A Small-Molecule Furin Inhibitor Inhibits Cancer Cell Motility and Invasiveness (original) (raw)
Abstract
Furin, a member the proprotein convertase (PC) family, processes inactive precursor proteins to functional proteins within the Golgi/_trans_-Golgi network secretory pathway. Furin and other PC family members (furin/PCs) activate proteins vital to proper physiological functioning, including growth factors and hormones, receptors, plasma proteins, and matrix metalloproteases (MMPs). Additionally, the expression and activity of furin/PC are necessary for processing substrates important for cell transformation and tumor progression, metastasis, and angiogenesis. Furin processing of the remodeling protease membrane type-1 matrix metalloproteinase (MT1-MMP) enhances cellular motility and invasiveness, contributing to aggression and metastatic potential cancer cells. Whereas overexpression and activity of furin/PC exacerbate the cancer phenotype, inhibition of its activity decreases or nullifies furin/PC-mediated effects, and thus, inhibition of furin may be a viable route to cancer therapy. Recently, we identified a small-molecule inhibitor of furin, named B3, by high-throughput screening with a _K_i and IC50 of 12µM. Here, we show that this cell-permeable, small-molecule compound inhibits furin-mediated cleavage of proMT1-MMP, resulting in decreased MMP-2 activation and cell motility in CHO cells expressing proMT1-MMP. Additionally, this molecule inhibited proMT1-MMP processing, complete MMP-2 maturation, and invasiveness of human fibrosarcoma cells (HT1080).
Introduction
Furin, a ubiquitously expressed member the proprotein convertase (PC) family, functions to process inactive precursor proteins to their functional or mature forms at the specific recognition motif RXK/RR↓ within the Golgi/_trans_-Golgi network secretory pathway [1,2]. Furin and other PC family members (furin/PC) process and activate many proteins vital to the proper physiological functioning, including growth factors and hormones, receptors, plasma proteins, and matrix metalloproteases (MMPs) [2]. Although furin/PC processing is essential, its activity contributes to several pathologic conditions such as Alzheimer's disease, arthritis, atherosclerosis, and cancer [1,3,4].
Furin/PC expression and processing can increase the incidence and severity of the cancer phenotype. Heightened furin expression and/or furin/PC processing of substrates lead to cellular transformation and promote tumor progression [5,6]. In fact, cancers found in the ovary [7], breast [8], head and neck [9], and brain [10], as well as non-small cell lung carcinomas [11], have increased furin expression compared to their normal cell counterpart. This elevated expression often results in an increase in tumor cell aggression and promotes formation of metastases, resulting in decreased patient survival. Furin/PC processing of substrates also contributes to tumor progression, aggressiveness, metastasis, and angiogenesis [12–15]. Furin/PC processing of substrates such as platelet-derived growth factor A [16] and B [17], insulin growth factor and its receptor [3], tumor necrosis factor α [18], and transforming growth factor β [5,19] facilitates cancer progression and invasiveness [3,20]. Furin processing of MMPs, such as intracellular remodeling proteases stromelysin-3 [21] and promembrane type-1 matrix metalloproteinase (proMT1-MMP) [22], increases cell motility and invasion thus promoting metastases [12]. Specifically, proMT1-MMP processing by furin is required for MT1-MMP activation [23], which results in the degradation of extracellular matrix components such as fibronectin, fibrin, collagens, and laminin-1 [24]. MT1-MMP also degrades adhesion molecules such as CD44, integrins, and tissue transglutaminase [24]. Active MT1-MMP activates other MMPs responsible for degrading extracellular matrix components such as proMMP-2 (gelatinase) and proMMP-13, further contributing to the metastatic potential of the cell [24]. Thus, the expression and activity of furin/PC directly enhances progression, aggression, and metastatic potential of tumor cells [21].
Existing evidence indicates that furin inhibition can be a viable route to cancer therapy [6,20]. For example, treatment of cultured human fibrosarcoma cells with the furin inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethylketone (decRVKR-CMK) decreased MT1-MMP maturation and invasive ability of these cells [25]. Moreover, transfection of the furin inhibitor, alpha 1-antitrypsin Portland (α1-PDX), in MT1-MMP-expressing cells inhibited MMP-related cell motility [23], cell growth, and invasiveness of glioma tumor cell lines [10] and of colon adenocarcinoma cells [26] both in vitro and in vivo. Although these furin inhibitors are effective in combating cancer in vitro (decRVKR-CMK and α1-PDX) and in vivo (α1-PDX) [6], these and other furin inhibitors [27–32] have not been developed as pharmaceuticals due to issues with size, stability, and toxicity [6,33,34]. Further, to combat furin/PC-mediated MT1-MMP-related motility and invasiveness, an inhibitor must be cell-permeable, as furin/PC cleavage of proMT1-MMP occurs primarily within the Golgi/_trans_-Golgi network [35–37]. The search for furin/PC inhibitors with the desired characteristics in terms of stability, toxicity, and cell permeability is ongoing. These properties may be found in a small-molecule inhibitor.
