Premature bifurcation of the apical dendritic trunk of vibrissa-responding pyramidal neurones of X-irradiated rat neocortex (original) (raw)
Abstract
- Electrophysiological and morphological experiments were performed on the pyramidal neurones of the somatosensory neocortex of the adult rat that had been exposed in utero to 200 rad of X-irradiation. Under urethane anaesthesia, evoked gross potentials, extracellular and intracellular neuronal responses, and laminar field potentials were recorded. The evoked epicortical potential to deflection of a single whisker was a long-lasting positive wave which contrasted with a biphasic positive-negative complex in unirradiated controls. Neurones were initially tested during extracellular recording for responses to whisker deflections. This was followed by intracellular injection with horseradish peroxidase (HRP), and the HRP-stained neuronal elements were visualized using diaminobenzidine as a chromagen.
- Fifty neurones were recorded and stained through the soma. The mean response latency was 15.1 ms. Of these, thirty-six showed premature bifurcation of the apical dendrite. The angle of bifurcation of the apical dendrite was measured in the coronal plane and was found to be a function of the depth at which the soma was located and the distance between the soma and bifurcation point. It was suggested that the apical dendrites of these neurones underwent premature bifurcation due to an imbalance between the growth of neurones and skull.
- Fifteen neurones were recorded and stained through the presumed apical dendritic shaft. The mean response latency was 19.5 ms. The electrode track left in the histological specimen pointed to the stained apical dendrite. Dendritic elements distal to the entry point were preferentially filled with HRP. In no cases were somata stained. There was, in turn, no evidence of recording from dendritic elements distal to the bifurcation since no electrode track was found to point to these elements. That dendritic spike responses may not travel beyond the bifurcation was further substantiated by laminar analysis of field potentials.
Neocortical pyramidal neurones (Fig. 1) normally have a long apical dendrite ending in a terminal tuft near the pial surface, and four to five primary basal dendrites emanating from the soma (Larkman, 1991; Ito, 1992; Schiller et al. 1995). In addition, there are oblique dendrites that branch off from the apical dendritic trunk, but division of the apical dendritic trunk into two parts of the same calibre seldom occurs except near the pial surface.
Figure 1. An HRP-filled pyramidal neurone in a coronal section from a normal rat.
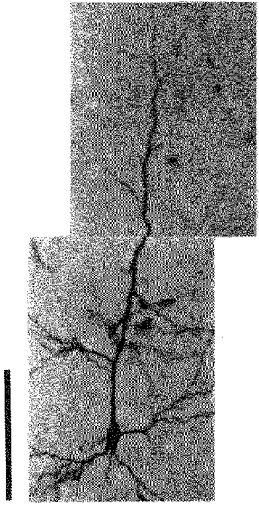
The calibration bar denotes 100 μm.
Unlike the sensory receptors in the periphery, whose shape and physical nature are adapted to respond to a specific aspect of the stimuli (for example, sustained vs. transient responses), the shape of central neurones is generally not considered to bear a causal relation to the response characteristics. Although there is an apparent correlation between the structure and function of cortical neurones (Connors & Gutnick, 1990; Mason & Larkman, 1990), the output firing of the central neurones is primarily dependent on the input firing of the incoming afferents, and the interaction between excitatory and inhibitory connections is more dominant in the determination of the time course of the response. However, with the recent demonstration of active dendritic currents, particularly the backpropagation of action potentials into the dendrites, the morphology of the dendritic arbor is now thought to be more intimately related to function. This issue is important because of the possible role of these action potentials in the control of long-term synaptic plasticity and the possible role of branch points (oblique branches, bifurcation, terminal tuft) of apical dendrites in the gating control of somatodendritic spike conduction (Spruston et al. 1995; Svoboda et al. 1997).
Recently, we noticed that layer V pyramidal neurones from micrencephalic rat barrel cortex often showed premature bifurcation of the apical dendritic trunk at a point remote from the pial surface (Ito, 1995). We also showed that these aberrant pyramidal neurones are functional in that they responded to vibrissa displacement, albeit with a lower than normal sensitivity. In this study, intracellular staining of vibrissa-responsive pyramidal neurones was continued and the angle of premature bifurcation was measured. The objectives of the experiments were twofold. Firstly, to understand how the apical dendritic trunk bifurcates in these animals, for which a detailed description of bifurcation was thought necessary. Secondly, to determine the functional aspects of the premature bifurcation, for which the response features of these neurones were noted. During the course of the study, we encountered cases of recording/staining of the apical dendritic segment proximal to bifurcation. Cortical evoked potential and current source density analysis (Mitzdorf & Singer, 1977) were carried out to test the interpretation that action potentials are prevented from travelling to the pial surface beyond the bifurcations in these animals.
