Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella (original) (raw)
Abstract
Filopodia are rodlike extensions generally attributed with a guidance role in cell migration. We now show in fish fibroblasts that filopodia play a major role in generating contractile bundles in the lamella region behind the migrating front. Filopodia that developed adhesion to the substrate via paxillin containing focal complexes contributed their proximal part to stress fiber assembly, and filopodia that folded laterally contributed to the construction of contractile bundles parallel to the cell edge. Correlated light and electron microscopy of cells labeled for actin and fascin confirmed integration of filopodia bundles into the lamella network. Inhibition of myosin II did not subdue the waving and folding motions of filopodia or their entry into the lamella, but filopodia were not then integrated into contractile arrays. Comparable results were obtained with B16 melanoma cells. These and other findings support the idea that filaments generated in filopodia and lamellipodia for protrusion are recycled for seeding actomyosin arrays for use in retraction.
Introduction
The protrusion of cytoplasm during cell migration occurs in the form of lamellipodia and filopodia. Filopodia were first described as “extremely fine filamentous pseudopodia” on the surface of neuronal growth cones (Harrison, 1910) and the same projections were observed much later on all types of migratory cells (Albrecht-Buehler, 1976). Filopodia are rodlike extensions of 0.1–0.2-μm diameter composed of a bundle of actin filaments (Goldman and Knipe, 1973; Small and Celis, 1978; Lindberg et al., 1981) oriented with their fast-growing filament “+ ends” toward the tip (Small et al., 1978). As a general rule, lamellipodia and filopodia coexist at the protruding front of migrating cells but the numbers of filopodia are highly variable depending on cell type and environmental conditions. Filopodia cannot drive cell motility alone, as lamellipodia can (Euteneuer and Schliwa, 1984), and have been attributed a number of roles (Wood and Martin, 2002), particularly in cell guidance. Axons become disoriented when filopodia are suppressed (Bentley and Toroian-Raymond, 1986; Chien et al., 1993) and the guidance of endothelial tip cells during angiogenesis requires orientation of filopodia along growth factor gradients (Gerhardt et al., 2003). Likewise, filopodia promote the establishment of cell contacts between epithelial cell layers (Wood and Martin, 2002), and during dorsal closure in Drosophila melanogaster, filopodia serve to align the merging cell boundaries (Jacinto et al., 2002). Consistent with a guidance role in vivo, filopodia have been implicated in the initiation of cell matrix adhesion during the in vitro migration of neuronal growth cones (Letourneau, 1981) and fibroblasts (DePasquale and Izzard, 1987; Rinnerthaler et al., 1988). Rather like the struts of a tent, filopodia can also provide boundaries of lamellipodia protrusion (Nobes and Hall, 1995), a role particularly evident in the growth cone (Wessels and Luduena, 1973).
Filopodia generally extend from bundles formed within the lamellipodium through reorganizations of filaments within the lamellipodium meshwork (Hoglund et al., 1980; Small et al., 1980, 1982; Svitkina et al., 2003), although they can also form without lamellipodia (Steffen et al., 2006). Thus, although filopodia and lamellipodia are intimate collaborators in protrusion, selective but likely overlapping pathways signal their formation. The details are far from settled, but it is already evident that actin polymerization in lamellipodia requires the Arp2/3 complex (Pollard, 2007), whereas filopodia protrusion is Arp2/3-independent and signaled via formins (Faix and Rottner, 2006). Filopodia are also enriched in the actin bundling protein fascin (Adams, 1995; Otto et al., 1979) and the LIM and SH3 protein lasp (Nakagawa et al., 2006); in certain cell types they are marked by espin at their base (Loomis et al., 2003).
Rather than being concerned with the formation of filopodia and their role in cell guidance, the topic of the present study is the fate of filopodia in relation to cytoskeleton turnover. Using cells of a fibroblast line that protrude numerous filopodia during migration, we show here that filopodia are cycled back into the cytoskeleton to contribute to stress fiber bundle formation in the lamella region (Heath and Holifield, 1993) behind the lamellipodium. In B16 melanoma cells, the fascin-rich “microspikes” that are essentially equivalent to filopodia are likewise shown to contribute to lamella assembly. The findings provide new insights into the generation of the actin cytoskeleton.
Results
Actin cytoskeleton phenotypes in migrating fish fibroblasts
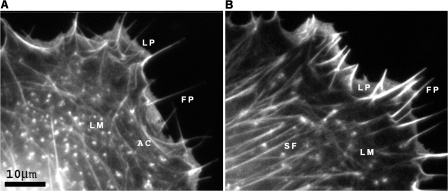
Goldfish (CAR) fibroblasts exhibit the same general characteristics of movement described for chick embryo fibroblasts by Abercrombie et al. (1970a), with phases of protrusion and withdrawal occurring in an unsynchronized fashion in segments along the cell front. Two extreme phenotypes of actin stress fiber organization could be recognized in migrating CAR cells (Fig. 1). In the first, stress fibers formed an interconnected polygonal network, commonly associated with crescent-shaped bundles at the base of lamellipodia segments (Fig. 1 A), and in the second, stress fibers were oriented more or less with their axis in the direction of movement (Fig. 1 B).
Figure 1.
Examples of actin cytoskeleton phenotypes in fish fibroblasts showing differences in the lamella region (LM) behind the anterior zone containing lamellipodia (LP) and filopodia (FP). Cells were transfected with mCherry-actin. Images show single frames from video sequences. (A) Lamella with bundles parallel to the cell front, together with arc shaped segments (AC). (B) Lamella with stress fiber bundles (SF) mainly perpendicular to the cell front. See Videos 1 and 2 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). Bar, 10 μm.
Imaging of cells transfected with mCherry-actin (Videos 1 and 2, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1) showed that filopodia projected up to 15 μm from the cell edge and translated laterally in both directions with their shafts embedded in lamellipodia at diverse angles; they underwent fusions as well as folded laterally or rearwards, as described for other cells (see Introduction).