To search for small-molecule furin inhibitors, we performed a high-throughput screening of several small-molecule libraries using a cell-based assay [38]. As a result of this screen, we identified a small-molecule inhibitor of furin, B3 (previously named CCG 8294 [38]), with a _K_i and IC50 of 12 µM. Here, we show that this cell-permeable, small-molecule inhibits furin processing of proMT1-MMP, resulting in decreased MMP-2 activity and cell motility in CHO cells expressing proMT1-MMP. Additionally, B3 inhibited proMT1-MMP processing and invasiveness of human fibrosarcoma cells (HT1080).
Materials and Methods
Reagents
decRVKR-CMK was purchased from Bachem (King of Prussia, PA). decRVKR-CMK and B3 were dissolved in DMSO. B3 was freshly dissolved in solution on the day of the experiment. BioCoat Matrigel Invasion Chambers were purchased from BD Biosciences (San Jose, CA). Concanavalin A (ConA) and polylysine were purchased from Sigma (St. Louis, MO). Reagents used in zymography were purchased from Invitrogen (Carlsbad, CA).
Compound Preparation (B3)
To prepare naphthofluorescein disodium salt (B3), a mixture of 1,6-dihydroxynaphthalene (9.6 g, 60 mmol) and phthalic anhydride (4.44 g, 30 mmol) was heated at 200°C with vigorous stirring for 16 hours under a nitrogen atmosphere followed by cooling to room temperature (RT). The resulting brown-black solid was treated with an excess of acetic anhydride (50 ml) and refluxed for 3 hours. The mixture was cooled to RT, treated with a mixture of crushed ice and CH2Cl2 (200 ml), and stirred for 3 hours. The organic layer was separated, washed with water (3 x 200 ml), and concentrated to give 12.35 g of black viscous oil. A 2.0-g portion was purified by repeated (twice) silica-gel flash chromatography using a gradient of methanol (1–3%) in CH2Cl2 to give 1.3 g (51.8%) as a tan solid. Next, a solution of NaOH in methanol (0.25 M, 4 ml, 1.0 mmol) was added dropwise with stirring (0.26 g, 0.5 mmol) under a nitrogen atmosphere. The intense dark blue suspension was stirred for an additional 30 minutes and concentrated. The residue was triturated with methyl _tert_-butyl ether (MTBE) (2 x 10 ml) and concentrated. A further portion of MTBE (20 ml) was added to the residue, the mixture stirred for 20 minutes and filtered. The product was rinsed with MTBE (2 x 5 ml) and dried (with protection from light) under high vacuum at 60°C for 3 hours to give 0.23 g (98.7%) of B3 as blue-black crystals: 1H nuclear magnetic resonance (DMSO-d6 + TMS, 400 MHz): σ 8.30 (d, J = 9.4 Hz, 2H), 8.12 (m, 1H), 7.54 (m, 2H), 7.15 (m, 1H), 6.95 (d, J = 9.2 Hz, 2H), 6.66 (dd, J = 9.3, 2.1 Hz, 2H), 6.49 (d, J = 9.0 Hz, 2H), 6.24 (d, J = 1.9 Hz, 2H); high-resolution mass spectrometry (electrospray ionization with HCOOH added): predicted m/z for C28H17O5 [(M - ′2Na+ + 2H+) + H]+ 433.1076, observed m/z 433.1073.
Cell Culture and Transfections
COS and HT1080 cells were maintained in DMEM containing 10% FBS, 1% l-glutamine, 100 µg/ml penicillin, and 100 µg/ml streptomycin (P/S/G) (Gibco, Carlsbad, CA). Chinese hamster ovary (CHO) cells were maintained in Ham's F12 media with 10% FBS, 1 µM MEM nonessential amino acids (Gibco), and P/S/G. COS and CHO cells were transfected with plasmid using Fugene 6 (Roche, Indianapolis, IN) according to the manufacturer's protocol. All cell lines were incubated at 37°C with 5% CO2.
Drug Treatment Assays
COS cells were plated in 10 cm plates 24 hours before transfection with plasmid-encoding proMMP-2. Twenty-four hours after transfection, the media was replaced with OptiMEM (Gibco), and the cells were allowed to incubate for 24 hours after which the conditioned media was retrieved and centrifuged at 1000_g_ for 10 minutes. Supernatant was collected and placed on CHO cells transfected with plasmid-encoding proMT1-MMP-HA ∼36 hours after transfection. The conditioned media from the CHO cells was collected after 8 hours of incubation. The media was centrifuged at 1000_g_ for 5 minutes, and the supernatant was used for zymography or Western blot analysis as indicated. For HT1080 experiments, 300,000 cells/well were plated in six-well plates. After 24 hours of incubation, cells were washed three times with media (no FBS) and were treated with 50 µg/ml ConA and the indicated concentration of drug. After an overnight incubation, conditioned media was collected and concentrated using a filter device (Microcon YM-10; Millipore, Billerica, MA). Cells were harvested for Western blot analysis as described.
Western Blot Analysis
Western blot analysis was performed as described [38]. Cells were washed in PBS and lysed with a buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, supplemented with Complete Protease Inhibitor Cocktail (Roche). Cells were sonicated briefly, and the supernatant was collected after centrifugation. Protein concentration was estimated using a detergent-compatible protein assay kit from Bio-Rad (Hercules, CA). Media and lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and protein expression was detected by Western blot analysis using appropriate primary antibody and HRP-conjugated secondary antibody followed by chemiluminescent HRP substrate (Pierce, Rockford, IL). solMT1-MMP expression was detected using a rabbit polyclonal antibody to MT1-MMP (Chemicon, Temecula, CA). proMT1-MMP-HA expression was detected using a mouse monoclonal antibody to HA (Covance, Princeton, NJ).
