Pancreatic Lkb1 Deletion Leads to Acinar Polarity Defects and Cystic Neoplasms (original) (raw)
Abstract
LKB1 is a key regulator of energy homeostasis through the activation of AMP-activated protein kinase (AMPK) and is functionally linked to vascular development, cell polarity, and tumor suppression. In humans, germ line LKB1 loss-of-function mutations cause Peutz-Jeghers syndrome (PJS), which is characterized by a predisposition to gastrointestinal neoplasms marked by a high risk of pancreatic cancer. To explore the developmental and physiological functions of Lkb1 in vivo, we examined the impact of conditional Lkb1 deletion in the pancreatic epithelium of the mouse. The Lkb1-deficient pancreas, although grossly normal at birth, demonstrates a defective acinar cell polarity, an abnormal cytoskeletal organization, a loss of tight junctions, and an inactivation of the AMPK/MARK/SAD family kinases. Rapid and progressive postnatal acinar cell degeneration and acinar-to-ductal metaplasia occur, culminating in marked pancreatic insufficiency and the development of pancreatic serous cystadenomas, a tumor type associated with PJS. Lkb1 deficiency also impacts the pancreas endocrine compartment, characterized by smaller and scattered islets and transient alterations in glucose control. These genetic studies provide in vivo evidence of a key role for LKB1 in the establishment of epithelial cell polarity that is vital for pancreatic acinar cell function and viability and for the suppression of neoplasia.
LKB1 encodes an evolutionarily conserved serine/threonine kinase that is involved in the regulation of cellular responses to energy stress and in the establishment of cell polarity (1, 3, 23). In response to an increase in the AMP/ATP ratio, LKB1 phosphorylates and activates AMP-activated protein kinase (AMPK), a key negative regulator of mTOR. Activation of the LKB1-AMPK axis results in a decrease in ATP-consuming processes and an increase in ATP production via inactivated mTOR, diminished fatty acid and glucose metabolism, and enhanced glucose transport. LKB1 also activates other members of the AMPK-related kinase subfamily including the microtubule affinity-regulating kinases 1 to 4 (MARK1, -2, -3 and -4) and the SAD/Brsk kinases (SAD-A and SAD-B) that induce cell polarity (30). Notably, LKB1 is required for the establishment of cell polarity in Drosophila melanogaster and Caenorhabditis elegans, as well as in cultured mammalian epithelial cells (2, 31, 48). Along similar lines, LKB1 and the SAD kinase define a pathway required for the polarization of neurons (6, 43). Recent studies have demonstrated that AMPK regulates tight junction assembly and cell polarity in mammalian cells and in Drosophila in a manner linked to the response to energy stress (28, 51, 52). Mouse knockouts of MARK2 show metabolic defects (19). Collectively, these data support the view that energy sensing and cell polarity may be broadly integrated under the control of LKB1-AMPK signaling.
In humans, germ line loss-of-function mutations of LKB1 are associated with Peutz-Jeghers syndrome (PJS), a disease characterized by benign gastrointestinal polyps (hamartomas) and an ∼30-fold increased risk of gastrointestinal malignancy at age 60 (17). In PJS, there is a range of pancreatic neoplasms including pancreatic ductal adenocarcinoma and two types of cystic tumors, intraductal papillary mucinous neoplasia and serous cystadenoma (39, 46, 50). Studies of Lkb1 mutant mice have confirmed the role of LKB1 as a tumor suppressor and a regulator of cellular energy metabolism. With respect to tumor suppression, Lkb1 heterozygous mice develop gastrointestinal polyps associated with deregulated AMPK-TSC-mTOR signaling, and concurrent mutation of the RAS oncogene and deletion of lkb1 in the lung lead to adenocarcinoma formation (5, 21, 41). With regard to energy metabolism, somatic deletion of Lkb1 in the skeletal muscle produces defects in glucose uptake and the loss of AMPK activation (25). Liver-specific Lkb1 deletion also causes metabolic defects and the loss of activity of both the AMPK and the AMPK-related kinase family member SIK2 (42).
The pancreas regulates energy balance on two levels. Its exocrine component produces enzymes for the digestion and absorption of nutrients from the gastrointestinal tract, and its endocrine lineages enable the regulation of energy homeostasis on the organismal level. In the mature pancreas, the acinar cells possess a highly polarized structure that is critical to their ability to direct the apical secretion of digestive enzymes into the acinar lumen. The pancreatic endocrine cells reside in the islets of Langerhans, designated beta, alpha, delta, and pancreatic polypeptide (PP) cells. Beta cells have a critical energy-sensing function and maintain glucose homeostasis via the regulated secretion of insulin into the bloodstream. Alpha cells secrete glucagon, which counteracts insulin, and delta cells secrete somatostatin, which may function in a paracrine manner to regulate islet function.
In this study, we sought to determine the physiological functions of Lkb1 in vivo through an analysis of its conditional ablation from the developing and the mature pancreas. Our efforts focused on the pancreas, given the highly polarized nature of its acinar cells, its critical physiological exocrine and endocrine roles in organismal metabolism, and the strikingly high risk of development of pancreatic neoplasms in individuals with PJS.
MATERIALS AND METHODS
Genetically engineered mouse strains: generation of the study cohort.
Lkb1L/+ and Pdx1-Cre Lkb1L/+ mice (where Lkb1L indicates mice homozygous for a conditional null allele of Lkb1 and Pdx1-Cre indicates transgenic mice) were crossed, yielding Pdx1-Cre; Lkb1L/L mice and littermate controls. All analyses included an evaluation of both the Pdx1-Cre; Lkb1L/+ and the Pdx1-Cre; Lkb1L/L animals. The physiologic, histologic, biochemical, and structural phenotypes of the wild-type (WT) mice, the Pdx1-Cre; Lkb1L/+ mice, and the Pdx1-Cre mice were indistinguishable and are represented below as the WT. To generate the colony, Lkb1L/L mice on an FVB/n background were crossed to Pdx1-Cre mice (ICR background). Animals were backcrossed at least four generations onto an FVB/n background for the experiments.
Immunohistochemistry.
All immunohistochemistry assays were performed with formalin-fixed samples, using antibodies described below. To ascertain the contribution of cellular constituents, the number of stained cells counted over 20 high-power fields were divided by the total number of nuclei. Sections for analysis were made along the long axis of the pancreas, near the main duct, to sample equal numbers of islets. Rates of cell death and proliferation were determined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) and by Ki67 staining and counting positive cells over 20 high-powered fields among at least three independent samples of each respective genotype.
Immunofluorescence.
Pancreases were fixed for 2 to 4 h in 4% paraformaldehyde and then immediately frozen in OCT. Ten-micrometer sections were used for all experiments. Confocal images were obtained with a Leica confocal microscope using an ×40 oil immersion objective, using the same laser intensity and z settings and analyzed using LCS advanced software. For each marker, at least three independent samples were evaluated for each genotype.
