Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis (original) (raw)
Abstract
We set out to determine the effects of pharmacist-led medication review in older people by means of a systematic review and meta-analysis covering 11 electronic databases. Randomized controlled trials in any setting, concerning older people (mean age > 60 years), were considered, aimed at optimizing drug regimens and improving patient outcomes. Our primary outcome was emergency hospital admission (all cause). Secondary outcomes were mortality and numbers of drugs prescribed. We also recorded data on drug knowledge, adherence and adverse drug reactions. We retrieved 32 studies which fitted the inclusion criteria. Meta-analysis of 17 trials revealed no significant effect on all-cause admission, relative risk (RR) of 0.99 [95% confidence interval (CI) 0.87, 1.14, P = 0.92], with moderate heterogeneity (I2 = 49.5, P = 0.01). Meta-analysis of mortality data from 22 trials found no significant benefit, with a RR of mortality of 0.96 (95% CI 0.82, 1.13, P = 0.62), with no heterogeneity (I2 = 0%). Pharmacist-led medication review may slightly decrease numbers of drugs prescribed (weighted mean difference = −0.48, 95% CI −0.89, −0.07), but significant heterogeneity was found (I2 = 85.9%, P < 0.001). Results for additional outcomes could not be pooled, but suggested that interventions could improve knowledge and adherence. Pharmacist-led medication review interventions do not have any effect on reducing mortality or hospital admission in older people, and can not be assumed to provide substantial clinical benefit. Such interventions may improve drug knowledge and adherence, but there are insufficient data to know whether quality of life is improved.
Keywords: medication review, meta-analysis, pharmacist, systematic review
Introduction
Older people are often affected by multiple ailments, and it is no surprise that they end up taking numerous medications. The complexity and toxicity of some drug regimens means that care must be taken to promote adherence, minimize harm and overcome problems with storage and stock-piling. Therefore, medication review by a pharmacist may have important benefits for older people.
Medication review is a structured evaluation of a patient's medicines, aimed at reaching agreement with the patient about drug therapy, optimizing the impact of medicines, and minimizing the number of medication-related problems. However, medication review is a relatively new intervention and we do not know whether such reviews have a definite impact on important outcomes such as reducing hospital admissions and mortality. Much of the research has so far tended to focus on prescribing outcomes rather than hospital admissions [1], but an Australian study of home-based medication review has demonstrated a reduction in hospital admissions of 25%, and also a reduction in out-of-hospital deaths [2]. A comprehensive overview of related research would help to determine if these findings were reproducible, and generalizable to other settings.
However, existing literature reviews in this area have concentrated on pharmacists' roles, rather than investigating key questions as to whether these new interventions actually have any worthwhile impact on important patient outcomes [3]. One Cochrane review of outpatient pharmacist roles, which reviewed trials up to March 1999, had great difficulty drawing conclusions due to the limited quality of the research available at that time [4]. More recently, a systematic review of clinical pharmacists and inpatient medical care concluded that there was generally improved care and no evidence of harm, but the authors did not attempt to pool the results statistically [5]. Another meta-analysis looked at medication review in the primary care setting and identified only weak evidence for an effect on admissions [6]. We therefore aimed systematically to evaluate and quantify the effects of medication review by pharmacists on substantive clinical outcomes (namely, hospital admissions and mortality) for older people across all care settings. We also aimed to evaluate, as secondary outcomes, the effects of medication review on qualitative outcomes such as quality of life and patient satisfaction.
Methods
Searching
Our search strategy identified research on medication review interventions involving pharmacists. Interventions were identified using a broad range of search terms and Medical Subject Headings (MeSH), including: medicine/medication review, drug review, medicine management, drug adherence/compliance/concordance, and pharmaceutical care planning. We developed our search with reference to the indexing of previously identified studies (full search available from the authors). The following databases were searched from their inception to 1 September 2005: MEDLINE, EMBASE, Cumulative index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine (AMED), Cochrane Controlled Trials Register, Web of Science (including a citation search of key papers), Pharmline, International Pharmaceutical Abstracts, the Royal Pharmaceutical Society's Electronic Pharmacy Information Coverage (EPIC) database, Cochrane Database of Systematic Reviews, and Database of Abstracts of Reviews of Effects (DARE). Reference lists of included articles and relevant review articles were searched.
Study selection
All titles retrieved by the literature search were reviewed by one of six investigators. Titles needed to appear potentially relevant to the study area. Pairs of investigators assessed abstracts independently against five criteria: (i) intervention involved pharmacist-led review of patient's medication; (ii) intervention delivered primarily by a pharmacist; (iii) randomized controlled trial; (iv) mainly included older people (mean age of subjects > 60 years); and (v) encompassed patients with a range of diseases (more than one diagnostic category). Studies could be carried out in any setting (hospital, clinic, etc.), but needed to have a minimum follow-up period of 1 month and be reported in English. Full papers from potential studies were assessed independently by the two investigators for their suitability for inclusion. Differences were resolved by discussion with reference to a third reviewer if necessary.