Zymography
The supernatant (10 µl) from cell assays was combined with running buffer without reducing agents and was applied to an agarose gel containing 1% gelatin. After separation by electrophoresis, the gel was placed in 1x renaturing buffer for 30 minutes and was then washed with 1x developing buffer for 30 minutes after which the buffer was replaced with fresh developing buffer and gel-incubated for an additional 24 hours. The gels were stained with Coomassie brilliant blue R250, and gelatinolytic activities were detected as clear bands against a blue background.
Motility Assays
A 2-mg gelatin was combined with AlexaFluor 544 nm (Invitrogen), 900 µl of H2O, and 100 µl of 1 M NaHCO3. The components were allowed to incubate for 1 hour and were then diluted 1:8 with H2O. The labeled gelatin was then dialyzed against PBS at 4°C for 24 hours with two-to-three PBS changes. Slides were then prepared by first coating with polylysine for 1 hour at RT. The labeled gelatin was then diluted 1:20 in H2O, added to the slide, and allowed to incubate for 2 hours at RT. The slide was fixed with glutaraldehyde (1.5% in PBS) for 4 minutes at RT and was then washed three times with PBS. The slide was blocked with NH4Cl (50 µM in PBS) for 10 minutes at RT and then washed three times with PBS. Ham's F12 media was then added to the slide for incubation at 37°C. CHO cells transfected with plasmid-encoding MT1-MMP-HA/green fluorescent protein (GFP) were trypsinized and washed 36 hours after transfection and applied to slides containing OptiMEM media with vehicle or drug. The cells and proteolytic tracks were visualized using a fluorescence microscope (Eclipse TE2000-U; Nikon, Melville, NY). Fluorescent images were acquired with Metamorph software (Molecular Devices Corp, Sunnyvale, CA) using identical exposure times. Proteolytic tracks were identified using Segmentation and Thresholding tools, and track area (pixels) was measured using Region Measurement tools in Metamorph software version 9 (Molecular Devices, Downingtown, PA). Tracks were measured from a minimum of 10 random fields and were added together to provide total proteolytic area. Proteolytic area was then expressed as percentage of control. This experiment was repeated eight times.
Toxicity Assays
CHO cells were plated in 96-well plates, treated with vehicle (DMSO), 5, 10, 15, or 20 µM B3, and were allowed to incubate for 16 hours. After incubation, a cell viability assay was performed using WST-1 reagent (Roche) according to the manufacturer's instructions. WST-1 reagent was added at the indicated time after drug treatment and assayed. Absorbance was monitored at 460 nm and background was monitored at 600 nm using a plate reader (Fluorostar Optima; BMG Labtech, Chicago, IL). Final absorbance units were computed by background subtraction.
Invasion Assays
Invasion assays were conducted using BioCoat Matrigel Invasion Chambers (8-µm pore size) according to the manufacturer's instructions. HT1080 cells (2.5 x 104 cells per well) were plated on inserts in 24-well tissue culture plates using DMEM without FBS (and indicated concentration of drug or vehicle) in upper chambers and DMEM with 10% FBS as chemoattractant in lower chambers (and indicated concentration of drug or vehicle). After 16 hours of incubation, noninvading cells were removed. Remaining cells were stained using Diff-Quik stain set (Dade Behring, Newark, DE) according to the manufacturer's instructions. Membrane inserts were placed on slides and visualized using a light microscope (Eclipse TE2000-U; Nikon) (objective, x10). Images were acquired with Metamorph software (Molecular Devices Corp, Sunnyvale, CA). Images of 10 random fields were taken, and cells were counted. Each cell treatment condition was done in triplicate. Percent invasion was determined by dividing the mean number of invading cells for the indicated treatment group by the mean number of invading cells of control group.
