Enhancement of Tumor-specific Immune Response with Plasmid DNA Replicon Vectors (original) (raw)
. Author manuscript; available in PMC: 2008 Feb 22.
Published in final edited form as: Cancer Res. 2000 Jan 1;60(1):51–55.
Abstract
To enhance the immunogenicity of nucleic acid vaccines, we used plasmid DNA vectors that contained replicons derived from the prototype alphavirus, Sindbis, and another alphavirus, Semliki Forest virus. When transfected into cells or injected directly into animal muscle, these plasmids launch a self-replicating RNA vector (replicon) which in turn directs the expression of a model tumor antigen. Immunization with plasmid DNA replicons elicited immune responses at doses 100 to 1000-fold lower than conventional DNA plasmids and effectively treated mice bearing an experimental tumor expressing the model antigen. Significantly, replicon-based DNA plasmids did not produce a greater quantity of antigen; instead, antigen production differed qualitatively. Plasmid DNA replicons mediated antigen production that was homogeneous in all transfected cells and associated with the apoptotic death of the host cells. Because of their safety and efficacy, plasmid DNA replicons may be useful in the development of recombinant vaccines for infectious diseases and cancer.
Introduction
“Naked” nucleic acids are attractive candidate vectors for the development of cancer vaccines encoding tumor-associated antigens. They are relatively simple to generate and safe to administer. Because they are not associated with a viral coat, “naked” nucleic acids are not generally subject to neutralizing antibody reactions that can hamper the clinical efficacy of vaccines based on recombinant viruses such as adeno- and vaccinia viruses (reviewed in Refs. 1 and 2).
In preclinical tumor models, nucleic acid vaccines encoding model antigens can elicit antigen-specific antibody and CD8+ T-cell responses and can be therapeutically effective when combined with immunomodulatory molecules such as CD40 ligand or interleukin 2 or interleukin 12 (3-6). DNA vectors have also been enhanced by optimizing promoters, introns, and polyadenylation signals. Immunostimulatory sequences have also been shown to enhance the function of some plasmid DNA immunization (7). One improvement upon plasmid DNA vectors was the incorporation of alphavirus replicons (8, 9). In animal models of infectious disease, these plasmid DNA replicons are substantially more efficient at stimulating antigen-specific immune responses, particularly cellular responses, as compared with conventional plasmid DNA expression vectors (10, 11). Alphavirus replicons, in the form of RNA, DNA, or infectious particles, are generally potent inducers of broad immune responses in both rodents and primates (reviewed in Refs. 12).
We have demonstrated recently that an RNA vaccine encoding a model tumor-associated antigen together with the gene for RNA replicase from the Semliki Forest virus was effective in the treatment of an experimental tumor (13). In the present study, we extend this work to DNA plasmids, using a CMV3 promoter to “prime the pump” and generate a long positive strand of RNA (replicon) which, like the alphaviral genome itself, is then capable of self-replication. We then compared these vectors, quantitatively and qualitatively, to conventional plasmid-based vaccines.
Materials and Methods
Vaccines
Plasmid DNA replicons encoding _β_-gal and conventional CMV promoter-based _β_-gal plasmids are shown in Fig. 1A. pSIN1.5-_β_-gal (Chiron Technologies, San Diego, CA) uses the Sindbis virus replicon, whereas a Semliki Forest virus replicase drives mRNA replication in the pRep-LacZ plasmid. pSPORT-_β_-gal was obtained from Life Technologies, Inc. (Bethesda, MD), and pCMV-_β_-gal was constructed at Powderject (Middleton, WI) by cloning a CMV-_β_-gal-cassette into the PGEM plasmid backbone (Promega Corp., Madison, WI; Ref. 4). A Semliki Forest virus replicase-based self-replicating RNA construct encoding _β_-gal (Rep-RNA) was used in some in vitro studies as a positive control (13). To monitor antigen expression on a cellular level, two plasmids were used that express EGPF either under the control of a CMV promoter (pEGFP-C1; Clontech Laboratories, Palo Alto, CA) or encode a Sindbis replicon (pSIN1.5-EGFP, provided by Dr. Rong-Fu Wang, National Cancer Institute, Bethesda, MD).
