions in cytokinesis (Liu et al, 1998; Wang et al, 2005). The C-terminal domain of FtsK (FtsKC) uses ATP-dependent DNA translocation to synapse two sister dif sites in a conformation that is activated for dimer resolution by XerCD (Steiner et al, 1999; Aussel et al, 2002).
Faithful and efficient chromosome segregation requires that the processes of decatenation and dimer resolution are coordinated in time and space. Consistent with this, TopoIV associates with the dif region (Hojgaard et al, 1999) and interacts with FtsK to stimulate TopoIV decatenation (Espeli et al, 2003a). In E. coli, TopoIV, FtsK, the dif site and the replication machinery all appear to be associated with midcell in the late stages of chromosome replication (Espeli and Marians, 2004; Wang et al, 2005).
We have previously shown that XerCD-_dif_-FtsK recombination can unlink catenanes formed by site-specific recombination in vitro (Ip et al, 2003). Here we extend that result by showing that XerCD-_dif_-FtsK recombination can unlink in vitro the range of replication catenanes accumulated in vivo during TopoIV impairment. We also show mathematically that efficient unlinking must occur in a stepwise fashion with catenanes and knotted dimers being interconverted at each step, a mechanism supported by experiments. We provide support for the direct involvement of XerCD-_dif_-FtsK recombination in in vivo chromosome unlinking when TopoIV activity is compromised. Further, Cre-loxP site-specific recombination can substitute for XerCD-dif recombination in chromosome unlinking, both reactions requiring KOPS-directed translocation by FtsK. Finally we show that the expression of cytoplasmic FtsK is sufficient to allow chromosome segregation in both parEts and parCts backgrounds independently of the previously identified suppressor, DnaX(γ). Taken together, this is compelling evidence that site-specific recombination can act to unlink chromosomes following replication independently of TopoIV, the major cellular decatenase.
Results
XerCD-dif-FtsK recombination unlinks plasmid replication catenanes formed in vivo
XerCD-_dif_-FtsK recombination converts unknotted supercoiled DNA dimers exclusively into monomeric free circles. This requires that ATP hydrolysis-dependent FtsK translocation along DNA directs the formation of simply synapsed dif sites, whose recombination leads to topologically simple products (Aussel et al, 2002). This selectivity of XerCD-FtsK recombination for topologically simple dif synaptic complexes suggested that such recombination might unlink catenated DNA, a prediction that was confirmed experimentally using simple catenanes with either parallel or antiparallel dif sites (Ip et al, 2003).
To explore whether XerCD-_dif_-FtsK recombination might be directly involved in vivo in the unlinking of newly replicated chromosomal DNA, we tested first whether this recombination can unlink the range of interlinked products formed by replication in vivo. To obtain these, we used a temperature-sensitive parCts mutant of TopoIV (Kato et al, 1988), harbouring a multicopy plasmid with a dif site (pUC-dif). Newly replicated _dif_-containing plasmid catenanes accumulated in vivo within minutes after the switch of parCts cells containing pUC-dif to 42°C (Adams et al, 1992) (Figure 1A). Because _dif_-plasmids are not good in vivo substrates for XerCD-FtsK recombination unless a cytoplasmic form of FtsK is overexpressed (unpublished data), we did not expect that such catenanes would be efficiently unlinked in vivo by XerCD-dif recombination. After isolation of supercoiled plasmid DNA from such a culture, the catenane band was gel extracted and shown to contain molecules with 2–14 catenation links after DNaseI nicking (Figure 1B, lane 1). A low level of dimeric knots was also present. The supercoiled replication catenane population was reacted in vitro with XerCD and FtsK50C, a biochemically active form of FtsK (Aussel et al, 2002; Ip et al, 2003). In the presence of ATP, the catenane population was efficiently converted to free circles in a 30 min 37°C reaction (lane 6). This reaction was dependent on ATP hydrolysis, since no reaction was observed with the nonhydrolysable analogue, AMPPNP or poorly hydrolysable ATPγS (not shown).
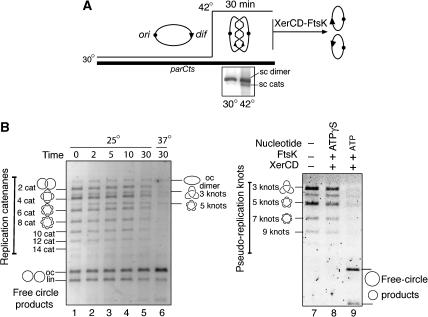
Figure 1.
Unlinking of replication catenanes by Xer recombination. (A) The strategy used to isolate plasmid replication catenanes. parCts cells harbouring a pUC-dif plasmid were grown at 30°C and then were subjected to a 30 min 42°C temperature shift. The supercoiled catenated _dif_-plasmid DNA (sc cats) was gel-extracted. In this and subsequent figures, double-stranded DNA is represented by a single line, and dif sites by a triangle. (B) Unlinking by XerCD-_dif_-FtsK50C recombination of replication pUC-dif catenanes formed in vivo. Product topology is revealed after DNaseI nicking (all lanes). Unlinked monomers are labelled as free circle products: oc, open circle; and lin, linear. Left panel: time course of XerCD-_dif_-FtsK50C recombination of replication catenanes at 25°C, alongside a 37°C control reaction. Dimeric DNA knots and unknotted dimeric DNA (oc dimer) appear as transient reaction intermediates in the course of catenane unlinking. Right panel: XerCD-_dif_-FtsK50C conversion of DNA dimer torus knots to free circles.
Initial considerations suggested that the unravelling of replication catenanes to free circles by XerCD-FtsK50C would proceed in a stepwise manner with one linking node removed per recombination event, with catenated monomers and knotted dimers being interconverted at each recombination step (Ip et al, 2003) (also see Figure 2D). In an attempt to slow the reaction and resolve these intermediates, a time course of unlinking was carried out at 25°C. Examination of the unlinking patterns of replication catenanes at 25°C was consistent with this stepwise unlinking (Figure 1B; lanes 2–5). In particular, knotted dimers appeared as transient recombination intermediates as the reaction progressed. While the majority of transient knotted forms may be difficult to discern, the appearance of the five-noded knot is the most apparent.