Antibodies.
The antibodies used were as follows: insulin (catalog no. A056401; DAKO); glucagon (GKU-001; BCBC collection); somatostatin (catalog no. 18-0078; Zymed); amylase (Sigma); biotin anti-GP, biotin anti-Rb, Cy2 anti-GP, Cy3 anti-Rb, and Cy5 anti-mouse antibody (Jackson); Hes-1 (0.6 μg/ml; a gift from Tetsuo Sudo); biotinylated Dolichos biflorus A (DBA) lectin (Vector); Lkb1 (Santa Cruz Biotechnology); Ki67 (Novocastra); E-cadherin (Zymed); beta-tubulin (Abcam); phalloidin (Sigma); MLC2-P (Ser19; catalog no. 3675; Cell Signaling); MLC2 (Cell Signaling); Map4-P (Ser768; BioLegend); MARK1 (catalog no. AP7144; Abgent); MARK2 (catalog no. H00002011-M01; Novus); MARK3 (catalog no. 05-680; Upstate); phospho-MARK (catalog no. 4836; Cell Signaling); AMPKalpha (catalog no. 2532; Cell Signaling); rabbit anti-phospho-AMPK (catalog no. 2535; Cell Signaling); SAD-A, SAD-B, and phospho-SAD (provided by Joshua Sanes, Harvard University). TUNEL staining was performed with a DeadEnd apoptosis detection kit (Promega) according to the manufacturer's instructions.
Electron microscopy.
Mice were perfused with 2% glutaraldehyde in 0.1 M sodium cacodylate, rinsed in phosphate-buffered saline, and then embedded in Epon for morphology. Blocks of tissue (0.1 to 1.5 mm3) were osmicated in 1% OsO4 in 0.1 M sodium cacodylate for 1 h at room temperature, rinsed in cacodylate buffer, and then rinsed in distilled water before they were stained in 2% uranyl acetate-double-distilled water for 1 h. Sections were dehydrated in graded alcohol and placed in 100% propylene oxide. The blocks were incubated in Epon-PO 1:1 overnight at room temperature and then changed to 100% Epon and polymerized overnight. Sections (90 nm) were cut on a Reichert-Jung Ultracut E microtome, collected on slot grids, and stained with 2% uranyl acetate and lead citrate before they were viewed on a Philips CM 10 electron microscope.
Western blotting.
Whole pancreases were snap frozen at the time of dissection and placed in standard sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis lysis buffer. Protein (25 to 40 μg) was loaded onto a 4 to 12% gradient SDS gel (Invitrogen) and transferred onto polyvinylidene difluoride, using standard techniques.
RESULTS
Lkb1 deficiency in the pancreas leads to the development of serous cystadenomas.
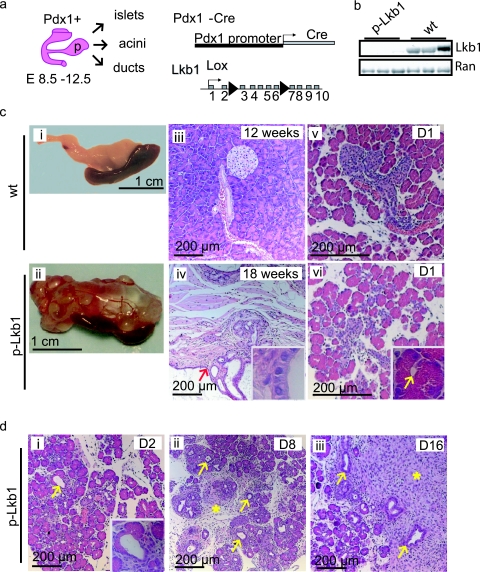
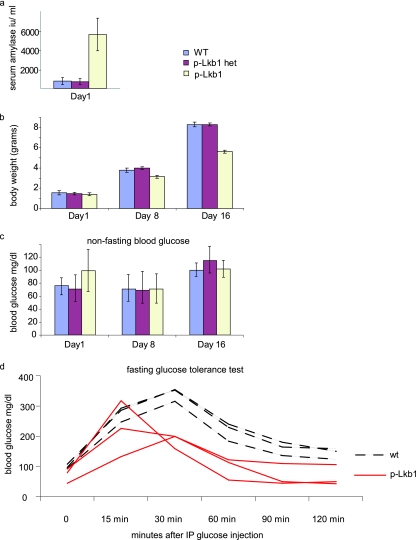
Lkb1 is expressed throughout the pancreatic epithelium from embryonic day 16.5 (E16.5). To eliminate Lkb1 in the developing pancreas, we generated mice homozygous for a conditional null allele of Lkb1 (Lkb1L) and transgenic for Pdx1-Cre. Pdx1-Cre is expressed at E8.5 in the common pancreas progenitors that give rise to the epithelial cells of the islets, ducts, and acini (Fig. 1a) (5, 18). The Pdx1-Cre transgenic mice homozygous for Lkb1 are designated hereafter as “p-Lkb1” mice. We documented the complete recombination and extinction of Lkb1 expression on postnatal day 1 (PD1) p-Lkb1 pancreases by Western blotting, immunohistochemistry, and Southern blotting analyses (Fig. 1b and 7a; data not shown). At birth, p-Lkb1 mice were present at the expected frequencies and were grossly indistinguishable from wild-type littermates. Thereafter, p-Lkb1 mice exhibited diminished weight gain, elevated serum amylase, and excess fat excretion in the stool (steatorrhea, a sign of exocrine pancreas dysfunction) (Fig. 2a and b). p-Lkb1 mice had elevated nonfasting blood glucose levels on PD1, which normalized by PD8, pointing to a possible impairment in the maturation of islet function (Fig. 2c; also see Discussion). By 10 to 30 weeks of age, all p-Lkb1 mice became increasingly cachectic, developed abdominal bloating, and required euthanasia. Necropsy revealed the total replacement of normal pancreases with large, fluid-filled, cystic masses (Fig. 1c, panel ii). Microscopic examination revealed cysts lined with a single layer of benign columnar epithelium separated by stromal tissue (Fig. 1c, panel iv). These cystic lesions closely resembled human serous cystadenoma, a pancreatic neoplasm observed with PJS (9). There was minimal normal pancreas, although present at the edges of the cystic regions were islets and focal areas of intact acinar tissue intermixed with ductal metaplasia. There were also significant areas of fibrosis and adipose replacement suggestive of chronic pancreatic damage, inflammation, and tissue remodeling, as is seen with pancreatitis. The penetrance of defects was 100% in p-Lkb1 mice. A detailed analysis of Pdx1-Cre mice and heterozygous p-Lkb1 mice did not reveal gross defects for up to 1 year or defects in islet function for up to 20 weeks of age. Together, these observations indicate that Lkb1 plays an essential role in the development and maintenance of the normal pancreas.
FIG. 1.