Filopodia contribute to the formation of actin bundles in the lamella
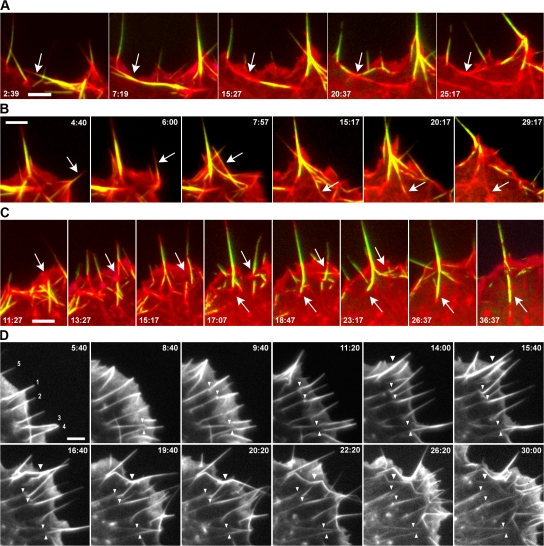
We focused in the present analysis on cells in which the front moved significantly during the video sequence so as to deduce developments relevant to the migration process. In cells expressing mCherry-actin, several fates of filopodia could be recognized. First, concave bundles seen at the base of lamellipodia received the major part of their building material from laterally translating filopodia that folded into the cell edge (Video 1). This activity of filopodia was especially highlighted in cells cotransfected with mCherry-actin and EGFP-fascin (Fig. 2, A and B; and Video 3, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). Measurements indicated that fascin transfection had no significant influence on the number of filopodia expressed at the cell edge. The formation of concave bundles at the base of lamellipodia involved one or several filopodia translating in the same or opposite directions (see Videos 1 and 3). The same bundles either dispersed into the lamella, or survived there as bundles that extended in length and interconnected with adjacent bundles (Fig. 2, A and B; and Video 3). Second, radially oriented filopodia were observed to persist in the same position as the cell front advanced over them and to merge as bundles into the stress fiber network (Fig. 2 D and Video 2). A significant part of this activity was the separation of the top part of the filopodium that was still embedded in the lamellipodium at a point sometimes corresponding to a kink in the filopodium bundle (Fig. 2 D, arrowheads; and Video 2). In such cases, the position of the kink corresponded to the end of the stress fiber bundle that later developed; four examples of filopodia that underwent these transitions are shown in Fig. 2 D (filopodia 1–4 at time 5:40). We shall return to this activity of filopodia in the context of adhesion formation at the cell front. More dramatic kinking of filopodia sometimes occurred as a consequence of retrograde flow or retraction of the lamellipodium, and multiple kinks gave rise to filopodium fragments that dissolved in the lamella as the cell front advanced (Fig. 2 C, top arrow; and Video 3). Finally, filopodia were observed to fold upwards and back into the lamella (Video 3) or, in cells where lamellipodia were depleted, to fold back directly into stress fiber bundles that extended to the cell edge (unpublished data).
Figure 2.
Variable fates of filopodia. (A and B) Development of actin bundles in the lamella from lateral folding of filopodia into the base of the lamellipodium (transitions marked with arrows). Cells were transfected with mCherry-actin (red) and EGFP-fascin (green; see Video 3, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). Times are given in minutes and seconds. (C) Folding into, kinking (top arrow), and withdrawal (bottom arrow) of filopodia into lamella. The same cell as in B is shown. See Video 3. (D) Transition of radially oriented filopodia into stress fiber bundles. The filopodia marked 1–4 at time 5:40 remain essentially stationary as the cell front advances and finally appear as bundles in the lamella (30:00). Arrowheads mark equivalent positions on the filopodia through the sequence. At the position marked, the filopodia become kinked and separate from the lamellipodium/filopodium boundary, except filopodium 2, which continues extending and contributes a further bundle to the lamella. Filopodium 5 (5:40) fuses with two other filopodia; the resulting filopodium subsequently bends in two places and flows into the lamella (26:20; see Video 1). Bars, 5 μm.
Total internal reflection fluorescence (TIRF) microscopy showed that the filopodia folding back into the cell entered the region of the evanescent wave within 200 nm of the coverslip surface, which corresponds to the level of the ventral cytoskeleton (Fig. 3 and Video 4, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). In Fig. 3 (A and B), the superimposed and simultaneous wide field (red) and TIRF images (green) of a cell transfected with EGFP-fascin demonstrate the lifting of filopodia before their entry into the base of the lamellipodium. And in Fig. 3 C, dual wavelength TIRF of mCherry-actin (pseudocolor green) and EGFP-fascin (pseudocolor red) likewise shows the contribution of filopodia to the formation of a stress fiber in the ventral cytoskeleton of the lamella.
Figure 3.
Entry of filopodia into ventral layer of the cytoskeleton. (A and B) Simultaneous wide-field (red) and TIRF (green) microscopy of a cell expressing EGFP-fascin. Filopodia fold down into the zone of the evanescent wave to within 200 nm from the substrate. (A) Filopodium marked with arrowhead folds upwards and then backward into the cell; filopodia marked with arrows fold laterally and down into the cell edge. (B) The numbered filopodia fold in opposite directions into the cell edge. See Video 4 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). (C) Periphery of a cell transfected with EGFP-fascin (pseudocolor red) and mCherry-actin (pseudocolor green) imaged simultaneously by TIRF microscopy. Note the generation of a ventral stress fiber bundle (arrow) from filopodia that fold bilaterally into the cell edge. Times are given in minutes and seconds. Bars, 5 μm.
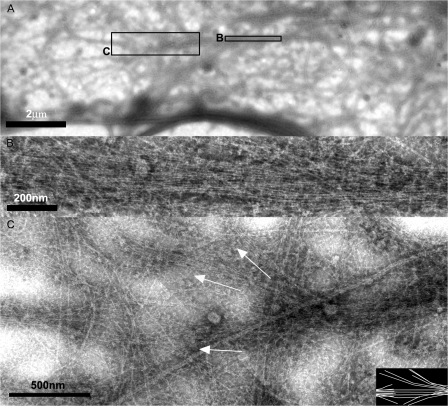
In cytoskeletons of fish fibroblasts processed for negative staining electron microscopy, filopodia appeared as tight bundles of actin filaments 0.1–0.2 μm in diameter (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). By correlated live cell microscopy and electron microscopy of the same cells, we could confirm that filopodia became integrated into the lamella cytoskeleton. In the cell shown in Fig. 4, two regions are highlighted where filopodia moved into the lamella just before fixation. In the first case (Fig. 4 C, which corresponds with inset C in Fig. 4 A; and Video 5), a filopodium folded up and back into the cell, ending up with its axis parallel to the cell edge. In the electron microscope (Fig. 5), the close density of filament packing, characteristic of extending filopodia bundles (Fig. S1), was still evident in the central region of the filopodium that entered the lamella (Fig. 5 B). At the ends of the filopodium bundle, filaments were observed to splay into other parallel arrays of actin filaments of the lamella (Figs. 5 C and S2), showing that the filopodium bundle had integrated into the lamella cytoskeleton. The second region (Fig. 4 D, which corresponds to inset D in Fig. 4 A; and Video 5) includes three filopodia that entered the lamella, contributing to the formation of two parallel actin bundles. One filopodium kinked and folded into the lamella forming the inner bundle (Fig. 4 D, right arrow). The second, outer bundle was formed as a result of the fusion of a laterally translating filopodium with a radial filopodium (Fig. 4 D, left arrow; and Video 5). Fig. 6 (A and B; see also Fig. S3) shows the linkage of actin filaments between the latter two filopodia, building the second bundle in the lamella.
Figure 4.
Correlative live cell imaging and electron microscopy of filopodia transitions. (A) Image of a cell transfected with EGFP-fascin (green) and mCherry-actin (red) after fixation at the end of the video sequence. (B) Electron micrograph of region shown in A after negative staining. Boxes and arrows in A and B indicate regions corresponding to the video sequences shown in C and D. Arrows in C and D indicate transition steps of filopodia into lamella, with the eventual depletion of fascin; final panels show actin label alone in the fixed cell. Times are given in minutes and seconds. See Video 5 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1).