METHODS
Animals and prenatal X-irradiation
The main body of neuronal data was collected from a total of thirty-four prenatally irradiated albino Wistar rats derived from nine litters. X-irradiation was applied as described in detail elsewhere (Ito et al. 1979; Ito, 1995). Briefly, a pregnant mother was constrained in an individual sector compartment of a circular Plexiglass container. Fetuses were exposed to a total of 200 rad through the body wall of the mother at a dose rate of 27.9 rad min−1 using a type KXC radiation apparatus (Toshiba, Tokyo, Japan) at noon on embryonic day 17. Offspring were subjected to electrophysiological analyses at the age of 2–3 months. There was no sign of pain or discomfort from the mother during or after irradiation. Offspring were somewhat more mobile during the first 2 months of age than unirradiated controls, but no differences were detectable after this time. Feeding was normal, and growth was normal except for the skull and brain. Experimental procedures including those described below were approved by the Ethics Committee on Animal Experiments of the Institute for Developmental Research.
Recording of neuronal activity and intracellular staining
Techniques of surgery, whisker stimulation, intra- and extracellular recording, intracellular injection of horseradish peroxidase (HRP) and histology have been described previously (Ito, 1992). Whiskers were trimmed to a length of 10 mm from the base. The vibrissa cortex was exposed under urethane anaesthesia (1.4 g (kg body weight)−1i.p.) to locate the centre of the barrel cortex by recording the maximum evoked potentials to deflection of whisker C2. Once the representation of whisker C2 was identified, recording/injecting electrodes pulled from glass micropipettes (o.d., 2.0 mm; Clark, Reading, UK) were introduced into the cortex vertically. The glass pipettes were backfilled with a solution of 4 % HRP (Wako, Osaka, Japan) and 0.2 m KCl in 0.05 m Tris buffer at pH 8.6 (50–100 MΩ). Once a whisker-responsive neurone was encountered, the most effective whisker was determined (receptive field centre) with a hand-held probe. The tip of the whisker was connected to a quantitative whisker deflector (Ito et al. 1979) and the response latency was measured using our standard short-pulsed deflection (a deflection of 500 μm as measured at the tip over a period of 5 ms, i.e. at a velocity of 100 mm s−1). The electrode tip was then made to penetrate the cell by passing 200 ms positive pulses of 3 nA at 0.3 Hz, and HRP was injected intracellularly by switching the repetition rate to 3 Hz and the current level to 6 nA. Intracellular injection lasted for 3 min. The animal was subsequently killed by an overdose of Nembutal and perfused through the heart with 1.5 % glutaraldehyde and 1.5 % paraformaldehyde in 0.1 m phospate buffer at pH 7.4. The brain was removed from the skull and postfixed in the same fixative overnight at 4°C. Coronal sections were cut at a thickness of 100 μm on a freezing microtome and incubated in a diaminobenzidine solution (DAB; Nacarai, Kyoto, Japan) for visualization of HRP staining according to the method of Adams (1981). In some cases, cytochrome C was added to visualize cytochrome oxidase-stained cortical barrels simultaneously (Wong-Riley & Welt, 1980; Ito, 1992). Sections were immersed briefly in 1 % gelatin, mounted on slides and air dried, then cleared in xylene. Entellan (Merck, Darmstadt, Germany) was added and coverslips were placed over the samples.
Measurement of bifurcation angle
HRP-stained neurones were drawn on a sheet of paper at a magnification of × 400 using a camera lucida. The centre of a template with a polar co-ordinate was placed on the bifurcating point and four angles subtended by intersections made by two dendritic branches with concentric rings at 5, 10, 15 and 20 μm were measured (Fig. 2). The mean of these four values was defined as the bifurcation angle. In cases where the bifurcation was not parallel to the plane of the section, the angle may have been underestimated. Since there was no reason to assume that the plane of bifurcation deviated more from the coronal plane for neurones of specific depths, conclusions based on the present measurements may not have been seriously affected by underestimation of the true angle.
Figure 2. Camera lucida drawing of an HRP-stained neurone illustrating how the bifurcation angle was measured.
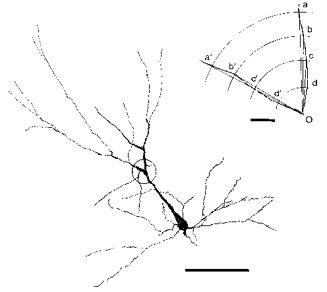
The bifurcation is encircled and enlarged in the inset in the upper right corner. Point O is on the centre of the soma, and the mean of angles aOa′, bOb′, cOc′ and dOd′ is defined as the bifurcation angle. Calibration bar for the neurone: 100 μm. Calibration bar for the inset: 5 μm.