Fig. 1.
Plasmid DNA replicons have superior immunogenicity compared with two conventional plasmids. A, structure of plasmids. The two plasmid DNA replicons, pSIN and pRep, encode alphaviral replicons from Sindbis virus and Semliki Forest virus, respectively. The LacZ gene is inserted between the subgenomic start sequence and a 3′ replicase-recognition sequence plus intron. Because the plasmid DNA replicons are twice the size of the conventional plasmids used, only half the number of plasmid copies of plasmid DNA replicon were injected compared with conventional plasmids. _B, β_-gal-specific IgG responses elicited by a single i.m. immunization with plasmid DNA replicons versus conventional plasmid DNA. Sera were collected from four mice/group 21 days after immunization and diluted 1:20, 1:100, 1:250, 1:2500. _A_490 nm shown are averages of individual determinations. Similar results were obtained in two additional experiments. C, plasmid DNA replicons elicit superior cellular immune responses. Pooled splenocytes (four mice/group) from mice immunized 21 days earlier were cultured for 6 days in the presence of _β_-gal876–884 peptide and then tested for antigen-specific recognition as measured by IFN-γ released/105 cells. Shown are values obtained in response to CT26 pulsed with _β_-gal peptide, but similar results were obtained using the _LacZ_-transfected target CT26.CL25 (not shown). The response to control targets (CT26.WT alone or pulsed with the P-1A35–43 peptide) was < 100 pg for all groups. This experiment was repeated with similar results.
Peptides and Cell Lines
The synthetic peptide TPHPARIGL, representing the naturally processed H-2 Ld-restricted peptide 876–884 of _β_-gal, and the peptide LPYLGWLVF, representing residues 35–43 of the P815A protein (P-1A), were synthesized and purified by Peptide Technologies (Washington, DC) and assayed by high-performance liquid chromatography and amino acid analysis. A _β_-gal-expressing clone (CT26.CL25) was generated from CT26.WT transduced with a LacZ retrovirus and maintained in complete medium based on RPMI 1640 with 10% fetal bovine serum, as described (14). BHK-21 (American Type Culture Collection, Manassas, VA) cells were used for in vitro transfection experiments.
Immunizations
Female BALB/c mice (6–10 weeks of age; Jackson Laboratories, Bar Harbor, ME) were immunized with 50 _μ_l of plasmid DNA in PBS into each quadricep, and immune responses were evaluated 3 weeks later. Sera antibody titers were determined individually in four mice/group (mean titers are shown) in a _β_-gal-ELISA assay as described previously (13). CD81 T-cell function was assessed in pooled spleen cells (four mice/group) cultured in the presence of 1 _μ_g/ml _β_-gal-peptide for 6 days. Subsequently, 105 effector cells/well were incubated with 105 target cells (CT26.WT alone or pulsed with 1 _μ_g/ml peptide, or CT26.CL25) for 24 h. Supernatants were diluted 1:10 and tested for IFN-γ release using a mIFN-γ ELISA kit (Endogen, Cambridge, MA). For tumor prevention experiments, at least five mice/group were injected i.v. with 5 × 105 CT26.CL25 cells 3 weeks after a single immunization with DNA. For treatment experiments, 10 mice/group were injected with 1 × 105 CT26.CL25 cells 2 days prior to treatment with DNA. The number of pulmonary metastases was determined in a blinded fashion 12 days after challenge, and data were analyzed using the Kruskal-Wallis test.