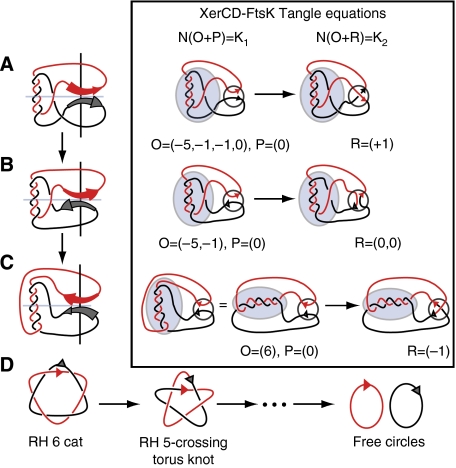
Figure 2.
Tangle methodology for stepwise unlinking by XerCD recombination at dif. The three possible topological mechanisms, which eventually yield unlinked products, are shown for the reaction from a RH 6-crossing catenane to a (+)5-torus knot (A–C). The tangle notation is presented in the framed figure. O, P and R are tangles, one round of recombination at dif by XerCD-FtsK is represented by a system of two tangle equations: N (O+P)=K1, N (O+R)=K2. Here _K_1 is a RH 6-cat with parallel sites, and the product _K_2 is assumed a knot or catenane of six or less crossings. The pre- and post-recombination synapse are trapped inside P and R, respectively. The tangle O encloses all nontrivial topology outside the synapse. Panels A, B and C illustrate the only three plausible solutions to this system of equations. The three proposed solution mechanisms recombine the 6-catenane into the RH 5-crossing torus knot; repeated rounds of recombination result in a stepwise unlinking of the substrate (D). In the leftmost diagrams viewing the synapse along the light blue axis converts A into B. Similarly a left-handed 180° rotation of the synapse in A around the black axis converts this to C. Therefore, these three solutions correspond to different projections of the same three-dimensional object. Note also that the three products shown in the right-hand panel are all the same five-noded knot product.
Catenane unlinking through DNA knot intermediates demands that XerCD-FtsK50C must be efficient at unlinking both DNA knots and catenanes. To demonstrate this, XerCD-FtsK50C recombination was assayed on torus knots that have equivalent topology and arrangement of dif sites to knot intermediates derived from replication catenanes. A population of supercoiled right-handed torus knots with dif sites in direct repeat was made by Cre-loxP site-specific recombination of a plasmid containing directly repeated dif sites and inverted loxP sites. A population of such supercoiled knots containing three-, five-, seven- and nine nodes was recombined to free circles when treated with XerCD-FtsK50C and ATP (Figure 1B; lanes 7–9). Note that this is a different substrate from the catenanes used in the left-hand panel (lanes 1–6), and so yields two nicked circular products of different sizes.
The demonstration that XerCD-FtsK50C recombination in vitro efficiently unlinks the range of replication catenanes accumulated in vivo after TopoIV inactivation strengthens the hypothesis that XerCD-FtsK recombination at dif may assist TopoIV in unlinking newly replicated DNA.
Tangle analysis supports a stepwise unlinking mechanism by XerCD-FtsK
We applied the tangle method, under assumptions suited for the Xer system, to assess rigorously whether stepwise unlinking is the only pathway for unlinking of replication catenanes by XerCD (Sumners et al, 1995). The software TangleSolve (Saka and Vazquez, 2002) was used to compute the topological mechanisms producing prime knots or catenanes with six or less crossings from a right-hand (RH) 6-cat substrate with sites in parallel orientation. There were only three plausible mechanisms with the correct site orientation, which are interpreted as three different projections of the same three-dimensional object (Figure 2). In other words, a single three-dimensional pathway satisfies the model. Iterating any of these mechanisms results in a stepwise unlinking taking the RH 6-cat to (+)5-torus knot, to RH 4-cat, to (+) trefoil, to 2-cat, to unknot, to free circles. Other pathways producing more complex intermediates containing six or more crossings were also considered, however the stepwise reduction in complexity of replication (torus) catenanes, via (torus) knotted intermediates, was the only route to efficiently produce free circles (Figure 2 and Supplementary data).
In summary, rigorous mathematical analysis supports the experimental evidence that unlinking of catenated newly replicated DNA by XerCD-dif recombination occurs by a stepwise reaction involving interconversion of catenated and knotted intermediates, with the topology being simplified at each step, until two free circles are produced.
The rescue of TopoIV temperature sensitivity by DnaX overexpression is dependent on XerCD-dif-FtsK recombination
Given that XerCD-_dif_-FtsK recombination in vitro unlinks plasmid replication catenanes, we addressed whether this recombination can remove catenation links from the chromosome at the completion of DNA replication, in addition to resolving chromosome dimers at dif. XerCD-_dif-_FtsK action is confined to the replication termination region (ter), at a time when TopoIV, FtsK, the replisome and ter are all localised to midcell and when completion of both DNA unlinking and dimer resolution must be achieved for chromosome segregation to occur successfully (Espeli et al, 2003b; Wang et al, 2005). TopoIV associates with dif and with the replisome at a time when they are all positioned in the midcell region and appears to have its unlinking activity stimulated by FtsK (Hojgaard et al, 1999; Espeli et al, 2003a, 2003b).
To assess the direct involvement of XerCD-_dif-_FtsK recombination in chromosome unlinking, TopoIV decatenation activity had to be impaired. Therefore, we exploited the fact that the temperature sensitivity of a parEts strain is suppressed by overexpressing the DnaX γ clamp loader (Levine and Marians, 1998). This suppression, which was proposed to arise by the stabilisation of ParEts by γ, is also dependent on FtsK, a result used to support experimental evidence for a physical interaction between FtsK and TopoIV (Espeli et al, 2003a). In light of the unlinking activity displayed by XerCD-FtsK50C in vitro, we addressed whether suppression of parEts temperature sensitivity by γ overexpression is a direct consequence of chromosomal decatenation by XerCD-FtsK recombination at chromosomal dif.