(a) Pdx1-Cre is expressed in common pancreas progenitors at E8 to E12, leading to the deletion of the conditional Lkb1 null allele in all pancreas epithelial cell types, acini, ducts, and islets. (b) Western blotting analysis of Lkb1 in PD1 p-Lkb1 and WT pancreas. (c) Gross and histological appearances of adult WT (i, iii, and v), p-Lkb1 pancreas (ii, iv, and vi); note the spleen adherent to the inferior border of the cystic mass, which involved the entire pancreas. Simple cystic lining epithelium is shown with red arrowhead and inset (iv). p-Lkb1 PD1 pancreases show intact acinar cells and occasional luminal dilatation (panel vi inset, yellow arrow) compared to that of WT (v), as well as apoptotic bodies (panel vi, blue arrow). (d) Loss of acini and replacement with ductal epithelial structures (arrows) and reactive fibrosis (asterisk) are seen at PD2 to PD8 in p-Lkb1 pancreases.
FIG. 7.
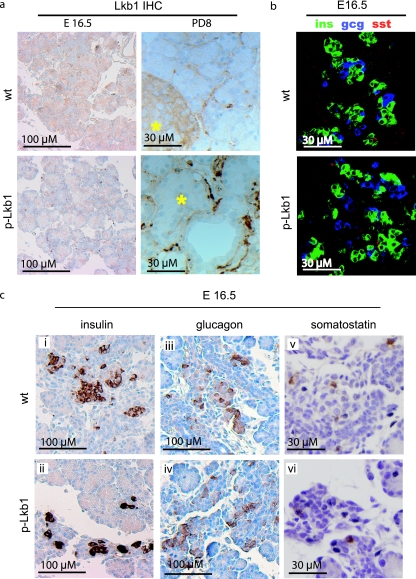
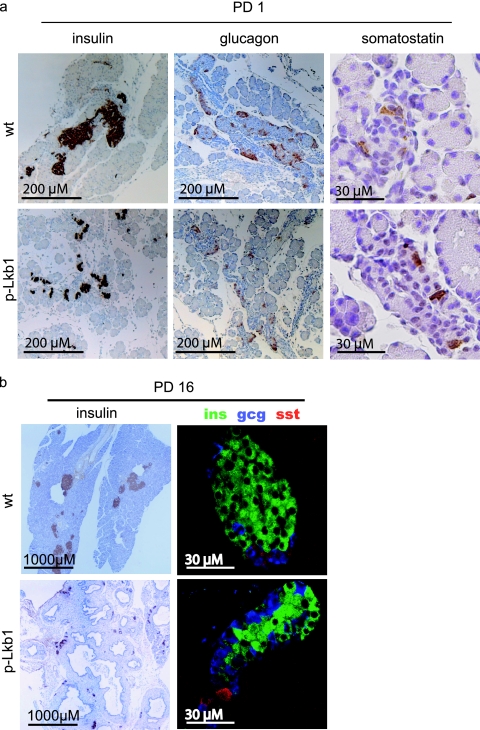
(a) Immunohistochemistry analysis of Lkb1 in E16.5 and PD8 pancreases. Lkb1 is expressed in both islet and acinar cells at E16.5 but is restricted in adulthood to islets (asterisks mark islets at PD8). Intense staining between acinar units and islets of p-Lkb1 at PD8 represents macrophages, fibroblasts, and infiltrative reactive stroma. (b) Immunofluorescence demonstrates similar distributions and contributions of α, β, and δ cells in E16.5 p-Lkb1 pancreases. (c) Immunohistochemistry analysis of endocrine markers at E16.5 demonstrates the presence of α, β, and δ cells. Abbreviations: ins, insulin; gcg, glucagon; sst, somatostatin.
FIG. 2.
Metabolic profiles of p-Lkb1. (a and b) PD1 serum amylase levels are increased (a), and perinatal weight gain is diminished (b) in p-Lkb1 mice. (c) Nonfasting blood glucose levels are slightly elevated following birth and are then comparable by PD8. (d) Six-week-old p-Lkb1 mice demonstrate improved glucose tolerance compared to their littermate controls. IP, intraperitoneal.
Lkb1 deletion impairs acinar cell structural integrity and survival and leads to acinar-to-ductal metaplasia.
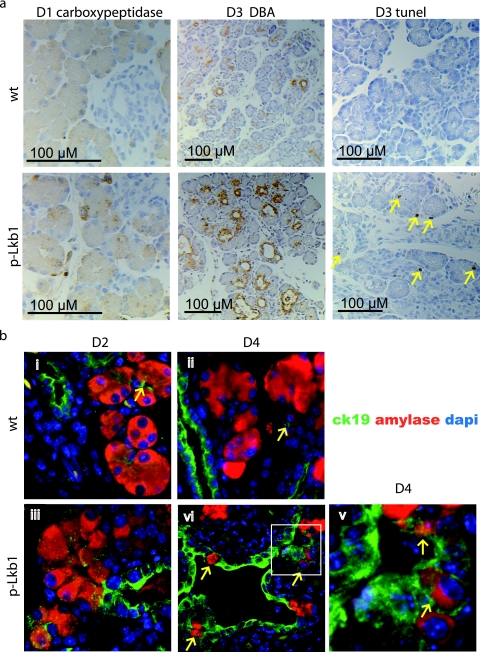
Serial histological and molecular analyses were performed to better define the nature and evolution of the p-Lkb1 phenotype. At PD1, the p-Lkb1 pancreas was smaller in size than that of the littermate controls (data not shown) but had grossly intact ductal and acinar structures (Fig. 1c, panels v and vi) and normal expression of the exocrine markers cytokeratin-19 and DBA-lectin stains for ducts and carboxypeptidase and chymotrypsin stains for acini (Fig. 3a; data not shown). High-power examination of the p-Lkb1 pancreas showed less compact acinar units, often with distended lumens (Fig. 1c, panel vi inset), and in some regions, the acinar units were fragmented and intermixed with endocrine cells (Fig. 1c, panel vi; also see Fig. 8a). Notably, the p-Lkb1 islets were smaller and less well aggregated (Fig. 1c, panel vi; see Fig. 8a), pointing to abnormalities in endocrine lineage development (see below).
FIG. 3.
(a) Immunohistochemistry analysis of PD1 carboxypeptidase, PD3 DBA, and PD3 TUNEL (arrows highlight TUNEL-positive cells in p-Lkb1). (b) Immunofluorescence analysis of PD2 and PD4, demonstrating both acinar and ductal cell contributions to metaplastic lesions. Terminal CK19 staining ducts (arrows in panels i and ii) are seen closely associated with acinar units in the indicated WT specimens; otherwise, acinar cells with cytoplasmic amylase staining do not exhibit membranous CK19 staining. Both amylase (acinar and cytoplasmic) and CK19 (ductal and membranous) marking as seen within rare cells within metaplastic lesions (arrows in panels iii, iv, and v and inset). DAPI, 4′,6′-diamidino-2-phenylindole.
FIG. 8.
