p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus (original) (raw)
Abstract
The subgranular zone (SGZ) of the dentate gyrus of the hippocampus is a brain region where robust neurogenesis continues throughout adulthood. Cyclin-dependent kinases (CDKs) have a primary role in controlling cell division and cellular proliferation. p21Cip1 (p21) is a CDK inhibitor that restrains cell cycle progression. Confocal microscopy revealed that p21 is abundantly expressed in the nuclei of cells in the SGZ and is colocalized with NeuN, a marker for neurons. Doublecortin (DCX) is a cytoskeletal protein that is primarily expressed by neuroblasts. By using FACS analysis it was found that, among DCX-positive cells, 42.8% stained for p21, indicating that p21 is expressed in neuroblasts and in newly developing neurons. p21-null (_p21_−/−) mice were examined, and the rate of cellular proliferation, as measured by BrdU incorporation, was increased in the SGZ of _p21_−/− compared with WT mice. In addition, the levels of both DCX and NeuN protein were increased in _p21_−/− mice, further demonstrating increased hippocampal neuron proliferation. Chronic treatment with the tricyclic antidepressant imipramine (10 mg/kg per day i.p. for 21 days) markedly decreased hippocampal p21 mRNA and protein levels, produced antidepressant-like behavioral changes in the forced swim test, and stimulated neurogenesis in the hippocampus. These results suggest that p21 restrains neurogenesis in the SGZ and imipramine-induced stimulation of neurogenesis might be a consequence of decreased p21 expression and the subsequent release of neuronal progenitor cells from the blockade of proliferation. Because many antidepressants stimulate neurogenesis, it is possible that their shared common mechanism of action is suppression of p21.
Keywords: antidepressant, neurogenesis, depression, cyclin-dependent kinase inhibitor
In the central nervous system, developing neurons are derived from quiescent multipotent or neural stem cells and progenitors (1). In the hippocampus, the neural progenitor cells are located in the subgranular zone (SGZ) of the dentate gyrus, at the border between the hilus and the granular cell layer (GCL) (2, 3). Newborn cells proliferate in SGZ, migrate into the GCL, develop the morphological and functional properties of granule cell neurons, and become integrated into existing neuronal circuitry (4). This suggests an important role of intrinsic stimulatory and inhibitory factors in the regulation of proliferation of neuronal precursor cells.
In mammalian cells, the control of cellular proliferation primarily is achieved in the G1 phase of the cell cycle. Cyclin-dependent kinases (CDKs) tightly control the cell cycle process. Cell cycle progression is negatively regulated by two families of CDK inhibitors: Ink4/ARF type (p16, p15, p18, and p19) and Cip/Kip type (p21, p27, and p57). p21Cip1 (p21) acts in the G1 phase of the cell cycle and delays or blocks the progression of the cell into the S phase (5). p21 maintains cell quiescence, and chronic activation of p21 can drive the cell into irreversible cell growth arrest and senescence (5, 6). Conversely, inhibition of p21 increases cellular proliferation (7).
The induction of neurogenesis might be part of the molecular mechanisms underlying the therapeutic effects of antidepressant treatment (8, 9). All major classes of antidepressants stimulate neurogenesis in rodents (10–12) and nonhuman primates (13). Irradiation of the brain blocks hippocampal neurogenesis and abolishes the behavioral effects of antidepressants (14). Furthermore, antidepressant-induced neurogenesis requires chronic rather than acute drug treatment, consistent with the time course of the therapeutic effects of these medications (15). The present study evaluated the role of the CDK inhibitor p21 in neurogenesis. The results suggest that antidepressants may stimulate SGZ neurogenesis by inhibiting p21 expression.
Results
p21 Is Expressed in Neuroblasts and Newly Developing Neurons in the SGZ of the Hippocampus.