Validity assessment
Many quality scales have been created to judge trials. Juni has recommended assessing trial quality by assessing three key components: concealment of allocation, use of intention-to-treat (ITT) analysis, and blinding of outcome assessment [7]. As blinding of outcome assessors is not particularly relevant to the end-points of hospital admissions or deaths, we therefore assessed whether studies confirmed outcome data by using at least two sources (e.g. hospital data and self-report). In addition, trial quality was assessed against criteria recommended by the York Centre for Reviews Dissemination [8]: explicit statement of inclusion criteria; baseline comparability between groups; a clearly defined primary outcome; and sample size calculation reported. The review team also considered the following criteria important in reference to this study area: length of follow-up (where = 6 months was considered adequate), >80% of patients retained in the trial, and reporting the training or selection of pharmacists. This gave a total of 10 quality criteria against which studies were assessed.
Data abstraction
We extracted data on a previously piloted data extraction form. Second reviewers checked extracted data. Data included type of participants, intervention details, outcomes and trial quality characteristics.
Study characteristics
Classification of interventions
Interventions had to be principally delivered by a pharmacist. Interventions were excluded which were delivered by combinations of health professionals (e.g. physician, nurses) where the pharmacist was only partly involved. Interventions needed to be targeted at patients and not health professionals, but could involve reviewing a patient's records. All forms of medication review for checking and optimizing the patients' drug regimens (i.e. ability to make recommendations on altering the regimen) were considered, provided that the interventions were not limited simply to increasing patients' knowledge and/or adherence. Studies were categorized by: the predominant setting of review; type of pharmacist involved; number of pharmacists involved; access to patient medical records; intervention components; contact between pharmacist and prescriber; ability of pharmacist to enact recommendations; and the extent of contact of the pharmacist with the patient (based on number of review opportunities).
Outcomes
The primary outcome was proportion of patients with one or more hospital emergency admission (all-cause). Secondary outcomes were all-cause mortality and mean drugs prescribed. Outcome data were extracted at the study's prespecified last follow-up point. Data on quality of life, patient satisfaction, drug-related problems, drug knowledge, adherence, adverse drug reactions, storage problems, removal of unnecessary drugs and cost data were also extracted. The latter included extracting the form of economic evaluation undertaken (e.g. cost-effectiveness analysis). Formal pooling was not possible for these additional outcomes due to the diversity of scales used. Nevertheless, informal pooling of results was undertaken in terms of number of studies showing a significant positive effect, a nonsignificant positive effect, no effect or a negative effect.
Quantitative data synthesis
We included all trials reporting appropriate data in the meta-analyses if interventions were compared with usual care. Authors of trials were contacted to provide additional data where needed if published after 2000. It was assumed that additional result data were unlikely to be available from articles published prior to this cut-off. For data on ‘proportion admitted’ and ‘mortality’ the effect of the intervention was reported as a relative risk. For ‘mean drugs’ a weighted mean difference was calculated. Meta-analyses were carried out using random effects methods using RevMan version 4.2.8. Funnel plots were used to assess possible publication bias.
The robustness of the findings in relation to admission and mortality were investigated in the following sensitivity analyses. First, we investigated reasons for any heterogeneity found and repeated analyses using fixed effects methods. We then explored the effect of excluding poorer quality studies. This was investigated in two ways: (i) comparing those demonstrating, or not, components recommended by Juni [7]; and (ii) removing those achieving ≤50% vs. those achieving >50% of the 10 criteria above. Finally, the effect of hospital or specialist pharmacists was compared against community pharmacists; and the effect of the studies with higher levels of patient contact (defined as three or more medication review opportunities) compared against less intense interventions.
Results
Search results and study characteristics
A total of 17 272 titles were identified, yielding 889 potentially relevant studies, of which 32 fitted the inclusion criteria (Figure 1). The first identified trial was published in 1990, but few trials were published in the following decade. Since 2000 numbers have increased rapidly, with almost two-thirds (n = 22) of identified trials published since then. The majority of trials were conducted in either the UK (n = 13, 41%) or USA (n = 10, 31%); four were conducted in Australia, three in Canada, one across several European countries and one in Singapore. The mean age of subjects in the studies varied between 61 and 85 years (average across trials was 71 years), with the proportion of male subjects varying from 20% to 99% (the latter recruited from a Veterans hospital [9]). Only one study limited inclusion to specific diagnoses (either chronic obstructive pulmonary disease or hypertension) [10].