(a) Immunohistochemistry analysis of PD1 pancreases for insulin (ins), glucagon (gcg), and somatostatin (sst) demonstrates scattered endocrine cells among p-Lkb1 pancreases compared with those that are coalesced at islets. (b) Evaluation of PD16 p-Lkb1 pancreases demonstrates smaller islets compared with those of the WT, however, with normal organization and contribution of α, β, and δ cells.
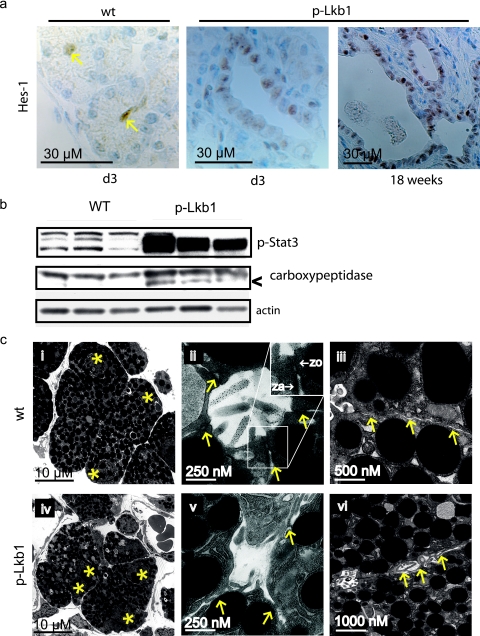
From PD2 to PD8, there was a rapid loss of acinar cells, associated with the progressive appearance of abnormal DBA-positive ductal structures and reactive fibrosis (Fig. 1d, panels i to iii). This replacement of acini by ductal structures (ductal metaplasia) is observed with a number of contexts including damage to the exocrine pancreas through oncogenic stress or physical insult (40, 44, 47). Ductal metaplasia also occurs in association with the earliest precursors to pancreatic adenocarcinoma (pancreatic intraepithelial neoplasms [PanINs]) in both humans and genetically engineered mouse models of the disease (7, 13, 53). In this setting, ductal metaplasia has been linked with a process involving acinar, ductal, and biphenotypic cells harboring both acinar and ductal features (53). More recently, lineage tracing has demonstrated acinar-to-ductal transdifferentiation as the mechanism underlying ductal metaplasia (45). In the p-Lkb1 pancreas, we assessed the extent to which the ductal metaplasia stems from alterations in cell proliferation and cell death and characterized acinar and ductal contributions to emerging cystic lesions. Proliferation rates of ductal and acinar cells were essentially identical across all genotypes from PD1 to PD3, as measured by Ki-67 staining (data not shown). In contrast, at PD3, TUNEL assays showed an ∼18-fold increase in acinar cell death in p-Lkb1 mice relative to that of controls (Fig. 3a). Coimmunofluorescence with the ductal marker ck-19 and the acinar marker amylase demonstrated the contribution of both cell types to metaplastic lesions (Fig. 3b). The existence of cells that exhibit colocalization of markers is consistent with an active acinar-ductal transdifferentiation process (Fig. 3b, panel v) (45, 53). Additionally, as described in other models of ductal metaplasia, we also observed increased expression of the Notch target gene HES-1 and the activated STAT3 gene (Fig. 4a and b) (24, 33, 34, 44).
FIG. 4.
(a) Immunohistochemistry demonstrates HES-1 staining in p-Lkb1 PD3 ductal structures and adult cystadenomas (arrows). (b) Western blotting analysis demonstrates increased phosphor-STAT3 and activated carboxypeptidase (arrowhead, middle gel) in the PD1 p-Lkb1 pancreas. (c) TEM of E18.5 p-Lkb1 pancreas demonstrates grossly abnormal acinar units with a loss of basal nuclear location (panel iv, nuclei marked with asterisks), the absence of apically located tight and adherens junctions (panels ii and v, arrows indicate the junction in the WT and the expected location in p-Lkb1) and lateralization of microvilli (panels iii and vi, arrows indicate lateral surface).
Autophagy is critical to maintaining energy stores in the immediate postnatal period (27). Given the role of the LKB1-AMPK pathway in directing autophagy and the possibility that deregulation could contribute to acinar cell loss, we evaluated levels of the LC3-II isoform (which is associated with autophagosomes) among PD1 p-Lkb1 pancreases (22, 29). Pancreas LC3-II levels were comparable for the genotypes of both the starved and the fed mice at PD1, suggesting similar levels of autophagy in both the stressed (fasted) and the unstressed (fed) state (Fig. 6d). Additionally, transmission electron microscopy (TEM) did not demonstrate appreciable differences between the number of autophagosomes among E18.5 and that of the PD1 WT and p-Lkb1 pancreases (data not shown). On the other hand, lysosomal fusion with exosomes (crinophagy), apoptotic bodies, and necrotic cellular debris were observed, consistent with early stages of pancreatitis and associated acinar cell death (37). PD2 pancreases showed the presence of a proteolytically cleaved, inappropriately activated form of carboxypeptidase, indicating ongoing pancreatic acinar cell injury (Fig. 4b). Together these studies indicate that the inactivation of Lkb1 during pancreatic organogenesis leads to acinar cell death, pancreatitis, and a rapid and progressive postnatal acinar-ductal metaplasia with corresponding Notch and Stat pathway activation.
FIG. 6.
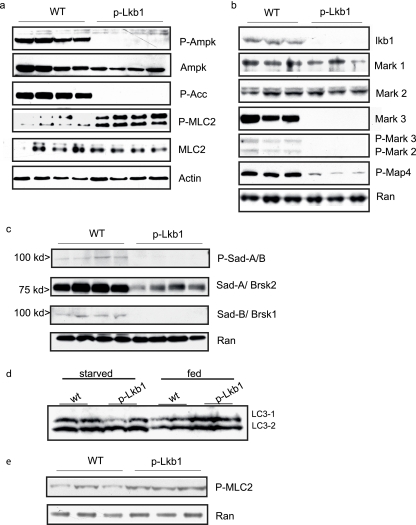
Biochemical evaluation of PD1 whole-pancreas lysates. (a to c) Mutant pancreases demonstrated a loss of phosphorylation of AMPK, MARK2 and MARK3, and SAD-B compared with that of the WT, as well as downstream targets ACC and MAP4. While AMPK and MARK2 levels were equivalent in the WT and mutant animals, MARK3 protein levels diminished in mutants. (d) Western blotting analysis of LC3 at PD1 in starved and fed WT and p-LKB1 whole-pancreas lysates. The p-Lkb1 and WT pancreases have similar LC3 ratios under both fed and starved conditions. (e) MLC2 Ser19 phosphorylation remains elevated among p-Lkb1 pancreases and compared with WT controls among PD1 animals starved for 16 h following birth.
Lkb1 regulates acinar cell polarity.