Current source density analysis
Rats were anaesthetized and underwent surgery as described above. Recording electrodes were low-impedance (2–5 MΩ) glass micropipettes filled with a saturated solution of Methylblue (Chroma, Stuttgart, Germany) in 1 m potassium acetate. Electrodes were inserted into the vibrissa cortex perpendicular to the pial surface. Field potentials were recorded in response to deflection of the C2 whisker. The whisker had been cut to 10 mm from the base and its tip was connected to the quantitative whisker deflector. The velocity and amplitude (measured at the tip) of deflection were 100 mm s−1 and 500 μm, respectively. Potentials were recorded at 50 μm intervals in depth. The repetition rate was 0.3 s−1. The deepest recording site was marked by an extracellular deposit of the dye Methylblue, made by passing 10 μA of negative DC current for 3 min.
Field potentials were amplified and stored on a floppy disk using a type DR-F1 digital recorder (TEAC, Tokyo, Japan) for later analysis. The time resolution (bin width) was 100 μs and ten traces were averaged. Computation of the one-dimensional current source density was done according to Mitzdorf & Singer (1977):
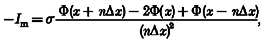
where I_m is the source density, Φ is the recorded potential, x is the depth, Δ_x is the recording depth interval, and σ is the conductivity. Δ_x_ was 50 μm and integer n was 4, and σ was assumed constant and regarded as unity in the present study (Mitzdorf & Singer, 1977; Barth et al. 1989; Ito, 1995). With this formula, current density profiles could be obtained for depths ranging from 200 μm below the pial surface to 200 μm above the deepest recording point.
Statistics
Results of the experiments are expressed as means ±s.d. For statistical comparison of the depth of neurones and the thickness of the cortex among three severity groups, ANOVA was used first, and after the overall difference among the three means was ascertained (F test; Steel & Torrie, 1980), Student's t test was used to assess the significance of the difference of two means (Fisher, 1958). For statistical analysis of correlation between parameters, Pearson's correlation coefficients were computed (Fisher, 1958). Values of P < 0.05 were considered significant.
RESULTS
Severity of the effect of X-irradiation
X-irradiation of rats with 200 rad on embryonic day 17 (hereafter referred to as X-irradiated or irradiated) resulted in micrencephaly in adulthood. The neocortex was maldeveloped; the posterior aspect of each hemisphere was particularly affected, and crossing of the corpus callosum was typically lacking. The occurrence of malformation was constant but there was a slight variation in the degree of severity of sequelae, which was arbitrarily divided into mild, moderate and severe (Fig. 3). The judgement was based on the presence or absence of the corpus callosum on the mid-line at the level of the anterior commissure. The mild case thus refers to a rat in which crossing of the corpus callosum (CC) was reduced in size but present at the anterior commissure (CA) level and anterior to it. In the moderate case the CC did not appear to cross at the CA level but appeared somewhere anterior to it. The severe case was designated by the total lack of CC crossing; CC crossing did not occur at any antero-posterior level. There were in total thirteen mild, seven moderate and fourteen severe cases. Mean values of the cortical thickness measured in the coronal plane including vibrissa-sensitive neurones for mild, moderate and severe cases were 1444 ± 145, 1157 ± 197 and 1018 ± 191 μm, respectively. The overall difference in the thickness among the three groups was significant at P < 0.005 (_F_2,31 = 20.2). The differences in the thickness between the mild and severe, and the mild and moderate groups were significant at P < 0.001 (t = 6.50, d.f. = 25) and P < 0.01 (t = 3.74, d.f. = 18), respectively. The difference in the thickness between the moderate and severe groups was not significant.
Figure 3. The degree of severity of the effect of prenatal X-irradiation on embryonic day 17.
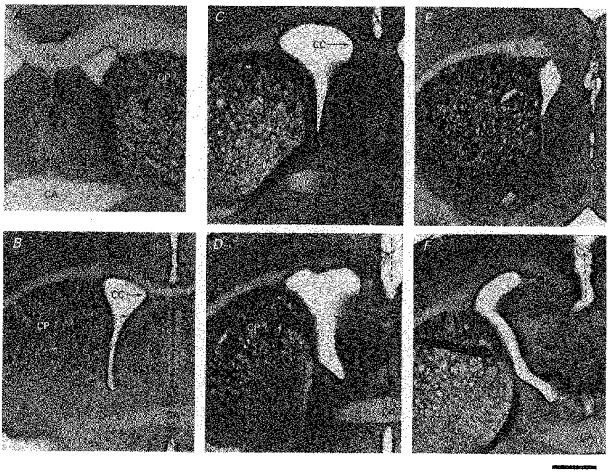
Peroxidase-stained, 100 μm-thick coronal sections are shown. A, control brain. Corpus callosum (CC) crossing is present at the level of the anterior commissure (CA). B, mild effect. CC is reduced in thickness but crosses at the coronal level of CA. C and D, moderate effect (two frames from the same brain). CC does not cross at the level of CA (D) but CC appears somewhere anterior to it (C). E and F, severe effect (two frames from the same brain). CC does not cross anywhere (F at CA level and E anteriorly). Dotted lines show the failure of crossing. CP, nucleus caudate putamen. Calibration bar, 1 mm.