Strains containing parEts in combination with mutant alleles of xerD, xerC, xerC YF and dif were transformed with plasmid pLex-dnaX(γ) that overexpresses γ in the presence of IPTG (Espeli et al, 2003b). To monitor the ability of γ overexpression to support survival at restrictive temperatures, growth of these strains was examined in liquid media at the permissive and nonpermissive temperatures in the presence and absence of γ overexpression (Figure 3A and B). All strains grew well at 30°, with similar growth rates, irrespective of the presence of IPTG. As expected from previous experiments, γ overexpression suppressed the temperature sensitivity of parEts at 43° as judged by _A_600 increase (Figure 3A) and by colony formation (Figure 3B). However, overexpression of γ did not restore the growth of parEts strains that did not produce XerC or XerD, or that contained a catalytic xerC YF mutation. As reported previously, deletion of dif also led to a lack of suppression of temperature sensitivity by γ overexpression (Espeli et al, 2003a). The inability of γ overexpression to support the continued growth of the parEts xerC YF strain leads us to favour a model in which the failure to suppress is due to the lack of Xer recombination at dif, since XerCYF forms a functional synapse, capable of strand exchange by XerD (Bregu et al, 2002), and is therefore likely to interact functionally with its partners.
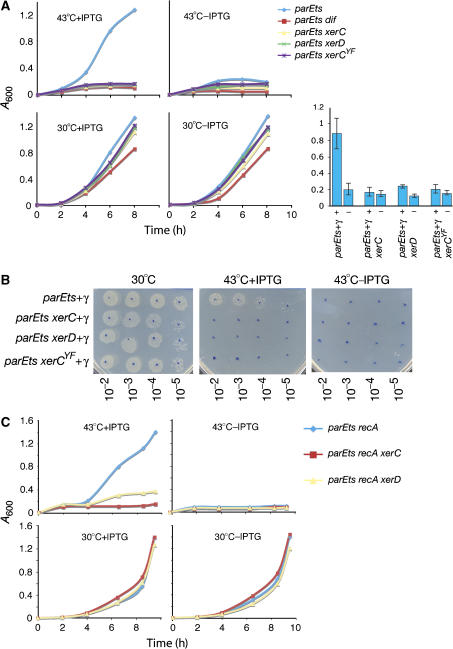
Figure 3.
Suppression of parEts temperature sensitivity by γ overexpression is dependent on XerCD-dif recombination. (A) Typical growth curves at 30 or 43°C for strains that are isogenic except for the indicated mutations, with or without IPTG to induce γ overexpression, as indicated. Each contained the γ overexpression plasmid, pLexA-dnaX(γ). To the right are histograms showing the mean values +/− 1 standard deviation of _A_600 of five independent cultures for each strain. (B) Colony-forming units for the strains at 30°C (left panel) and 43°C with IPTG (middle panel) and 43° without IPTG (right panel). Cultures were grown to an _A_600∼0.6, serially diluted (as indicated), plated and grown in the indicated conditions. (C) Typical growth curves at 30°C or 43°C, with and without IPTG, of the indicated recA strains.
XerCD-dif-FtsK recombination rescues parEts cells in the absence of dimer formation by homologous recombination
To rule out the possibility that the failure of XerCD-dif recombination-deficient cells to suppress the temperature sensitivity of parEts cells during γ overexpression was a consequence of an inability to resolve chromosome dimers, an recA mutation was introduced into the test strains and growth was examined (Figure 3C). γ overexpression supported the 43°C growth of parEts recA strains proficient for XerCD recombination at dif, but not in those deficient for XerCD-dif recombination. Therefore, XerCD-dif recombination is needed to suppress ParEts temperature sensitivity by γ overexpression in the absence of chromosome dimers formed by homologous recombination.
Chromosome separation is impaired in parEts _γ_-overexpressing cells at 42°C when Xer recombination is compromised
The role of Xer recombination in the rescue of parEts by overexpression of γ was examined by flow cytometry. First it had to be established whether TopoIV impairment affects ongoing replication. To test this, strains were grown in minimal media at the permissive temperature and then treated with cephalexin and rifampicin, replication ‘run-out' conditions, at 30 and 42°C, where replication is completed, but replication re-initiation and cell division are blocked. The analysis revealed almost identical profiles for the parEts strain at both temperatures, with most cells having two chromosome equivalents at the end of the run-out. This shows that ongoing replication of the bulk of the chromosome continues during TopoIV impairment (Figure 4A), consistent with an earlier report (Espeli et al, 2003b). Any effect upon very late stages of replication cannot be assessed by this technique. The xerC and recA derivatives of the parEts strain also showed the ability to complete replication in run-out conditions at nonpermissive temperature, as did impairment of TopoIV by a parCts mutation (data not shown).
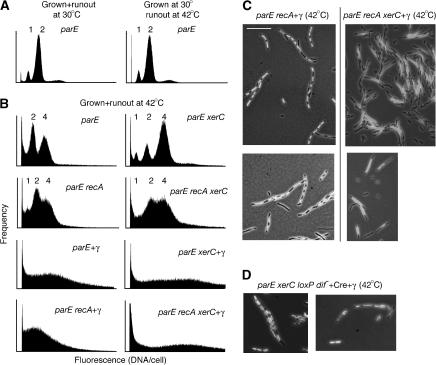
Figure 4.
Chromosome segregation requires an active site-specific recombination system at the terminus. (A) Flow cytometry histograms of replication run-out at 30°C compared to run-out at 42°C of a parEts strain initially grown at 30°C. Chromosome equivalents are indicated above each peak. DNA staining is with syto-16. (B) Flow cytometry histograms showing replication run-out of strains following growth for 3 h at 42°C; the left-hand set of panels shows the Xer+ strains, and the right-hand panels are the corresponding xerC derivative strains. DNA staining is with syto-16. Chromosome content was determined by comparison of multiple experiments with both syto-16 and propidium iodide staining and is marked above each peak. Individual peaks are not observed in the strains overexpressing γ. (C) Micrographs of DAPI-stained cells grown at 42°C for 3 h. Fields from two independent experiments are shown. Note the absence of segregated nucleoids in the xerC derivative strain (right hand panels) as well as numerous anucleate cells: classical Par− phenotype. The bar represents 10 μm. (D) Micrographs of DAPI-stained parEts xerC cells where the dif site is replaced by loxP, and both γ and the Cre recombinase are expressed, grown in LB at 42°C for 3 h. The cells are filamentous but have well separated nucleoids.