Results
MT1-MMP-Dependent Activation of MMP-2 Is Inhibited By Furin Inhibitor, B3
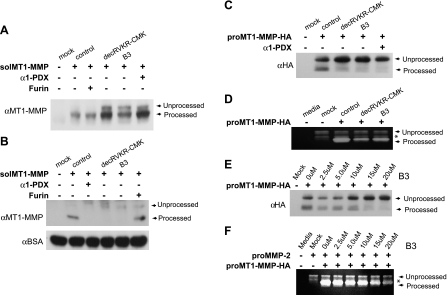
To evaluate the ability of the furin inhibitor, B3, to alter MT1-MMP maturation, we used CHO cells expressing a secreted, soluble form of proMT1-MMP (solMT1-MMP) [39] and performed Western blot analysis using cell lysates. Expression of solMT1-MMP in these cells resulted in the appearance of mature solMT1-MMP (∼57 kDa) (Figure 1_A_). Coexpression of furin in solMT1-MMP-expressing cells had no effect on solMT1-MMP maturation as solMT1-MMP was completely processed by CHO cells. Treatment of CHO cells expressing solMT1-MMP with the well-characterized furin inhibitor, decRVKR-CMK (20 µM), resulted in a decrease in processed solMT1-MMP and appearance of unprocessed solMT1-MMP (∼60 kDa) and served as a positive control. Similarly, treatment of cells with 15 µM B3 resulted in a decrease in processed solMT1-MMP and the appearance of unprocessed solMT1-MMP. Coexpression of another well-characterized furin inhibitor, α1-PDX [27], in solMT1-MMP-expressing cells resulted in the appearance of unprocessed solMT1-MMP in cell lysates and served as an additional positive control. We also performed Western blot analysis using the conditioned media of these same cells to confirm B3's ability to inhibit solMT1-MMP maturation. Expression of solMT1-MMP in these cells resulted in the appearance of mature solMT1-MMP (∼54 kDa) in the conditioned media (Figure 1_B_). Treatment of cells expressing solMT1-MMP with decRVKR-CMK (20 µM) or coexpression of α1-PDX resulted in a decrease in mature solMT1-MMP and the appearance of unprocessed solMT1-MMP (∼64 kDa) in the conditioned media. Similarly, treatment of cells with 15 µM B3 resulted in a decrease in mature solMT1-MMP and the appearance of unprocessed solMT1-MMP. As previously mentioned, overexpression of furin in solMT1-MMP-expressing cells had no further effect on MT1-MMP maturation. Next, we investigated whether B3 would inhibit furin-mediated maturation of membrane-bound proMT1-MMP. For this, we performed Western blot analysis using cell lysates of CHO cells expressing an HA-tagged proMT1-MMP (proMT1-MMP-HA) (Figure 1_C_). CHO cells expressing empty vector (mock) did not express detectable levels of MT1-MMP. In contrast, expression of proMT1-MMP-HA plasmid in CHO cells (control) resulted in the appearance of both the unprocessed (∼63 kDa) and processed forms (∼60 kDa) of MT1-MMP. Treatment of these cells with decRVKR-CMK (20 µM) resulted in a decrease in processed MT1-MMP and increase in the pro-form of MT1-MMP and served as a positive control. Similarly, treatment of cells with 15 µM B3 resulted in a decrease in processed MT1-MMP and an increase in the proMT1-MMP compared to control. Expression of α1-PDX in proMT1-MMP-HA expressing cells also resulted in a decrease in processed proMT1-MMP compared to control cells. Furin cleaves MT1-MMP processes and activates its downstream substrate, progelatinase (proMMP-2), to enzymatically active/mature forms. To determine whether furin inhibition of MT1-MMP processing would have a concomitant decrease in MMP-2 enzymatic activity, we incubated proMT1-MMP-HA-expressing CHO cells with conditioned media containing proMMP-2 in the absence or presence of a furin inhibitor (Figure 1_D_). Zymographic analysis revealed that conditioned media alone (media) had no MMP-2-related enzymatic activity. When conditioned media containing proMMP-2 was added to CHO cells expressing empty vector (mock), only the unprocessed (∼66 kDa) and intermediate (∼62 kDa) forms of MMP-2 were detected. In contrast, proMMP-2-containing conditioned media added to CHO cells expressing proMT1-MMP-HA resulted in the detection of unprocessed, intermediate, and mature forms (∼59 kDa) of MMP-2. In the presence of B3 (15 µM) or decRVKR-CMK (20 µM), a substantial decrease in mature MMP-2 was detected. To determine the dose dependence of furin inhibition on proMT1-MMP processing and MMP-2 activation, we repeated the previously mentioned experiment with various doses of B3. A dose-dependent decrease in proMT1-MMP-HA maturation was observed on the analysis of cell lysates (Figure 1_E_). Concomitant zymographic analysis of MMP-2 activity in the conditioned media also demonstrated a dose-dependant decrease in MMP-2 activation (Figure 1_F_). Some toxicity was observed with cells treated with 20 µM B3.
Figure 1.
MT1-MMP-dependent activation of MMP-2 is inhibited by furin inhibitor, B3. (A) A representative Western blot of lysates or (B) conditioned media of CHO cells expressing empty vector (-) or sol proMT1-MMP plasmid alone or in combination with α1-PDX or furin or treated with decRVKR-CMK (20 µM) or B3 (15 µM) and probed with MT1-MMP antibody. An anti-BSA antibody was used as a loading control for conditioned media. (C) A representative Western blot of lysates of CHO cells expressing empty vector (-) or proMT1-MMP-HA plasmid alone or in combination with α1-PDX or treated with furin inhibitors decRVKR-CMK (20 µM) or B3 (15 µM) and probed with HA antibody. (D) Zymographic analysis of conditioned media alone (media) or conditioned media containing proMMP-2 on CHO cells expressing empty vector (-) (mock) or CHO cells expressing proMT1-MMP-HA plasmid (control) and treated with either decRVKR-CMK (20 µM) or B3 (15 µM). Asterisk (*) indicates the intermediate form of MMP-2. (E) A representative Western blot of lysates of CHO cells transfected with empty vector (mock) or MT1-MMP-HA plasmid alone or in combination with indicated dose of B3 and probed with HA antibody. (F) Zymogram analysis of conditioned media (media) or conditioned media containing proMMP-2 on CHO cells expressing empty vector (-) (mock) or proMT1-MMP-HA plasmid and treated indicated concentration of B3. Asterisk (*) indicates the form of MMP-2.