Figure 5.
Integration of filopodia into lamella cytoskeleton. (A) Electron micrograph of region corresponding to the terminal frame in Fig. 4 C. (B) Enlargement of A (small box) showing central region of filopodium bundle in the lamella. (C) End region of the filopodia bundle (large box in A) showing splaying of filaments into adjacent bundles of the lamella (indicated by white arrows; also depicted in the inset). See Fig. S2 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1).
Figure 6.
Integration of filopodia into lamella cytoskeleton. (A) Electron micrograph of the two bundles (1 and 2) marked by arrows in the terminal frame of Fig. 4 D. (B) Enlargement of the region boxed in A showing interconnection of the filopodium bundle with adjacent bundles of the lamella cytoskeleton. See also Fig. S3 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1).
Myosin is not required for filopodium protrusion folding and kinking but promotes integration into contractile bundles in the lamella
After entering the lamella domain, filopodia bundles in untreated cells became populated with myosin to form contractile arrays (Fig. 7 A and Video 6, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). In the region shown in Fig. 7 A, the kinked end of a filopodium was drawn inwards by its forked linkage into two actomyosin-containing bundles in the lamella. The filopodium bundle was then straightened and became progressively populated with myosin, finally becoming integrated into the contractile network of the lamella (Video 6, central arrow). Bundles that formed at the cell periphery by laterally folding filopodia likewise accumulated myosin as they were taken up into the actin cytoskeleton behind the cell front (Video 6, upper arrow). These findings showed that myosin contractility was required for integration of filopodia filaments into continuous actin bundles in the lamella.
Figure 7.
Coupling with myosin in the lamella. (A) Withdrawal and integration of a filopodium into actomyosin network of lamella. Cell was transfected with mCherry-actin and EGFP-myosin regulatory light chain. Arrows indicate a filopodium that first became kinked and was then drawn into the lamella with the progressive accumulation of myosin and the eventual transition into a stress fiber bundle. Times are given in minutes and seconds. See Video 6 (available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). (B) Inhibition of myosin contractility with 50 μM blebbistatin. CAR fibroblast transfected with mCherry-actin. Filopodia formation was not inhibited and filopodia translocated into the lamella (arrowheads) but did not form contractile bundles. See Video 7.
In cells treated with blebbistatin (30–50 μM; Fig. 7 B and Video 7, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1) or Rho-kinase inhibitor (Y27632, 30 μM; Video 8), filopodia protrusion and folding was not inhibited. In early phases of inhibitor treatment (30–60 min), filopodia number did not change significantly in blebbistatin and either increased or decreased in Rho-kinase inhibitor. The kinking of filopodia was unaffected by myosin inhibition, suggesting that it may occur as a result of the abrupt change in retrograde flow rate at the lamellipodium–lamella boundary. In the electron microscope, no change in filament packing in filopodia was evident but curling of actin filaments at the base of filopodia was observed in blebbistatin (unpublished data). Although myosin contractility was required for the integration of filaments derived from filopodia into contractile bundles, the inhibition of myosin by blebbistatin did not prevent the entry of filopodia into the lamella. Fibroblasts treated with blebbistatin exhibited random protrusions and filopodia generated in these protrusions translocated into the lamella as the cell edge advanced beyond them; the filopodia bundles then persisted and either contributed to a random filament array (Fig. 7 B and Video 7) or dispersed in the cytoplasm.
Substrate adhesions initiated under a subset of filopodia mark division of the filopodia bundles into proximal and distal domains
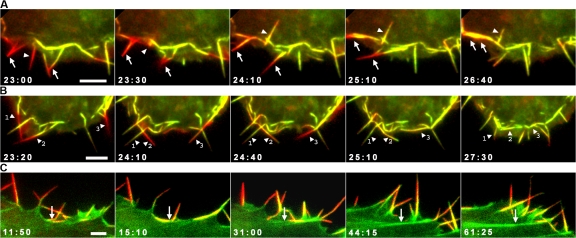
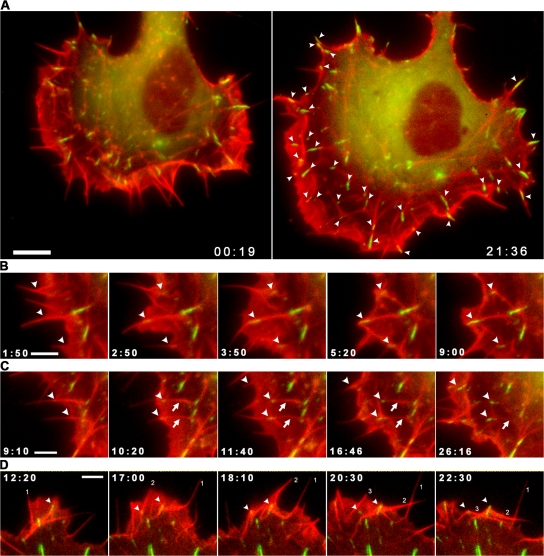
Colabeling of cells for actin and an adhesion component (vinculin, vasodilator-stimulated phosphoprotein [VASP], paxillin, or zyxin) revealed a spatial correspondence of filopodia with early sites of substrate adhesion, referred to here as focal complexes (Nobes and Hall, 1995). In fish fibroblasts, a prominent class of focal complexes was observed to appear at some point along the length of a formed or forming filopodium (Fig. 8). Only a subpopulation of filopodia were involved in adhesion formation, the proportion varying widely from cell to cell. Measurements of focal complex initiation along the edge of three cells revealed, respectively, 63, 40, and 16% of filopodia engaged in focal complex formation (total of 174 filopodia). It was notable that the appearance of adhesion components at a site along the shaft of a filopodium coincided with the advance of the lamellipodium up to or past this site (Fig. 8, B and C; and Video 9, available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1). By tracing the history of focal adhesions in the time-lapse sequence back to their origin at the cell front, we established that their position coincided at an earlier time point, without exception, with focal complexes localized on filopodia. Fig. 8 A shows two frames of a video of a cell separated by 21 min, where the arrowheads in the later frame indicate those adhesions whose origin could be definitively traced to a filopodium.
Figure 8.
A subpopulation of filopodia are involved in the initiation of substrate adhesion. (A) Two video frames of a CAR fibroblast expressing mCherry-actin and EGFP-paxillin separated by 21 min and showing (marked on the right with arrowheads) those adhesion sites whose origin could be traced to a position along a filopodium. (B and C) Video frames of selected regions taken from the cell in A showing the appearance of focal complexes along filopodia (arrowheads; see Video 9). Small arrows in C indicate development of filopodia into bundles in the lamella, with focal adhesions at their ends. (D) Video frames of a cell transfected as in A and showing the bending of filopodia (marked 1–3) around adhesion foci (arrowheads). Times are given in minutes and seconds. Bars: (A) 10 μm; (B–D) 5 μm.