Pancreatitis is typically associated with an initial mechanical, biochemical, or chemical insult to acinar cells, leading to an inappropriate release of digestive enzymes into the tissue parenchyma. Subsequently, a progressive process of apoptosis, necrosis, and subsequent organ remodeling ensues. Following birth, and stimulated by feeding, acinar cells undergo CCK-mediated exocytosis of zymogens into the apical lumen, allowing for the digestion of enteral nutrients. Thus, acinar cells require a highly polarized organization for the appropriately directed secretion of zymogen granules into the ductal network. Given the temporal onset of pancreatitis with exocrine organ activation and the reported role of Lkb1 in establishing cell polarity in cultured cells, we conducted a detailed assessment of cytoskeletal organization in the p-Lkb1 acinar cell compartment. TEM and cytoskeletal marker studies focused on the time points E18.5 and PD1, given that the p-Lkb1 pancreas showed intact morphology by light microscopy analysis.
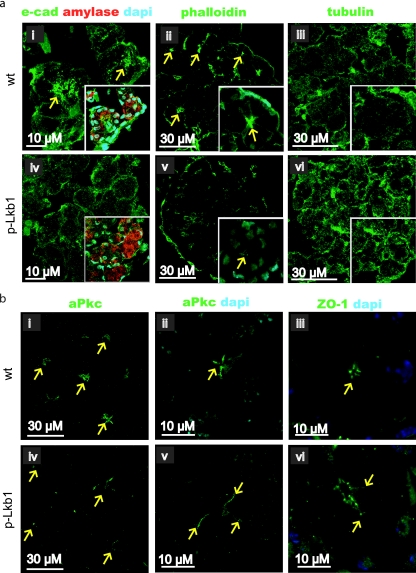
TEM revealed a clear loss of acinar polarity in the E18.5 p-Lkb1 pancreas, as reflected by the loss of basal positioning of the nucleus, the lateralization of microvilli, and the frequent absence of tight and adherens junctions (Fig. 4c). Correspondingly, these ultrastructural defects were associated with abnormalities in biochemical mediators of cell polarity and adhesion. Total E-cadherin levels were similar in the p-Lkb1 and the WT pancreas (data not shown); however, E-cadherin, which normally exhibits a laterally enriched apical distribution and serves to anchor the actin cytoskeleton, was distributed throughout the plasma membrane of p-Lkb1 acinar cells (Fig. 5a, panels i and iv). p-Lkb1 acini also showed diminished formation of actin caps (Fig. 5a, panels ii and v). Beta-tubulin, which stains evenly throughout WT acinar cells, exhibited a punctate, granular, and coarse appearance in p-Lkb1 acinar cells (Fig. 5a, panels iii and vi). A complex containing aPKC, Par-3, Par-6, and cdc42 is essential for tight junction regulation (49). Lkb1 function has been shown to be critical for normal apical aPKC localization in Drosophila (28, 31, 32). Among p-Lkb1 acinar units, aPKC, as well as the tight junction marker, ZO1, extended basolaterally away from the apices, demonstrating lateralization of tight junctions (Fig. 5b).
FIG. 5.
(a) Apical localization of e-cadherin (e-cad) (panel i, arrows) is absent in p-Lkb1 pancreas; costaining (insets) for amylase and DAPI (4′,6′-diamidino-2-phenylindole) demonstrates full acinar differentiation and an associated loss of basal nuclear localization. Phalloidin marks the apical capping and localization of actin in the WT (panel ii, arrows) that is markedly reduced in p-Lkb1 (v). Tubulin has a punctate and irregular distribution in p-Lkb1 (vi) compared with that of the WT (iii). (b) aPKC is seen laterally displaced from apices in p-Lkb1 PD1 animals (panels iv and v, arrows) as is the TJ marker, ZO1 (vi).
Next we examined Lkb1 signaling molecules implicated in cell polarity. The AMPK/MARK/SAD subfamily kinases can be activated by the Lkb1-mediated phosphorylation of a threonine residue in their activation loops (30). AMPKs, MARKs, and SADs are potential mediators of the Lkb1-dependent regulation of epithelial polarity, through their roles in the establishment of tight junctions and the regulation of tubulin dynamics (14, 15, 19, 28, 51, 52). Western blotting analyses of PD1 pancreatic lysates demonstrated greatly reduced phosphorylation of AMPK, MARK2 and MARK3, and SAD-B in the p-Lkb1 pancreas. While total AMPK and MARK1 and MARK2 levels were unaffected, total MARK3, SAD-A, and SAD-B levels were markedly diminished in the p-Lkb1 pancreas. Loss of phosphorylation of the AMPK target and the acetyl-coenzyme A carboxylase and the diminished phosphorylation of the MARK target MAP4 were observed (Fig. 6). Notably, MCL2, a recently described downstream AMPK target, demonstrated increased phosphorylation in p-LKB1 pancreases following birth (Fig. 6). Given the demonstration that nutrient deprivation induces MLC2 phosphorylation, we evaluated whole lysates from newborns starved for 16 h following birth. Among WT pancreases, MLC2 phosphorylation was indeed induced by starvation (Fig. 6e), although still at levels that were less than those among p-Lkb1 animals, a finding that runs counter to data implicating MCL2 in Drosophila and cultured intestinal epithelial lines (see Discussion).
Collectively, these data provide in vivo evidence for a critical role for Lkb1 in the regulation of acinar cell polarity and cytoskeletal organization. Furthermore, molecular analysis supports a role for AMPK, MARK, and SAD proteins as physiological substrates of Lkb1 in the pancreas and suggests that Lkb1 regulates the stability of MARK3, SAD-A, and SAD-2 proteins. We propose that the failure to establish normal acinar cell polarity in the developing pancreas is a major cause of acinar cell death, chronic pancreatitis, and ductal metaplasia, ultimately with neoplastic consequences during postnatal life.
Impaired islet formation and delta cell differentiation in the Lkb1-deficient pancreas.
The abnormal dispersion of p-Lkb1 islet cells prompted a detailed analysis of the endocrine lineages in the pancreas. In the normal pancreas, glucagon-positive and insulin-positive cells are evident at E12.5, while somatostatin-positive cells emerge at E15.5 (12). At E16.5 p-Lkb1 pancreases exhibited a similar organization and distribution of α, β, and δ cells (Fig. 7b and c). However, at PD1, p-Lkb1 islets were significantly smaller, less compact, and distributed more diffusely compared to WT islets (Fig. 8a), and the p-Lkb1 pancreas showed an overall decrease in insulin-positive, glucagon-positive, and somatostatin-positive cells (∼75% of WT) (Fig. 8a). Serial histological analysis showed a delayed aggregation of islets, with an eventual accumulation of smaller but comparably organized islets by PD16 (Fig. 8b). At PD16, p-Lkb1 islets exhibited appropriate peripheral localization of α and δ cells surrounding β cells. Correspondingly, there was evidence of impaired β-cell function, as reflected by elevated blood glucose levels in PD1 mice, although these levels returned to normal by PD8. Notably, adult p-Lkb1 mice showed improved β-cell function compared to that of WT animals, as reflected by a more rapid clearance of blood glucose in glucose tolerance tests (Fig. 2d). Together, these results show that Lkb1 is required for the normal structural organization of islets yet dispensable for the generation of the various islet cell types.