Inhibition of Furin-Mediated MT1-MMP-dependent Motility By B3
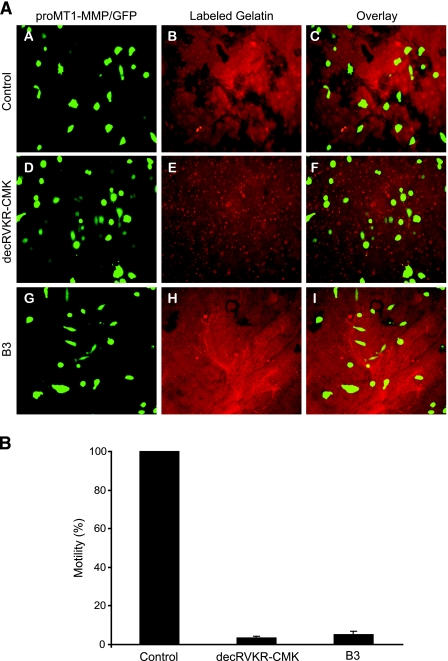
To functionally assess MT1-MMP-dependent activation of MMP-2, we next performed a motility assay, monitoring the ability of CHO cells expressing proMT1-MMP-HA/GFP to activate endogenous MMP-2 (gelatinase). Active MMP-2 proteolyses its substrate, gelatin, as the cell travels along a gelatin substratum. Wild-type CHO cells do not degrade gelatin; however, CHO cells expressing MT-MMP proteolyse a gelatin substrate [23]. To test this, we transfected CHO cells with an expression plasmid containing both proMT1-MMP-HA and GFP, placed these cells on fluorescently labeled gelatin-plated slides with or without furin inhibitory molecules, and monitored their ability to degrade the gelatin substratum as they migrated across the slide surface using fluorescence microscopy as described in Materials and Methods. CHO cells expressing proMT1-MMP-HA/GFP (control) degraded fluorescently labeled gelatin, resulting in dark proteolytic tracks (Figure 2A, subpanels A, B, and C). proMT1-MMP-HA/GFP-expressing CHO cells treated with the furin inhibitor, decRVKR-CMK, had a significant decrease in motility compared to control cells as indicated by the absence of proteolytic tracks (Figure 2A, subpanels D, E, and F) and served as positive control. proMT1-MMP-HA/GFP-expressing CHO cells treated with 15 µM B3 had a significant decrease in motility compared to control cells, although some slight motility remained (Figure 2A, subpanels G, H, and I). Ten randomly chosen fields were photographed from eight replicate slides and proteolytic track area was measured as described in Materials and Methods. Motility (total proteolytic area) was then computed and expressed as a percentage of control (Figure 2_B_). proMT1-MMP-HA/GFP-expressing CHO cells treated with decRVKR-CMK (positive control) or B3 both had a significant reduction in motility (2–5%) compared to control cells.
Figure 2.
Inhibition of furin mediated MT1-MMP-dependent motility by B3. (A) Representative images of proteolysis of gelatin substrate by CHO cells expressing proMT1-MMP and GFP (control, subpanels A, B, and C) and treated with decRVKR-CMK (20 µM) (subpanels D, E, and F) or B3 (15 µM) (subpanels G, H, and I) as indicated and visualized by fluorescent microscopy. (B) Quantification of motility (%) for each of the conditions shown in (A). For this, proteolytic track area (motility) was measured in 10 images of each treatment group as described in Materials and Methods and was expressed as percentage of control. This experiment was replicated eight times (mean ± SD).
Inhibition of Furin-Mediated MT1-MMP Maturation and MMP-2 Activation By B3 in HT1080 Cells
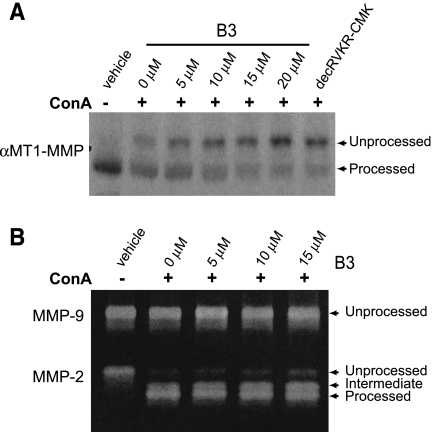
To investigate whether B3 would affect proMT1-MMP processing and subsequent MMP-2 activity in a human cancer cell line, we performed the next series of experiments using the highly invasive human fibrosarcoma cell line, HT1080. HT1080 cells have increased levels of processed MT1-MMP compared with other cell lines [40], which is thought to contribute to their invasive ability. Additionally, treatment of HT1080 cells with ConA enhances conversion of proMMP-2 into its active forms [25]. Treatment of HT1080 cells with ConA (50 µg/ml) and increasing concentrations of B3 resulted in a decrease in the mature MT1-MMP and a concomitant increase in proMT1-MMP in a dose dependent manner, as observed by Western blot analysis (Figure 3_A_). To determine whether this decrease in active MT1-MMP would result in an increase in proMMP-2 in this cell line, we performed zymography using the conditioned media of these same cells. Zymogram analysis (Figure 3_B_) revealed conditioned media from HT1080 cells treated with vehicle alone contained only unprocessed MMP-2. Addition of ConA (50 µg/ml) to cells resulted in processing of MMP-2 to intermediate and mature forms. Treatment of these cells with both ConA and increasing concentrations of B3 as previously mentioned resulted in a decrease in the mature form and an increase in the intermediate and unprocessed forms of MMP-2 in a dose-dependent manner with no alternations in MMP-9 activity (∼92 kDa, loading control).