In Vitro Transfection and Analysis of Antigen Expression
BHK cells seeded at 1 × 105 cells/well in a six-well plate were cultured for 24 h and transfected with 1 _μ_g of DNA/well using LipofectAMINE PLUS (Life Technologies, Inc.), according to the manufacturer's instructions. Twenty-four h after transfection, cells were washed, fixed in 0.5% glutaraldehyde for 10 min, and incubated in 5-bromo-4-chloro-3-indolyl-_β_-D-galactopyranoside (Life Technologies, Inc.; 1:50) at 37°C for 1–2 h to determine transfection efficiency. Total _β_-gal production in lysed cells was determined using a _β_-gal ELISA (Boehringer Mannheim, Mannheim, Germany) as described (15). Expression of _β_-gal is reported as percentage of _β_-gal protein corrected for the transfection efficiency for the particular plasmid based on the total amount of cellular protein, determined with a BCA Protein Assay (Pierce, Rockford, IL). Cells transfected with EGFP plasmids were analyzed for antigen expression on a FACScan (Becton Dickinson, San Jose, CA).
Apoptosis in Transfected Cells in Vitro
BHK cells were transfected with SIN-EGFP as described above. After 24 h in the presence or absence of 20 _μ_m/ml caspase inhibitors (peptides z-VAD.fmk and z-FA.fmk; Enzyme Systems Products, Livermore, CA), cells were harvested and seeded in quadruplicates at 50–100 cells/well in 96-well plates. Fresh caspase inhibitor was added every 24 h, and living cells were counted daily using fluorescence microscopy.
Results and Discussion
Plasmid DNA Replicons Induce Stronger Humoral and Cellular Immune Responses Than Conventional DNA Vaccines
To evaluate the effects of the alpha-viral replicon on immune responses elicited by DNA vaccines encoding LacZ, we compared two plasmid DNA replicons encoding LacZ and two conventional CMV promoter-based LacZ plasmids. pSIN uses the Sindbis nonstructural proteins, whereas pRep uses nonstructural proteins derived from Semliki Forest virus. The conventional plasmids had been optimized for antigen expression (pSPORT-_β_-gal) or have successfully been used as a vaccine before in preclinical tumor vaccine trials (pCMV-_β_-gal; Ref. 4). After a single immunization, pSIN-_β_-gal produced strong antibody responses at all doses, whereas detectable humoral responses with all other plasmids were lost with between 10 and 1 _μ_g (Fig. 1B). Note that the plasmid DNA replicons, pSIN and pRep, are about two times larger than the conventional CMV-based plasmids, pSPORT and pCMV, and thus only half the number of moles of plasmid DNA replicon were delivered per _μ_g of DNA.
Cellular responses were superior in mice immunized with the plasmid DNA replicon, as measured by antigen-specific IFN-γ release (Fig. 1C). At the 1-_μ_g dose, only mice immunized with the plasmid DNA replicons, pSIN and pRep, produced an IFN-γ response. Conventional plasmids were not immunogenic at doses <10 _μ_g in repeated experiments. Interestingly, the strongest response of all groups was obtained with the lowest dose of pSIN, and similar to the antibody response, no direct correlation between the injected dose and the strength of the immune response was observed.
Plasmid DNA Replicon Vaccines Effectively Immunize against Tumor Challenge
Protection from tumor challenge was evaluated in experiments in which mice were immunized with a single dose of vector injected i.m. Immunization with 50 _μ_g resulted in complete prevention with all plasmids used (not shown) and protection that was nearly complete at the 10-_μ_g dose (Fig. 2A). However, at 1 and 0.1 _μ_g/mouse, only immunization with pSIN completely protected from tumor challenge. Although pRep showed some protection, the two non-replicon-coding plasmid vaccines completely lost their protective effect.
Fig. 2.