Figure 1.
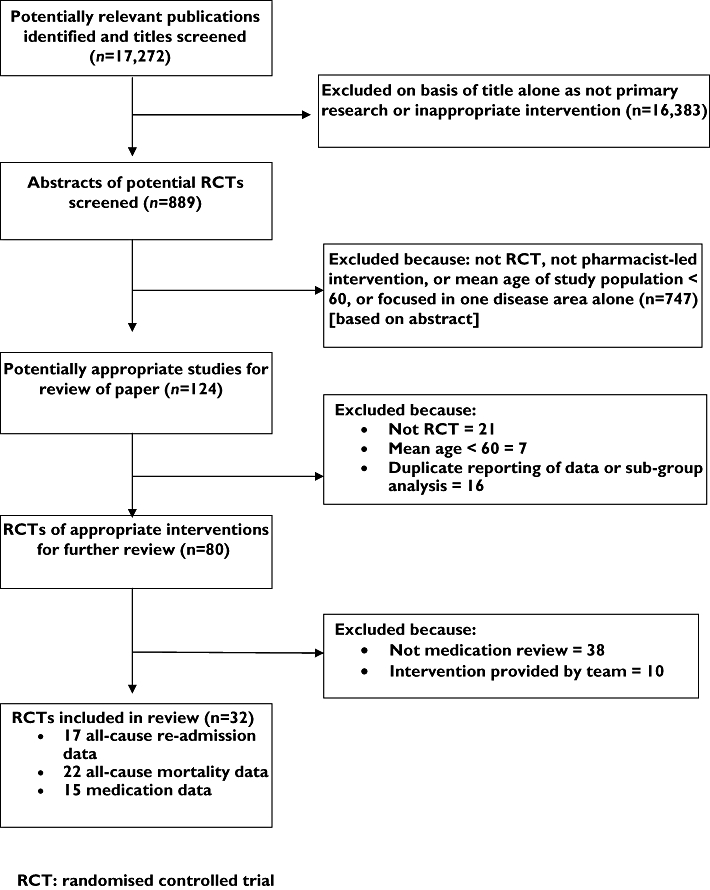
Flowchart describing study selection and excluded studies
Trial quality
For the three key quality components, only 18 (56%) clearly described a form of concealed allocation, 15 (47%) definitely or probably used an ITT analysis and 12 (38%) used some form of data checking. In total, five studies (16%) satisfied all three key quality components together [9, 11–14], three of which were published since 2001. When trials were considered against all 10 quality criteria, the majority (17/32) met at least six. Quality issues often lacking were reporting a sample size calculation and defining a primary outcome.
Interventions(Table 1)
Table 1.
Description of studies and interventions
| Study author | Date | Country | No. of patients | Mean age, years | % male | Type of pharmacist | No. of pharmacists | Intervention | Patient data | Ability to enact advice | Contact with prescriber | Setting | Extent of patient contact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Begley [1] | 1997 | UK | 222 | 82 | 39.4 | Research pharmacist | Unclear | Home visits and counselling by a pharmacist after hospital discharge | Discharge letter | Unable to enact | Unclear | Own home | Four detailed visits over a year |
| Bernsten [18] | 2001 | Europe | 2454 | 74 | 42.4 | Community pharmacist | 104 | Community pharmacy assessment of drug-related problems and implementation of a pharmaceutical care plan | Repeat prescribing data | Unable to enact | Unclear | Pharmacy | Unclear |
| Bolas [34] | 2004 | Northern Ireland | 243 | 74 | 39.5 | Hospital/ clinical pharmacist | 1 | Full history, preparation of discharge letter. Medication review (stated in abstract but not method). | Full notes | Unable to enact | Close contact | Hospital | Inpatient ward visit plus discharge plan |
| Bond [17] | 2000 | UK | 3074 | 66 | 41.6 | Community pharmacist | 62 | Pharmacist-controlled repeat prescription system where pharmacist checked if medication needed. Review of side-effects and interactions | Repeat prescribing data | Unable to enact | Contact by letter | Pharmacy | Limited contact, mainly review of repeat scripts |
| Carter [35, 36] | 1998 | USA | 1054 | 66.7 | 96.3 | Hospital/ clinical pharmacist | >4 | Medication assessment and adherence, change of nonformulary to formulary drugs, and education | Full notes | Partly enact | Close contact | Primary care or clinic | Detailed enquiry, mean 3.5 visits over a year |
| Furniss [37] | 2000 | UK | 330 | 81.2 | 27 | Research pharmacist | 1 | Medication review with patient | Drug chart in nursing home | Unable to enact | Unclear | Nursing home | Detailed review, with second brief visit at 8 months |
| Gourley [10] | 1998 | USA | 231 | 68.05 | 97.8 | Hospital/ clinical pharmacist | 45 | Pharmacists involvement in healthcare team in the management of patient's drug therapy | Full notes | Partly enact | Unclear | Hospital | Clinical, review, at least 5 visits over 6 months |
| Graffen [38] | 2004 | Australia | 402 | 77.7 | 38.8 | Research pharmacist | 1 | Clinic-based medication review | Full notes | Unable to enact | Close contact | Primary care or clinic | One visit with brief enquiry |
| Granas [14] | 1999 | UK | 500 | 65 | 38 | Community pharmacist | Probably 1 | Community pharmacist identified a drug-related problem and this was then discussed with pt's GP | Full notes | Unable to enact | Close contact | Primary care or clinic | Review of repeat prescription only |
| Grymonpre [39] | 2001 | USA | 135 | 77 | 20.74 | Hospital/ clinical pharmacist | 1 | Home medication history taken by ‘lay person’ and reviewed by a pharmacy consultant | Lay person report | Unable to enact | Contact by letter | Primary care or clinic | Single visit over a year |
| Hanlon [9] | 1996 | USA | 208 | 69.8 | 99 | Hospital/ clinical pharmacist | 1 | Monitored drug therapy, patient outcomes, medication use & drug-related problems | Full notes | Unable to enact | Close contact | Primary care or clinic | At least two visits and option for multiple visits over a year |