Figure 3.
Inhibition of furin-mediated MT1-MMP maturation and MMP-2 activation by B3 in HT1080 cells. (A) A representative Western blot of HT1080 lysates treated with vehicle or ConA (50 µg/ml) and indicated dose of B3 and probed with MT1-MMP antibody. (B) Zymographic analysis of conditioned media from HT1080 cells treated with vehicle or ConA (50 ng/ml) and indicated dose of B3.
B3 Decreases HT1080 Invasion
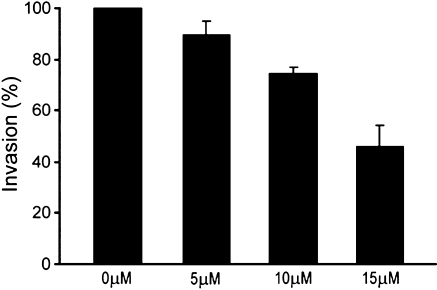
To functionally validate that inhibition of furin activity by B3 results in decreased invasive capacity, HT1080 cells were treated with indicated doses of B3 or vehicle (control) (Figure 4). HT1080 control cells treated with vehicle alone freely invaded the membrane (100% invasion). Cells treated with 5 µM B3 showed a slight decrease in membrane penetration. Cells treated with 10 µM B3 had invasive ability reduced to ∼80% of control. 15 µM B3 was required to inhibit invasive activity to ∼50% of control cells. Because doses more than 20 µM resulted in some toxicity, higher doses of B3 were not evaluated in this assay.
Figure 4.
B3 decreases HT1080 invasion. HT1080 cells were seeded in transwell cell culture inserts coated with Matrigel and treated with 0, 5, 10, or 15 µM B3. Cells that had invaded through the membrane were counted as described in Materials and Methods. Results are expressed as percentage of control cells (mean ± SD).
Discussion
Activation of MT1-MMP by furin directly contributes to the motility and invasiveness of the tumor cell [23,24]. Here, we have shown that inhibition of furin activity prevents cleavage of proMT1-MMP (Figures 1, B and D, and 3_A_) and subsequent activation of MMP-2 (Figures 1 C and 3_B_). Clearly, inhibition of furin activity did not completely prevent the activity of mature MMP-2 as shown by zymography, but MMP-2 activity is often contingent on other factors [35,41]. Here, we show that B3 can inhibit furin cleavage of proMT1-MMP and that this inhibition reduces the motility and invasiveness of cells. Addition of B3 to CHO cells expressing proMT1-MMP resulted in an almost complete inhibition of MMP-2-related cell motility (Figure 2, A and B) and a 50% decrease in the invasiveness of the HT1080 cell line (Figure 4). These functional experiments provide a direct means to assess MT1-MMP-dependent MMP-2 activity and confirm furin inhibition as a means to combat tumor cell motility and invasiveness in vitro.
B3 was discovered by high-throughput screening with small-molecule libraries using a cell-based assay and was confirmed as a furin inhibitor in a series of secondary assays [38]. However, cellular experiments indicate some toxicity associated with this molecule, which becomes apparent when applied at concentrations of 20 µM or more (data not shown). Preliminary experiments using small molecules with structural similarity to B3 have indicated that these molecules have inhibitory function toward furin, and other PC family members, with IC50 values in the micromolar range. These molecules also inhibit processing of the furin substrates pro-von Willebrand Factor [42] and anthrax PA toxin [43], some with less toxicity than that observed with B3 (unpublished data). In light of this, we believe that B3 serves as an ideal candidate as a lead molecule for a new class of cell-permeable furin inhibitors.
In addition to processing and activating proMT1-MMP, furin activates a myriad of other proteins involved in cancer progression, such as transforming growth factor β [5], insulin growth factor 1 and the insulin receptor [3], and vascular endothelial growth factor C/D [12,13]. Because of this, we believe that inhibition of furin may be a more viable route to cancer therapy than the use of MT1-MMP inhibitors. Recently, phase III clinical trials of MT1-MMP inhibitors demonstrated no clinical efficacy [44]. Because furin/PCs act upstream of MT1-MMP and furin/PC activity is associated with activation of other substrates that contribute to cancer progression, furin may be a more effective pharmaceutical target than MT1-MMP. Future experiments will determine whether B3 can inhibit cleavage of other furin substrates involved in tumorigenesis. Additionally, B3 inhibits other PC family members (unpublished results). Because other PCs (PACE4 [21], PC5/6 [23], and PC7 [12,13]) are involved in processing substrates necessary for tumor progression and invasion [3], inhibition of multiple PCs may be necessary for complete suppression of the cancer phenotype.
Furin activity is also necessary for the development of degenerative diseases, such as arthritis, atherosclerosis, and Alzheimer's disease [1], and the propagation of viruses, such as avian influenza, human immunodeficiency virus 1, Ebola, measles, cytomegalovirus, and yellow fever [2,45]. Like MT1-MMP and other cancer-promoting substrates, furin processing and activation of these substrates occur primarily within the Golgi [35], which necessitate the use of a cell-permeable inhibitor. Furin inhibition also thwarts the activation of bacterial toxins, such as anthrax toxin, pseudomonas exotoxin A, Shiga toxin, diphtheria toxin, and tetanus and botulism neurotoxins, which are necessary for infectivity, and the virulence of these bacteria [4,10,46–50]. Furin/PC inhibition may be a route to manage or cure these debilitating and often fatal diseases. Future studies with this inhibitory molecule, or a structurally modified derivative, will enable verification of efficacy against cancer and other furin-mediated disease.