DISCUSSION
Here, we describe the complex pathophysiological and neoplastic consequences of somatic Lkb1 deletion in the murine pancreas. While pancreatic epithelial lineages and structures are established during organogenesis and are grossly normal at birth, the postnatal Lkb1-deficient pancreas shows defective acinar cell polarity, abnormal cytoskeletal organization, loss of tight junctions, and inactivation of AMPK/MARK/SAD family kinases. Correspondingly, rapid and progressive postnatal acinar degeneration occurs shortly after birth, in association with cell death and progressive ductal metaplasia, leading to the development of serous cystadenoma. Lkb1 deficiency also affects the endocrine compartment as evidenced by smaller and scattered islets and transient alterations in glucose control. These studies provide in vivo evidence that Lkb1 is a critical regulator of epithelial cell polarity, as well as essential for the maintenance of acinar cell viability and overall morphological stability of the pancreas.
A striking observation from this study is that while alterations in acinar polarity were observed for the embryonic p-Lkb1 pancreas, acinar degeneration did not occur until after birth. We reason that this timing is consistent with the absence of a role for the pancreatic energy balance and nutrient uptake during embryogenesis, whereas following birth, there is an initiation of exocrine function with the apically directed exocytosis of accumulated zymogen granules to enable the digestion of enteric nutrients. The newborn pancreas, as well as other organs, also experiences the loss of maternally supplied nutrients, creating a state of energy stress. The ensuing induction of cellular autophagy contributes to continued nutrient availability (27). Although Lkb1 has been implicated in controlling autophagy in cultured cells, an analysis of the Lkb1-deficient pancreas did not reveal evidence of an autophagic process by TEM analysis and measurement of LC3-1 and LC3-2 protein ratios (29). Taken together, we conclude that acinar polarity defects in association with postnatal activation of acinar secretion of digestive enzymes contribute directly to progressive acinar cell destruction in the p-Lkb1 mice.
The defects noted in acinar cells provided an opportunity to examine in vivo the signaling components of the Lkb1-AMPK pathway, which have been implicated as the key regulators of cell polarity in cell culture-based systems and genetic model organisms. Along these lines, the activation of Lkb1 by forced expression of the pseudokinase STRAD induces full polarization of isolated LS174T intestinal epithelial cells, and, correspondingly, energy stress induces polarization of LS174T cells in an AMPK-dependent manner (2, 28). In addition, AMPK is necessary for the efficient establishment of tight junctions in cultured MDCK renal epithelial cells in response to calcium administration (51). Reinforcing the importance of AMPK as the major target of Lkb1 in the establishment of polarity are the similarity of the phenotypes of the Lkb1 and the Ampk Drosophila mutants and the rescue of polarity defects in the Lkb1-Ampk double mutants. MLC2, the regulatory subunit of myosin II, appears to be a critical substrate for AMPK-mediated changes in cell structure in both Drosophila and LS174T cells (28). The MARKs promote epithelial polarization via the regulation of tubulin dynamics, which is mediated in part through the phosphorylation of MT binding protein, and involves alteration in the cell contacts and in E-cadherin localization (10, 11, 16). Finally, Lkb1 is essential for the axon specification of mammalian neurons and mediates this effect through the activation of SAD-B and SAD-A. In our in vivo study, we observed that the activation of the AMPK, MARK, and SAD-A/B kinases were all markedly compromised in the PD1 p-Lkb1 pancreas. Consistent with the role found for MARKs in the polarization of the pancreatic epithelium, we documented the fact that MAP4 phosphorylation, tubulin structure, and E-cadherin localization are all altered in the p-Lkb1 pancreas, findings that corroborate observations from studies of cultured mammalian epithelial cells. Importantly, however, the AMPK target, MLC2, shows increased rather than decreased phosphorylation in the p-Lkb1 pancreas, both immediately following birth and with prolonged starvation, suggesting that an Lkb1-AMPK-MLC2 axis is not operative in vivo in the induction of acinar polarity. Together, our study findings are consistent with the contributions of AMPKs, MARKs, and SADs to Lkb1-mediated induction of acinar polarity and provide a genetic framework for the further systematic dissection of the relative roles of these components in this biological process. The absence of tight and adherens junctions among mutant acinar cells may reflect a failure of de novo formation in proliferating tissues, a loss of established junctions, or a combination of these two mechanisms. Lkb1 is strongly expressed in both acinar and islet compartments through development and early postpartum tissue expansion, whereas in the adults, Lkb1 is expressed primarily in islets. This expression pattern is consistent with a primary role for Lkb1 in the establishment of polarity rather than maintenance of cell structure and orientation. The presence of small areas of normal acinar tissue lasting into adulthood supports this view.
Serous cystadenomas in the p-Lkb1 pancreas emerge from ductal metaplasia. Ductal metaplasia refers to the progressive replacement of acinar tissue with ductal structures and may be due to either acinar-to-ductal transdifferentiation or to expansion of the ductal compartment concurrently with acinar cell death (26, 36, 45). Ductal metaplasia is seen in a number of settings including oncogenic stress, inactivation of genes required for the formation of primary cilia of the ducts, and forced expression of Pdx1 and Notch1 (8, 20, 34, 35, 38). Serous cystadenomas have been associated specifically with ductal metaplasia in the setting of acinar cell transforming growth factor-alpha overexpression and concurrent p53 and Ink4a/Arf mutations (4). Recently, the role of acinar-to-ductal transdifferentiation as the mechanism underlying ductal metaplasia has been established in vivo, with lineage tracing (45). Importantly, pancreatic ductal adenocarcinoma precursor lesions, PanINs, also appear to arise from regions of acinar-to-ductal transdifferentiation (53). Taken together, these studies support the view that acinar cells, sustaining oncogenic mutation or injury, may contribute to ductal adenocarcinoma formation through a pathway of transdifferentiation. Our immunofluorescence studies of the Lkb1 mutant pancreas reveal costaining for acinar and ductal markers in early metaplastic lesions, and the progressive loss of acinar markers in more established lesions appears consistent with a process of acinar-to-ductal transdifferentiation. As observed with some of these other models, we also see activation of pathways such as STAT3 and Notch associated with metaplastic change. We speculate that the impact of the Lkb1 deletion on acinar integrity could underlie the pronounced pancreatic cancer risk observed with PJS patients.
An outstanding question in cancer biology has been how to define the role Lkb1 plays in regulating epithelial cell structure and polarity in vivo and how to relate this to the suppression of neoplasia. Our current work establishes a role for Lkb1 in cellular polarity and cytoskeletal structure in the pancreas and show that the loss of cell polarity control is associated with the development of ductal metaplasia and serous cystadenomas. Together, these findings underscore the importance of Lkb1 in the maintenance of epithelial cell polarity and strongly implicates the loss of acinar polarity as a contributor to the increased risk of pancreatic neoplasia for patients with PJSs.
