Human CUL1 forms an evolutionarily conserved ubiquitin ligase complex (SCF) with SKP1 and an F-box protein (original) (raw)
Abstract
The SCF ubiquitin ligase complex of budding yeast triggers DNA replication by catalyzing ubiquitination of the S phase cyclin-dependent kinase inhibitor SIC1. SCF is composed of three proteins—ySKP1, CDC53 (Cullin), and the F-box protein CDC4—that are conserved from yeast to humans. As part of an effort to identify components and substrates of a putative human SCF complex, we isolated hSKP1 in a two-hybrid screen with hCUL1, the closest human homologue of CDC53. Here, we show that hCUL1 associates with hSKP1 in vivo and directly interacts with both hSKP1 and the human F-box protein SKP2 in vitro, forming an SCF-like particle. Moreover, hCUL1 complements the growth defect of yeast _cdc53_ts mutants, associates with ubiquitination-promoting activity in human cell extracts, and can assemble into functional, chimeric ubiquitin ligase complexes with yeast SCF components. Taken together, these data suggest that hCUL1 functions as part of an SCF ubiquitin ligase complex in human cells. Further application of biochemical assays similar to those described here can now be used to identify regulators/components of hCUL1-based SCF complexes, to determine whether the hCUL2–hCUL5 proteins also are components of ubiquitin ligase complexes in human cells, and to screen for chemical compounds that modulate the activities of the hSKP1 and hCUL1 proteins.
The irreversible nature of proteolysis makes it well suited to serve as a regulatory switch for controlling unidirectional processes. This principle is clearly evident in the organization of the cell division cycle, where initiation of DNA replication, chromosome segregation, and exit from mitosis are triggered by the destruction of key regulatory proteins (1–3).
Proteins typically are marked for proteolytic degradation by attachment of multiubiquitin chains. This process is initiated by a ubiquitin-activating enzyme (E1), which activates ubiquitin by adenylation and becomes linked to it via a thiolester bond. Ubiquitin then is transferred to a ubiquitin-conjugating enzyme, E2. Whereas E2s can attach ubiquitin directly to lysine residues in a substrate, most physiological ubiquitination reactions probably require a ubiquitin ligase, or E3 (4). E3s have been implicated in substrate recognition and, in one case, transfer of ubiquitin from E2 to a substrate via an E3–ubiquitin-thiolester intermediate (5). Once the substrate is multiubiquitinated, it then is recognized and degraded by the 26S proteasome.
A ubiquitination pathway recently has been discovered in budding yeast (1, 6, 7). Components of this pathway include the CDC53, CDC4, and ySKP1 gene products, which assemble into a ubiquitin ligase complex known as SCFCDC4 (for SKP1, Cullin, F-box protein CDC4); because several of the yeast and human subunits have identical names—e.g., SKP1—we distinguish them with the letters y or h, respectively. SCFCDC4 collaborates with the E2 enzyme yCDC34 to catalyze ubiquitination of the cyclin-dependent kinase (CDK) inhibitor SIC1. The specificity of SCFCDC4 is thought to be governed by ySKP1 and the F-box-containing subunit CDC4, which together form a substrate receptor that tethers SIC1 to the complex. The assembly of this receptor is thought to be mediated by a direct interaction between ySKP1 and the F-box domain of CDC4 (6, 7).
Whereas genetic analysis has revealed that SIC1 proteolysis requires CDC4, G1 cyclin proteolysis appears to depend on a distinct F-box-containing protein known as GRR1 (8). Alternative SCF complexes (SCFGRR1) assembled with GRR1 instead of CDC4 bind G1 cyclins but not SIC1, suggesting that there exist multiple SCF complexes in yeast whose substrate specificities are dictated by the identity of the F-box subunit (7).
Components of the SCF ubiquitination pathway have been highly conserved during evolution. Human homologues of yCDC34 and ySKP1 have been reported (9, 10), and F-box-containing proteins like CDC4 and GRR1 have been identified in many eukaryotes (11). Many of these F-box proteins also contain either WD-40 repeats (such as CDC4) or leucine-rich repeats (such as GRR1). A potential human counterpart of GRR1, SKP2, has been identified along with hSKP1 as a Cyclin A/CDK2-associated protein that is necessary for S-phase progression (10). Homologues of CDC53, which are known as Cullins, also are present in many eukaryotes, including humans and nematodes (12, 13).
Studies in budding yeast suggest that SCF substrates must be phosphorylated before they can be ubiquitinated (14, 15). Several human cell cycle regulators are targeted for ubiquitination after their phosphorylation by CDKs, implicating them as potential substrates of an SCF pathway(s) in human cells. Among them is the CDK inhibitor p27, the abundance of which may be regulated by CDC34-dependent ubiquitination (16, 17). In addition, Cyclins E and D1 are degraded by a ubiquitin-dependent pathway after phosphorylation at a specific site (18–20). The observation that Cyclin A/CDK2 associates preferentially with hSKP1 and SKP2 in transformed cells to the exclusion of a proliferating cell nuclear antigen and p21 (10) raises the possibility that Cyclin A is also a target of an SCF pathway. Alternatively, SCF-bound Cyclin A/CDK2 may phosphorylate SCF subunits or potential substrates such as E2F-1/DP-1, thereby activating SCF-dependent ubiquitination (21, 22).