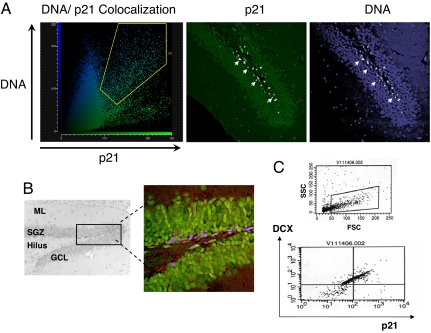
Confocal microscopy images revealed that p21 is abundantly expressed in the nuclei of cells in the SGZ (Fig. 1A) and that it is colocalized with NeuN, a marker for neurons. (Fig. 1B). p21 is not colocalized with NeuN in the GCL, a region where postmitotic neurons reside. Some p21-positive cells were negative for NeuN. Because p21 was not found in glial cells (data not shown), this indicates that p21 also is likely expressed in premature, NeuN-negative neuroblasts. Thus, p21 expression might be confined to neuronal precursors or/and newly developing neurons.
Fig. 1.
p21 is expressed in newly developing neurons in the SGZ of hippocampus. (A) Intranuclear p21 expression in the SGZ of the dentate gyrus. Double-labeling analysis with color-coded fluorogram (58) is shown. The confocal images show intranuclear expression of p21 as demonstrated by colocalization of DNA and p21 staining (DNA, blue; p21, green). Colocalized pixels in the fluorogram are marked with a yellow gate. Colocalization of DNA and p21 is marked white in the corresponding images. (B) Black and white picture showing an H&E-stained 5-μm section of the dentate gyrus of the hippocampus. SGZ, subgranular zone; GCL, granular cell layer; ML, molecular layer. The box delineates the region imaged by confocal microscopy. The confocal image illustrates p21-positive cells (red), neurons (green), and areas of colocalization (blue) in the SGZ. The areas imaged in the confocal micrographs are 500 × 500 μm. (C) Dot plots of DCX/p21 double-expressing cells derived from WT hippocampi. Cells were stained with DCX antibodies, sorted, collected, and then costained with p21 antibodies. (Upper) Scatter plot obtained from dissociated hippocampi with the cell size on the x axis (forward scatter, FSC) and cell granularity on the y axis (side scatter, SSC). DCX-positive cells are gated. (Lower) Gated DCX-positive cells were collected and analyzed for p21 fluorescence. Dual-parameter fluorescence intensity of DCX versus p21 is shown. Cells appearing in the upper right quadrant are strongly positive for both antigens (42.8%), whereas the upper left quadrant represents DCX-positive cells negative for p21 (48.8%). The experiment was performed two times with similar results, and a representative graph is shown.
Doublecortin (DCX) is a cytoskeletal protein that is primarily expressed by neuroblasts, and a subpopulation of NeuN/DCX-positive cells is found in the SGZ (16, 17). FACS analysis was used to determine whether p21 is expressed in DCX-positive cells. Whole hippocampi from 10 mice were pooled and dissociated, and DCX-positive cells were selected by sorting. Approximately 5 × 104 DCX-positive cells were obtained. They were small in size (Fig. 1C Upper), in agreement with data showing that DCX is expressed in small, compact cells corresponding to neuroblasts (17). After sorting, both DCX-positive and DCX-negative cells were stained for p21. Among the DCX-positive cells, 42.8% stained for p21 (Fig. 1C Lower). In a separate experiment, among the DCX-negative cells, <1% stained for p21 (data not shown). These results demonstrate that p21 is expressed in neuroblasts and in newly developing neurons.
p21 Deletion Increases Proliferation of Hippocampal Neurons.