For those filopodia involved in adhesion formation, the focal complex site divided the associated filopodium into an inner and outer segment, with different fates of the two segments. The inner, proximal segment typically became immobile, remained associated with the adhesion site, and seeded a stress fiber bundle (Fig. 8 C and Video 9). The distal, outer segment experienced different fates: (a) it could depolymerise or (b) more typically bend around the adhesion site, folding back into the cell, or (c) move laterally in the lamellipodium, sometimes remaining linked to the adhesion by a looped connection. The outer segment could contribute to the construction of a bundle at the base of a lamellipodium, sometimes remaining attached to the adhesion site (unpublished data). A focal complex site formed on one filopodium was also seen to serve as a fulcrum for bending other filopodia that contacted the focal complex during lateral translation in the lamellipodium (Fig. 8 D). The folding of other filopodia back into the cell was not however dependent on the presence of an adhesion at the bending site.
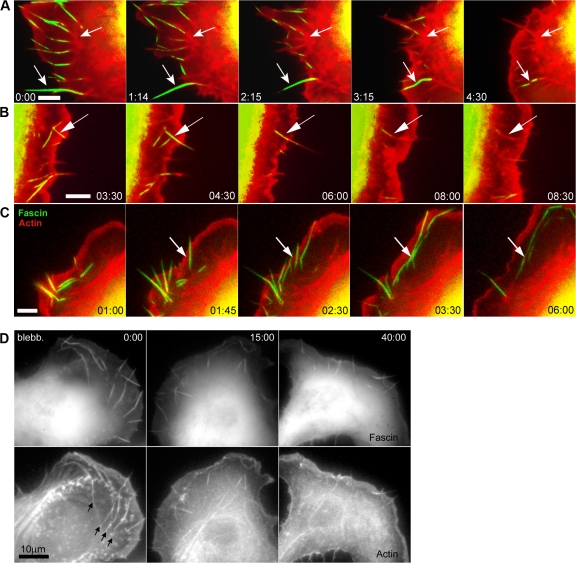
Microspikes in B16 melanoma cells contribute to lamella assembly
B16 melanoma cells characteristically exhibit actin bundles that traverse the lamellipodium, most of which however do not extend significantly beyond the cell edge (Ballestrem et al., 1998) but are fascin-rich and indistinguishable in structure from filopodia (Rottner et al., 1999a; Vignjevic et al., 2006). Such bundles have also been referred to as “ribs” or “microspikes” (Adams, 1997; Small et al., 2002). Observations of B16 melanoma cells transfected with mCherry-actin and GFP-fascin revealed that microspikes became integrated into the lamella in essentially the same way as filopodia (Fig. 9). Radial microspikes were observed to seed the formation of radial bundles in the lamella (Fig. 9, A and B) and arc-shaped bundles behind the lamellipodium were generated via the lateral translation of microspikes along the cell edge (Fig. 9 C and Video 10, before blebbistatin). The same transitions of microspikes into arcs were observed in cells transfected only with GFP-actin or mCherry-actin (not depicted; Koestler et al., 2008). As with fibroblasts, fascin-rich bundles entered the lamella in B16 cells treated with blebbistatin but then dissolved into the diffuse and relaxed actin network (Fig. 9 D and Video 10). The number of microspikes varied widely in control B16 melanoma cells (15 ± 7 per cell; range 7–28) and within the same cell showed up to a twofold change within a 30-min period. After blebbistatin treatment, the number of microspikes showed no consistent increase or decrease and varied within the same range observed in control cells.
Figure 9.
Contribution of microspikes in B16 melanoma cells to construction of actin bundles in the lamella network. (A–C) Video sequences of cells expressing GFP-fascin and mCherry-actin showing transition of fascin-positive microspikes (arrows) into radial (A and B) and transverse bundles (C, arcs) in the lamella. (D) Inhibition of myosin contractility by 30 μM blebbistatin in a B16 melanoma cell expressing GFP-fascin and mCherry-actin (see Video 10). The fascin images were obtained only at the given time points shown in the video sequence to avoid inactivation of blebbistastin. The arc-shaped bundles arising from the lateral translation of microspikes (arrows at time 0:00) dispersed in blebbistatin, but microspike segments continued to enter the lamella (times 15:00 and 40:00). Times are given in minutes and seconds.
Discussion
We here reveal a hitherto unrecognized role of filopodia in cell migration, namely as primary contributors to the generation of the actin cytoskeleton. A link between substrate adhesion formation and filopodia has been described before in fibroblasts and in neuronal growth cones (see Introduction), but a direct contribution of filopodia to actin bundle formation in the lamella has not previously been appreciated. In a study by phase-contrast microscopy, Bray and Chapman (1985) described a folding back of growth cone filopodia into the axon shaft and postulated that they contributed to the cortex of the axon cylinder, which is generally consistent with the present findings. It is likewise noteworthy that Adams (1997) reported the localization of fascin in stress fibers of myoblasts and epithelial cells, although the significance was unclear (Adams, 2004). And Tilney et al. (2004) have identified the filopodium as the building block of bristles in D. melanogaster, an extreme example of actin bundle assembly. As we show, migrating cells also produce filopodia with construction in mind.
The present findings complement and extend observations by ourselves (Heath and Holifield, 1993; Small et al., 1993, 1995; Small and Resch, 2005; Koestler et al., 2008) and Hotulainen and Lappalainen (2006) and allow us to propose overlapping roles of lamellipodia and filopodia in the generation of the actin cytoskeleton of migrating cells (Fig. 10). In all cases, protrusive, polar assemblies of actin at the cell front (lamellipodia and filopodia) are reorganized into contractile, bipolar assemblies of actin filaments in the lamella behind. We suggest that this transition is effected in different ways: through the bilateral polymerization of single filaments and filament bundles along the front of the lamellipodium as a result of their diagonal organization (Small et al., 1998; Small and Resch, 2005); via the bilateral translation, bending, and kinking of filopodia; and through ruffling activity (Small and Resch, 2005), during which both lamellipodia and filopodia fold back into the lamella (Ingram, 1969; Abercrombie et al., 1970b; Harris, 1973). We consider these modes of producing pools of actin filaments of mixed polarity more feasible than a flipping of actin filaments by dynamic cross-linking through α actinin (Hotulainen and Lappalainen, 2006). Myosin and actin cross-linking proteins resident in stress fibers (Gordon and Bushnell, 1979) presumably play a key role in contractile bundle formation, likely involving the sorting of actin filaments into bipolar assemblies (Verkhovsky et al., 1995), but, as recently reemphasized, without the periodic I-band–like organization found in myofibrils (Peterson et al., 2004). As shown previously, the recruitment of actin monomer into stress fiber bundles is very slow compared with lamellipodia (Glacy, 1983; Wang, 1984, 1985; Zicha et al., 2003) and stress fibers can form in starved cells in response to Rho activation before significant incorporation of monomeric actin (Machesky and Hall, 1997). Preexisting stress fibers can also anneal longitudinally to form longer bundles (Zimerman et al., 2004). Thus, the recruitment of filaments from a preexisting filament pool appears to play a significant role in stress fiber assembly.