Despite the conservation of SCF components from yeast to humans, several observations raise the question of whether the metazoan homologues are actually components of SCF-like ubiquitin ligases. First, whereas _Saccharomyces cerevisiae cdc53_ts mutants arrest at the G1/S transition, Caenorhabditis elegans cul-1 mutants fail to exit the cell cycle, resulting in hyperplasia of most larval tissues (12). It is unclear whether this discrepancy arises because cul-1 and CDC53 have different functions or because they are components of distinct ubiquitin ligase complexes with different substrate specificities. Second, the recent discovery of ubiquitin-like proteins (RUB1/NEDD8 and SMT3/SUMO1) that are conjugated to proteins by pathways that involve E1 and E2 homologs (23) suggests that some homologs of SCF components might function in these alternative pathways. Indeed, attachment of RUB1 to CDC53 fails to occur in skp1 mutants, suggesting that ySKP1 may be involved directly in the “rubinylation” of CDC53 (24). Third, the best characterized human Cullin, CUL2, assembles with the von Hippel–Lindau tumor suppressor protein/Elongin B/Elongin C complex that has been suggested to regulate mRNA transcript elongation and accumulation of hypoxia-inducible mRNAs (25, 26). Fourth, ySKP1 is a subunit of the centromere-binding CBF3 complex, suggesting that vertebrate SCF subunits may serve as components of a variety of unrelated molecular machines (34).
To address whether SCF-like activities are present in animal cells, we sought hCUL1 binding partners, and we tested whether putative human SCF subunits can assemble together to yield complexes with ubiquitin ligase activity. We report here that hCUL1 is a direct functional homologue of CDC53 because it can suppress the temperature-sensitive growth of cdc53 mutants, can associate with ubiquitin-conjugation activity in human cell lysates, and can substitute for CDC53 in the reconstitution of SIC1 ubiquitination with purified components. Moreover, hCUL1 directly binds to the putative SCF subunits hSKP1 and SKP2. Taken together, these data provide strong evidence that an SCF-dependent ubiquitination pathway is conserved from yeast to mammals.
MATERIALS AND METHODS
Yeast Strains and Reagents.
Yeast strains, plasmids, and a HeLa cDNA library for the two-hybrid screen were a generous gift from R. Brent (Massachusetts General Hospital). Wx131.2c _cdc53–2_ts strain was obtained from M. Goebl (Indiana University). Baculoviruses expressing hCDK2HA, hCyclin A (D. Morgan, University of California, San Francisco), SKP2 (H. Zhang, Yale University), hSKP1 (P. Sorger, Massachusetts Institute of Technology) and plasmids pGEX-KG-hSKP1 and pGEX-KG-SKP2 (P. Jackson, Stanford University) and pCS2+nβgal and pCS2+HA-SMC1 (S. Handeli, Fred Hutchinson Cancer Research Center) were kindly provided by the indicated investigators. Other baculoviruses have been described (6). Ubiquitin and the Protein Biotinylation Kit were purchased from Sigma, and biotinylated ubiquitin (BUb) was prepared according to the manufacturer’s instructions. Ubiquitin aldehyde was a generous gift from R. Cohen (University of Iowa).
Plasmid and Baculovirus Construction.
Full-length hCUL1 ORF was assembled from expressed sequence tags HE2AB96 and HSVAD74 and subcloned into pRS316 and pMALc (New England Biolabs). The same hCUL1 fragment also was subcloned into pVL1393 (PharMingen) to generate a hCUL1-expressing baculovirus. An N-terminal epitope-tagged version of hCUL1 was constructed by inserting a DNA cassette that contains two tandem repeats of the Polyoma epitope (MEYMPME) followed by six histidine residues (designated as PHis6) into pRS316-hCUL1. PHis6hCUL1 fragment then was subcloned into pFASTBAC1 (GIBCO/BRL) to generate a PHis6hCUL1 baculovirus and was subcloned into pDNA3.1/Zeo (Invitrogen) to generate pcDNA3.1-PHis6-hCUL1. pCS2+HA-hSKP1 was generated by subcloning a hSKP1 fragment from pGEX-KG-hSKP1 into pCS2+HA-SMC1.
Antibodies.
Anti-hCUL1 antibodies were generated in rabbits immunized with either a fusion protein containing the first 41 residues of hCUL1 followed by glutathione _S_-transferase (GST) (Babco, Richmond, CA) or a fusion protein containing GST followed by the last 86 residues of hCUL1 (California Institute of Technology antibody facility). Antibodies against hCUL1 and GST were affinity purified by using maltose binding protein (MBP) fusions of the corresponding peptides and GST, respectively, as described (27). Monoclonal anti-Polyoma antibodies were bound to protein A-Sepharose beads and crosslinked to protein A with dimethylpimilimadate (27) at a concentration of ≈2 mg of antibodies per ml of protein A resin. Anti-HA resin was generated by coupling 1 ml of anti-HA ascites to 1 ml of CNBr activated agarose (Pharmacia) according to the manufacturer’s protocol.
Expression and Purification of Proteins.
Proteins expressed in bacteria or yeast were purified according to standard protocols and as described (6). For the expression and purification of chimeric SCF complexes, Hi5 insect cells were infected with baculoviruses expressing PHACDC4 (PHA designates an epitope-tag consisting of two tandem repeats of the Polyoma epitope followed by three hemagglutinin epitopes), CDC53PHA, PHis6hCUL1 (multiplicities of infection of 6), ySKP1His6, or hSKP1 (multiplicities of infection of 4). Cells were collected 72 hr post-infection, and lysates were prepared as described (6). The Polyoma tagged proteins were affinity purified from these lysates (6) to yield the various SCF complexes.
Cell Cultures and Transfections.