Therapeutic efficacy of “naked” DNA immunization. A, prevention of tumor challenge with plasmid DNA replicons is superior to conventional plasmids at limiting amounts of DNA. Mice were immunized once and challenged 21 days later with 5 × 105 CT26.CL25 tumor cells. Pulmonary metastasis were enumerated 12 days after challenge. B, only p-SIN-_β_-gal treats established pulmonary metastasis after challenge with 1 × 105 CT26.CL25. The mean number of pulmonary metastasis is indicated. C, a single treatment with 1 _μ_g of p-SIN-_β_-gal significantly prolongs survival of CT26.CL25 tumor-bearing mice. *, P compares pSIN-_β_-gal with PBS control. There was no significant difference between PBS and pSPORT-_β_-gal. Treatment with 10 _μ_g of either plasmid yielded almost identical results as with 1 _μ_g (not shown).
A Plasmid DNA Replicon Treats Established Tumor
A more challenging evaluation of the in vivo efficacy than tumor prevention is the treatment of established tumor. On the basis of the immunogenicity of the plasmids in the above studies, pSIN was selected for treatment studies. At all doses, the plasmid DNA replicon pSIN yielded significant treatment of established CT26.CL25 tumors. Of the conventional DNA plasmids, only pSPORT treated tumors and only at doses <10 _μ_g (Fig. 2B; only 10-_μ_g dose shown), whereas pCMV-_β_-gal had no therapeutic effect at any dose used (0.1 to 50 _μ_g, not shown). pSIN was most efficacious at the lowest dose (median number of metastases at 0.1-_μ_g dose, 43), whereas its efficacy slightly decreased with increased dose (median at 1 _μ_g, 76; median at 10 _μ_g, 124). To study the increase in survival after tumor challenge, mice with established lung metastasis were treated with a single dose of pSIN or pSPORT (Fig. 2C). Although both plasmids prolonged survival at very high doses (50 or 100 _μ_g), there was no significant difference between the plasmids (data not shown). However, survival was significantly prolonged by treatment with 10 or 1 _μ_g pSIN (compared with control P < 0.00001 and P = 0.0001, respectively), whereas the same doses of pSPORT were ineffective compared with control (P = 0.12 and P = 0.09, respectively).
In Vitro Antigen Expression Does Not Correlate with in Vivo Immunogenicity of the Individual Plasmids
One possible explanation for the enhanced immunogenicity of replicon-based systems was that they mediated increased expression of the encoded antigen. However, we found no correlation between antigen expression and immunogenicity (Fig. 3). Despite the presence of the alphaviral replicon in pSIN and pRep, a conventional CMV-based plasmid yielded the highest _β_-gal production. To test antigen expression on a single-cell basis, cells were transfected with a conventional, CMV (pEGFP-C1)- or replicon (pSIN1.5-EGFP)-based plasmid encoding EGFP for analysis of the transfected cells by flow cytometry. pEGFP-C1-transfected cells varied widely in their expression of the transgene, whereas pSIN-transfected cells displayed a homogenous EGFP expression profile (Fig. 4A). This phenomenon is a direct result of the plasmid-encoded alphaviral replicon, because the same staining pattern was obtained with a self-replicating RNA construct (Rep-RNA-EGFP, data not shown). To test whether this striking effect was merely attributable to a choice of a particular concentration of DNA, we assessed antigen expression in cells transfected with limiting amounts of plasmid. For the conventional CMV-based plasmid, pEGFP-C1, the overall expression level directly correlated with the amount of DNA used, leading to the disappearance of highly transfected cells at low DNA concentrations. In contrast, pSIN-EGFP-transfected cells express the same amount of antigen independent of the amount of plasmid used, establishing a quantitative as well as a qualitative difference between the two systems (Fig. 4B). This phenomenon could explain why immune responses induced by CMV-based plasmids correlate with the dose of injected DNA, whereas plasmid DNA replicons do not show a dose titration.
Fig. 3.