Having established that ParEts inactivation does not stop ongoing chromosome replication, the effects of ParEts inactivation on chromosome segregation were compared in Xer+and Xer− backgrounds. Cells grown in minimal media at the permissive temperature were shifted to 42°C for 3 h, followed by a further 3 h at 42°C with cephalexin and rifampicin, prior to analysis by flow cytometry (Figure 4B). With all the strains tested, growth and run-out at 30°C showed very little variation between the Xer+ and Xer− strains (data not shown). After growth at nonpermissive temperature, replication run-out revealed a larger proportion of cells with higher chromosome content in the parEts xerC strain than in parEts Xer+. The patterns observed are consistent with a failure to segregate chromosomes in both strains, but seemingly worse in the Xer− background. A similar trend toward higher chromosome content in an xerC derivative was also seen with a recA background, showing that the higher chromosome content is not related to a failure to resolve chromosome dimers.
To examine the effect of γ overexpression, a parEts recA strain and its xerC derivative were used to remove any complication of chromosome dimer formation. After 3 h growth at 42°C, both these strains were filamentous. The parEts recA cells overexpressing γ went from a mean length of 5.0 μm (σ=1.4, _n_=50) at 30° to a mean length of 19.4 μm (σ=7.9, _n_=64) at 42°C. A similar difference was seen with the xerC derivative. This increase in length is in the range expected if cell division stopped at 42°C, but the cells continued growing at a similar rate; 3 h growth reflects about two doublings in this growth media. Without γ overexpression, the parEts cells rarely achieved this filament length, because of cessation of cell growth and/or ongoing division to produce the many anucleate cells observed.
The propensity to filament masks any possible effects of Xer mutation on the chromosome content of γ-overexpressing cells, as judged by chromosome content during flow cytometry; presence or absence of homologous recombination had little overall effect on the chromosome content (Figure 4B). However, when DAPI-stained cells were examined microscopically, it was apparent that the filaments produced by γ overexpression contained smaller well-separated nucleoids in an Xer+ background, but had unseparated DNA in an Xer− background (Figure 4C). The average length of nucleoids in γ-overexpressing parEts recA cells was 3.05 μm (σ=0.94, _N_=89), whereas in the xerC derivative the average length was 6.39 μm (σ=1.78, _N_=56), indicative of a failure to segregate the chromosome in the absence of Xer recombination. For comparison, the nucleoid lengths at 30°C were 3.10 μm (σ=0.65, _N_=100) and 3.16 μm (σ=0.82, _N_=100), respectively, for the parE recA and parE recA xerC strains overexpressing γ.
The finding that in the absence of γ overexpression an Xer− derivative accumulates higher chromosome content than an Xer+ cell implies that Xer recombination is going some way to allow segregation in parEts cells at 42°C, but that without γ overexpression, it is not sufficient to allow cell survival. It is plausible that DnaX overexpression allows more time for the cells to decatenate their chromosomes by recombination, leading to inhibition of cell division in the short term, but viability in the long term.
Cre-loxP site-specific recombination can substitute for XerCD-dif in _γ_-mediated suppression of the parEts phenotype
Suppression of the parEts phenotype by overexpression of γ is thus dependent on XerCD, FtsK and dif. If this suppression is through iterated rounds of site-specific recombination, which reduce the catenation crossings of the chromosomes until they are unlinked from each other, then it might be possible that another recombination system could substitute for XerCD-dif in the terminus. To test this, a parEts strain in which the dif site was replaced by loxP was analysed. FtsK-dependent replacement of XerCD-dif by Cre-loxP for chromosome dimer resolution has been demonstrated previously (Leslie and Sherratt, 1995; Capiaux et al, 2002).
Cells expressing a low constitutive level of the Cre recombinase and containing pLex-dnaX(γ) were grown on LB plates with either glucose or IPTG at 30 or 42°C. Cells grew well at 30°C, but none survived to give colonies at 42° with glucose, or when the Cre expression vector was absent. Conversely, at the nonpermissive temperature in the presence of IPTG, growth was observed with a mixture of several large colonies, as well as many small colonies. When re-streaked at 42°C, the cells again showed variable growth, with some clones again giving variable-sized colonies and some giving larger colonies. An xerC derivative of the same strain containing both the Cre and γ expression vectors exhibited the same behaviour, thereby demonstrating that suppression of the temperature sensitivity is XerC-independent when Cre-loxP recombination can occur.
Individual large and small clones on the 42°C plates were grown in liquid culture at 42°C in the presence of IPTG. Dilutions were plated onto LB agar with IPTG, alongside a parEts+pLex-dnaX(γ) control. All cultures yielded similar numbers of viable colonies upon growth at either 30 or 42°C. Also, samples were DAPI stained and examined microscopically (Figure 4D). The filamentous cells (as previously observed when γ was overexpressed at 42°C), had well-segregated nucleoids along their length, similar to those in a strain competent for XerCD-dif recombination, confirming that unlinking of chromosomes was occurring within the cell filaments. We conclude that iterative Cre-loxP recombination can substitute for XerCD-dif recombination in unlinking chromosomes, thereby rescuing the ParEts phenotype when γ is overexpressed.
The failure of the parEts loxP_Δ_dif cells expressing γ and Cre to give uniform growth at 42°C may be related to the requirement to maintain two separate plasmids within each cell. When TopoIV is impaired, plasmid replication catenanes accumulate (Zechiedrich and Cozzarelli, 1995).
FtsK mutants incompetent for KOPS-directed translocation towards the terminus fail to suppress parEts
FtsK is able to translocate directionally on the chromosome, guided by polarised 8 bp sequences, KOPS, that point towards the dif site (Bigot et al, 2005; Ptacin et al, 2006). parEts strains were constructed that contain chromosomal variants of FtsK that are translocation-competent but are impaired in their ability to be guided by these KOPS sequences (Sivanathan et al, 2006). FtsKΔγ is deleted for the FtsK γ domain and is therefore unable to support XerCD-dif recombination (Yates et al, 2006), while FtsK-Blind contains three substitutions (R1300A, E1303A, E1306A) within the DNA-binding helix of the γ domain, changes that do not compromise XerCD-dif recombination on plasmid substrates (Sivanathan et al, 2006) or on the chromosome (data not shown). The expectation is that FtsK-Blind will retain its ability to interact with the same range of protein partners as the wild-type protein (Sivanathan et al, 2006).
The two FtsK variants were tested for their ability to suppress the parEts phenotype when γ was overexpressed in strains competent for either XerCD-dif or Cre-loxP recombination. None of the four strains were able to support growth or colony formation at 42°C, whereas growth was normal at 30°C. Therefore, both Cre-loxP and XerCD-dif recombination at the chromosome terminus require KOPS-directed translocation by FtsK.