WI-38 human lung fibroblasts were purchased from ATCC. HeLa S3 cells were a gift from S. Handeli. Cells were grown in DMEM-F12 (GIBCO/BRL) supplemented with 10% fetal bovine serum (GIBCO/BRL) at 37°C/5%CO2. Cells were transfected in 100-mm dishes by the modified calcium phosphate method (28). 10 μg pCS2+HA-hSKP1 and 7.5 μg pcDNA3.1-PHis6-hCUL1 vectors were used per transfection plate. Transfection efficiency was monitored by cotransfection of 2.5 μg pCS2+nβgal plasmid per transfection plate followed by standard colorimetric βgal assays (29). Total DNA concentration was 20 μg/100-mm dish and was adjusted for every transfection plate by adding empty vectors. Cells were harvested and lysed 24 hr post-transfection.
Immunoprecipitations and Western Blotting.
Baculovirus-infected insect cells were harvested and lysed at 48 hr (for Sf9 cells) or 72 hr (for Hi5 cells) post-infection in 0.8 ml of lysis buffer per 100-mm plate (as described in ref. 6). Metabolic labeling was done by incubating insect cells for 3 hr in methionine-deficient medium plus 20 μCi/ml of Tran[35S]-label before lysis. WI-38 and HeLa S3 cells were lysed in 0.4 ml of lysis buffer per 100-mm plate. Lysates were cleared by centrifugation at 14,000 × g for 15 min, were adjusted to 10% glycerol, were frozen in liquid nitrogen, and were stored at −80°C. Cell lysates (1 mg) were incubated with 50 μl of antibody-coupled beads (1:1 suspension in lysis buffer) for 2 hr at 4°C. Precipitates were washed five times with 1 ml of lysis buffer and were analyzed by SDS/PAGE followed by Western blotting or autoradiography. Western blotting was performed as described (27). PHis6hCUL1 and HAhSKP1 were detected by rabbit polyclonal anti-hCUL1 and biotinylated anti-HA (12CA5) primary antibodies and were visualized by incubation with goat anti-rabbit-horseradish peroxidase (HRP) and streptavidin-HRP conjugates followed by ECL detection (Amersham).
Ubiquitination Reactions.
Crude Sf9 cell lysates (500 μg) prepared from cells infected with Phis6hCUL1 baculovirus were incubated with 20 μl anti-Polyoma beads for 2 hr at 4°C to allow Phis6hCUL1 binding. Beads were washed three times with lysis buffer and were incubated with 1 mg of crude HeLa S3 lysate overnight at 4°C. Beads then were washed three times with lysis buffer and were supplemented with 6 μg BUb, 500 ng hCDC34, 25 ng His6yUBA1, 1 μl of 10× ATP-regenerating system (6), 1 μl of 10× reaction buffer (6), and 0.5 μM ubiquitin aldehyde. Reactions were adjusted to 10 μl by adding 20 mM Hepes (pH 7.6), 100 mM potassium acetate, 1 mM DTT, were incubated for 90 min at 30°C, and were terminated by adding Laemmli sample buffer. Samples were analyzed by Western blotting with streptavidin–HRP conjugate. All ubiquitination reactions with chimeric SCF complexes were performed as described (6).
RESULTS
Human CUL1-Interacting Proteins.
To identify human proteins that interact with hCUL1, we performed a two-hybrid screen (30, 31). A full-length hCUL1 cDNA, fused to the LexA DNA-binding domain, was used as a bait to identify cDNAs from a HeLa library that encode hCUL1 interactors. This screen yielded clones encoding hSKP1, protein phosphatase 2A catalytic subunit, and the 20S proteasome subunit HsN3. None of these clones interacted with LexA-hCDK2 or LexA-Lamin C baits, suggesting that their interaction with LexA-hCUL1 was specific. Here, we examine in detail the interaction of hCUL1 with hSKP1 (see below). The physiological significance of the interaction of hCUL1 with HsN3 or protein phosphatase 2A has not been evaluated yet.
Human CUL1 Interacts with hSKP1 in Vivo.
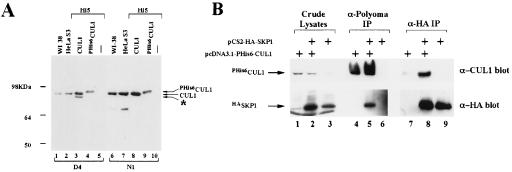
The identification of hSKP1 as a hCUL1-interacting protein suggested that these proteins may be subunits of a complex in human cells that is similar to the SCF ubiquitin ligase of budding yeast. To test whether hCUL1 interacts with hSKP1 in vivo, we prepared affinity-purified rabbit polyclonal antibodies directed against the N and C termini of hCUL1. Fig. 1A shows specificity of the affinity-purified antibodies. Both antibodies recognized one major polypeptide of ≈80 kDa in transformed (HeLa S3) and nontransformed (WI-38) cell lines (Fig. 1A, lanes 1, 2, 6, and 7). This species comigrated with hCUL1 produced in Hi5 cells infected with a baculovirus that contains full length hCUL1 cDNA (Fig. 1A, lanes 3 and 8). A more rapidly migrating species of recombinant hCUL1 detected in Hi5 cells by the anti-C-terminal antibodies (Fig. 1A, lane 3) presumably represents a breakdown product or initiation of translation downstream of the normal start codon because this species was not detected by the anti-N-terminal antibodies. As expected, addition of a Polyoma antigen-hexahistidine tag to hCUL1 (PHis6hCUL1) yielded a more slowly migrating hCUL1 band (Fig. 1A, lanes 4 and 9).