Acknowledgments
We thank the gifts proMT1-MMP-HA/GFP and proMMP-2 expression plasmids from Stephen Weiss (Life Sciences Institute, University of Michigan). The solMT1-MMP expression plasmid was kindly provided by Jian Cao and Stanley Zucker (Stony Brook, New York). Thanks to Xiao-Li Yan from the Weiss Laboratory for helpful discussions regarding the cell motility and zymogram assays. Thanks to Mike Dugre for careful reading of the manuscript.
Abbreviations
α1-PDX
alpha 1-antitrypsin Portland
ConA
concanavalin A
decRVKR-CMK
decanoyl-Arg-Val-Lys-Arg-chloromethylketone
GFP
green fluorescent protein
MMP
matrix metalloprotease
MT1-MMP
membrane type 1-matrix metalloprotease
MTBE
methyl _tert_-butyl ether
PC
proprotein convertase
RT
room temperature
TGN
_trans_-Golgi network
Footnotes
1
This work was supported by the National Institutes of Health/National Cancer Institute grants P01CA85878, R24CA83099, and P50CA093990.
References
- 1.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327(Pt 3):625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 4.Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 5.Blanchette F, Day R, Dong W, Laprise MH, Dubois CM. TGFβ1 regulates gene expression of its own converting enzyme furin. J Clin Invest. 1997;99:1974–1983. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Cicco RL, Bassi DE, Benavides F, Conti CJ, Klein-Szanto AJ. Inhibition of proprotein convertases: approaches to block squamous carcinoma development and progression. Mol Carcinog. 2007;46:654–659. doi: 10.1002/mc.20331. [DOI] [PubMed] [Google Scholar]
- 7.Page RE, Klein-Szanto AJ, Litwin S, Nicolas E, Al-Jumaily R, Alexander P, Godwin AK, Ross EA, Schilder RJ, Bassi DE. Increased expression of the pro-protein convertase furin predicts decreased survival in ovarian cancer. Cell Oncol. 2007;29:289–299. doi: 10.1155/2007/930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng M, Watson PH, Paterson JA, Seidah N, Chretien M, Shiu RP. Pro-protein convertase gene expression in human breast cancer. Int J Cancer. 1997;71:966–971. doi: 10.1002/(sici)1097-0215(19970611)71:6<966::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog. 2001;31:224–232. doi: 10.1002/mc.1057. [DOI] [PubMed] [Google Scholar]
- 10.Mercapide J, Lopez De Cicco R, Bassi DE, Castresana JS, Thomas G, Klein-Szanto AJ. Inhibition of furin-mediated processing results in suppression of astrocytoma cell growth and invasiveness. Clin Cancer Res. 2002;8:1740–1746. [PubMed] [Google Scholar]
- 11.Schalken JA, Roebroek AJ, Oomen PP, Wagenaar SS, Debruyne FM, Bloemers HP, Van de Ven WJ. fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest. 1987;80:1545–1549. doi: 10.1172/JCI113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chretien M, Seidah NG, Khatib AM. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McColl BK, Paavonen K, Karnezis T, Harris NC, Davydova N, Rothacker J, Nice EC, Harder KW, Roufail S, Hibbs ML, et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 14.Seiki M, Yana I. Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci. 2003;94:569–574. doi: 10.1111/j.1349-7006.2003.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci USA. 2001;98:10326–10331. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegfried G, Khatib AM, Benjannet S, Chretien M, Seidah NG. The proteolytic processing of pro-platelet-derived growth factor-A at RRKR(86) by members of the proprotein convertase family is functionally correlated to platelet-derived growth factor-A-induced functions and tumorigenicity. Cancer Res. 2003;63:1458–1463. [PubMed] [Google Scholar]
- 17.Siegfried G, Basak A, Prichett-Pejic W, Scamuffa N, Ma L, Benjannet S, Veinot JP, Calvo F, Seidah N, Khatib AM. Regulation of the stepwise proteolytic cleavage and secretion of PDGF-B by the proprotein convertases. Oncogene. 2005;24:6925–6935. doi: 10.1038/sj.onc.1208838. [DOI] [PubMed] [Google Scholar]
- 18.Tellier E, Negre-Salvayre A, Bocquet B, Itohara S, Hannun YA, Salvayre R, Auge N. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Mol Cell Biol. 2007;27:2997–3007. doi: 10.1128/MCB.01485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon S, Laprise MH, Dubois CM. Alternative pathway for the role of furin in tumor cell invasion process. Enhanced MMP-2 levels through bioactive TGFβ. Exp Cell Res. 2003;291:326–339. doi: 10.1016/s0014-4827(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 20.Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG. Pro-protein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am J Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassi DE, Mahloogi H, Klein-Szanto AJ. The proprotein convertases furin and PACE4 play a significant role in tumor progression. Mol Carcinog. 2000;28:63–69. [PubMed] [Google Scholar]
- 22.Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996;393:101–104. doi: 10.1016/0014-5793(96)00861-7. [DOI] [PubMed] [Google Scholar]