| Holland [13] | 2005 | UK | 872 | 85.4 | 37.6 | Mixture | 22 | 2 home visits to check adherence, ability to self-medicate, evaluate patients and carer, remove out of date drugs, report ADRs to GP, and need for interventions such as compliance aid | Discharge letter | Unable to enact | Contact by letter | Own home | Two visits over 6 months |
| Jameson [15] | 1995 | USA | 64 | 60.5 | 20 | Specialist pharmacist | Probably 1 | Pharmacist performed a chart review and medication history then interviewed pt on current regimen and side-effects. A new regimen was developed by pharmacist and physician, which pharmacist then explained to the pt with additional info on drug therapy | Full notes | Fully enact | Close contact | Primary care or clinic | Two visits over 6 months |
| Kassam [25, 40] | 2001 | Canada | 363 | 73.5 | 33.1 | Community pharmacist | >4 | Interview and follow-up to identify drug-related problems | Repeat prescribing data | Unable to enact | Unclear | Pharmacy | At least two visits over a year |
| Krska [41] | 2001 | UK | 381 | 75.1 | 39.5 | Hospital/ clinical pharmacist | 1–5 | Detailed profile of pt formed from med notes by pharmacist who then interviewed pt in own home about use of and response to meds and use of health and soc. services. Care plan drawn up, given to GP; pharmacist agreed actions assisted by practice staff | Full notes | Partly enact | Contact by letter | Own home | One visit over 3 months |
| Lenaghan [24] | 2004 | UK | 136 | 84.3 | 34.3 | Community pharmacist | 1 | Home based medication review | Full notes | Partly enact | Close contact | Own home | Two visits over 6 months |
| Lim [16] | 2004 | Singapore | 136 | 80 | 35 | Hospital/ clinical pharmacist | Probably 1 | Each patient was evaluated with the aim of simplifying regimen, improving effectiveness and decreasing ADRs. Recommendation discussed with doctor and those accepted were implemented | Full notes | Fully enact | Unclear | Hospital | One visit plus follow-up at two months |
| Lipton [22, 42] | 1992 | USA | 706 | 74.5 | 49 | Hospital/ clinical pharmacist | 2 | Clinical pharmacist reviews of hospital records and drug regimens of participants and consultation with these patients and their physicians | Full notes | Unclear | Close contact | Hospital | Five visits in 3 months |
| Lowe [43] | 2000 | UK | 181 | 76.25 | 33 | Hospital/ clinical pharmacist | 1 | Assessment and rationalizing of medication, med education and knowledge and compliance assessed at the end of the study period | Full notes | Partly enact | Close contact | Primary care or clinic | Three visits in 7 weeks |
| Mackie [44] | 2001 | UK | 3025 | 67 | 36.6 | Research pharmacist | 4 | Problems identified during patient interview in GP/home, discussed with GP and enacted. | Full notes | Partly enact | Contact by letter | Primary care or clinic | One visit over 40 weeks |
| McMullin [45] | 1999 | USA | 259 | 61 | 30 | Specialist pharmacist | 6 | Review to identify cost saving interventions e.g. removing unnecessary drugs, modifying route of administration, or use of cheaper agents | Full notes | Unable to enact | Contact via telephone | Hospital | Ward review only |
| Naunton [19] | 2003 | Australia | 136 | 75.5 | 37.5 | Research pharmacist | 1 | Medication review and counselling by home visit | Full notes | Partly enact | Contact via telephone | Own home | One visit over 3 months |
| Nazareth [12] | 2001 | UK | 362 | 84 | 36 | Community pharmacist | 34 | Hospital interview with initial medication assessment and rationalization, check ability to self-medicate, provide advice. Then patient visit by a pharmacist to check discrepancies, understanding, adherence | Full notes | Partly enact | Contact by letter | Hospital | Two visits over 6 months |
| Sellors [46] | 2001 | Canada | 132 | 76 | 35 | Community pharmacist | 1 | Clinic based medication review, then telephone interviews | Full notes | Unable to enact | Close contact | Primary care or clinic | One visit and several telephone calls over 6 months |