Figure 1.
hCUL1 and hSKP1 interact in vivo. (A) hCUL1 detection by affinity-purified anti-hCUL1 antibodies. Crude human cell lysates (50 μg) (lanes 1, 2, 6, and 7) and 0.5 μg of crude lysates from Hi5 insect cells, uninfected (lanes 5 and 10) or infected with hCUL1 (lanes 3 and 8) or PHis6hCUL1 (lanes 4 and 9) viruses, were resolved on an 8% SDS-polyacrylamide gel, transferred to a PVDF membrane, and probed with anti-hCUL1 antibodies. D4, serum raised against C-terminal part of hCUL1; N1, serum raised against N-terminal part of hCUL1. Asterisk designates putative N-terminally truncated hCUL1 that is recognized by C-terminal antibody. (B) HeLa S3 cells were transfected with pcDNA3.1-PHis6-hCUL1 (lanes 1, 4, and 7), pCS2+HA-hSKP1 (lanes 3, 6, and 9), or both plasmids (lanes 2, 5, and 8). Lysates (1 mg) were prepared 24 hr post-transfection and were immunoprecipitated with anti-Polyoma (lanes 4–6) or anti-HA (lanes 7–9) beads. Proteins retained on the beads were analyzed by Western blotting. Lanes 1–3 contained 20 μg of crude lysates.
Neither polyclonal antibody precipitated hCUL1 from crude human cell lysates, precluding analysis of hCUL1 complexes in nontransfected cells. Thus, to evaluate the potential interaction of hCUL1 with hSKP1 in vivo, we transfected HeLa S3 cells with PHis6hCUL1 and HAhSKP1 expression vectors. Lysates were prepared from these cells 24 hr post-transfection and were immunoprecipitated by using crosslinked anti-Polyoma or anti-HA antibody beads. Proteins bound to the beads were separated by SDS/PAGE and were analyzed by immunoblotting with anti-hCUL1 and anti-HA antibodies (Fig. 1B). Consistent with the two-hybrid data, hCUL1 was detected specifically in hSKP1 immunoprecipitates and vice versa.
Human CUL1, hSKP1, and SKP2 Assemble into an SCF-like Complex that Can Associate with Cyclin A/CDK2 Kinase.
Human SKP1 was initially identified as a Cyclin A/CDK2-associated protein in transformed human cells (10). This association is mediated by SKP2, a human F-box protein with leucine-rich repeats, reminiscent of the GRR1 protein. CDC53 and ySKP1, together with the F-box protein GRR1, constitute a putative SCFGRR1 ubiquitin ligase complex that targets G1 cyclins for degradation (6, 7). The homology of hSKP1, SKP2, and hCUL1 proteins with components of the ySCF complex suggests that the human proteins may form a similar complex. We addressed this possibility by immunoprecipitating PHis6hCUL1 from [35S]-labeled insect cells infected with baculoviruses that express PHis6hCUL1, hSKP1, and SKP2 (Fig. 2A) and by testing whether hCUL1 can assemble with a previously described complex containing Cyclin A/CDK2, hSKP1, and SKP2 (Fig. 2B). The interaction of hCUL1 with the Cyclin A/CDK2HA/hSKP1/SKP2 complex was monitored by immunoprecipitating CDK2HA from [35S]-labeled insect cells infected with all five viruses in various combinations. As shown in Fig. 2A, PHis6hCUL1 efficiently assembled with hSKP1 and SKP2, suggesting that these proteins form a ternary complex similar to ySCF. Surprisingly, hCUL1 interacted with Cyclin A/CDK2HA complexes in the absence of SKP2 or hSKP1 (Fig. 2B, lane 6; note that hSKP1 does not associate with Cyclin A/CDK2HA complex in the absence of SKP2). This interaction may be caused by either a direct interaction between hCUL1 and Cyclin A/CDK2HA or the presence of a bridging protein in insect cells (e.g., see ref. 6). Regardless, the ability of hCUL1 expressed in insect cells to assemble into complexes containing a cyclin-dependent kinase is likely to be physiologically significant because PHis6hCUL1 immunoprecipitates prepared from HeLa S3 cells contained histone H1 kinase activity (data not shown).
Figure 2.
Human CUL1 can interact with human SKP1, SKP2, and Cyclin A/CDK2. Sf9 insect cells were infected with baculovirus constructs that express various human proteins as indicated. Cells were labeled with Tran[35S]-label for 3 hr before harvesting. PHis6hCUL1 (A) and CDK2HA (B) together with associated proteins were immunoprecipitated by anti-Polyoma and anti-HA beads, respectively. The composition of the protein complexes in the immunoprecipitates was analyzed by SDS/PAGE followed by autoradiography.
Human CUL1 Directly Interacts with hSKP1 and SKP2.