Expression of cytoplasmic FtsK50C facilitates chromosome segregation in parEts and parCts cells
In order to investigate whether efficient site-specific recombination could suppress the parEts phenotype in the absence of overexpression of γ, parEts cells were transformed with a plasmid that expresses cytoplasmic FtsK50C from the arabinose promoter (Aussel et al, 2002). Transformed cells grown at 30°C overnight were subcultured into fresh media containing varying amounts of arabinose, grown initially at 30°C followed by a shift to 42°C. Substantive overexpression of FtsK50C results in cessation of growth followed by cell death. Cells supplemented with a low level of arabinose (⩽0.01%) grew steadily under these conditions, albeit more slowly than a control parEts pLex-dnaX(γ) strain with IPTG. This continued growth was not seen when an xerC derivative was used, confirming the dependence of growth upon site-specific recombination.
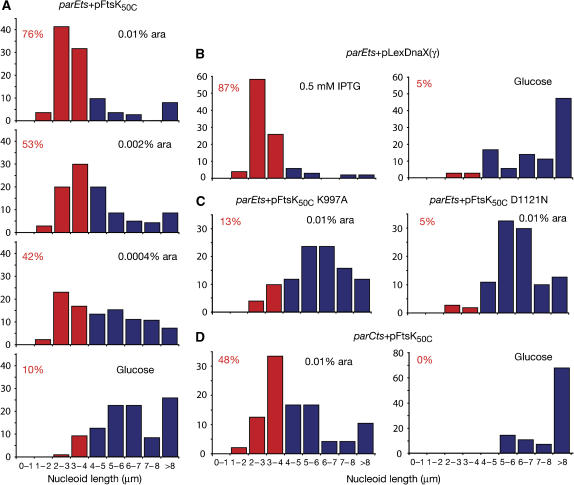
DAPI-treated cells from these strains were examined microscopically. With subtoxic levels of FtsK50C expression, a proportion of the cells contained well-segregated nucleoids, while the remainder showed a Par− phenotype. To quantify this suppression, the DAPI-stained nucleoid length distributions were determined (Figure 5A). The length distribution of nucleoids when 0.01% arabinose was present is similar to that seen in the control γ overexpression parEts strain (Figure 5B). The proportion of segregated nucleoids decreases as the arabinose level is decreased, and with glucose present to repress FtsK50C expression, the nucleoid length distribution resembles the control parEts strain where γ expression is repressed.
Figure 5.
FtsK50C expression allows nucleoid segregation when TopoIV is inactivated. Cells grown in LB at 42°C for 2 h were stained with DAPI and individual nucleoid lengths measured. Each graph shows 50–200 individual measurements from a single growth experiment. Multiple repeats of the experiment gave the same trends. Nucleoid lengths are grouped into 1 μm intervals, and bars representing those measuring 4 μm or less are coloured red, those longer than this are coloured blue. The percentage of nucleoids that are ⩽4 μm is shown in red at the top left of each graph. Growth conditions are indicated at the top right. (A) parEts cells grown with a plasmid expressing wild-type FtsK50C from a plasmid under the control of the arabinose promoter. (B) Control cells showing the nucleoid size distribution when γ is overexpressed from pLexDnaX(γ). (C) parEts cells expressing the Walker A mutant (K997A) or Walker B mutant (D1121N) of FtsK50C from a plasmid, under control of the arabinose promoter. (D) parCts cells with the vector for expression of FtsK50C.
When γ was overexpressed (Figure 5B), 87% of cells had nucleoid lengths of 4 μm less, whereas without γ overexpression, only 5% of cells fell into this category. Using a nucleoid length of 4 μm or less as an indicator of segregated chromosomes (Figure 5; red bar), 76% of cells expressing FtsK50C with 0.01% arabinose fall into this category. This decreases to 10% of cells when glucose was present. When a plasmid expressing a catalytically inactive Walker A (K997A) or Walker B (D1121N) mutant of FtsK50C was expressed under these same conditions, the majority of cells displayed a central unsegregated nucleoid, with only 13 or 5%, respectively, having nucleoids under 4 μm long (Figure 5C). This confirms that active XerCD and FtsK are required to suppress the parE temperature sensitivity, and that this effect can be seen independently of overexpression of γ.
parCts cells were also transformed with the FtsK50C expression plasmid and grown as above. When the length distribution of DAPI-stained nucleoids was examined, it was seen that the presence of 0.01% arabinose led to 48% of the cells having nucleoids of 4 μm or less. In the presence of glucose, no cells were observed with these smaller segregated nucleoids. Thus, the nucleoid segregation effect of overexpression of FtsK50C is seen in both parEts and parCts backgrounds, unlike suppression by γ overexpression, which is _parE_-specific (Levine and Marians, 1998).
Discussion
We have presented the first evidence for chromosome decatenation in vivo by tyrosine site-specific recombinases. Catenation of the daughter chromosomes arises as a result of replication of helical DNA and prevents chromosome segregation unless every single link is removed. TopoIV efficiently removes these catenation crossings (Kato et al, 1990), while DNA gyrase, the other type II topoisomerase present in E. coli, is at least 100- to 1000-fold less efficient in decatenation (Peng and Marians, 1993; Zechiedrich and Cozzarelli, 1995). At 42°C, TopoIVts cells are unable to propagate because of accumulation of catenation leading to a failure to segregate daughter chromosomes (Figure 6). Although no other mechanism present in wild-type cells can fully compensate for the loss of TopoIV activity, we have shown that efficient iterated rounds of XerCD-dif, or Cre-loxP site-specific recombination, acting in the terminus region in vivo, can decatenate daughter chromosomes when either cytoplasmic FtsK50C is expressed, or DnaX(γ) is overexpressed (Levine and Marians, 1998). XerCD-dif recombination requires FtsK to directly activate recombination through interaction with XerCD, and to ensure that all recombination occurs on simple synapses with no trapped links, so that complex catenated and knotted products of recombination are not formed. The ability of Cre-loxP recombination to replace XerCD-dif during TopoIV impairment, and indeed to act in chromosome dimer resolution (Capiaux et al, 2002), is also FtsK-dependent, because FtsK translocation directs Cre recombination to give simple products (Ip et al, 2003). KOPS-directed translocation by FtsK may also indirectly stimulate XerCD-dif and Cre-loxP recombination as a result of facilitating synapsis by colocalising the two chromosomal termini within the cell.