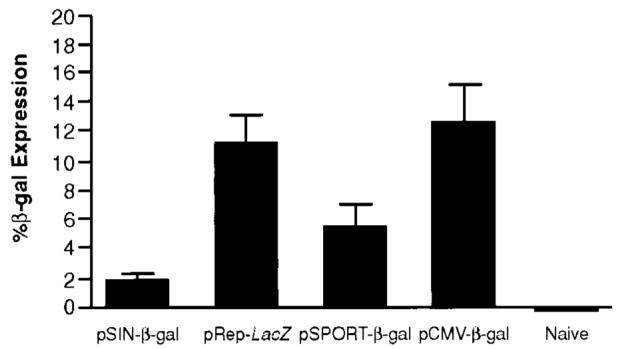
Plasmid DNA replicons do not necessarily induce more antigen expression than conventional plasmids. The amount of _β_-gal produced by transfected BHK-21 cells within 24 h is expressed as percentage of _β_-gal based on the total cellular protein of the transfected cells, after correcting for the different transfection efficiencies of the various plasmids. SDs (bars) were calculated from replicate transfections. The highest expression was obtained with the least immunogenic plasmid, pCMV-_β_-gal, whereas the most immunogenic plasmid, pSIN-_β_-gal, yielded the lowest antigen production.
Fig. 4.
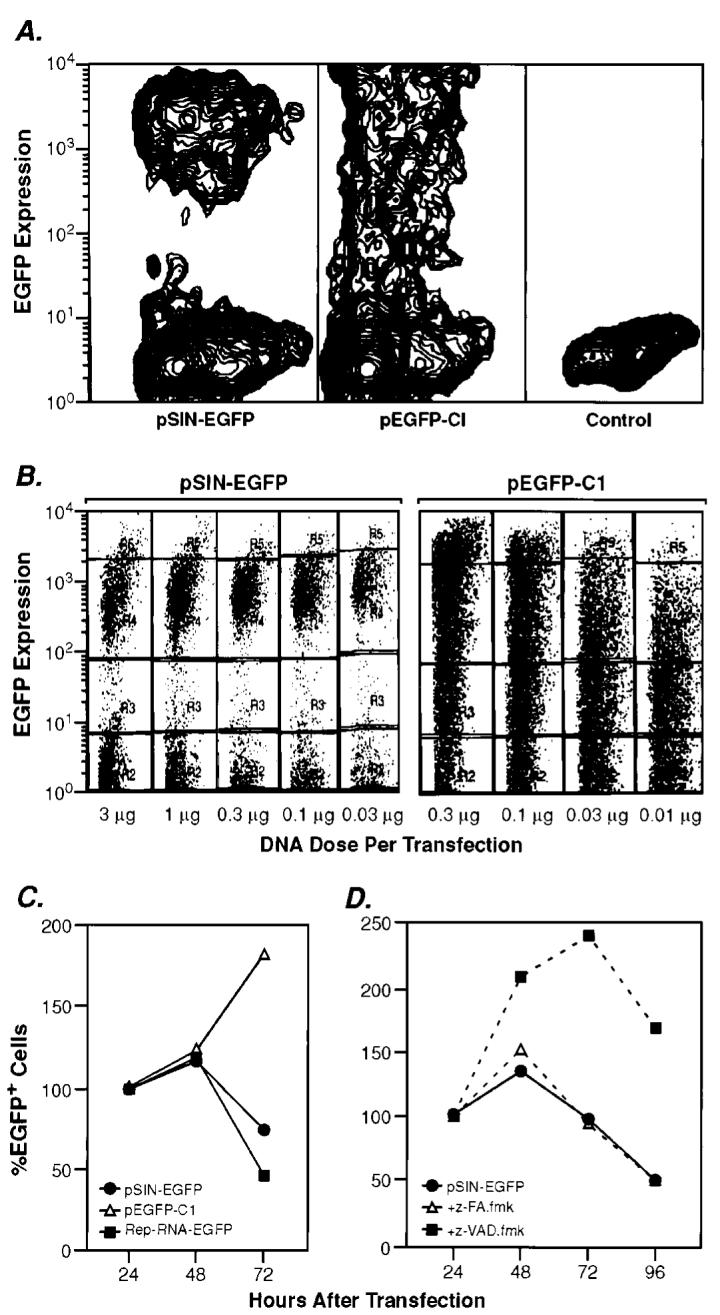
Plasmid DNA replicons are qualitatively different than conventional plasmids. A, BHK-21 cells transfected with pEGFP-C1 or pSIN-EGFP produce a distinct and significantly different staining pattern, as analyzed by flow cytometry. B, altering the amount of pSIN-EGFP used for transfection does not affect the EGFP expression profile. However, transfection with various amounts of pEGFP-C1 plasmid causes a shift in the subpopulations of EGFP-expressing cells, resulting in a decrease in the high-expressor cell population when reducing the amount of plasmid. Transfected cells are grouped into low, medium, and high expressors. Categories are based on expression level of EGFP by pSIN-EGFP-transfected cells, which equals medium expressors. C, alphaviral replicase causes cell death in transfected cells in vitro, whereas cells transfected with a conventional DNA construct continue to proliferate. BHK-21 cells were transfected with pSIN-EGFP, pEGFP-C1, or a self-replicating RNA construct expressing EGFP (Rep-RNA-EGFP). D, the decrease in the number of pSIN-transfected cells is attributable to apoptosis, which can be delayed but not completely prevented by using the caspase inhibitor z-VAD.fmk. The control-peptide z-FA.fmk does not affect cell survival. The number of cells is reported as %EGFP+ cells based on the number of cells plated 24 h after transfection (= 100%).
Plasmid Transfection of Cells in Vitro Induces Apoptosis
Alphaviral infection of cells is known to induce their apoptotic death. Likewise, transfection with “self-replicating” RNA constructs cause the quantitative death of cells by apoptosis (16-18). This death is likely caused by the requisite double-stranded RNA intermediates that are generated during the replication of the alphavirus. To test whether the DNA constructs also mediated apoptotic death, BHK cells were transfected with the plasmid DNA replicon, pSIN-EGFP (Fig. 4C). As a positive control, cells were transfected with a self-replicating RNA construct, Rep-RNA-EGFP (13). Cells transfected with pSIN-EGFP start to disappear after a brief proliferation phase with a peak at 48 h after transfection, following the same kinetics as cells transfected with a self-replicating RNA construct. Survival of transfected BHK cells can be significantly prolonged, but not completely prevented, by the addition of the caspase inhibitor z-VAD.fmk but not the control fluro-methyl-ketone peptide inhibitor, z-FA.fmk (Fig. 4D).
Implications for Vaccine Development
On the basis of the notion that “more antigen is better,” vaccinologists have spent a great deal of effort optimizing antigen production by recombinant vectors. However, we have shown previously that the optimization of antigen expression is best conducted in dendritic cells and that late promoters, which mediate the highest expression of antigens by vaccinia viral constructs, were not the most immunogenic (15). The findings presented here suggest that the qualitative aspects of antigen expression may be more important than high-level antigen production. Thus, although we found that the replicon-based vectors did not necessarily produce more antigen (Fig. 3), the immunogen was produced at consistent levels in all transfected cells and was associated with the apoptotic death of host cells (Fig. 4). This caspase-dependent death is likely mediated by the double-stranded RNA-dependent kinase PKR, as well as RNaseL, both enzymes involved in cellular defense against viral infection (19, 20).
Apoptotic death associated with the production of double-stranded RNA intermediates is likely to have immunological consequences that are of benefit to the vaccinologist: (a) death by apoptosis may increase the uptake of antigen by dendritic cells for subsequent processing and class I-restricted presentation to CD8+ T cells (21). This uptake is mediated primarily by _α_V_β_5 receptors on the surfaces of dendritic cells and is restricted to dendritic cells (22). We have demonstrated previously an increased uptake of cells undergoing apoptosis as a result of transfection with “self-replicating” RNA (13), confirming these observations; and (b) double-stranded RNA itself is a “danger signal,” functioning as an adjuvant to the T-cell-specific stimulus of the encoded antigen (23). Plasmid DNA replicons thus appear to mimic a host cell infection by an alphavirus because the replicons mediate the production of double-stranded RNA, which in turn activates dendritic cells (24).