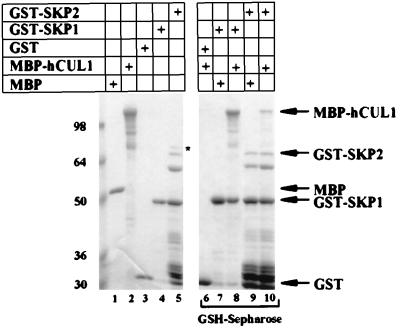
The results in Fig. 2 suggest that hCUL1, hSKP1, and SKP2 can assemble into an SCF-like particle when coexpressed in insect cells. Because of the strong conservation of SCF components, however, these interactions might be mediated by other proteins provided by the host cells (for an example, see ref. 6). To test whether the observed interactions are direct, we produced GST-hSKP1, GST-SKP2, and MBP-hCUL1 in bacteria. The GST fusions (or unfused GST control) were mixed with MBP-hCUL1 or MBP and were recovered by binding to glutathione-Sepharose beads. Bound proteins were resolved by SDS/PAGE and were visualized by Coomassie blue staining (Fig. 3). MBP-hCUL1 but not MBP bound specifically and efficiently to GST-hSKP1 and GST-SKP2 but not GST. This result demonstrates that hCUL1 can bind to both hSKP1 and SKP2 without the participation of other proteins.
Figure 3.
Human CUL1 binds directly to hSKP1 and SKP2. MBP, MBP-hCUL1, GST, GST-hSKP1, and GST-SKP2 were expressed individually in and purified from bacteria. Each protein was present in the binding reactions at 65 μg/ml. Proteins (4 μg of each) were loaded in lanes 1–5, which represents 1/5 of the input for the binding reactions. Proteins were mixed as indicated (lanes 6–10) and incubated on ice for 1 hr. GST and GST fusions were collected on glutathione-Sepharose (GSH-Sepharose) for 1 hr at 4°C, and the beads then were washed three times with 20 mM Hepes (pH 7.6), 150 mM NaCl, 0.1% Triton X-100, 5 mM MgCl2, 5 mM EDTA, and 2 mM DTT. Proteins bound to glutathione-Sepharose were resolved by SDS/PAGE and were visualized by staining with Coomassie blue. Positions of the full-length fusion proteins are indicated by the arrows. An ≈70-kDa band that copurified with GST-SKP2 from bacteria is marked by an asterisk.
Human CUL1 Is Functionally Homologous to CDC53 and Can Form an Active Chimeric SCF Complex with ySKP1 and CDC4.
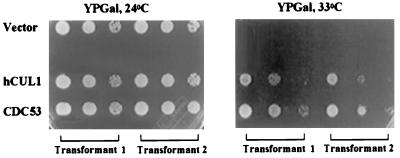
The above observations indicate that hCUL1, the closest human homologue of CDC53, can assemble with hSKP1 and the F-box protein SKP2 into a complex reminiscent of the yeast SCFGRR1 complex. We next tested whether this complex—in the presence of hCDC34, E1 enzyme, and ubiquitin—was able to ubiquitinate proteins that either bind to it (Cyclin A; ref. 10), are known to be degraded in S phase (Cyclin E, E2F-1; refs. 18, 19, 21, and 22), or have been implicated as substrates of hCDC34 (p27; refs. 16, 17). These efforts were unsuccessful (data not shown), raising the question of whether SCFSKP2 complexes possess ubiquitin ligase activity. Moreover, ubiquitin ligase activity of the analogous yeast SCFGRR1 complex has not been demonstrated yet and might require additional unidentified components. However, we were able to address whether hCUL1 is a functional component of a ubiquitin ligase complex genetically and biochemically by taking advantage of the considerable knowledge of this pathway in yeast. First, we asked whether hCUL1 can complement the _cdc53_ts mutation. We introduced hCUL1 and CDC53 under the control of the GAL1 promoter into a yeast strain carrying a temperature-sensitive mutation in the CDC53 gene. Individual transformants were spotted at different dilutions on glucose (noninducing conditions, data not shown) and galactose (inducing conditions) media at permissive (24°C) and restrictive (33°C) temperatures (Fig. 4). Only transformants that expressed wild-type CDC53 or hCUL1 proteins were able to grow at the restrictive temperature. However, hCUL1 failed to complement a cdc53 null strain (data not shown).
Figure 4.
hCUL1 complements the _cdc53_ts mutant phenotype. A _cdc53–2_ts mutant strain was transformed with pTS161-CDC53 and pTS161-hCUL1 plasmids that allow controlled expression of CDC53 and hCUL1 from the galactose-inducible GAL1 promoter. The empty vector alone was used as a negative control. Serial dilutions 1/10 of the individual transformants were spotted on synthetic galactose medium and were incubated for 5 days at restrictive (33°C) and permissive (24°C) temperatures.
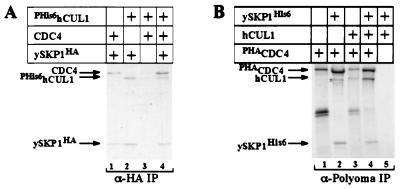
The ability of hCUL1 to complement the _cdc53_ts mutation implied that hCUL1 can assemble into functional SCF complexes with yeast proteins. To test this idea, we examined whether hCUL1 can interact with the budding yeast SCF subunits ySKP1 and CDC4. All three proteins were coexpressed in [35S]-methionine-radiolabeled insect cells in various combinations, as indicated in Fig. 5. Human CUL1 specifically coimmunoprecipitated with ySKP1HA (Fig. 5A, lane 2) or PHACDC4/ySKP1 (Fig. 5B, lane 4), indicating that it can form a chimeric SCFCDC4 complex with yeast proteins.
Figure 5.
Human CUL1 can interact with the yeast SCF components ySKP1 and CDC4. Hi5 insect cells were infected with baculovirus constructs expressing various proteins as indicated. Cells were labeled with Tran[35S]-label for 3 hr before harvesting. Yeast SKP1HA (A) and PHACDC4 (B) together with associated proteins were immunoprecipitated by anti-HA and anti-Polyoma beads, respectively. The composition of the protein complexes in the immunoprecipitates was analyzed by SDS/PAGE followed by autoradiography. The asterisk marks an unidentified contaminant that migrates reproducibly faster than the authentic hCUL1.