Evoked potentials in response to vibrissa displacement
Figure 4 compares superimposed records of potentials evoked by quick displacement of whisker C2 in the surface of the somatosensory cortex of normal (control) and prenatally irradiated animals. Evoked potentials in the control animal had a biphasic appearance with an initial positive wave beginning 10 ms after the stimulus, followed by a negative wave, the entire complex having a time course of 10–15 ms. In contrast, those in the irradiated animal showed a long-lasting monophasic positive wave of low amplitude. The surface monophasic long-lasting positivity in the irradiated cortex was always observed irrespective of the degree of severity described in the previous section.
Figure 4. Cortical evoked potentials to deflection of a single whisker.
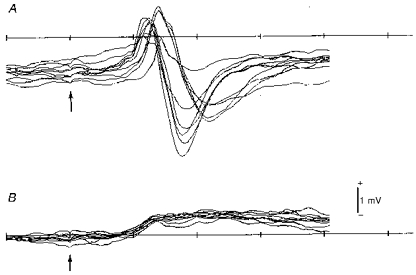
A, control rat. B, X-irradiated rat. Note biphasic positive-negative waves in the control animal and a low-amplitude, long-lasting positive wave in the irradiated animal. Arrows indicate the time point of short-pulsed whisker deflection applied 10 ms after the sweep onset. Time scale shows 10 ms intervals. Nine and eight superimposed sweeps for A and B, respectively.
Response feature and depth of somata of stained vibrissa neurones in the irradiated cortex
Fifty neurones were found to respond to whisker displacement and their somata were stained intracellularly with horseradish peroxidase (HRP). The widths of the somata were between 12 and 25 μm, and these values were well within the range of those of irradiated neurones in the previous study (Ito, 1995). The response latencies ranged from 10 to 20 ms (Fig. 5_A, B, D_ and E) and the mean (n = 50) was 15.1 ± 3.0 ms. There were burst responses as well as single-spike responses. Although the velocity threshold was not determined quantitatively in this series of experiments, these neurones appeared to be slightly less responsive than those of the unirradiated controls (see also Ito et al. 1981). This judgement was based on audio-monitoring of spike responses. Hyperpolarization appeared after regenerative spikes deteriorated (Fig. 5_C_). Stained somata were distributed more superficially in severe cases of micrencephaly than in mild ones, with moderate cases in between (Fig. 6). The mean depth of recovered neurones in the mild, moderate and severe groups was 522 ± 164, 396 ± 113 and 329 ± 153 μm, respectively. The overall difference in the depth of somata among the three severity groups was significant at P < 0.005 (_F_2,47= 8.01). The mean depth differed significantly between the mild and moderate groups at P < 0.05 (t = 2.30, d.f. = 28), and the mild and severe groups at P < 0.001 (t = 3.73, d.f. = 36). These differences reflected a variability in the thickness of the irradiated vibrissa cortex, paralleling the degree of sequelae among three severity groups. In fact, four out of five normal apical dendrites were found among mild cases of micrencephaly. As far as the response latency was concerned, there was no difference between these five normal-looking neurones and thirty-six neurones with premature apical dendritic bifurcations (see below). Nor were there any differences in the response latency among the three severity groups.
Figure 5. Examples of intracellular responses to whisker deflection.
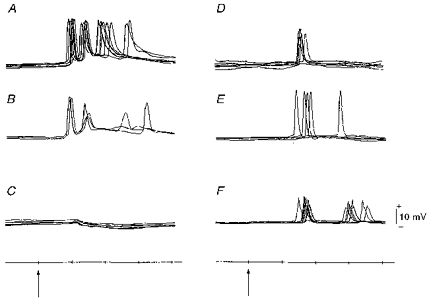
Recordings from somata (A-E) and presumed apical dendritic shaft (F). A and B, burst responses. C, hyperpolarization appeared after spike potentials in B deteriorated. D and E, single responses (one spike only was elicited each time). F, responses obtained from the portion of the stained dendrite pointed to by the electrode track in Fig. 9_C_. Arrows and time scale as in Fig. 4.