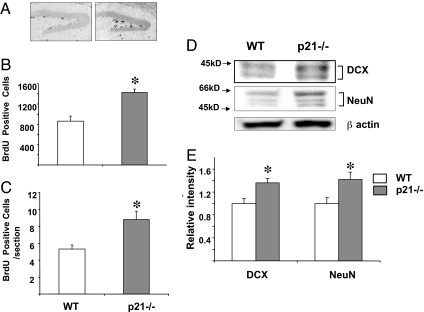
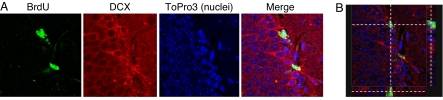
To further evaluate the role of p21 in hippocampal neurogenesis, p21-null (_p21_−/−) mice were examined. Heterozygous (p21+/−) male and female mice were bred to obtain WT and _p21_−/− mice from the same breeding. BrdU, which incorporates into nuclear DNA during the S phase of the cell cycle, was used to assess cellular proliferation. WT and _p21_−/− mice were injected with BrdU every 2 h for a total of three injections and then killed 24 h after the first injection. In the SGZ, BrdU incorporation was higher in _p21_−/− mice than in WT mice (1,424 ± 68 vs. 862 ± 167, P < 0.05) (Fig. 2 A and B). The average number of BrdU-positive cells per section also was higher in _p21_−/− mice than in WT mice (9.6 ± 1.04 vs. 5.03 ± 0.48, P < 0.05) (Fig. 2C). These results indicate that the rate of cellular proliferation was increased in the SGZ of _p21_−/− mice. Separate groups of mice were killed, and protein levels of DCX and NeuN were measured in whole hippocampi by Western blot. The levels of both DCX and NeuN were increased in _p21_−/− mice, further demonstrating increased neuronal proliferation in these animals (Fig. 2 D and E). To confirm that the neuroblasts were proliferating, slides were double-stained for BrdU and DCX. BrdU was colocalized with DCX in the SGZ of _p21_−/− mice (Fig. 3), showing that the increased cellular proliferation was due to enhanced neurogenesis.
Fig. 2.
p21 deletion increases neuronal proliferation in the dentate gyrus. (A) Immunoperoxidase-labeled BrdU in representative sections from the dentate gyrus of WT and _p21_−/− WT mice. (B) Quantification of BrdU-positive cells in the SGZ of the hippocampus in _p21_−/− and WT mice. Three mice per group were analyzed. (C) Average numbers of BrdU-positive cells per section in the SGZ of _p21_−/− and WT mice. (D) Western blot analysis of NeuN and DCX in whole hippocampus of WT and _p21_−/− mice. Mouse DCX monoclonal antibodies recognize two bands in the 41- to 44-kDa range. Mouse NeuN monoclonal antibodies recognize two to three bands in the 46- to 48-kDa range and a band at ≈66 kDa. For each sample, hippocampi from three mice per group were pooled. The experiment was repeated three times, and a representative blot is shown. (E) Quantitative analysis of three independent experiments. The intensity of the DCX and NeuN bands in each experiment was quantified and corrected with respect to the loading control (β-actin), and the ratios were corrected to WT mice to calculate relative changes. *, P < 0.05.
Fig. 3.
BrdU is incorporated in proliferating neuroblasts in SGZ of _p21_−/− mice. (A) Representative images of SGZ cells of _p21_−/− mice coexpressing BrdU (green) and DCX (red). Cell nuclei were stained with the DNA-specific dye ToPro3 (blue). (B) The orthogonal slices are shown to confirm double labeling throughout the extent of two positive cells. The area imaged in the confocal micrographs is 100 × 100 μm.
The number of DCX-positive cells in the hippocampi of _p21_−/− mice also was assessed. Ten whole hippocampi from WT and _p21_−/− mice were dissociated, and DCX-positive cells were identified by FACS analysis. In the WT mice 2.6% of hippocampal cells were positive for DCX, whereas in the _p21_−/− mice 4.1% of cells were DCX-positive (data not shown). The increased number of DCX-positive cells in the SGZ of _p21_−/− mice confirms that the enhanced neurogenesis was the result of the increased proliferation of neuroblasts.
Chronic Treatment with Imipramine Decreases p21 Expression in the SGZ of the Hippocampus.