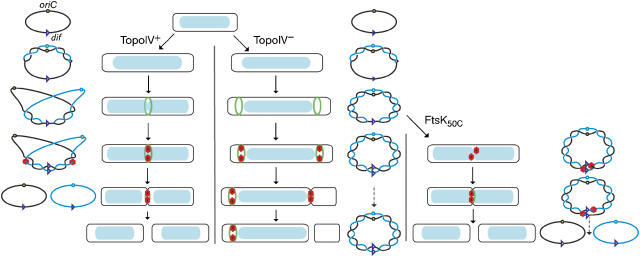
Figure 6.
Model for chromosomal decatenation and segregation. A cell is represented here with a light blue nucleoid inside it, alongside a representation of the (pre-)catenation state of the chromosome (as previously, a single line represents double-stranded DNA). On the cellular level, the green ring represents the FtsZ ring and the subsequent divisome ring components with FtsK being a red hexamer. When TopoIV is active (left-hand side), any initial pre-catenation (i.e. interlinks between newly replicated sisters) formed during replication is quickly removed by topoisomerase action or moved away from the oriC region allowing it to segregate. Towards the end of replication, the FtsZ ring is formed at midcell over the invaginating nuceloid. When replication is completed, the action of TopoIV and/or XerCD-FtsK-dif resolves the remaining interlinks. When TopoIV is inactive, (pre-)catenation accumulates and FtsZ, and subsequently FtsK, is excluded from the nucleoid. As growth continues, anucleate cells are produced. FtsK50C is not dependent on localisation by FtsZ, but can translocate on the catenated nucleoid. FtsK/XerCD can then function in de-catenation, but only after dif has been replicated. However, it is possible that translocation of FtsK towards the terminus can function to localise catenation to this region and so could aid in segregation of oriC proximal markers. On the right-hand side, a single round of FtsK-XerCD-dif recombination to produce a knotted dimer is shown. Iterated rounds of recombination eventually yield unlinked chromosomes.
Mathematical analysis of Xer recombination demonstrates that the only tenable pathway for unlinking of replication catenanes is a multistep mechanism interconverting catenated monomers and knotted dimeric forms, with intermediates becoming topologically simpler by one link at each step, until the two chromosomes are finally unlinked. Consistent with this, knotted forms are seen as reaction intermediates on the pathway to free circles. Therefore, the number of site-specific recombination events needed to decatenate the chromosome is equal to the number of catenation nodes present before the reaction, and each step of this process must be carried out in a simple synapse, a process dictated by FtsK.
The observation that expression of cytoplasmic FtsK50C allows efficient chromosome segregation in both parCts and parEts backgrounds suggests that recombination at dif is sufficient to overcome the absence of TopoIV. We conclude that without expression of γ or FtsK50C, endogenous site-specific recombination cannot remove all chromosomal catenation, either because the FtsK levels are too low, or because access of FtsK and the recombination machinery to a nucleoid containing extensively catenated daughter chromosomes is limited. We favour the latter explanation, since if extensive catenation arises as a consequence of TopoIV impairment, the daughter nucleoids will not be able to initiate separation and FtsK action at XerCD-dif will be prevented (Figure 6). This is because nucleoid occlusion prevents an FtsZ ring formation at midcell when chromosome separation is inhibited (Sun et al, 1998); FtsK is recruited to the septum by interaction with FtsZ (Liu et al, 1998) and is active only when septum formation is well advanced and daughter nucleoids are largely separated (Steiner and Kuempel, 1998; Steiner et al, 1999). The presence of many anucleate cells and smooth filaments in TopoIV-impaired cells shows that FtsZ ring formation and subsequent septation are either inhibited, or occur inappropriately away from the central nucleoid mass. This may explain why native FtsK is unable to promote efficient unlinking by XerCD recombination under these conditions. Because cytoplasmic FtsK50C can access DNA independently of FtsZ and septation, it will facilitate XerCD-dif recombination even when chromosome separation and normal septation have been inhibited.
The mechanism by which γ overexpression supports the viability of parEts cells when FtsK and site-specific recombination function in chromosome unlinking is unclear. We are attracted to the idea that excess γ restricts formation of precatenanes, because its association with the replisome limits dissociation or rotation of the replication machinery, a prerequisite for precatenane formation during replication. This would enable gyrase to play a greater role in parental strand unlinking as replication proceeds, resulting in fewer catenation links holding the unsegregated nucleoids together. This reduced level of trapped DNA may allow active FtsK rings to access a partially segregated nucleoid and facilitate the action of XerCD, which would then have to proceed through fewer iterations to complete chromosome unlinking. Nevertheless, such multiple rounds of FtsK-XerCD-dif unlinking could lead to a delay in cell division, as observed here and in a previous study (Levine and Marians, 1998).
There may be additional compensatory effects of γ overexpression that are not revealed by these studies. For example, we cannot eliminate the possibility that interactions between TopoIV and FtsK-XerCD at midcell also concentrate and stabilise TopoIVts and thereby contribute to the suppression when γ is overexpressed, as proposed by Espeli et al (2003a). Nevertheless, the demonstration here that survival of parEts cells overexpressing γ is dependent on active Cre-loxP or XerCD-dif recombination rather than simply the presence of the recombination proteins, and is independent of homologous recombination, argues against stabilisation of TopoIVts activity playing a central role in the suppression. Similarly, the demonstration that suppression is dependent on not only the presence of FtsK, but also that its translocation be KOPS-directed, argues strongly for site-specific recombination underlying the suppression mechanism. Most compelling is the finding that expression of FtsK50C can support chromosome segregation in the absence of γ overexpression both in a parEts and a parCts strain.