Figure 10.
Schematic illustration of the different fates of filopodia. Large arrow indicates direction of movement. (1 and 1′) Filopodia fold laterally in opposite directions into the cell edge, forming a bundle with antiparallel filaments that integrates into the lamella as the cell edge advances. Integration is coupled with the incorporation of myosin and actin cross-linkers into the bundle. Single filaments originating from the lamellipodium can also contribute to these bundles. (2) A few filopodia kink as a result of lamellipodial retrograde flow and can release fragments into the lamella. (3 and 3′). An early adhesion (focal complex) forms underneath a filopodium. The part of the filopodium distal to the adhesion retracts or folds away, finally separating from the point of adhesion. The proximal part of the filopodium links with oppositely polarized filaments in the lamella via interaction with myosin and actin cross-linkers, forming a contractile stress fiber and resulting in the maturation of the focal complex into a focal adhesion. Again, filaments originating from the lamellipodium can potentially be recruited into the bundle. (4 and 4′). A filopodium folds up and back into the lamella.
Using interference microscopy, DePasquale and Izzard (1987) noted a correlation in the position of immobilized ribs (the shafts of filopodia, corresponding to microspikes) in lamellipodia, with newly formed focal adhesions. And Wang and Glacy (Glacy, 1983; Wang, 1984) were the first to observe the polarized growth of actin filaments from foci at the cell periphery (focal adhesions) into stress fiber bundles, later confirmed by others (Turnacioglu et al., 1998; Hotulainen and Lappalainen, 2006). So why was a direct contribution of filopodia to stress fiber bundle formation not previously detected? At least two factors would explain this discrepancy: (1) the improved resolution and sensitivity offered by current imaging systems; and (2) the observation of cells over extended periods of forward migration of the cell front. In principle, the segment of a filopodium competent to initiate stress fiber assembly need only be short and could be readily overlooked in cells undergoing slow movement with intense ruffling activity. Medeiros et al. (2006) have recently described a contribution of lamellipodia/filopodia retrograde flow to the construction of the cytoskeleton of Aplysia californica growth cones. In this model system, the large fan-shaped growth cones are immobilized on polylysine and filopodia are constrained to the plane of the substrate, so these cells are not migratory, nor do they develop stress fibers. However, a similar kinking and severing of filopodia roots was observed in these cells, as we describe in fibroblasts and melanoma cells, but whether or not these filopodia fragments contributed to arc formation could not be determined (Medeiros et al., 2006).
Overexpression of either Rac or Cdc42 in cells depleted of Rho stimulates the formation of “focal complexes” under lamellipodia and filopodia, respectively, and these focal complexes serve as precursors of focal adhesions (Nobes and Hall, 1995; Rottner et al., 1999b; Kaverina et al., 2002; Zaidel-Bar et al., 2003). Substrate adhesion formation in migrating CAR fibroblasts is initiated primarily under filopodia, presumably signaled via Cdc42 or alternative pathways that induce filopodia (Czuchra et al., 2005; Pellegrin and Mellor, 2005; Faix and Rottner, 2006). As we have noted, the development of adhesion sites under filopodia in CAR cells commonly coincided with the advance of the lamellipodium boundary, prompting the suggestion that components of the lamellipodium tip complex may contribute to adhesion development. Among these, VASP would be an obvious candidate because it resides both at lamellipodia tips and adhesion sites. What the present results show is that the filopodium not only seeds an adhesion site but can surrender its base for stress fiber assembly. Hence, the first segment of the stress fiber is composed of filaments produced by the filopodia pathway. Similar observations have been made with primary chick embryo fibroblasts (Cramer, L., and T. Anderson, personal communication). Because stress fiber assembly is eventually dependent on Rho (Ridley and Hall, 1992), the maturation of an adhesion initiated underneath a filopodium presumably involves a switch in the actin polymerization machinery. Formins have already been implicated in filopodia (mDia2; Pellegrin and Mellor, 2005; Schirenbeck et al., 2005; Eisenmann et al., 2007) and stress fiber assembly (mDia1; Watanabe et al., 1999; Hotulainen and Lappalainen, 2006), but the localization of a formin at focal adhesions has not been described. The possibility exists that once the work of nucleation has been done, subsequent polymerization becomes formin-independent, analogous to the switch from Arp-dependent nucleation to Arp-independent elongation described for Listeria monocytogenes propulsion in the presence of fascin (Brieher et al., 2004). Actin monomer insertion at the adhesion site is presumably driven through the engagement of Ena/VASP proteins (Laurent et al., 1999). Given that focal adhesion maintenance depends on contractility (Chrzanowska-Wodnicka and Burridge, 1996), the switch in signaling could be induced by mechanosensory interactions at adhesion foci (Bershadsky et al., 2006; Clark et al., 2007) resulting from recruitment by myosin of antiparallel arrays of actin into the anchored segment of the filopodium bundle.
Different cell types adopt alternative strategies to model and remodel their actin cytoskeleton during cell movement that is also reflected in the patterns of adhesion they develop with the underlying matrix (Kaverina et al., 2002). Epidermal keratocytes move effectively without filopodia, and neuronal processes can extend with or without them (Bray and Chapman, 1985; Leeuwen et al., 1997). We suggest that these are variations on an underlying theme, whereby the protrusive activity at the cell front supplies filaments to seed contractile assemblies in the cytoskeleton behind, that are eventually required in moving cells for tail retraction.
Materials and methods
Cell culture and transfection
Goldfish fin fibroblasts (line CAR, No. CCL71; American Type Culture Collection) were maintained in basal Eagle's medium (Sigma-Aldrich) supplemented with 1% nonessential amino acids, 2.5% Hepes (Invitrogen), 1% penicillin/streptomycin, 1% glutamine, and with 12% fetal bovine serum (Thermo Fisher Scientific) at 27°C.
For transient transfection, subconfluent monolayer cultures on 30-mm Petri dishes were used. The transfection mixture was prepared as follows: 2 μg of DNA and 12 μl of Superfect lipofection agent (QIAGEN) were mixed in 200 μl Optimem (Invitrogen). After a 15-min incubation at room temperature, a further 5% serum-containing medium with transfection mixture was added to the cells for 5 h. The cells were then washed and returned to normal medium. Transfected cells were replated after 24 h on coverslips coated with human fibronectin (Roche) at a concentration of 50 μg/ml.
B16-F1 mouse melanoma cells were maintained and transfected as described previously (Rottner et al., 1999a; Koestler et al., 2008).