Figure 6. Depth distribution of stained somata in three severity groups.
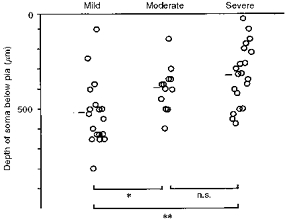
Short bars denote the mean depth for each group. Overall difference (ANOVA) was significant at P < 0.005. Significance of mean difference between groups is marked: **P < 0.001, *P < 0.05, or n.s. (not significant).
Premature bifurcation of the apical dendrite after prenatal irradiation
The apical dendritic shaft often divided into two branches of the same calibre in the prenatally X-irradiated cortex. Of the fifty intracellularly stained neurones in the irradiated cortex, thirty-six neurones showed this abnormality; only five neurones were normal. For the remaining nine neurones, the apical dendrites were not stained enough to allow judgement. Figure 7 depicts three examples of premature bifurcation of the apical dendritic shaft observed in the irradiated cortex. In Fig. 8_A_ an inverse relationship between the bifurcation angle of an apical dendrite and the depth of the soma of its origin is shown. The correlation coefficient (r =−0.58) was significant at P < 0.001 (n = 36, t = 4.20). The bifurcation angle of an irradiated apical dendrite tended to be larger the sooner it bifurcated, i.e. the smaller the distance between the soma and the bifurcation point (the depth of soma minus the depth of bifurcation). Figure 8_B_ shows this relationship. The correlation coefficient (r =−0.56) was significant at P < 0.001 (n = 36, t = 3.96). The most significant correlation was found between the depth of the soma and the depth of bifurcation (r = 0.77, P < 0.001, n = 36, t = 6.94; not shown). Accordingly, the angle of bifurcation was also inversely correlated with the depth of bifurcation, but the degree of correlation was the lowest among the parameters tested (r =−0.33, P < 0.05, n = 36, t = 2.10; not shown).
Figure 7. Three examples of premature bifurcation of the main apical dendrite in the irradiated cortex.
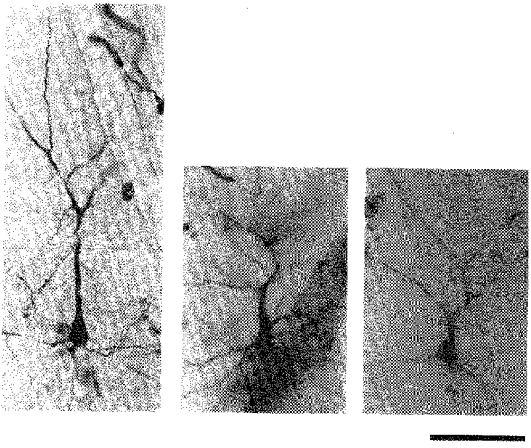
Photomicrographs of HRP-stained neurones in coronal sections. In each panel the soma is in the lower half and the apical dendrite extends upwards, and soon divides. Scale bar denotes 100 μm.
Figure 8. Measurement of the bifurcation angle.
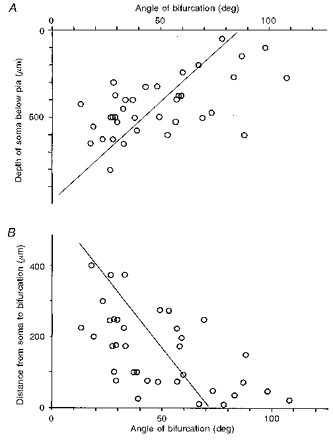
A, correlation between the subpial somal depth of neurones and the bifurcation angle of the apical dendrite. r =−0.58, P < 0.001 (n = 36). Regression line was drawn with the depth as the independent variable. B, correlation between the distance from the soma of the bifurcation and the angle of bifurcation. r =−0.56, P < 0.001 (n = 36). Regression line was drawn with the distance as the independent variable.
Possible apical dendritic responses to whisker deflection
In addition to the above-mentioned fifty vibrissa-sensitive neurones that were intrasomatically injected with HRP (Fig. 9_A_), there were fifteen cases in which vibrissa responses were identified before staining (e.g. Fig. 5_F_), but HRP reaction was confined to the apical dendritic shaft and the distal branches (Fig. 9_B–E_). Based on the following findings we assume that these recordings were made from the apical dendritic shaft. Firstly, as Fig. 9_C, D_ and E show, electrode tracks pointed to the stained apical dendritic shaft (except for Fig. 9_B_). Conversely, in none of the somatic stainings was the electrode track seen to point to the dendritic shaft. Secondly, spikes were never followed by hyperpolarization as was frequently the case in the somatic recording. The averaged latency of ‘apical dendritic’ responses was 19.5 ± 3.7 ms (n = 15). The difference in the latency between the ‘somatic’ responses (15.1 ± 3.0 ms, n = 50) and the ‘apical dendritic’ responses was significant at P < 0.001 (t = 4.79, d.f. = 63). The entry point of HRP was proximal to the premature bifurcation, suggesting that recording/staining in the segment distal to bifurcation never occurred.