Acknowledgments
We thank Lyle Lopez for technical expertise, Ben Stanger, Andy McClatchey, and Susan Bonner-Weir for discussion and comments, and Brendan Lillis and Josh Sanes for the use of SAD antibodies and technical expertise.
This work was support by a grant from NIH, K08CA122835, and ASCO/PanCAN to A.F.H.; by the Barbara Goodman-ICRF pancreatic cancer career development award and JDRF; by NIH grant K01CA104647 and NIH P01 CA117969-01; and by grants from the Samuel Waxman Foundation and the Linda Verville Foundation to N.B. R.A.D. is supported by NIH grants P01CA117969-03 and U01CA084313-09, the LeBow Fund to Cure Myeloma, and by the Center for Applied Cancer Science of the Belfer Institute for Innovative Cancer Science. R.A.D. is an Ellison Foundation for Medical Research Senior Scholar and an American Cancer Society Research Professor.
Footnotes
▿
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Alessi, D. R., K. Sakamoto, and J. R. Bayascas. 2006. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75137-163. [DOI] [PubMed] [Google Scholar]
- 2.Baas, A. F., J. Kuipers, N. N. van der Wel, E. Batlle, H. K. Koerten, P. J. Peters, and H. C. Clevers. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116457-466. [DOI] [PubMed] [Google Scholar]
- 3.Baas, A. F., L. Smit, and H. Clevers. 2004. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 14312-319. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy, N., J. Morgan, M. Sinha, S. Signoretti, S. Srivastava, M. Loda, G. Merlino, and R. A. DePinho. 2002. Obligate roles for p16Ink4a and p19Arf-p53 in the suppression of murine pancreatic neoplasia. Mol. Cell. Biol. 22635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardeesy, N., M. Sinha, A. F. Hezel, S. Signoretti, N. A. Hathaway, N. E. Sharpless, M. Loda, D. R. Carrasco, and R. A. DePinho. 2002. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419162-167. [DOI] [PubMed] [Google Scholar]
- 6.Barnes, A. P., B. N. Lilley, Y. A. Pan, L. J. Plummer, A. W. Powell, A. N. Raines, J. R. Sanes, and F. Polleux. 2007. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129549-563. [DOI] [PubMed] [Google Scholar]
- 7.Brune, K., T. Abe, M. Canto, L. O'Malley, A. P. Klein, A. Maitra, N. Volkan Adsay, E. K. Fishman, J. L. Cameron, C. J. Yeo, S. E. Kern, M. Goggins, and R. H. Hruban. 2006. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 301067-1076. [PMC free article] [PubMed] [Google Scholar]
- 8.Cano, D. A., S. Sekine, and M. Hebrok. 2006. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 1311856-1869. [DOI] [PubMed] [Google Scholar]
- 9.Canto, M. I., M. Goggins, C. J. Yeo, C. Griffin, J. E. Axilbund, K. Brune, S. Z. Ali, S. Jagannath, G. M. Petersen, E. K. Fishman, S. Piantadosi, F. M. Giardiello, and R. H. Hruban. 2004. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin. Gastroenterol. Hepatol. 2606-621. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, D., P. J. Brennwald, E. Rodriguez-Boulan, and A. Musch. 2004. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 164717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, D., Y. Tian, and A. Musch. 2007. Par1b promotes hepatic-type lumen polarity in Madin Darby canine kidney cells via myosin II- and E-cadherin-dependent signaling. Mol. Biol. Cell 182203-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collombat, P., J. Hecksher-Sorensen, P. Serup, and A. Mansouri. 2006. Specifying pancreatic endocrine cell fates. Mech. Dev. 123501-512. [DOI] [PubMed] [Google Scholar]
- 13.Detlefsen, S., B. Sipos, B. Feyerabend, and G. Kloppel. 2005. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 447800-805. [DOI] [PubMed] [Google Scholar]
- 14.Drewes, G., A. Ebneth, and E. M. Mandelkow. 1998. MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci. 23307-311. [DOI] [PubMed] [Google Scholar]
- 15.Drewes, G., A. Ebneth, U. Preuss, E. M. Mandelkow, and E. Mandelkow. 1997. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89297-308. [DOI] [PubMed] [Google Scholar]
- 16.Elbert, M., D. Cohen, and A. Musch. 2006. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol. Biol. Cell 173345-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giardiello, F. M., and J. D. Trimbath. 2006. Peutz-Jeghers syndrome and management recommendations. Clin. Gastroenterol. Hepatol. 4408-415. [DOI] [PubMed] [Google Scholar]
- 18.Gu, G., J. R. Brown, and D. A. Melton. 2003. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech. Dev. 12035-43. [DOI] [PubMed] [Google Scholar]
- 19.Hurov, J. B., M. Huang, L. S. White, J. Lennerz, C. S. Choi, Y. R. Cho, H. J. Kim, J. L. Prior, D. Piwnica-Worms, L. C. Cantley, J. K. Kim, G. I. Shulman, and H. Piwnica-Worms. 2007. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc. Natl. Acad. Sci. USA 1045680-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jhappan, C., C. Stahle, R. N. Harkins, N. Fausto, G. H. Smith, and G. T. Merlino. 1990. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 611137-1146. [DOI] [PubMed] [Google Scholar]
- 21.Ji, H., M. R. Ramsey, D. N. Hayes, C. Fan, K. McNamara, P. Kozlowski, C. Torrice, M. C. Wu, T. Shimamura, S. A. Perera, M. C. Liang, D. Cai, G. N. Naumov, L. Bao, C. M. Contreras, D. Li, L. Chen, J. Krishnamurthy, J. Koivunen, L. R. Chirieac, R. F. Padera, R. T. Bronson, N. I. Lindeman, D. C. Christiani, X. Lin, G. I. Shapiro, P. A. Janne, B. E. Johnson, M. Meyerson, D. J. Kwiatkowski, D. H. Castrillon, N. Bardeesy, N. E. Sharpless, and K. K. Wong. 2007. LKB1 modulates lung cancer differentiation and metastasis. Nature 448807-810. [DOI] [PubMed] [Google Scholar]
- 22.Kabeya, Y., N. Mizushima, T. Ueno, A. Yamamoto, T. Kirisako, T. Noda, E. Kominami, Y. Ohsumi, and T. Yoshimori. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 195720-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katajisto, P., T. Vallenius, K. Vaahtomeri, N. Ekman, L. Udd, M. Tiainen, and T. P. Makela. 2007. The LKB1 tumor suppressor kinase in human disease. Biochim. Biophys. Acta 177563-75. [DOI] [PubMed] [Google Scholar]