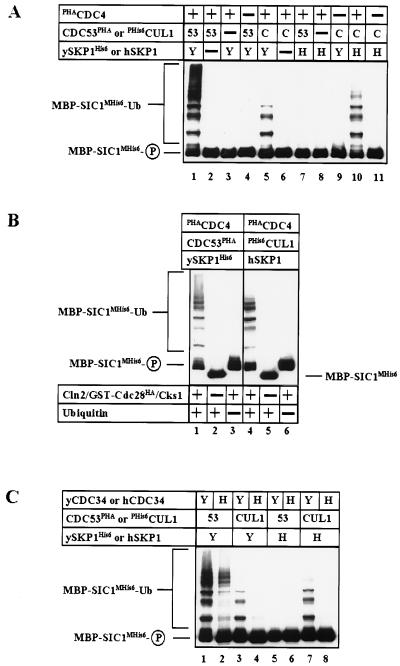
Our previous findings (6) identified SCFCDC4 as a functional E3 that required the presence of all three subunits (CDC4, CDC53, and ySKP1) to catalyze ubiquitination of phosphorylated SIC1 (6, 7). Preceding its ubiquitination, phosphorylated SIC1 is recruited to SCF CDC4 by binding to the CDC4/ySKP1 substrate receptor (6, 7). Given that hCUL1 and hSKP1 assembled with CDC4 (Fig. 5B), we sought to test whether these hybrid SCF complexes were able to promote ubiquitination of phosphorylated SIC1. Purified chimeric SCF complexes were incubated with MBP-SIC1MHis6 and purified ubiquitination components. In the presence of SCFCDC4 (Fig. 6A, lane 1), MBP-SIC1MHis6 was converted efficiently to high molecular weight forms. Omission of either CDC4, CDC53, or ySKP1 resulted in no activity (Fig. 6A, lanes 2–4). Replacement of CDC53PHA with PHis6hCUL1 resulted in an SCF complex with modest ubiquitination activity that depends on both CDC4 and ySKP1 (Fig. 6A, lanes 5, 6, and 9). Additionally, an SCF complex containing both PHis6hCUL1 and hSKP1 along with PHACDC4 also was able to catalyze ubiquitination of MBP-SIC1MHis6 (Fig. 6A, lane 10). The conversion of MBP-SIC1MHis6 to high molecular weight forms by hybrid CDC4/hCUL1/hSKP1 complexes required both substrate phosphorylation (Fig. 6B, lane 5) and the presence of ubiquitin (Fig. 6B, lane 6). Interestingly, coexpression of PHACDC4, CDC53PHA, and hSKP1 did not result in a functional SCF complex (Fig. 6A, lane 7).
Figure 6.
SIC1 is ubiquitinated by chimeric SCF complexes. (A) Hi5 insect cells were infected with various baculoviruses expressing PHACDC4, CDC53PHA (53), PHis6hCUL1 (C), ySKP1His6 (Y), or hSKP1 (H) as indicated. At 72 hr post-infection, lysates were prepared and SCF complexes were affinity purified on an anti-Polyoma matrix, and eluted complexes were incubated for 2 hr at 25°C with MBP-SIC1MHis6 in the presence of His6yUBA1 (E1), yCDC34 (E2), purified CLN2/GST-CDC28HA/CKS1, ubiquitin, and an ATP-regenerating system. At the end of the incubation, the samples were fractionated by SDS/PAGE and were immunoblotted with anti-myc antibodies to detect MBP-SIC1MHis6. Bound antibodies were visualized by ECL. (B) The indicated SCF complexes were purified from baculovirus-infected insect cell lysates and were incubated with the full set of ubiquitination components (lanes 1 and 4) or in the absence of CLN2/GST-CDC28HA/CKS1 (lanes 2 and 5) or ubiquitin (lanes 3 and 6). (C) Purified SCF complexes containing CDC53PHA (53), PHis6hCUL1 (CUL1), ySKP1His6 (Y), or hSKP1 (H) subunits were incubated with ubiquitination components containing either yCDC34 (Y) or hCDC34 (H).
CDC53 was shown to interact with yCDC34 (32). Thus, we presumed that an SCF complex containing hCUL1 would prefer to use hCDC34 as an E2 as opposed to yCDC34. However, SCFCDC4 complexes containing PHis6hCUL1 with either ySKP1His6 or hSKP1 appeared to work much more efficiently with yCDC34 than with hCDC34 serving as the E2 (Fig. 6C, lanes 3, 4, 7, and 8). Although we do not understand the basis for this preference, it is possible that there exist additional human CDC34-like E2s that interact preferentially with hCUL1-containing complexes. Alternatively, the interaction between an F-box subunit and an E2 enzyme might also contribute to the specificity for a particular E2 (38).
Human CUL1 Assembles with Ubiquitination-Promoting Activities in Human Cell Extracts.