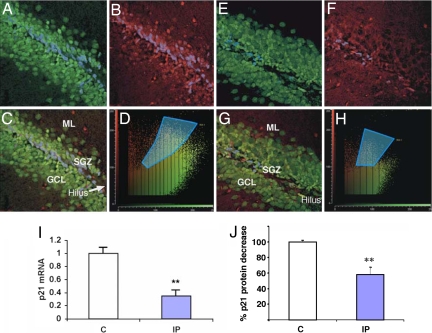
To investigate whether p21 is involved in the antidepressant-dependent increase of neuronal proliferation, p21 expression was measured in mice chronically treated with the tricyclic antidepressant imipramine. Mice were treated daily for 21 days with saline (0.9%) or imipramine hydrochloride (10 mg/kg per day i.p.) and killed 24 h after the last injection. Confocal microscopy indicated that treatment with imipramine markedly decreased the number of cells positive for p21 in the SGZ compared with saline-treated controls (Fig. 4 A–H). Other groups of mice were killed, and mRNA and protein were isolated from whole hippocampi. p21 gene expression was detected by quantitative real-time PCR, and levels of p21 protein were analyzed by ELISA. p21 mRNA levels were lower in the hippocampi after imipramine treatment (Fig. 4I), as were levels of p21 protein (Fig. 4J). There were no differences in p27Kip and p18INK4C mRNA or protein levels (data not shown). Thus, chronic treatment with imipramine specifically suppresses p21 expression in the SGZ of the hippocampus.
Fig. 4.
Chronic treatment with imipramine suppresses p21 expression in the hippocampus. (A–H) Double-labeling analysis of the hippocampal dentate gyrus with color-coded fluorograms (51). Confocal images show double immunofluorescence labeling of hippocampal dentate gyrus after chronic treatment with saline (A–D) or imipramine (E–H). p21-positive cells are red, and neurons (NeuN-labeled) are green. Cells where p21 and NeuN are colocalized are marked with a blue overlay. Colocalized pixels in the fluorograms are marked with blue (D and H). The area imaged in the confocal micrographs is 250 × 250 μm. SGZ, subgranular low; GCL; granular cell layer; ML, molecular layer. (I) Real-time PCR of p21 mRNA levels in the hippocampi of mice after chronic treatment with saline (C) or imipramine (IP) (seven to eight mice per group). *, P < 0.05. (J) p21 protein levels after chronic treatment with imipramine (IP) as measured by ELISA. p21 protein expression in normal saline-treated mice (C) was taken as 100%. Shown are results summarized from three independent experiments. For each experiment whole hippocampi from three mice per group were pooled. **, P < 0.01.
Chronic Treatment with Imipramine Increases Neurogenesis in the SGZ of the Hippocampus and Produces Antidepressant-Like Activity in the Forced Swim Test (FST).
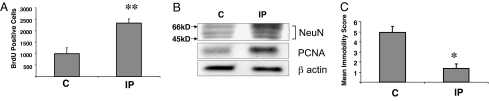
To confirm that the imipramine-induced decrease in p21 is associated with increased neurogenesis, separate groups of mice were chronically treated with imipramine (or saline) as described above. Twenty-four hours after the last injection of imipramine (or saline), the mice were injected every 2 h with BrdU for a total of three injections and then killed 24 h after the first BrdU injection. The number of BrdU-positive cells in the SGZ was significantly higher in mice treated with imipramine, indicating increased BrdU incorporation and increased cellular proliferation in this region (Fig. 5A).
Fig. 5.
Chronic treatment with imipramine increases neuronal proliferation in the hippocampus and decreases immobility. (A) Quantification of BrdU-positive cells in SGZ after chronic imipramine treatment (IP) compared with saline control (C). Three mice per group were analyzed. **, P < 0.01. (B) Western blot analysis of protein levels of NeuN and PCNA in whole hippocampus after chronic treatment with saline or imipramine. Mouse NeuN monoclonal antibodies recognize two to three bands in the 46- to 48-kDa range and a band at ≈66 kDa. For each sample hippocampi from three mice per group were pooled. The experiment was repeated twice with similar results. A representative blot is shown. (C) Mean immobility score in the FST in mice chronically treated with saline or imipramine. *, P < 0.05 (n = 15–17 mice per group).
Other mice were similarly treated with imipramine (or saline), and protein was isolated from whole hippocampi and analyzed by Western blot. The levels of PCNA, a marker of cellular proliferation, were higher in imipramine-treated mice, confirming increased cellular proliferation. Levels of NeuN also were elevated in the hippocampi of the imipramine-treated mice (Fig. 5B), demonstrating that there was an increase in the number of neurons in the hippocampus.