| Sellors [11] | 2003 | Canada | 889 | 74 | 47 | Community pharmacist | 24 | Clinic based medication review and telephone interview and recommendation to physician | Full notes | Unable to enact | Close contact | Primary care or clinic | One visit plus 1–2 telephone calls over 5 months |
| Sidel [47] | 1990 | USA | 284 | 65 | 33 | Unclear | Unclear | Explanations and contact physician | Survey by nonmedical staff | Unable to enact | Contact via telephone | Own home | Two home visits over 6 months |
| Smith [48] | 1997 | UK | 68 | 77.5 | Not stated | Hospital/ clinical pharmacist | 1–5 | Medication review in hospital with discharge care plan and access to telephone helpline | Full notes | Unable to enact | Contact by letter | Hospital | One pre- discharge visit, 1 week follow-up |
| Sorensen [27] | 2004 | Australia | 400 | 71.8 | 36.3 | Community pharmacist | 32 | Home visit and medication review by pharmacist and team conferences with GP and implementation at next patient/GP visit | Home visit and GP's report | Unable to enact | Close contact | Own home | One visit over 6 months |
| Stowasser [49] | 2002 | Australia | 240 | 66 | 55 | Hospital/ clinical pharmacist | Unclear | Medication review plus special discharge summary | Full notes | Unable to enact | Close contact | Hospital | One hospital visit, plus follow-up at 30 days |
| Taylor [20] | 2003 | USA | 81 | 65.6 | 31.9 | Community pharmacist | 4 | Rural education and drug information programme | Full notes | Unable to enact | Close contact | Primary care or clinic | Brief enquiry, number of visits not described |
| Williams [23] | 2004 | USA | 140 | 73 | 43 | Specialist pharmacist | 1 | Medication review | Full notes | Partly enact | Close contact | Primary care or clinic | One detailed review, with 6 weeks' follow-up |
| Zermansky [50] | 2001 | UK | 1188 | 73.5 | 44 | Specialist pharmacist | 1 | Record current drugs, medical problems, perform patient interview, confirms drug still needed, ADRs, consider costs, assess adherence & feedback to GP + assessing implementing agreed changes | Full notes | Partly enact | Close contact | Primary care or clinic | One review over 12 months |
The majority of interventions were delivered in either hospital (n = 8, 25%) or a clinic/primary care setting (n = 13, 41%). Three were delivered in a community pharmacy, seven in the patient's own home and one in a nursing home. Pharmacists were described as hospital or clinical pharmacists in a third of trials (n = 11), community pharmacists in a third of trials (n = 10), specialist or research pharmacists in nine trials, whereas one trial used a mixture. Sixteen trials (50%) used a single pharmacist to deliver their intervention, thus limiting generalizability.
In 23 trials (72%) intervention pharmacists had access to patient medical notes (either hospital or primary care records), whereas in three trials pharmacists had some form of detailed referral information. Information in the remaining trials was limited to either a discharge letter (two trials), repeat prescribing data (three trials), or patient self-report. Pharmacists delivered medication counselling, advice on adherence, checked drug benefit and adverse events, and aimed to optimize medication in >60% of the trials. Contact with the physician was considered close (i.e. face-to-face) in over half of trials (n = 17), telephone contact was used in four trials, and mail in seven trials (not described in four trials). Pharmacists were generally unable (n = 19, 59%) or only partly able to enact their own recommendations (n = 10, 36%). Only in two trials (6%) were pharmacists considered to be able to enact fully their recommendations [15, 16]. Overall, we found that the pharmacists generally had one or two review visits with the patients, but that there were seven trials where patients could be reviewed on three or more occasions (usually in person, but sometimes through regular telephone calls).