Plasmids
The mCherry-actin was created by K. Rottner (Helmholtz Centre for Infection Research, Braunschweig, Germany) using an mCherry construct provided by R. Tsien (University of California, San Diego, La Jolla, CA). The EGFP-myosin light chain was provided by R. Chisholm (Northwestern University, Chicago, IL), EGFP-fascin was provided by J. Adams (Cleveland Clinic Foundation, Cleveland, OH), and pEGFP-paxillin was provided by K. Rottner.
Drugs
For myosin II inhibition, blebbistatin (Toronto Research Chemicals) was used at concentrations between 30 and 50 μM, and the Rho-kinase inhibitor Y27632 (Sigma-Aldrich) was used at a concentration of 30 μM. Because blebbistatin is sensitive to radiation below 500 nm (Sakamoto et al., 2005), videos with this drug were made using mCherry-actin and, where appropriate, with only single frames to record GFP-fascin label at intermittent time points during the sequence.
Fluorescence microscopy
Live cell imaging was performed at room temperature with cells grown on 15-mm-diameter coverslips for light microscopy or 22 × 30-mm filmed coverslips for correlative light and electron microscopy (see following section) and samples were mounted in an open chamber fitting on a heating platform (Harvard Instruments). The cells were maintained on the microscope in culture medium. Imaging was performed on inverted microscopes (Axiovert 200M; Carl Zeiss, Inc.) equipped with cooled charge-coupled device (CCD) cameras together with filter wheels and shutters controlled with Metamorph software (MDS Analytical Technologies). For wide-field imaging, a halogen lamp was used for illumination in combination with a 100× TIRF 1.45 NA objective (Carl Zeiss, Inc.) and a Micromax 512 × 512 rear-illuminated, cooled CCD camera (13 μm/pixel; Roper Scientific). Images were collected every 10 s unless otherwise stated. TIRF microscopy was performed with a 1.45 or 1.46 NA objective (Carl Zeiss, Inc.) using a VisiTIRF condensor (Visitron Systems, GmbH), a rear-illuminated CCD camera (16 μm/pixel; Cascade 512B; Roper Scieintific) and a dual imager (Optical Insights) for simultaneous imaging in the EGFP and RFP channels. Illumination in TIRF was provided by 488- and 568-nm laser lines (Laser Physics USA) controlled via an acousto-optical tunable filter (VisiTech International). Wide-field illumination in combination with TIRF was achieved by illumination from a shuttered mercury lamp directed through a separate light pipe into the rear of the TIRF condenser.
Color and contrast in individual video frames was enhanced using Metamorph software.
Correlated light and electron microscopy
The technique used for correlated light and electron microscopy is described in detail by Auinger and Small (2008). In brief, the procedure was as follows. Formvar films cast on a glass slide were floated onto a water surface and coverslips (22 × 30 mm), placed onto the film, retrieved on parafilm, and dried. A grid pattern was then embossed on the film by evaporation of gold through tailor-made masks placed onto the coverslips. The transfected cells were plated onto the coverslips precoated with fibronectin. After video microscopy and fixation, the coverslips were transferred to a 9-cm Petri dish filled with cytoskeleton buffer and the film was gently peeled off the coverslip with forceps. At this stage, the film was inverted and brought to the buffer surface to spread out under surface tension. Under a dissecting microscope, the film was floated onto a stainless steel ring platform and the liquid was removed until the film was immobilized, with the grid pattern centered. An EM grid (50 mesh hexagonal, copper) was then placed onto the film with the central hole over the region containing the cell of interest. For this manipulation, the grid was mounted in forceps held in a modified dual pipette holder (Leica) to allow controlled release, with the pipette holder mounted on a micromanipulator (Narashiga; Auinger and Small, 2008). The film was then floated off the stand by addition of buffer, recovered with a piece of parafilm, rinsed with negative stain solution, and dried on the cell side. The negative stain was composed of a mixture of 2% sodium silicotungstate (Agar Scientific) and 1% aurothioglucose, pH 7.0 (Wako Chemicals). Cells were observed and imaged immediately in the electron microscope (Morgagni; FEI).
For correlated light microscopy, the film–coverslip combination carrying the cells was mounted in a home-made Plexiglas flow-through chamber that fitted on a temperature-controlled platform (Harvard Instruments). The chamber (40 × 20 × 8 mm) featured two syringe needles glued into each end that connected to a central channel 0.5 mm deep between the filmed coverslip on the base and an upper, round coverslip glued to a central depression in the chamber. Cells were fixed at the end of a selected video sequence by sucking the fixative/detergent mixture through the chamber. The composition of the mixture was: 0.5% Triton X-100, 0.25% glutaraldehyde in a cytoskeleton buffer (CB; 10 mM MES, 150 mM NaCl, 5 mM EGTA, 5 mM glucose, and 5 mM MgCl2, pH 6.1) with 1 μg/ml phalloidin added. An initial fixation of 1 min in this mixture was followed by a postfixation in 2% glutaraldehyde (in CB plus 1 μg/ml phalloidin) for 15 min. Final fluorescence images of the selected cell were recorded during the initial fixation period. The coverslip was removed from the chamber and stored in cytoskeleton buffer containing 2% glutaraldehyde and 10 μg/ml phalloidin at 4°C until processing for electron microscopy.
Online supplemental material
Fig. S1 shows electron micrographs of negatively stained filopodia in CAR fish fibroblasts. Figs. S2 and S3 include larger areas of the electron micrographs in Figs. 5 C and 6 B and show the interconnection of filaments derived from the filopodia into other actin bundles of the lamella. Video 1 corresponds to Fig. 1 A and shows the bilateral translation of filopodia along the cell edge as well as the folding of filopodia to form bundles at the base of the lamellipodium. Video 2 corresponds to Figs. 1 B and 2 D and shows the transition of radially oriented filopodia into actin bundles in the lamella. Video 3 corresponds to Fig. 2 (A, B, and C) and demonstrates different modes of entry of filopodia into the lamella, eventually forming stress fiber bundles as well as kinking and fragmentation of filopodia bundles. Video 4 corresponds to Fig. 3 (A and B) and demonstrates by TIRF microscopy the folding of filopodia into the ventral layer of the lamella at the base of the lamellipodium. Video 5 corresponds to Fig. 4 A and shows the folding of filopodia into a cell subsequently processed for electron microscopy. Video 6 corresponds to Fig. 7 A and shows the withdrawal of a filopodium into the lamella followed by the accumulation of myosin and integration into the stress fiber network. Another region of the video shows the integration into a stress fiber of a bundle formed by filopodia folding into the cell edge. Video 7 corresponds to Fig. 7 B and shows the effect of 50 μM blebbistatin on a CAR fibroblast transfected with mCherry-actin. Video 8 shows the effect of 30 μM of Rho-kinase inhibitor on a CAR fibroblast transfected with mCherry-actin and GFP-fascin (inhibitor was added at 25 min and imaging continued 6 min later). Video 9 corresponds to Fig. 8 C and shows the development of early adhesion sites underneath filopodia and stress fiber formation. Video 10 corresponds to Fig. 9 D and shows a B16 melanoma cell transfeced with mCherry-actin and GFP-fascin before and after treatment with 30 μM blebbistatin. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200709134/DC1.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We thank Guenter Resch for support in the electron microscopy facility and Stefan Koestler for discussion. The following colleagues are kindly thanked for providing probes for this study: Klemens Rottner, Roger Tsien, Rex Chisholm, and Josephine Adams.We thank Louise Cramer and Tom Anderson for sharing data on filopodia in primary chick embryo fibroblasts before publication.