To show that the imipramine treatment regimen produced a behavioral response, additional mice were treated for 21 days with imipramine (or saline), and 24 h after the last injection they were subjected to the FST, a widely accepted animal model used to screen drugs for antidepressant activity (18–20). In the FST, antidepressant activity is reflected by a reduction in time spent immobile and an increase in the time spent in active, escape-oriented behaviors. Immobility was significantly decreased after chronic treatment with imipramine (Fig. 5C), demonstrating that the imipramine treatment regimen produced antidepressant-like effects.
Discussion
In the adult, neurogenesis in the hippocampus is restricted to the SGZ of the dentate gyrus (21, 22). The results of the present study show that p21 immunoreactivity is prominent within the dentate gyrus and restricted to the SGZ. The small, compact morphology of cells expressing p21 is consistent with migrating neuroblasts that express DCX (17). The results of the flow cytometry studies show that 42% of newly developing neurons (DCX-positive cells) in the hippocampus are also positive for p21. Double-staining for p21 and NeuN indicates that p21 is localized in neurons in the SGZ. The absence of p21 in the GCL, the region where mature neurons reside, and the fact that mature postmitotic neurons do not express DCX, indicate that p21 is expressed in developing neurons. Although NeuN is considered to be a marker of mature neurons, a population of immature, still dividing neuronal cells (D1 cells) localized in the SGZ express both DCX and NeuN (17, 23). Others have also found type 2 neuroblasts (NB2) that express both DCX and NeuN and remain confined to the SGZ (16). Taken together, these results suggest that p21 expression in the hippocampus is confined to dividing, immature neurons. However, p21 also might be expressed at earlier stage of neuronal differentiation, including stem cells.
CDKs play a critical role in regulating the cell division cycle (6, 24, 25). Inhibitors of these kinases, such as p21, block the G1- to S-phase transition and restrain cellular proliferation (6, 7). For example, p21 has been shown to be an important regulator of hematopoietic stem cell quiescence and self-renewal (26). Therefore, decreased levels of p21 would be expected to be associated with increased cellular proliferation. The data from the present experiments indicate that neurogenesis was increased in the SGZ of _p21_−/− mice. BrdU incorporation was greater in the SGZ of the _p21_−/− mice, and there was up-regulation of DCX and NeuN proteins. In addition, there was a higher number of DCX–positive cells in the hippocampi of _p21_−/− mice compared with WT animals. In support of our finding, _p21_−/− mice have been found to exhibit increased forebrain neural stem cell proliferation and more cell divisions (27), as well as greater neurogenesis in response to experimental ischemia (28). In addition, p21 is a transcriptional target for p53. When p53 is absent, p21 is down-regulated, and there is increased proliferation and survival of neural stem cells (29).
Others have found that cell cycle inhibitors are expressed in the adult brain. For example, p15INK4B has been found in the forebrain, p21 has been found in the cerebellum (30, 31), and p27Kip has been found in the cerebellum and postmitotic cortical neurons (31, 32). It is likely that other CDK inhibitors also affect neurogenesis. Double knockout animals obtained from crossing p19-null and p27-null mice exhibit increased neuronal proliferation in all parts of the brain (33). Given the capacity of p21 to restrain the cell cycle, its localization in the SGZ, and its confinement to DCX-positive cells, p21 might play an important and unique role in regulating neuronal proliferation in the hippocampus of adult mice.
Previous studies have demonstrated that repeated treatment with antidepressants increases the total number of neurons in the hippocampus (8) and that SGZ neuronal progenitor cells are the target of fluoxetine treatment (16). Increased neurogenesis in the adult hippocampus occurs after chronic administration of different classes of antidepressants, including selective serotonin and norepinephrine reuptake inhibitors and monoamine oxidase inhibitors, and after electroconvulsive shock therapy, a procedure that is efficacious in the treatment of depression (4, 12, 34). Consistent with these findings, our data indicate that chronic administration of a tricyclic antidepressant stimulates neuronal proliferation in the hippocampus, as shown by increased BrdU incorporation and elevated levels of NeuN and PCNA. Imipramine treatment also decreased immobility in the FST. The increased neurogenesis and the behavioral effects were associated with decreased p21 mRNA and protein levels in the SGZ. Thus, suppression of p21 might mediate these effects of imipramine.