Figure 9. Electrode track and possible point of recording/HRP injection.
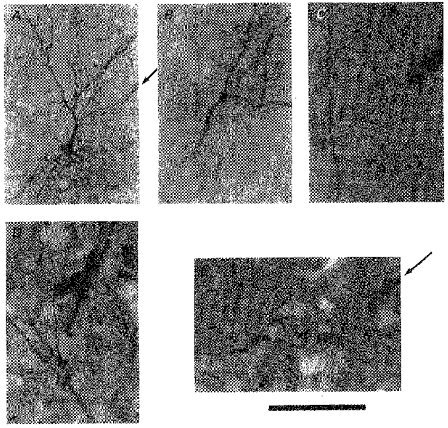
A, electrode track (arrow), visualized by peroxidase reaction of red blood cells, points to a stained soma. B-E, possible dendritic recording/HRP injection site. B, the soma was not filled, and neither was the electrode track visible, but the recording/injection point must have been on the apical dendrite. C, electrode track points to the apical dendrite. Soma was not filled. This neurone did not show bifurcation of the apical dendrite. D, electrode track is very close to the bifurcation. Apical dendrite was visible beyond the frame of photograph (beyond top, bottom and left margins), indicating that HRP was also transported somatopetally (beyond bottom margin) for some distance, but the soma was not stained. E, electrode track from upper right (arrow) points to the proximal end of the stained apical dendrite (bifurcation is seen at upper left). Calibration bar denotes 200 μm for A, and 100 μm for B-E. All the specimens are from irradiated animals.
Laminar and current source density analysis of vibrissa-evoked responses in the cortex
Figure 10 shows the cortical laminar profiles of field potentials (FP) and the extracellular current flowing in parallel to the columnar structure (CSD) after deflection of C2 whisker in the control (A) and prenatally irradiated animal (B). Whereas a positive wave in the superficial layers appears to be offset by a travelling negative wave from deep layers in the control laminar profile (Control FP), the long positive wave of the irradiated cortex is simply a phase reversal of a negativity in the intermediate layers (Irradiated FP). The current source density most probably represents the thalamocortical and translaminar synaptic current. In the control animal the sink is set up first in upper layer IV within the first 10 ms of whisker deflection, and propagates superficially. In the X-irradiated animal, however, there is only a slowly rising and long-lasting sink within the intermediate zone between layer I and layer Vb where the apical dendritic bifurcations were abundant.
Figure 10. Laminar profile of field potentials (FP) to whisker deflection and calculated current source density (CSD) of control and irradiated cortex.
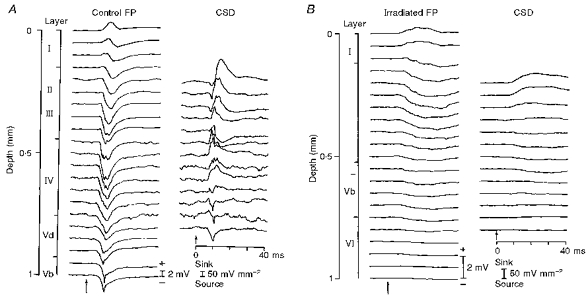
Recordings were made in steps of 50 μm. Calibrations of magnitude (positive up) for field potential and sink-source density are indicated. Time scale applies to both FP and CSD. Short-pulsed whisker deflection is indicated by the arrows.
DISCUSSION
Premature bifurcation of the apical dendritic shaft of neocortical pyramidal neurones
The neocortical pyramidal neurones normally have four to five main basal dendrites emanating radially from the soma in the laminar plane, and an apical dendritic shaft oriented towards the cortical surface, dividing first at the border of layers I and II/III, forming a terminal tuft (Larkman & Mason, 1990; Ito, 1992; Schiller et al. 1995). A premature bifurcation of the apical dendritic trunk, as observed in this study of prenatally X-irradiated rats, seldom occurs in the normal cortex, and the somata of such aberrant neurones are larger than those in the normal cortex (Ito, 1995). Chagnac-Amitai et al. (1990) did find this type of pyramidal neurone in layer V in the normal cortex but they stress that the neurones are atypical. Lübke et al. (1996) observed five cases of such bifurcation out of fifteen somatosensory neocortical pyramidal neurones from young rats (13–15 days old). Also, premature bifurcation of the apical dendritic trunk does not seem to be rare in the cat visual cortex (Fig. 11 in O'Leary, 1941), but in the rat visual cortex no mention was made of this phenomenon in Larkman's extensive experimental series on dendritic branching (Larkman, 1991). Larkman & Mason (1990) found only a single neurone with a premature bifurcation out of forty pyramidal neurones in the rat visual cortex. Therefore, it must be stressed that the present finding is not only unusual in the somatosensory barrel cortex of the rat, but also in the adult neocortex throughout the rodent species. Moreover, a bifurcation with an angle as large as 100 deg observed in the present report has never been described.