- 24.Kimura, K., K. Satoh, A. Kanno, S. Hamada, M. Hirota, M. Endoh, A. Masamune, and T. Shimosegawa. 2007. Activation of Notch signaling in tumorigenesis of experimental pancreatic cancer induced by dimethylbenzanthracene in mice. Cancer Sci. 98155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh, H. J., D. E. Arnolds, N. Fujii, T. T. Tran, M. J. Rogers, N. Jessen, Y. Li, C. W. Liew, R. C. Ho, M. F. Hirshman, R. N. Kulkarni, C. R. Kahn, and L. J. Goodyear. 2006. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol. Cell. Biol. 268217-8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konieczny, S. F., and S. D. Leach. 2007. Metaplastic metamorphoses in the mammalian pancreas. Gastroenterology 1332056-2059. [DOI] [PubMed] [Google Scholar]
- 27.Kuma, A., M. Hatano, M. Matsui, A. Yamamoto, H. Nakaya, T. Yoshimori, Y. Ohsumi, T. Tokuhisa, and N. Mizushima. 2004. The role of autophagy during the early neonatal starvation period. Nature 4321032-1036. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. H., H. Koh, M. Kim, Y. Kim, S. Y. Lee, R. E. Karess, S. H. Lee, M. Shong, J. M. Kim, J. Kim, and J. Chung. 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 4471017-1020. [DOI] [PubMed] [Google Scholar]
- 29.Liang, J., S. H. Shao, Z. X. Xu, B. Hennessy, Z. Ding, M. Larrea, S. Kondo, D. J. Dumont, J. U. Gutterman, C. L. Walker, J. M. Slingerland, and G. B. Mills. 2007. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 9218-224. [DOI] [PubMed] [Google Scholar]
- 30.Lizcano, J. M., O. Goransson, R. Toth, M. Deak, N. A. Morrice, J. Boudeau, S. A. Hawley, L. Udd, T. P. Makela, D. G. Hardie, and D. R. Alessi. 2004. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin, S. G., and D. St Johnston. 2003. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421379-384. [DOI] [PubMed] [Google Scholar]
- 32.Mirouse, V., L. L. Swick, N. Kazgan, D. St Johnston, and J. E. Brenman. 2007. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 177387-392. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Miyamoto, Y., A. Maitra, B. Ghosh, U. Zechner, P. Argani, C. A. Iacobuzio-Donahue, V. Sriuranpong, T. Iso, I. M. Meszoely, M. S. Wolfe, R. H. Hruban, D. W. Ball, R. M. Schmid, and S. D. Leach. 2003. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3565-576. [DOI] [PubMed] [Google Scholar]
- 34.Miyatsuka, T., H. Kaneto, T. Shiraiwa, T. A. Matsuoka, K. Yamamoto, K. Kato, Y. Nakamura, S. Akira, K. Takeda, Y. Kajimoto, Y. Yamasaki, E. P. Sandgren, Y. Kawaguchi, C. V. Wright, and Y. Fujitani. 2006. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 201435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murtaugh, L. C., B. Z. Stanger, K. M. Kwan, and D. A. Melton. 2003. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 10014920-14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oertel, J. E. 1989. The pancreas. Nonneoplastic alterations. Am. J. Surg. Pathol 13(Suppl. 1)50-65. [PubMed] [Google Scholar]
- 37.Saito, I., S. Hashimoto, A. Saluja, M. L. Steer, and J. Meldolesi. 1987. Intracellular transport of pancreatic zymogens during caerulein supramaximal stimulation. Am. J. Physiol. 253G517-G526. [DOI] [PubMed] [Google Scholar]
- 38.Sandgren, E. P., N. C. Luetteke, R. D. Palmiter, R. L. Brinster, and D. C. Lee. 1990. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 611121-1135. [DOI] [PubMed] [Google Scholar]
- 39.Sato, N., C. Rosty, M. Jansen, N. Fukushima, T. Ueki, C. J. Yeo, J. L. Cameron, C. A. Iacobuzio-Donahue, R. H. Hruban, and M. Goggins. 2001. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am. J. Pathol. 1592017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid, R. M. 2002. Acinar-to-ductal metaplasia in pancreatic cancer development. J. Clin. Investig. 1091403-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw, R. J., N. Bardeesy, B. D. Manning, L. Lopez, M. Kosmatka, R. A. DePinho, and L. C. Cantley. 2004. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 691-99. [DOI] [PubMed] [Google Scholar]
- 42.Shaw, R. J., K. A. Lamia, D. Vasquez, S. H. Koo, N. Bardeesy, R. A. Depinho, M. Montminy, and L. C. Cantley. 2005. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 3101642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shelly, M., L. Cancedda, S. Heilshorn, G. Sumbre, and M. M. Poo. 2007. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129565-577. [DOI] [PubMed] [Google Scholar]
- 44.Stanger, B. Z., B. Stiles, G. Y. Lauwers, N. Bardeesy, M. Mendoza, Y. Wang, A. Greenwood, K. H. Cheng, M. McLaughlin, D. Brown, R. A. Depinho, H. Wu, D. A. Melton, and Y. Dor. 2005. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8185-195. [DOI] [PubMed] [Google Scholar]
- 45.Strobel, O., Y. Dor, J. Alsina, A. Stirman, G. Lauwers, A. Trainor, C. F. Castillo, A. L. Warshaw, and S. P. Thayer. 2007. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 1331999-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su, G. H., R. H. Hruban, R. K. Bansal, G. S. Bova, D. J. Tang, M. C. Shekher, A. M. Westerman, M. M. Entius, M. Goggins, C. J. Yeo, and S. E. Kern. 1999. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am. J. Pathol. 1541835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuveson, D. A., L. Zhu, A. Gopinathan, N. A. Willis, L. Kachatrian, R. Grochow, C. L. Pin, N. Y. Mitin, E. J. Taparowsky, P. A. Gimotty, R. H. Hruban, T. Jacks, and S. F. Konieczny. 2006. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 66242-247. [DOI] [PubMed] [Google Scholar]
- 48.Watts, J. L., D. G. Morton, J. Bestman, and K. J. Kemphues. 2000. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 1271467-1475. [DOI] [PubMed] [Google Scholar]
- 49.Wodarz, A. 2002. Establishing cell polarity in development. Nat. Cell Biol. 4E39-44. [DOI] [PubMed] [Google Scholar]
- 50.Yee, N. S., E. E. Furth, and M. Pack. 2003. Clinicopathologic and molecular features of pancreatic adenocarcinoma associated with Peutz-Jeghers syndrome. Cancer Biol. Ther. 238-47. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, L., J. Li, L. H. Young, and M. J. Caplan. 2006. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl. Acad. Sci. USA 10317272-17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng, B., and L. C. Cantley. 2007. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 104819-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, L., G. Shi, C. M. Schmidt, R. H. Hruban, and S. F. Konieczny. 2007. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am. J. Pathol. 171263-273. [DOI] [PMC free article] [PubMed] [Google Scholar]