The requirement for KOPS-directed translocation by FtsK in Xer-mediated decatenation could be to promote the alignment of recombination sites present in the terminus region at midcell, facilitating simple synapsis and thereby mediating efficient unlinking by recombination. Even then, multiple rounds of recombination will be required to remove substantial catenation, since each pair of strand exchanges will remove a single link, compared to a linkage change of two per step for TopoIV. Each of the iterated recombination steps requires a resetting of the recombinase synapse, since processive recombination by tyrosine recombinases would lead to reaction reversal rather than unlinking (Ip et al, 2003). This, coupled to the fact that TopoIV is not limited by a single site of action and can remove catenation crossings wherever they may occur, suggests that most catenation in a wild-type cell is removed by TopoIV. Nevertheless, we note that whereas TopoIV action is limited to the site of a DNA crossing, rounds of recombination at dif can remove crossings that are located anywhere on the chromosomes. KOPS-directed translocation by FtsK in normal cells may also localise catenation crossings to the dif region, facilitating their removal by TopoIV associated with this region. Therefore, KOPS-directed translocation by FtsK may facilitate the action of both TopoIV and XerCD in chromosome unlinking and segregation, a view consistent with the observation that FtsK has been shown to interact with ParC (Espeli et al, 2003b). FtsK may therefore constitute the core of a chromosome segregation machine that acts to complete chromosome unlinking and segregation; complexes of XerCD-_dif_-FtsK-TopoIV would localise the activities necessary for segregation, decatenation and dimer-resolution, to a specific region of the chromosome terminus and to the developing septum. We note that dimer formation by homologous recombination during replication will convert any precatenane/catenane crossings to knot nodes in the dimer. The first round of recombination by XerCD at dif will convert knotted dimers to catenated monomers rather than free circles. Hence, chromosome dimer resolution will always produce catenated monomers unless the initial dimer has previously had all knot nodes removed. For segregation to occur, these crossings need to be unlinked by further rounds of XerCD recombination or by TopoIV action.
The action of FtsKC becomes crucial when normal chromosome segregation has not been completed in a timely manner; for example, when the presence of dimers or catenanes has prevented segregation away from the septum. We think it likely that the C-terminal translocase domain of FtsK forms hexameric rings around any trapped DNA and then functions in dimer resolution-decatenation without being able to distinguish whether it is operating on knotted dimers or catenated monomers, and interconverts the two. This blindness of XerCD-dif recombination to external DNA topology is also manifest in dif+ plasmids in vivo and in vitro. Consistent with this, XerCD recombination is not dependent on the presence of chromosomal dimers because decatenation by XerCD occurs in both RecA+ and RecA− cells. Sequential rounds of XerCD recombination will then occur until the two daughter chromosomes are unlinked and sister dif sites are separated by chromosome segregation. Future experiments need to characterize when and how functional FtsK is organised at the septum, how its activity is related to the progression of septation and chromosome segregation, and whether chromosomal DNA is transported before and/or after closure of the septal inner membranes.
Materials and methods
Strains and plasmids
parCts and parEts strains were C600_parC1215_ and W3110_parE10_ (Kato et al, 1988, 1990). parCts was used to isolate catenated newly replicated plasmids and parEts was used to monitor the effect of γ overexpression in supporting viability at restrictive temperatures (Adams et al, 1992; Espeli et al, 2003b). An _xerC_YF mutation was transferred into the xerC gene by allelic exchange, thereby replacing the insertion within xerC2 by the xerC YF allele. To produce _dif_-containing DNA knots, a plasmid containing directly repeated dif sites and inverted loxP sites was reacted with Cre as described (Ip et al, 2003). The IPTG-inducible γ expression plasmid, pLex-dnaX(γ), has been described (Espeli et al, 2003b). Cre expression plasmids pMS173 or pMS174, a kind gift from S Colloms and WM Stark, are based upon the Cre variants previously described (Gourlay and Colloms, 2004). FtsK50C and its catalytic mutants were expressed from a plasmid under control of the arabinose promoter (Aussel et al, 2002; Pease et al, 2005).
Isolation and recombination of replication plasmid catenanes
Overnight cultures of a pUC-dif parCts strain were diluted 1:200 into fresh media with ampicillin (Ap; 100 μg/ml) and grown at 30°C to _A_600 0.4. Cells were then grown for 30 min at 42°C. Cells were quickly transferred to ice and plasmid DNA was immediately extracted using QIAgen kits, then loaded onto 0.7% agarose gels in TBE. Gels were run at 4 V/cm for 18–24 h. Under these electrophoresis conditions, the catenated newly replicated sister plasmids run ahead of the supercoiled plasmid dimer. This band was excised from the gel and purified by a QIAquick gel extraction kit. The purified population of supercoiled replication plasmid catenanes was reacted in vitro with XerCD-FtsK50C as described (Ip et al, 2003).
Rescue of parEts derivatives by clamp loader γ overexpression
Cultures of W3110_parEts_ pLex-dnaX(γ) strains (Espeli et al, 2003b) were grown overnight at 30°C in LB+Ap and diluted 1:600 into fresh media to _A_600 0.002. Where indicated, IPTG (500 μM) was added to induce γ overexpression. Samples were grown with shaking at 30 and 43°C. The _A_600 was measured every 2 h for the growth curves, or after 8–9 h of incubation at respective temperatures for the comparative growths in liquid media. To determine colony formation, cultures were grown in LB overnight at 30°C, diluted into fresh media containing IPTG and grown at 30°C to _A_600 ∼0.6. The cultures were then adjusted to similar concentrations and serially diluted. Aliquots (10 μl) were then spotted on LB Ap plates with or without IPTG and incubated overnight at 30 and 43°C.
Flow cytometry and microscopy
Cells were grown overnight at 30°C in M9 minimal media supplemented with glycerol (0.2%) and casamino acids (0.1%). Cells were then diluted into fresh media and grown at 30°C to _A_600∼0.1. For immediate run-out, rifampicin (300 μg/ml) and cephalexin (100 μg/ml) were added (replication run-out conditions), and the cells kept at 30 or 42°C for a further 3 h. Alternatively, the culture was split and grown either at 30 or 42°C for 3 h. At this point, samples were taken for microscopy and the remaining culture treated for replication run-out, as above. For flow cytometry, cells were fixed with ethanol (70% final), washed with PBS and stained with syto-16 (0.1 μg/ml), or propidium iodide (1 μg/ml), and 100 000 events recorded in a Becton Dickinson FACScalibur machine using FL1-H for syto-16, and FL2-H for propidium iodide. Conditions for microscopy were as in Wang et al (2005).