The mechanism of action underlying imipramine-induced suppression of p21 is not known. It is possible that imipramine suppresses p21 promoter activity secondary to its effects on neurotransmitters such as serotonin and/or norepinephrine. Imipramine inhibits the reuptake of both neurotransmitters, and its effects on p21 expression could involve either or both neurotransmitters. Local hippocampal factors also could be involved in the regulation of p21 expression. For example, the interplay between TGFβ2 and brain-derived neurotrophic factor in the cerebellum has been suggested to account for antiproliferative and prodifferentiating activities observed in postmitotic cerebellar neurons (30).
If a mechanism of action of antidepressants is to reduce p21-induced suppression of neurogenesis, then the etiology of some forms of depression might involve stimulation or up-regulation of p21. Stress is thought to play an important role in the pathogenesis of depression (35, 36), and glucocorticoids released in response to stressors can stimulate p21 promoter activity (37). Inflammatory processes also have been implicated in the pathogenesis of major depression (38), and proinflammatory cytokines, including IL-6 (39) and INFα, can arrest neuronal proliferation (40). These proinflammatory cytokines activate the Jak–STAT signaling pathway (41), and several binding sites for STAT1 and STAT3 are present on the p21 promoter (37). Antidepressants might indirectly reduce p21 levels by decreasing hypothalamo-pituitary-adrenal axis activity (42, 43) or by restraining the expression of inflammatory cytokines (44).
The role of neurogenesis in the hippocampus is not completely clear, but it has been suggested to be involved in memory formation and mood regulation (14, 45, 46). In depressed patients, hippocampal volume is decreased, and the magnitude of the volume reduction is directly related to the length and severity of the illness (15, 47–49). In rodents, chronic stress-induced “depression” also leads to hippocampal atrophy (50–52). Because neurogenesis is required for some of the behavioral effects of chronic antidepressant treatment in rodents (14), the induction of neurogenesis in the adult might represent a common final target for different classes of drugs and treatments that produce antidepressant effects.
In summary, the present data demonstrate that p21 is expressed in the SGZ of the dentate gyrus of the hippocampus, where it might act to restrain neurogenesis. Chronic treatment with the antidepressant imipramine down-regulates p21, possibly releasing neuronal progenitor cells from the blockade of proliferation. Because many antidepressants have been found to stimulate neurogenesis (8), it is possible that a shared common mechanism of action of antidepressants is suppression of p21. It must be acknowledged that the causal linkages between decreases in hippocampal neurogenesis and depression, and between therapeutic effects of antidepressants and increases in neurogenesis, have not been proven (53, 54). However, if these relationships are confirmed, then the results of the present study suggest that new therapies for major depression could be designed to directly target the expression of CDK inhibitors such as p21.
Methods
Experimental Animals.
Two-month-old, male C57BL/6j mice were treated daily for 21 days with saline (0.9%) or imipramine hydrochloride (10 mg/kg per day i.p.; Sigma–Aldrich). Twenty-four hours after the last injection, the mice were subjected to BrdU injections and used for the behavioral experiment or killed for the other studies. Cdk1atm1Tyj (_p21_−/−) mice on 129S2 genetic background were obtained from The Jackson Laboratory. For real-time PCR, ELISA, and Western blot analyses, the mice were killed, the brains were removed and rapidly cooled in ice-cold saline, and the hippocampi were dissected out (55). The Institutional Animal Care and Use Committee approved all experimental procedures.
Immunohistochemistry.