Effect on all-cause admission (Figure 2)
Figure 2.
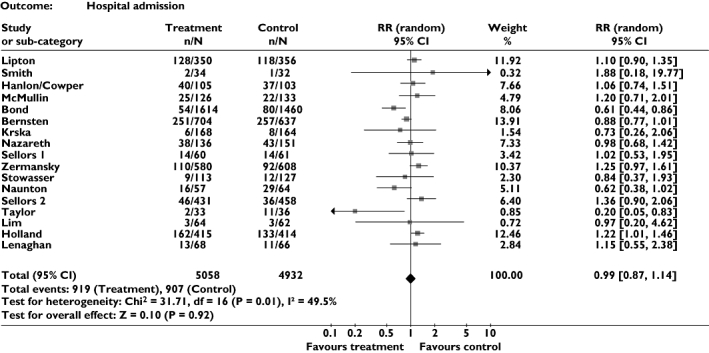
Meta-analysis showing relative risk for all-cause admission
Seventeen trials, including a total of >9900 patients, provided data on this outcome. Meta-analysis suggested that pharmacist-led medication review has no effect on all-cause admission, relative risk (RR) of 0.99 [95% confidence interval (CI) 0.87, 1.14, P = 0.91]. However, overall results were conflicting, with four trials providing significant or borderline significant results in favour of the intervention [17–20], whereas others provided evidence favouring the control [13, 21]. This variation in findings can be seen as moderate heterogeneity on statistical testing (P = 0.01, I2 = 49.5).
Effect on all-cause mortality (Figure 3)
Figure 3.
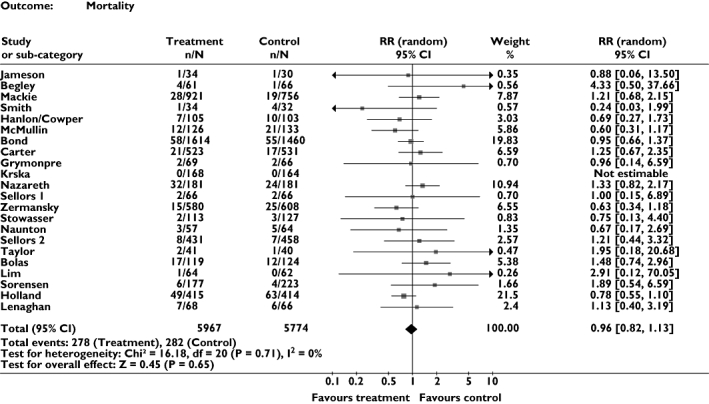
Meta-analysis showing relative risk for all-cause mortality
Twenty-two trials, which included a total of >11 700 patients, provided data on mortality. Meta-analysis suggested that pharmacist-led medication review has no effect on mortality (RR = 0.96, 95% CI 0.82, 1.13, P = 0.65). No heterogeneity was detected between trials (I2 = 0%).
Effect on prescribing (Figure 4)
Figure 4.
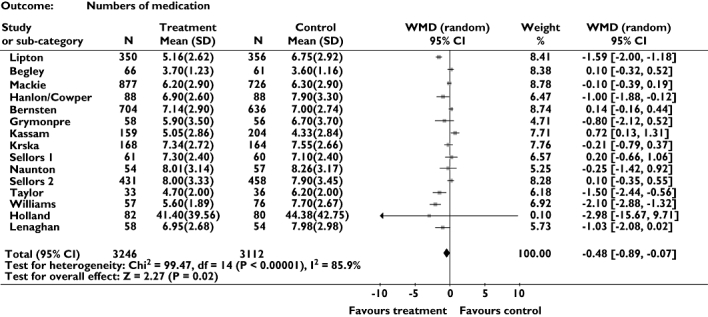
Meta-analysis showing weighted mean difference for number of drugs prescribed
Fifteen trials provided data on prescribing. Meta-analysis suggested that these interventions may reduce numbers of drugs prescribed (weighted mean difference = −0.48, 95% CI −0.89, −0.07). However, as with admissions, heterogeneity was identified, although in this case it was very marked (P < 0.001, I2 = 85.9%) with five positive trials [9, 20, 22–24] and one negative trial [25].
Effect on additional outcomes (Table 2)
Table 2.