A more general type of apical dendritic anomaly is observed in genetic, drug-induced and irradiation-induced neuronal migration disorders, and has been variously described as ‘not radial’, ‘bizarre’, ‘atypically oriented’, or ‘not uniformly oriented’ (Berry et al. 1963; Johnston & Coyle, 1979; Jensen & Killackey, 1984; Chae et al. 1997; Lee et al. 1997). In this study, we could highlight the premature bifurcation of the main apical dendrite of cortical neurones by using in vivo staining techniques in the classical model of migration disorders.
Correlations among measured parameters of bifurcated dendrites
The only parameter found to be correlated with the arbitrary severity classes of micrencephaly was the depth of somata; vibrissa-responding neurones were differentially distributed among the three severity groups (Fig. 6). There was no difference in the bifurcation angle of the apical dendrites or the response latency of these neurones among the three severity classes. Considering that the variable degree of corpus callosum development was the most remarkable semi-macroscopic variability of the prenatally X-irradiated micrencephalus, the failure to find severity-dependent parameters is in keeping with the earlier description that individual effects of irradiation are remarkably constant (Berry et al. 1963; Jensen & Altman, 1982). The bifurcation angle of the apical dendrite increased with the decreasing depth of location of its soma below the pial surface. At the risk of oversimplification we propose that within the developing cortex there is a gradient of increasing pressure towards the skull. This was probably caused by the lack of proliferating layer IV granular neurones, which would normally stimulate the growth of the skull. An apical dendritic trunk in the micrencephalic brain cannot continue to grow as one cylinder, or cope with the increasing pressure from the skull; it bifurcates into two cylinders of the same, but smaller calibre. Alternatively, the lack of cytoarchitectural barrels (Ito, 1995) may be responsible for not guiding the straight growth of the apical dendritic shafts in the irradiated cortex. However, the presence of histochemically detectable (but not cytoarchitectural) barrels suggests fasciculated ingrowth of the thalamocortical afferents.
Functional implication of apical dendritic bifurcation
The constant and most remarkable feature of the prenatal X-irradiation was a long-lasting positive wave recorded at the pial surface in response to vibrissa deflection. This is in sharp contrast to a biphasic positive-negative complex in the control rats. Regarding the relationship between the structure and genesis of electrocortical responses, the most severely changed parameters must be referred to elements which have been most dramatically affected. Such alteration occurred in the apical dendrites of the irradiated pyramidal neurones, and this must have caused the long-lasting positive waves recorded at the pial surface. Normally, the initial surface positivity is considered to reflect activation in the deeper layers, and as the activation travels towards the pia, the surface positivity is replaced by the negativity (Fig. 4) or at least soon terminated by offsetting (Fig. 10). Therefore, the long-lasting positive wave in the irradiated surface field potential may be accounted for by the non-propagating nature of ‘deep sink’ in these animals (Nicholson & Llinas, 1972).
We have been able to record spike potentials from an apical dendritic trunk, but not beyond the bifurcation point. This implies that spikes do not propagate into (or do not originate in) bifurcated branches (see also Spruston et al. 1995; Svoboda et al. 1997), or that the branches are too thin to record from. A question arises as to whether the recorded dendritic spikes were travelling somatofugally or somatopetally. Despite the paucity of possible dendritic recordings, a general delay (15.1 ms for somatic latency vs. 19.5 ms for dendritic latency, on average) favours the former possibility. Similar backpropagation of apical dendrites has recently been demonstrated by calcuim ion imaging in normal neocortical slice experiments (Schiller et al. 1995). However, in our earlier HRP staining of normal cortical neurones in vivo (Ito, 1992) we did not come across an electrode track pointing to a stained apical dendrite. It could well be that, for some unknown reason, apical dendrites in the irradiated cortex are more easily recorded and stained.