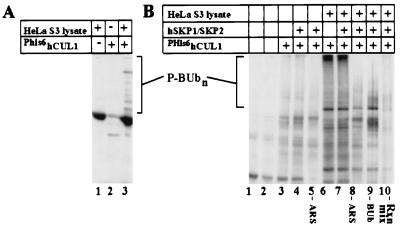
The data presented so far are consistent with hCUL1 functioning as a component of a ubiquitin ligase complex in human cells. Because we have failed so far to detect ubiquitination activity by using recombinant hCUL1/hSKP1/SKP2 complexes, we sought to develop an assay that would allow us to identify either substrates or cofactors of a hCUL1-dependent ubiquitination pathway. PHis6hCUL1 produced in insect cells in the presence or absence of hSKP1 plus SKP2 was bound to anti-Polyoma beads and incubated with crude HeLa S3 lysates to allow binding of other potential SCF components, regulators, and substrates. After washing away unbound proteins, E1, hCDC34, BUb, and an ATP-regenerating system then were added to the beads. After an incubation, reactions were fractionated by SDS/PAGE, were transferred to nitrocellulose, and were blotted with streptavidin-HRP to detect ubiquitin conjugates. Whereas Phis6hCUL1 or Phis6hCUL1/hSKP1/SKP2 complexes isolated from insect cells exhibited little ubiquitination activity (Fig. 7A, lane 2 and Fig. 7B, lanes 3 and 4), a high molecular weight smear characteristic of ubiquitinated proteins appeared (Fig. 7A, lane 3 and Fig. 7B, lanes 6 and 7) when these same components were preincubated with HeLa S3 lysate before the assay. In contrast, no signal was detected when naked polyoma beads were preincubated with HeLa S3 lysate (Fig. 7A, lane 1). The appearance of slowly migrating biotinylated proteins depended on the addition of ubiquitin and ATP-regenerating system to the reaction (Fig. 7B, lanes 8 and 9), indicating that the high molecular weight smear was caused by ubiquitination occurring during the in vitro incubation.
Figure 7.
Human CUL1 associates with ubiquitination activity in HeLa S3 lysates. (A) Anti-Polyoma beads were incubated in the presence of PHis6hCUL1, HeLa S3 lysates, or both as indicated, were washed five times with lysis buffer, and were mixed with the ubiquitination reaction components (Rxn mix) His6yUBA1, hCDC34, BUb, and ATP-regeneration system. Incorporation of BUb into proteins present in the reactions was monitored by probing with streptavidin-HRP conjugate followed by ECL detection. (B) PHis6hCUL1 alone or together with hSKP1 and SKP2 was produced in insect cells and was bound to anti-Polyoma beads that then were washed and incubated at 4°C in the presence or absence of crude HeLa S3 lysates to allow bead “activation”. Activated beads then were treated as in A. Dependence on the presence of ATP and BUb in the reactions was determined by omitting these components from the reaction mix (lanes 5, 8, and 9, respectively). The entire reaction mix was omitted in lane 10. Lane 1 contained reaction mix only. P-BUbn designates a ladder of ubiquitinated proteins produced in the reaction.
DISCUSSION
Multiple homologues of the ySKP1, CDC53, and F-box subunits of the SCF ubiquitin ligase complex have been identified (10–13) and implicated in various cellular processes, including kinetochore function (33, 34), S-phase progression (10), exit from the cell cycle (12), transcript elongation, regulation of hypoxia-inducible genes, and suppression of tumorigenesis (25, 26). Based on the close homology between hCUL1 and CDC53, we sought to address whether hCUL1 functions as part of an SCF-like ubiquitin ligase complex in human cells. A two-hybrid screen to identify proteins that interact with hCUL1 yielded hSKP1, suggesting that hCUL1 does indeed assemble into SCF-like complexes in human cells. Several other observations reported here support this hypothesis. First, hCUL1 associates with hSKP1 in transfected HeLa S3 cells. Second, hCUL1 assembles into complexes with both hSKP1 and the F-box protein SKP2 in vitro. Third, hCUL1 complements the growth defect of a _cdc53_ts mutant. Fourth, hCUL1 and hSKP1 can form chimeric SCF complexes with CDC4, and these complexes are able to ubiquitinate the SCFCDC4 substrate SIC1 in vitro. Fifth, hCUL1 associates with ubiquitination-promoting activity in HeLa S3 cell lysate. Taken together, these data strongly suggest that hCUL1 is a subunit of an SCF-like E3 complex in human cells.
What are the candidate substrates for hCUL1-dependent ubiquitination in human cells? SIC1, CLN2, and FAR1 must be phosphorylated before they can be ubiquitinated by the budding yeast SCF/CDC34 pathway (6, 7, 35). The stability of many mammalian regulatory proteins—including IκB, β-catenin, p27, Cyclin D, and Cyclin E—is known to be controlled by phosphorylation (16–20, 36, 37). Further work will be required to determine whether any of these proteins are substrates for human SCF complexes. SCF-associated Cyclin A might also be a substrate of the SCFSKP2 pathway. This is less likely, though, because Cyclin A is thought to be primarily destroyed via the antigen-presenting cell/cyclosome pathway, and both Cyclin A and SKP2 activities are essential for entry into S phase (10). Instead, the tight association of Cyclin A/CDK2 with SCF subunits both in vivo and in vitro might reflect an efficient coupling between substrate phosphorylation and ubiquitination in transformed cells.
While this manuscript was in preparation, a study that complements our findings was reported by Lisztwan et al. (38). These authors demonstrated that hCUL1, hSKP1, and SKP2 assemble into a complex both in unperturbed and transfected human cells. Moreover, SKP2 also was shown to bind the ubiquitin-conjugating enzyme hCDC34 in human cells, suggesting that SKP2 is part of an SCF-like ubiquitin ligase. SKP2 also was shown to associate with Cyclin A/CDK2, and mutational analysis suggested that Cyclin A/CDK2 binding might regulate SKP2/hSKP1 but not SKP2/hCUL1 interaction in vivo. Our data support these findings and extend them by establishing that hCUL1 interacts directly with hSKP1 and SKP2 without the participation of other eukaryotic proteins, and hCUL1 and hSKP1 can assemble into active ubiquitin ligase complexes either in insect cells or in HeLa S3 cell lysates.