The present work reveals that factors in addition to the amount of expressed antigen can profoundly affect the immunogenicity of the recombinant vaccine. Indeed, more antigen is not necessarily better. Instead, “danger signals” provided by the “naked” plasmid DNA replicons might be the key to their enhanced immunogenicity.
Acknowledgments
We thank M. Blalock for graphics, P. Spiess for help with animal experiments, Dr. Rong-Fu Wang for the construction of pSIN1.5-EGFP, and Dr. S. A. Rosenberg for helpful discussion.
Footnotes
3
The abbreviations used are: CMV, cytomegalovirus; _β_gal, _β_-galactosidase; EGFP, enhanced green fluorescent protein.
References
- 1.Hellstrom I, Hellstrom KE. Tumor vaccines–a reality at last? J. Immunother. 1998;21:119–126. [PubMed] [Google Scholar]
- 2.Minev BR, Chavez FL, Mitchell MS. Cancer vaccines: novel approaches and new promise. Pharmacol. Ther. 1999;81:121–139. doi: 10.1016/s0163-7258(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 4.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J. Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 5.Gurunathan S, Irvine KR, Wu CY, Cohen JI, Thomas E, Prussin C, Restifo NP, Seder RA. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SS, Eisenlohr LC, McCue PA, Mastrangelo MJ, Lattime EC. Intravesical gene therapy: in vivo gene transfer using recombinant vaccinia virus vectors. Cancer Res. 1994;54:3325–3328. [PubMed] [Google Scholar]
- 7.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu. Rev. Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Dubensky TWJ, Driver DA, Polo JM, Belli BA, Latham EM, Ibanez CE, Chada S, Brumm D, Banks TA, Mento SJ, Jolly DJ, Chang SM. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J. Virol. 1996;70:508–519. doi: 10.1128/jvi.70.1.508-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driver DA, Polo JM, Belli BA, Banks TA, Hariharan MJ, Dubensky TW. Plasmid DNA-based alphavirus expression vectors for nucleic acid immunization. Curr. Res. Mol. Therapeut. 1998;1:510–517. [PubMed] [Google Scholar]
- 10.Hariharan MJ, Driver DA, Townsend K, Brumm D, Polo JM, Belli BA, Catton DJ, Hsu D, Mittelstaedt D, McCormack JE, Karavodin L, Dubensky TWJ, Chang SM, Banks TA. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berglund P, Smerdou C, Fleeton MN, Tubulekas I, Liljestrom P. Enhancing immune responses using suicidal DNA vaccines. Nat. Biotechnol. 1998;16:562–565. doi: 10.1038/nbt0698-562. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger S, Dubensky TW. Alphavirus vectors for gene expression and vaccines. Curr. Opin. Biotechnol. 1999;10:434–439. doi: 10.1016/s0958-1669(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 13.Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, Leitner WW, Restifo NP. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 1999;5:823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 15.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc. Natl. Acad. Sci. USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow GM, McGee MM, Tarbatt CJ, Mooney DA, Sheahan BJ, Atkins GJ. The Semliki Forest virus vector induces p53-independent apoptosis. J. Gen. Virol. 1998;79:2405–2410. doi: 10.1099/0022-1317-79-10-2405. [DOI] [PubMed] [Google Scholar]
- 17.Nava VE, Rosen A, Veliuona MA, Clem RJ, Levine B, Hardwick JM. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J. Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka N, Sato M, Lamphier MS, Nozawa H, Oda E, Noguchi S, Schreiber RD, Tsujimoto Y, Taniguchi T. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998;3:29–37. doi: 10.1046/j.1365-2443.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Guerra M, Rivas C, Esteban M. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology. 1997;236:354–363. doi: 10.1006/viro.1997.8719. [DOI] [PubMed] [Google Scholar]
- 20.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature (Lond.) 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 22.Albert ML, Pearce SA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18:765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