Mice were anesthetized with isoflurane and perfused with paraformaldehyde (4%), and brain paraffin sections were processed as described previously (56). The slides were incubated with mouse anti-p21 monoclonal antibodies (BD Pharmingen) and conjugated with Alexa Fluor 488 fluorescent dye (Molecular Probes). The antibody for NeuN (Chemicon) was conjugated with Alexa Fluor 568 fluorescent dye (Molecular Probes) and used to determine colocalization with p21 expression. DNA (nuclei) was stained with ToPro3 (Molecular Probes). Multiparameter fluorescent microscopy and Leica Confocal Software were used to identify p21 intracellular localization and colocalization with the neuronal marker NeuN. For comparability, all images on confocal multiimage figure plates were adjusted with identical contrast and brightness settings.
BrdU Immunochemistry.
The entire left half of the brain was cut into 5-μm sagittal sections and processed by using a BrdU Labeling and Detection Kit (Roche Applied Biosystems). Sections were coded for blind observation. Unbiased random sampling from 0.36 to 0.6 mm lateral to the midline (55) was carried out. Every third section (of a total of 30 sections) was counted under a ×100 objective, and the sum was multiplied by 3 to estimate the total number of BrdU-positive cells in the region. Cells were counted if they were in or touching the SGZ, and cells were excluded if they were more than two cell diameters from the GCL (8). Some sections derived from p21-null mice were double-labeled to detect DCX (Santa Cruz Biotechnology), and at least 20 BrdU-positive cells were examined by confocal microscopy to determine colocalization with DCX.
FACS Analysis.
Hippocampi were dissociated with a Papain Dissociation System (Worthington Biochemical), and the cells were processed according to a Flow Cytometry Staining Protocol (Cell Signaling Technology). Fixed cells were incubated overnight with DCX antibodies (Cell Signaling Technology), washed, and treated with secondary antibodies (Alexa Fluor 586 fluorescent dye, red), and samples were analyzed in a FACSCalibur system (Becton Dickinson). The cells were gated on the basis of forward/side scatter plot to eliminate the debris. DCX-positive and DCX-negative cells were sorted. For the double-staining experiments, both DCX-positive and DCX-negative cells were stained for p21 (BD Pharmingen) for 2 h at 4°C, then stained with a secondary antibody (Alexa Fluor 488 fluorescent dye, green), and the number of DCX/p21-positive cells was determined. Control cells were stained with both secondary antibodies.
Protein Isolation and Western Blot Analysis.
Proteins were isolated (Immunoprecipitation Kit; Roche Diagnostics), separated by SDS/PAGE, electroblotted onto membranes (Millipore), incubated overnight with primary antibodies, and then incubated with corresponding secondary antibodies as described (56). Immunoreactive bands were detected by the ECL immunodetection system. NeuN (Chemicon) and PCNA, DCX, and β-actin (Santa Cruz Biotechnology) antibodies were used. For quantitative analysis blots were scanned by using an Epson V750 PRO and transferred to Adobe Photoshop Elements 3. Intensity analysis of the bands (all multiple bands simultaneously) was performed with ImageJ Software (ImageJ; W. S. Rosband, National Institutes of Health; http://rsb.info.nih.gov/ij).
ELISA.
p21 protein levels in the hippocampus were determined by using a PathScan Total p21 Waf1/Cip1 Sandwich ELISA kit (Cell Signaling Technology). Proteins were isolated by using an Immunoprecipitation Kit (Roche Diagnostics). The hippocampi from three mice per group were pooled, and lysates from three independent experiments were assessed in triplicate.
Quantitative Real-Time PCR.
Total RNA was isolated with TRIzol reagent according to the manufacturer's instructions (Invitrogen). Quantitative real-time PCR (56) was performed to detect p21 mRNA expression. Relative quantification of p21 mRNA in experimental samples was determined from the corresponding standard curve, normalized to the housekeeping gene 18S, and expressed as arbitrary units.
FST.
Twenty-four hours after the last injection of saline or imipramine, the FST was performed as described previously (57). Briefly, mice are individually placed into a glass cylinder (25-cm height, 10-cm diameter) containing 8 cm of water maintained at 22–24°C. Every 30 sec for a total of 6 min the mice are rated for immobility, defined as the absence of active, escape-oriented behaviors such as swimming, jumping, rearing, sniffing, or diving. At each time point the mice were observed for 10 sec, and immobility was recorded as being present or absent. The raters were blind to the experimental treatment. The number of time points where immobility was scored during the last 4 min of the experimental session was summed for each subject to yield a total immobility score (18).