- 23.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 25.Maquoi E, Noel A, Frankenne F, Angliker H, Murphy G, Foidart JM. Inhibition of matrix metalloproteinase 2 maturation and HT1080 invasiveness by a synthetic furin inhibitor. FEBS Lett. 1998;424:262–266. doi: 10.1016/s0014-5793(98)00187-2. [DOI] [PubMed] [Google Scholar]
- 26.Khatib AM, Siegfried G, Prat A, Luis J, Chretien M, Metrakos P, Seidah NG. Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J Biol Chem. 2001;276:30686–30693. doi: 10.1074/jbc.M101725200. [DOI] [PubMed] [Google Scholar]
- 27.Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc Natl Acad Sci USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron A, Appel J, Houghten RA, Lindberg I. Polyarginines are potent furin inhibitors. J Biol Chem. 2000;275:36741–36749. doi: 10.1074/jbc.M003848200. [DOI] [PubMed] [Google Scholar]
- 29.Kacprzak MM, Peinado JR, Than ME, Appel J, Henrich S, Lipkind G, Houghten RA, Bode W, Lindberg I. Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-d-arginine. J Biol Chem. 2004;279:36788–36794. doi: 10.1074/jbc.M400484200. [DOI] [PubMed] [Google Scholar]
- 30.Basak A, Cooper S, Roberge AG, Banik UK, Chretien M, Seidah NG. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem J. 1999;338(Pt 1):107–113. [PMC free article] [PubMed] [Google Scholar]
- 31.Podsiadlo P, Komiyama T, Fuller RS, Blum O. Furin inhibition by compounds of copper and zinc. J Biol Chem. 2004;279:36219–36227. doi: 10.1074/jbc.M400338200. [DOI] [PubMed] [Google Scholar]
- 32.Jiao GS, Cregar L, Wang J, Millis SZ, Tang C, O'Malley S, Johnson AT, Sareth S, Larson J, Thomas G. Synthetic small molecule furin inhibitors derived from 2,5-dideoxystreptamine. Proc Natl Acad Sci USA. 2006;103:19707–19712. doi: 10.1073/pnas.0606555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richer MJ, Keays CA, Waterhouse J, Minhas J, Hashimoto C, Jean F. The Spn4 gene of Drosophila encodes a potent furin-directed secretory pathway serpin. Proc Natl Acad Sci USA. 2004;101:10560–10565. doi: 10.1073/pnas.0401406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterwalder T, Kuhnen A, Leiserson WM, Kim YS, Keshishian H. Drosophila serpin 4 functions as a neuroserpin-like inhibitor of subtilisin-like proprotein convertases. J Neurosci. 2004;24:5482–5491. doi: 10.1523/JNEUROSCI.5577-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Rehemtulla A, Bahou W, Zucker S. Membrane type matrix metalloproteinase 1 activates pro-gelatinase A without furin cleavage of the N-terminal domain. J Biol Chem. 1996;271:30174–30180. doi: 10.1074/jbc.271.47.30174. [DOI] [PubMed] [Google Scholar]
- 36.Mazzone M, Baldassarre M, Beznoussenko G, Giacchetti G, Cao J, Zucker S, Luini A, Buccione R. Intracellular processing and activation of membrane type 1 matrix metalloprotease depends on its partitioning into lipid domains. J Cell Sci. 2004;117:6275–6287. doi: 10.1242/jcs.01563. [DOI] [PubMed] [Google Scholar]
- 37.Zucker S, Hymowitz M, Conner CE, DiYanni EA, Cao J. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest. 2002;82:1673–1684. doi: 10.1097/01.lab.0000041713.74852.2a. [DOI] [PubMed] [Google Scholar]
- 38.Coppola JM, Hamilton CA, Bhojani MS, Larsen MJ, Ross BD, Rehemtulla A. Identification of inhibitors using a cell-based assay for monitoring Golgi-resident protease activity. Anal Biochem. 2007;364:19–29. doi: 10.1016/j.ab.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J Biol Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 40.Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 41.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 42.Rehemtulla A, Kaufman RJ. Preferred sequence requirements for cleavage of pro-von Willebrand factor by propeptide-processing enzymes. Blood. 1992;79:2349–2355. [PubMed] [Google Scholar]
- 43.Beauregard KE, Collier RJ, Swanson JA. Proteolytic activation of receptor-bound anthrax protective antigen on macrophages promotes its internalization. Cell Microbiol. 2000;2:251–258. doi: 10.1046/j.1462-5822.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 44.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 45.Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 46.Gordon VM, Leppla SH. Proteolytic activation of bacterial toxins: role of bacterial and host cell proteases. Infect Immun. 1994;62:333–340. doi: 10.1128/iai.62.2.333-340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basak A, Zhong M, Munzer JS, Chretien M, Seidah NG. Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses: a comparative analysis with fluorogenic peptides. Biochem J. 2001;353:537–545. doi: 10.1042/0264-6021:3530537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- 49.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moulard M, Decroly E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim Biophys Acta. 2000;1469:121–132. doi: 10.1016/s0304-4157(00)00014-9. [DOI] [PubMed] [Google Scholar]