The authors thank the Human Frontier Science Program Organization, The Austrian Science Research Council, the Vienna Science Research and Technology Fund, and the City of Vienna/Zentrum für Innovation und Technologie via the Spot of Excellence grant “Center of Molecular and Cellular Nanostructure” for financial support.
Abbreviation used in this paper: TIRF, total internal reflection fluorescence; VASP, vasodilator-stimulated phosphoprotein.
References
- Abercrombie, M., J.E. Heaysman, and S.M. Pegrum. 1970. a. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp. Cell Res. 59:393–398. [DOI] [PubMed] [Google Scholar]
- Abercrombie, M., J.E. Heaysman, and S.M. Pegrum. 1970. b. The locomotion of fibroblasts in culture. II. “Ruffling”. Exp. Cell Res. 60:437–444. [DOI] [PubMed] [Google Scholar]
- Adams, J.C. 1995. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 108:1977–1990. [DOI] [PubMed] [Google Scholar]
- Adams, J.C. 1997. Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes. Mol. Biol. Cell. 8:2345–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, J.C. 2004. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 16:590–596. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler, G. 1976. The function of filopodia in spreading 3T3 mouse fibroblasts. In Cell Motility. A. Book, T. Pollard, R. Goldman, and J. Rosenbaum, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 247–264.
- Auinger, S., and J.V. Small. 2008. Correlated light and electron microscopy of the actin cytoskeleton. Methods Cell Biol. In press. [DOI] [PubMed]
- Ballestrem, C., B. Wehrle-Haller, and B.A. Imhof. 1998. Actin dynamics in living mammalian cells. J. Cell Sci. 111:1649–1658. [DOI] [PubMed] [Google Scholar]
- Bentley, D., and A. Toroian-Raymond. 1986. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 323:712–715. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A.D., C. Ballestrem, L. Carramusa, Y. Zilberman, B. Gilquin, S. Khochbin, A.Y. Alexandrova, A.B. Verkhovsky, T. Shemesh, and M.M. Kozlov. 2006. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 85:165–173. [DOI] [PubMed] [Google Scholar]
- Bray, D., and K. Chapman. 1985. Analysis of microspike movements on the neuronal growth cone. J. Neurosci. 5:3204–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher, W.M., M. Coughlin, and T.J. Mitchison. 2004. Fascin-mediated propulsion of Listeria monocytogenes independent of frequent nucleation by the Arp2/3 complex. J. Cell Biol. 165:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, C.B., D.E. Rosenthal, W.A. Harris, and C.E. Holt. 1993. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 11:237–251. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., and K. Burridge. 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133:1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K., M. Langeslag, C.G. Figdor, and F.N. van Leeuwen. 2007. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 17:178–186. [DOI] [PubMed] [Google Scholar]
- Czuchra, A., X. Wu, H. Meyer, J. van Hengel, T. Schroeder, R. Geffers, K. Rottner, and C. Brakebusch. 2005. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol. Biol. Cell. 16:4473–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale, J.A., and C.S. Izzard. 1987. Evidence for an actin-containing cytoplasmic precursor of the focal contact and the timing of incorporation of vinculin at the focal contact. J. Cell Biol. 105:2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann, K.M., E.S. Harris, S.M. Kitchen, H.A. Holman, H.N. Higgs, and A.S. Alberts. 2007. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr. Biol. 17:579–591. [DOI] [PubMed] [Google Scholar]
- Euteneuer, U., and M. Schliwa. 1984. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature. 310:58–61. [DOI] [PubMed] [Google Scholar]
- Faix, J., and K. Rottner. 2006. The making of filopodia. Curr. Opin. Cell Biol. 18:18–25. [DOI] [PubMed] [Google Scholar]
- Gerhardt, H., M. Golding, M. Fruttiger, C. Ruhrberg, A. Lundkvist, A. Abramsson, M. Jeltsch, C. Mitchell, K. Alitalo, D. Shima, and C. Betsholtz. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glacy, S.D. 1983. Subcellular distribution of rhodamine-actin microinjected into living fibroblastic cells. J. Cell Biol. 97:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, R.K., and D.M. Knipe. 1973. Functions of cytoplasmic fibers in non-muscle cell motility. In Cold Spring Harbor Symposium on Quantitative Biology. Vol. XXXVII. Cold Spring Harbor Laboratory, Cold Spring Harbor. 523-534.
- Gordon, W.E. III, and A. Bushnell. 1979. Immunofluorescent and ultrastructural studies of polygonal microfilament networks in respreading non-muscle cells. Exp. Cell Res. 120:335–348. [DOI] [PubMed] [Google Scholar]
- Harris, A.K. 1973. Cell surface movements related to cell locomotion. In Locomotion of Tissue Cells. Ciba Foundation Symposium. Vol. 14. R. Porter and D. W. Fitzsimmons, editors. Elsevier, Amsterdam. 3–26. [DOI] [PubMed]
- Harrison, R.G. 1910. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 9:787–848. [DOI] [PubMed] [Google Scholar]
- Heath, J.P., and B.F. Holifield. 1993. On the mechanisms of cortical actin flow and its role in cytoskeletal organisation of fibroblasts. Symp. Soc. Exp. Biol. 47:35–56. [PubMed] [Google Scholar]
- Hoglund, A.S., R. Karlsson, E. Arro, B.A. Fredriksson, and U. Lindberg. 1980. Visualization of the peripheral weave of microfilaments in glia cells. J. Muscle Res. Cell Motil. 1:127–146. [DOI] [PubMed] [Google Scholar]
- Hotulainen, P., and P. Lappalainen. 2006. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, V.M. 1969. A side view of moving fibroblasts. Nature. 222:641–644. [DOI] [PubMed] [Google Scholar]
- Jacinto, A., S. Woolner, and P. Martin. 2002. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev. Cell. 3:9–19. [DOI] [PubMed] [Google Scholar]
- Kaverina, I., O. Krylyshkina, and J.V. Small. 2002. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 34:746–761. [DOI] [PubMed] [Google Scholar]
- Koestler, S.A., S. Auinger, M. Vinzenz, K. Rottner, and J.V. Small. 2008. Differentially oriented populations of actin filaments generated in lamellipodia collaborate in pushing and pausing at the cell front. Nat. Cell Biol. 10:306–313. [DOI] [PubMed] [Google Scholar]
- Laurent, V., T.P. Loisel, B. Harbeck, A. Wehman, L. Grobe, B.M. Jockusch, J. Wehland, F.B. Gertler, and M.F. Carlier. 1999. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J. Cell Biol. 144:1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwen, F.N., H.E. Kain, R.A. Kammen, F. Michiels, O.W. Kranenburg, and J.G. Collard. 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau, P.C. 1981. Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell–substratum adhesions. Dev. Biol. 85:113–122. [DOI] [PubMed] [Google Scholar]