Qualitative pooling of secondary outcomes
| No. of trials reporting outcome compared with control | No. reporting a significant positive effect (%) | No. reporting a nonsignificant positive effect (%) | No. reporting no effect (%) | No. reporting either a nonsignificant or a significant negative effect (%) | |
|---|---|---|---|---|---|
| Quality of life | 12 | 0 | 4 (33) | 8 (66) | 0 |
| Patient satisfaction | 4 | 2 (50) | 1 (25) | 0 | 1 (25) |
| Drug-related problems | 4 | 4 (100) | 0 | 0 | 0 |
| Knowledge | 11 | 6 (55) | 2 (18) | 3 (27) | 0 |
| Adherence | 14 | 7 (50) | 4 (29) | 3 (21) | 0 |
| Adverse drug reactions | 9 | 1 (11) | 3 (33) | 3 (33) | 2 (22) |
| Storage problems | 3 | 2 (66) | 0 | 1 (33) | 0 |
| Unnecessary drugs | 7 | 5 (71) | 2 (29) | 0 | 0 |
| Cost analysis* | 14 | 4 (29) | 6 (43) | 2 (14) | 2 (14) |
Interventions appeared to have a positive effect on intermediate outcomes such as numbers of drug-related problems, knowledge, adherence, improving storage and reducing unnecessary drugs. Equally, both patient satisfaction and adverse drug reactions generally, but not always, were improved by these interventions. However, despite these positive effects, only one-third of those trials that measured quality of life found a benefit and these were not statistically significant. Finally, 14 trials collected data on cost, of which only three performed formal economic evaluations [13, 26, 27], the remainder being simple cost analyses focusing on prescribing costs. The cost analyses appeared promising, with nine of the 11 studies yielding a positive or nonsignificantly positive outcome. The cost effectiveness analyses used differing comparators: cost per gain in medication appropriateness index [26], and cost per reduction in adverse drug effects [27]. Only one study calculated an incremental cost per quality adjusted life year (QALY) and this found it to be very high [28].
Sensitivity analyses
Table 3 describes the various sensitivity analyses conducted on the primary outcome (all-cause admissions). The overall effect was very similar across all sensitivity analyses, with the exception that those studies which appeared to be of lower research quality appeared to provide more effective interventions than higher quality studies, although the CIs overlapped. Table 3 also describes the same sensitivity analyses conducted on mortality data. Again, the overall effect appeared reasonably similar across all sensitivity analyses, with the exception that those studies that did not appear to use concealed allocation for randomization appeared to have a more favourable effect on mortality (RR = 0.65, 95% CI 0.43, 0.97, P = 0.03). Finally, there was no evidence of publication bias evident from inspection of funnel plots (available from the authors).
Table 3.
Sensitivity analysis on primary outcome (admission) and mortality
| Admission No. of patients (no. of trials) | Relative risk (95% CI) | _P_-value for overall effect | I2-value for heterogeneity (%) | Mortality No. of patients (no. of trials) | Relative risk (95% CI) | _P_-value overall effect | I2-value for heterogeneity (%) | |
|---|---|---|---|---|---|---|---|---|
| Random vs. fixed effects | ||||||||
| Random effects | 9990 (17) | 0.99 (0.87, 1.14) | 0.92 | 49.5 | 11 741 (21) | 0.96 (0.82, 1.13) | 0.65 | 0 |
| Fixed effects | 9990 (17) | 1.00 (0.92, 1.08) | 0.95 | 49.5 | 11 741 (21) | 0.97 (0.82, 1.13) | 0.66 | 0 |
| Removing outliers | ||||||||
| Base case | 9990 (17) | 0.99 (0.87, 1.14) | 0.92 | 49.5 | – | – | – | – |
| Removing extreme trials* | 5831 (12) | 1.02 (0.93, 1.11) | 0.74 | 0 | NA | – | – | – |
| Quality component | ||||||||
| 1. Concealed allocation | 5542 (7) | 1.02 (0.82, 1.27) | 0.84 | 57.5 | 9 264 (13) | 1.04 (0.87, 1.24) | 0.66 | 0 |
| No clear concealment | 4448 (10) | 0.97 (0.87, 1.14) | 0.92 | 45.5 | 2 477 (8) | 0.65 (0.43, 0.97) | 0.03 | 0 |
| 2. Intention to treat (ITT) | 7326 (10) | 1.06 (0.90, 1.26) | 0.47 | 43.9 | 9 721 (12) | 0.95 (0.80, 1.13) | 0.57 | 2.4 |
| Unclear if ITT used | 2664 (7) | 0.89 (0.72, 1.10) | 0.29 | 44.6 | 2 020 (9) | 1.08 (0.68, 1.72) | 0.74 | 0 |
| 3. Data cross-checked | 2877 (9) | 1.09 (0.95, 1.26) | 0.21 | 4.3 | 2 973 (9) | 0.90 (0.70, 1.16) | 0.42 | 0 |
| No clear cross-check | 7103 (8) | 0.94 (0.76, 1.17) | 0.59 | 65.5 | 8 768 (12) | 1.01 (0.82, 1.25) | 0.93 | 0 |
| Overall quality score | ||||||||
| High score (> 5 out of 10) | 7894 (11) | 1.07 (0.93, 1.23) | 0.37 | 37.4 | 10 540 (13) | 0.97 (0.82, 1.16) | 0.76 | 0 |
| Low score (≤ 5 out of 10) | 2096 (6) | 0.83 (0.62, 1.11) | 0.20 | 36.5 | 1201 (8) | 0.96 (0.59, 1.43) | 0.70 | 6.5 |
| Extent of patient contact | ||||||||
| Three or more opportunities | 1035 (3) | 1.09 (0.92, 1.29) | 0.33 | 0 | 1521 (4) | 1.11 (0.68, 1.82) | 0.67 | 0 |
| Less than three opportunities | 8834 (13) | 1.00 (0.84, 1.19) | 0.77 | 46 | 10 220 (18) | 0.95 (0.80, 1.12) | 0.53 | 0 |