Further characterization of the SCF pathway in human cells will require the identification of functional F-box subunits and physiological substrates. The ability to stimulate the E3 activity of insect cell-derived hCUL1 with HeLa S3 cell lysate provides a strategy for identifying these proteins. Moreover, this assay can be adapted readily to test whether the related hCUL2-hCUL5 proteins also assemble into ubiquitin ligase complexes in human cells. Lastly, by converting either the chimeric SCF complex assay (Fig. 6) or the biotin-ubiquitin-based assay (Fig. 7) to a microtiter plate format, it should be feasible to screen chemical libraries to identify compounds that modulate the activities of hSKP1 and hCUL1. Given its critical role in cell division in budding yeast, inhibitors of human SCF might be valuable lead compounds for the development of novel anti-cancer chemotherapeutics.
Acknowledgments
We thank R. Brent, M. Goebl, D. Morgan, H. Zhang, P. Sorger, W. Harper, P. Jackson, S. Handeli, and R. Cohen for generously providing strains, libraries, clones, antibodies, recombinant baculoviruses, and other reagents. We also thank W. Dunphy, R. Feldman, J. Kroll, and R. Verma for critically reading the manuscript and members of the laboratory for helpful discussions. R.J.D. was supported by a grant from the National Institutes of Health (GM52466–01) and by Scholar Awards from Searle/Chicago Community Trust and the Burroughs-Wellcome Foundation. C.C.C. is a recipient of a Postdoctoral Fellowship Award from the Leukemia Society of America (Ref. # 5172–97).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CDK, cyclin-dependent kinase; GST, glutathione _S_-transferase; MBP, maltose binding protein; BUb, biotinylated ubiquitin; HRP, horseradish peroxidase.
References
- 1.Schwob E, Böhm T, Mendenhall M, Nasmyth K. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 2.Glotzer M, Murray A W, Kirschner M W. Nature (London) 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Fix O, Peters J-M, Kirschner M W, Koshland D. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 4.Hershko A, Heller H, Elias S, Ciechanover A. J Biol Chem. 1983;258:8206–8214. [PubMed] [Google Scholar]
- 5.Scheffner M, Nuber U, Huibregtse J M. Nature (London) 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 6.Feldman R M R, Correll C C, Kaplan K B, Deshaies R J. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 7.Skowyra D, Craig K L, Tyers M, Elledge S J, Harper J W. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 8.Barral Y, Jentsch S, Mann C. Genes Dev. 1995;9:399–409. doi: 10.1101/gad.9.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Plon S E, Leppig K A, Do H N, Groudine M. Proc Natl Acad Sci USA. 1993;90:10484–10488. doi: 10.1073/pnas.90.22.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Kobayashi R, Galaktionov K, Beach D. Cell. 1995;82:912–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 11.Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper J W, Elledge S J. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 12.Kipreos E T, Lander L E, Wing J P, He W W, Hedgecock E M. Cell. 1996;85:1–20. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 13.Mathias N, Johnson S L, Winey M, Adams A E, Goetsch L, Pringle J R, Byers B, Goebl M G. Mol Cell Biol. 1996;16:6634–6643. doi: 10.1128/mcb.16.12.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanker S, Valdivieso M H, Wittenberg C. Science. 1996;271:1597–1601. doi: 10.1126/science.271.5255.1597. [DOI] [PubMed] [Google Scholar]
- 15.Verma R, Annan R, Huddleston M, Carr S, Reynard G, Deshaies R J. Science. 1997;278:455–460. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- 16.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G, Rolfe M. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 17.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 18.Won K A, Reed S I. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]
- 19.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 20.Diehl J A, Zindy F, Sherr C J. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 21.Dynlacht B D, Flores O, Lees J A, Harlow E. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann F, Martelli F, Livingston D M, Wang Z. Genes Dev. 1996;10:2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]
- 23.Hochstrasser M. Genes Dev. 1998;12:908–913. [Google Scholar]
- 24.Lammer D, Mathias N, Laplaza J M, Jiang W, Liu Y, Callis J, Goebl M, Estelle M. Genes Dev. 1998;12:914–926. doi: 10.1101/gad.12.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pause A, Lee S, Worrell R A, Chen D Y T, Burgess W H, Linehan W M, Klausner R D. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lonergan K M, Iliopoulos O, Ohh M, Kamura T, Conaway R C, Conaway L W, Kaelin W G. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 28.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: Assay for β-galactosidase in Extracts of Mammalian Cells. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 30.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 31.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 32.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Kobayashi R, Wittenberg C, Tyers M. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 33.Connelly C, Hieter P. Cell. 1996;86:275–285. doi: 10.1016/S0092-8674(00)80099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan K B, Hyman A A, Sorger P K. Cell. 1997;91:491–500. doi: 10.1016/s0092-8674(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 35.Henchoz S, Chi Y, Catarin B, Herskowitz I, Deshaies R J, Peter M. Genes Dev. 1997;11:3046–3060. doi: 10.1101/gad.11.22.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;22:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 37.Aberle H, Baueer A, Stappert J, Kispert A, Kemler R. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisztwan J, Marti A, Sutterluty H, Gstaiger M, Wirbelauer C, Krek W. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]