An irradiation-induced aberrant cortical neurone is readily accessible to the microelectrode. Overall sensitivity of these neurones is lower than normal and yet most of the basic response features are preserved (Ito et al. 1981). The use of this animal model in future studies should be relevant to elucidating other aspects of cortical neuronal dendritic function.
References
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. Journal of Histochemistry and Cytochemistry. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Barth DS, Di S, Baumgartner C. Laminar cortical interactions during epileptic spikes studied with principal component analysis and physiological modeling. Brain Research. 1989;484:13–35. doi: 10.1016/0006-8993(89)90344-2. [DOI] [PubMed] [Google Scholar]
- Berry M, Clendinnen BG, Eayrs JT. Electrocortical activity in the rat X-irradiated during early development. Electroencephalography and Clinical Neurophysiology. 1963;15:91–104. [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai L-H. Mice lacking p35, a neuronal specific activator of cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. Journal of Comparative Neurology. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends in Neurosciences. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. 13. Edinburgh: Oliver and Boyd; 1958. [Google Scholar]
- Ito M. Simultaneous visualization of cortical barrels and horseradish peroxidase-injected layer 5b vibrissa neurones in the rat. The Journal of Physiology. 1992;454:247–265. doi: 10.1113/jphysiol.1992.sp019263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Barrelfield of the prenatally X-irradiated rat somatosensory cortex: A histochemical and electrophysiological study. Journal of Comparative Neurology. 1995;352:248–262. doi: 10.1002/cne.903520208. [DOI] [PubMed] [Google Scholar]
- Ito M, Kawabata M, Shoji R. Responses of vibrissa-sensitive cortical neurons in normal and prenatally X-irradiated rat. Journal of Neurophysiology. 1979;42:1711–1726. doi: 10.1152/jn.1979.42.6.1711. [DOI] [PubMed] [Google Scholar]
- Ito M, Kawabata M, Shoji R. Effect of prenatal X-irradiation on sensitivity of cortical neurons responding to vibrissa stimulation. Journal of Neurophysiology. 1981;46:716–724. doi: 10.1152/jn.1981.46.4.716. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Altman J. The contribution of late-generated neurons to the callosal projection in the rat: a study with prenatal x-irradiation. Journal of Comparative Neurology. 1982;209:113–122. doi: 10.1002/cne.902090202. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Subcortical projections from ectopic neocortical neurons. Proceedings of the National Academy of Sciences of the USA. 1984;81:964–968. doi: 10.1073/pnas.81.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Coyle JT. Histological and neurochemical effects of fetal treatment with methylazoxymethanol on rat neocortex in adulthood. Brain Research. 1979;170:135–155. doi: 10.1016/0006-8993(79)90946-6. 10.1016/0006-8993(79)90946-6. [DOI] [PubMed] [Google Scholar]
- Larkman A. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: I. Branching patterns. Journal of Comparative Neurology. 1991;306:307–319. doi: 10.1002/cne.903060207. [DOI] [PubMed] [Google Scholar]
- Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. Journal of Neuroscience. 1990;10:1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Schottler F, Collins JL, Lanzino G, Couture D, Rao A, Hiramatsu K-I, Goto Y, Hong S-C, Caner H, Yamamoto H, Chen Z-F, Bertram E, Berr S, Omary R, Scrable H, Jackson T, Goble J, Eisenman L. A genetic animal model of human neocortical heterotopia associated with seizures. Journal of Neuroscience. 1997;17:6236–6242. doi: 10.1523/JNEUROSCI.17-16-06236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke J, Markram H, Frotcher M, Sakmann B. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. Journal of Neuroscience. 1996;16:3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Larkman A. Correlation between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. Journal of Neuroscience. 1990;10:1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U, Singer W. Laminar segregation of afferents to lateral geniculate nucleus of the cat: an analysis of current source density. Journal of Neurophysiology. 1977;40:1227–1244. doi: 10.1152/jn.1977.40.6.1227. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Llinas R. Field potentials in the alligator cerebellum and theory of their relationship to Purkinje dendritic spikes. Journal of Neurophysiology. 1972;34:509–531. doi: 10.1152/jn.1971.34.4.509. [DOI] [PubMed] [Google Scholar]
- O'Leary JL. Structure of the area striata of the cat. Journal of Comparative Neurology. 1941;75:131–164. [Google Scholar]
- Schiller J, Helmchen F, Sakmann B. Spatial profile of dendritic calcium transients evoked by action potentials in rat neocortical pyramidal neurones. The Journal of Physiology. 1995;487:585–600. doi: 10.1113/jphysiol.1995.sp020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and Procedures of Statistics. 2. Tokyo, Japan: McGraw-Hill Kogakusha; 1980. [Google Scholar]
- Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proceedings of the National Academy of Sciences of the USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]