- Lindberg, U., A.S. Hoglund, and R. Karlsson. 1981. On the ultrastructural organization of the microfilament system and the possible role of profilactin. Biochimie. 63:307–323. [DOI] [PubMed] [Google Scholar]
- Loomis, P.A., L. Zheng, G. Sekerkova, B. Changyaleket, E. Mugnaini, and J.R. Bartles. 2003. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky, L.M., and A. Hall. 1997. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. 138:913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros, N.A., D.T. Burnette, and P. Forscher. 2006. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 8:215–226. [DOI] [PubMed] [Google Scholar]
- Nakagawa, H., A.G. Terasaki, H. Suzuki, K. Ohashi, and S. Miyamoto. 2006. Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett. 580:3223–3228. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 81:53–62. [DOI] [PubMed] [Google Scholar]
- Otto, J.J., R.E. Kane, and J. Bryan. 1979. Formation of filopodia in coelomocytes: localization of fascin, a 58,000 dalton actin cross-linking protein. Cell. 17:285–293. [DOI] [PubMed] [Google Scholar]
- Pellegrin, S., and H. Mellor. 2005. The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 15:129–133. [DOI] [PubMed] [Google Scholar]
- Peterson, L.J., Z. Rajfur, A.S. Maddox, C.D. Freel, Y. Chen, M. Edlund, C. Otey, and K. Burridge. 2004. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell. 15:3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T.D. 2007. Regulation of actin filament assembly by arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36:451–477. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., and A. Hall. 1992. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 70:389–399. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler, G., B. Geiger, and J.V. Small. 1988. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J. Cell Biol. 106:747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner, K., B. Behrendt, J.V. Small, and J. Wehland. 1999. a. VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1:321–322. [DOI] [PubMed] [Google Scholar]
- Rottner, K., A. Hall, and J.V. Small. 1999. b. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9:640–648. [DOI] [PubMed] [Google Scholar]
- Sakamoto, T., J. Limouze, C.A. Combs, A.F. Straight, and J.R. Sellers. 2005. Blebbistatin, a myosin II inhibitor, is photoinactivated by blue light. Biochemistry. 44:584–588. [DOI] [PubMed] [Google Scholar]
- Schirenbeck, A., T. Bretschneider, R. Arasada, M. Schleicher, and J. Faix. 2005. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat. Cell Biol. 7:619–625. [DOI] [PubMed] [Google Scholar]
- Small, J.V., and J.E. Celis. 1978. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 16:308–325. [PubMed] [Google Scholar]
- Small, J.V., and G.P. Resch. 2005. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr. Opin. Cell Biol. 17:517–523. [DOI] [PubMed] [Google Scholar]
- Small, J.V., G. Isenberg, and J.E. Celis. 1978. Polarity of actin at the leading edge of cultured cells. Nature. 272:638–639. [DOI] [PubMed] [Google Scholar]
- Small, J.V., J.E. Celis, and Isenberg, G. 1980. Aspects of cell architecture and locomotion. In Transfer of Cell Constituents into Eukaryotic Cells. J.E. Celis, A. Graessmann, and A. Loyter, editors. Plenum Press, N.Y. 75–111.
- Small, J.V., G. Rinnerthaler, and H. Hinssen. 1982. Organization of actin meshworks in cultured cells: the leading edge. Cold Spring Harb. Symp. Quant. Biol. 46:599–611. [DOI] [PubMed] [Google Scholar]
- Small, J.V., A. Rohlfs, and M. Herzog. 1993. Actin and cell movement. In Cell Behaviour: Adhesion and Motility. G.W. Jones, C. Wigley, and R. Warn, editors. The Company of Biologists Ltd, Bath, England, UK. 57–71.
- Small, J.V., M. Herzog, and K. Anderson. 1995. Actin filament organization in the fish keratocyte lamellipodium. J. Cell Biol. 129:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, J.V., K. Rottner, I. Kaverina, and K.I. Anderson. 1998. Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta. 1404:271–281. [DOI] [PubMed] [Google Scholar]
- Small, J.V., T. Stradal, E. Vignal, and K. Rottner. 2002. The lamellipodium: where motility begins. Trends Cell Biol. 12:112–120. [DOI] [PubMed] [Google Scholar]
- Steffen, A., J. Faix, G.P. Resch, J. Linkner, J. Wehland, J.V. Small, K. Rottner, and T.E. Stradal. 2006. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol. Biol. Cell. 17:2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T.M., E.A. Bulanova, O.Y. Chaga, D.M. Vignjevic, S. Kojima, J.M. Vasiliev, and G.G. Borisy. 2003. Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160:409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L.G., P.S. Connelly, and G.M. Guild. 2004. Microvilli appear to represent the first step in actin bundle formation in Drosophila bristles. J. Cell Sci. 117:3531–3538. [DOI] [PubMed] [Google Scholar]
- Turnacioglu, K.K., J.W. Sanger, and J.M. Sanger. 1998. Sites of monomeric actin incorporation in living PtK2 and REF-52 cells. Cell Motil. Cytoskeleton. 40:59–70. [DOI] [PubMed] [Google Scholar]
- Verkhovsky, A.B., T.M. Svitkina, and G.G. Borisy. 1995. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 131:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic, D., S. Kojima, Y. Aratyn, O. Danciu, T. Svitkina, and G.G. Borisy. 2006. Role of fascin in filopodial protrusion. J. Cell Biol. 174:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.L. 1984. Reorganization of actin filament bundles in living fibroblasts. J. Cell Biol. 99:1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.L. 1985. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J. Cell Biol. 101:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., T. Kato, A. Fujita, T. Ishizaki, and S. Narumiya. 1999. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1:136–143. [DOI] [PubMed] [Google Scholar]
- Wessels, N.K., B.S. Spooner, and M.A. Luduena. 1973. Surface movements, microfilaments and cell locomotion. In Locomotion of tissue cells. Ciba Foundation Symposium. Vol. 14. Elsevier, Amsterdam. 53–82. [DOI] [PubMed]
- Wood, W., and P. Martin. 2002. Structures in focus–filopodia. Int. J. Biochem. Cell Biol. 34:726–730. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar, R., C. Ballestrem, Z. Kam, and B. Geiger. 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116:4605–4613. [DOI] [PubMed] [Google Scholar]
- Zicha, D., I.M. Dobbie, M.R. Holt, J. Monypenny, D.Y. Soong, C. Gray, and G.A. Dunn. 2003. Rapid actin transport during cell protrusion. Science. 300:142–145. [DOI] [PubMed] [Google Scholar]
- Zimerman, B., T. Volberg, and B. Geiger. 2004. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil. Cytoskeleton. 58:143–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]