Supplementary Material
Supplementary data
Acknowledgments
We thank K Marians for supplying the pLex-dnaX(γ) plasmid, O Espeli for assistance with the parEts rescue protocol, Sean Colloms and Marshall Stark for the Cre expression vectors pMS173 and pMS174, and L Zechiederich for the C600_parC_1215 and W3110_parE_10 strains. We thank S Trigueros for valuable discussion. The research was supported by the Wellcome Trust. VS was supported by a Clarendon scholarship. MV was supported by Grant P20 MD000262 from RIMI (NCMHD, NIH) and by NIH SCORE 506 GM052588.
References
- Adams DE, Shekhtman EM, Zechiedrich EL, Schmid MB, Cozzarelli NR (1992) The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell 71: 277–288 [DOI] [PubMed] [Google Scholar]
- Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt D (2002) FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108: 195–205 [DOI] [PubMed] [Google Scholar]
- Barre FX, Sherratt DJ (2005) Chromosome dimer resolution. In The Bacterial Chromosome, Higgins NP (ed), pp 513–524. Washington, DC: ASM Press [Google Scholar]
- Bigot S, Saleh OA, Lesterlin C, Pages C, El Karoui M, Dennis C, Grigoriev M, Allemand JF, Barre FX, Cornet F (2005) KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J 24: 3770–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregu M, Sherratt DJ, Colloms SD (2002) Accessory factors determine the order of strand exchange in Xer recombination at psi. EMBO J 21: 3888–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiaux H, Lesterlin C, Perals K, Louarn JM, Cornet F (2002) A dual role for the FtsK protein in Escherichia coli chromosome segregation. EMBO Rep 3: 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli O, Marians KJ (2004) Untangling intracellular DNA topology. Mol Microbiol 52: 925–931 [DOI] [PubMed] [Google Scholar]
- Espeli O, Lee C, Marians KJ (2003a) A physical and functional interaction between Escherichia coli FtsK and topoisomerase IV. J Biol Chem 278: 44639–44644 [DOI] [PubMed] [Google Scholar]
- Espeli O, Levine C, Hassing H, Marians KJ (2003b) Temporal regulation of topoisomerase IV activity in E. coli. Mol Cell 11: 189–201 [DOI] [PubMed] [Google Scholar]
- Gourlay SC, Colloms SD (2004) Control of Cre recombination by regulatory elements from Xer recombination systems. Mol Microbiol 52: 53–65 [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Szerlong H, Tabor C, Kuempel P (1999) Norfloxacin-induced DNA cleavage occurs at the dif resolvase locus in Escherichia coli and is the result of interaction with topoisomerase IV. Mol Microbiol 33: 1027–1036 [DOI] [PubMed] [Google Scholar]
- Ip SC, Bregu M, Barre FX, Sherratt DJ (2003) Decatenation of DNA circles by FtsK-dependent Xer site-specific recombination. EMBO J 22: 6399–6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Nishimura Y, Imamura R, Niki H, Hiraga S, Suzuki H (1990) New topoisomerase essential for chromosome segregation in E. coli. Cell 63: 393–404 [DOI] [PubMed] [Google Scholar]
- Kato J, Nishimura Y, Yamada M, Suzuki H, Hirota Y (1988) Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J Bacteriol 170: 3967–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Sherratt DJ (1995) Site-specific recombination in the replication terminus region of Escherichia coli: functional replacement of dif. EMBO J 14: 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine C, Marians KJ (1998) Identification of dnaX as a high-copy suppressor of the conditional lethal and partition phenotypes of the parE10 allele. J Bacteriol 180: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Draper GC, Donachie WD (1998) FtsK is a bifunctional protein involved in cell division and chromosome localization in Escherichia coli. Mol Microbiol 29: 893–903 [DOI] [PubMed] [Google Scholar]
- Pease PJ, Levy O, Cost GJ, Gore J, Ptacin JL, Sherratt D, Bustamante C, Cozzarelli NR (2005) Sequence-directed DNA translocation by purified FtsK. Science 307: 586–590 [DOI] [PubMed] [Google Scholar]
- Peng H, Marians KJ (1993) Decatenation activity of topoisomerase IV during oriC and pBR322 DNA replication in vitro. Proc Natl Acad Sci USA 90: 8571–8575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacin JL, Nollmann M, Bustamante C, Cozzarelli NR (2006) Identification of the FtsK sequence-recognition domain. Nat Struct Mol Biol 13: 1023–1025 [DOI] [PubMed] [Google Scholar]
- Saka Y, Vazquez M (2002) TangleSolve: topological analysis of site-specific recombination. Bioinformatics 18: 1011–1012 [DOI] [PubMed] [Google Scholar]
- Schvartzman JB, Stasiak A (2004) A topological view of the replicon. EMBO Rep 5: 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt DJ, Arciszewska LK, Blakely G, Colloms S, Grant K, Leslie N, McCulloch R (1995) Site-specific recombination and circular chromosome segregation. Philos Trans R Soc Lond B Biol Sci 347: 37–42 [DOI] [PubMed] [Google Scholar]
- Sivanathan V, Allen MD, de Bekker C, Baker R, Arciszewska LK, Freund SM, Bycroft M, Lowe J, Sherratt DJ (2006) The FtsK gamma domain directs oriented DNA translocation by interacting with KOPS. Nat Struct Mol Biol 13: 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner W, Liu G, Donachie WD, Kuempel P (1999) The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol 31: 579–583 [DOI] [PubMed] [Google Scholar]
- Steiner WW, Kuempel PL (1998) Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli. Mol Microbiol 27: 257–268 [DOI] [PubMed] [Google Scholar]
- Sumners DW, Ernst C, Spengler SJ, Cozzarelli NR (1995) Analysis of the mechanism of DNA recombination using tangles. Q Rev Biophys 28: 253–313 [DOI] [PubMed] [Google Scholar]
- Sun Q, Yu XC, Margolin W (1998) Assembly of the FtsZ ring at the central division site in the absence of the chromosome. Mol Microbiol 29: 491–503 [DOI] [PubMed] [Google Scholar]
- Wang X, Possoz C, Sherratt DJ (2005) Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev 19: 2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Crick FH (1953) Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171: 737–738 [DOI] [PubMed] [Google Scholar]
- Yates J, Zhekov I, Baker R, Eklund B, Sherratt DJ, Arciszewska LK (2006) Dissection of a functional interaction between the DNA translocase, FtsK, and the XerD recombinase. Mol Microbiol 59: 1754–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechiedrich EL, Cozzarelli NR (1995) Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev 9: 2859–2869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data