Statistical Analysis.
The total immobility scores for the FST were analyzed by one-way ANOVA. The data for the real-time PCR, Western blot quantification, ELISA, and BrdU injection were analyzed by using unpaired t tests.
ACKNOWLEDGMENTS.
We are grateful to Dr. Shlomo Melmed for his continuing support and for the generous gift of the p21+/− mice. We thank Drs. Mark H. Rapaport and Robert M. Cohen. This work was partially supported by a NARSAD Young Investigator Award and National Institutes of Health Grant MH 079988 (to V.C.) and by the Levine Family Fund Research Endowment (R.N.P.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–168. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 8.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- 11.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Perera TD, Lisanby SH. Neurogenesis and depression. J Psychiatr Pract. 2000;6:322–333. doi: 10.1097/00131746-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 15.Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 18.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 19.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berlin) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 20.Willner P, Wilkes M, Orwin A. Attributional style and perceived stress in endogenous and reactive depression. J Affect Disord. 1990;18:281–287. doi: 10.1016/0165-0327(90)90080-r. [DOI] [PubMed] [Google Scholar]
- 21.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 22.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 24.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 25.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 26.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 27.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, et al. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meletis K, et al. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Wu Y, Sousa N, Almeida OF. SMAD pathway mediation of BDNF and TGF beta 2 regulation of proliferation and differentiation of hippocampal granule neurons. Development. 2005;132:3231–3242. doi: 10.1242/dev.01893. [DOI] [PubMed] [Google Scholar]
- 31.Legrier ME, Ducray A, Propper A, Kastner A. Region-specific expression of cell cycle inhibitors in the adult brain. NeuroReport. 2001;12:3127–3131. doi: 10.1097/00001756-200110080-00029. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa K. Cell cycle regulators in neural stem cells and postmitotic neurons. Neurosci Res. 2000;37:1–14. doi: 10.1016/s0168-0102(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 33.Zindy F, et al. Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1999;96:13462–13467. doi: 10.1073/pnas.96.23.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malberg JE, Blendy JA. Antidepressant action: To the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 36.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 37.Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 38.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 39.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe T, Kominsky SL, Subramaniam PS, Johnson HM, Torres BA. Inhibition of the glioblastoma cell cycle by type I IFNs occurs at both the G1 and S phases and correlates with the upregulation of p21(WAF1/CIP1). J Neurooncol. 2000;48:225–232. doi: 10.1023/a:1006476408190. [DOI] [PubMed] [Google Scholar]
- 41.Ihle JN. STATs: Signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: Tianeptine. Mol Psychiatry. 2005;10:525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- 43.Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacol Res. 2004;49:119–131. doi: 10.1016/j.phrs.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Shors TJ. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 46.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajkowska G. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- 51.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 52.Stein-Behrens BA, Sapolsky RM. Stress, glucocorticoids, and aging. Aging (Milan) 1992;4:197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- 53.Feldmann RE, Jr, Sawa A, Seidler GH. Causality of stem cell based neurogenesis and depression—to be or not to be, is that the question? J Psychiatr Res. 2007;41:713–723. doi: 10.1016/j.jpsychires.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic; 1997. [Google Scholar]
- 56.Chesnokova V, Kovacs K, Castro AV, Zonis S, Melmed S. Pituitary hypoplasia in Pttg−/− mice is protective for Rb+/− pituitary tumorigenesis. Mol Endocrinol. 2005;19:2371–2379. doi: 10.1210/me.2005-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pechnick RN, et al. Reduced immobility in the forced swim test in mice with a targeted deletion of the leukemia inhibitory factor (LIF) gene. Neuropsychopharmacology. 2004;29:770–776. doi: 10.1038/sj.npp.1300402. [DOI] [PubMed] [Google Scholar]
- 58.Demandolx D, Davoust J. J Microscopy. 1997;185:21–36. [Google Scholar]