| Type of pharmacist | ||||||||
| Hospital/clinical | 3246 (9) | 1.08 (0.95, 1.23) | 0.23 | 0 | 5840 (13) | 0.94 (0.73, 1.21) | 0.61 | 0 |
| Community/other | 6744 (8) | 0.95 (0.76, 1.19) | 0.66 | 68.5 | 5901 (8) | 0.98 (0.80, 1.21) | 0.65 | 0 |
Discussion
Main study findings
This systematic review has identified a large and rapidly growing body of trial evidence investigating the effectiveness of pharmacist-led medication review. This evidence is of increasingly high quality, suggesting that researchers in this area have responded to earlier criticism [4, 29]. However, our review has not identified any clear effect on either hospital admission or mortality. Importantly, this lack of effect did not seem to be related to type of pharmacist performing the review, or the intensity of their review.
Our review has identified that these forms of intervention may be able to reduce numbers of prescribed drugs slightly, and would appear to have positive effects on a wide range of intermediate outcomes, such as drug knowledge, adherence and drug storage. Despite this, few studies identified any important effect on quality of life, and those that suggested positive effects were not statistically significant.
Strengths and weaknesses of the study
This study searched all key databases relevant to this study area. The database search was supplemented by checking study references, review articles and conducting a citation search of identified studies. In addition, authors of included studies were contacted where additional data were required. Finally, conducting this review across a team of researchers allowed data extraction to be checked and decisions agreed. We acknowledge that there may be unpublished data that we have not been able to retrieve, but given the number of studies already in our meta-analysis, the results are likely to be significantly changed only if the unpublished studies consisted of several large-scale randomized trials. Although nonrandomized studies may provide additional information, we excluded such studies due to their susceptibility to bias.
The main focus of this study was to quantify the direct impact of medication review on hard outcomes such as hospital admissions and death. As such, we did not evaluate interventions which were designed solely to improve knowledge or medication adherence. It is possible that interventions of this nature may have substantial qualitative effects on older patients, and that such outcomes may be better evaluated using descriptive methods, rather than the statistical techniques used here.
The review was to some extent limited by reporting of the primary outcome. In total, only 17 of the 32 studies provided data on this outcome. Some authors reported total admissions (as opposed to proportion admitted or not), which could not be entered into the meta-analysis, or reported that there was no difference observed but omitted numerical data in their paper. Efforts to contact these authors added four additional data points. As a result, data on the primary outcome were provided for almost 10 000 patients.
The meta-analysis of our primary outcome (all-cause admission) demonstrated moderate heterogeneity, suggesting that some differences existed between the studies included in that analysis. Nevertheless, it should be stressed that our sensitivity analysis (Table 3) did not identify changes to our main finding of no effect, with the exception that poorer quality studies seemed to yield greater effect than higher quality studies, which is not an unusual finding. Only a small proportion of studies fulfilled all the quality criteria, thus indicating that some of the studies may have been susceptible to bias.
We have also attempted to evaluate other patient-related measures such as medication-related problems, adherence and quality of life, given that global outcomes such as hospital admissions may not relate specifically to the interventions delivered, or particular patient's needs. However, these other outcomes are not consistently reported in the trials (Table 2), thus limiting our ability to draw any robust conclusions on the effectiveness of the intervention on such outcomes.
Heterogeneity in the results for all-cause admission may also have arisen from factors that are difficult to quantify in a precise way. The baseline risk for medication problems of the patients may have differed amongst the trials, as may have the care that the patients' received from other sources or teams within that particular healthcare system. Differences in the delivery of the pharmacist-led medication review and care settings of the trials may have masked findings of beneficial effects in certain situations. However, it is also important to note that there was no heterogeneity in the mortality measure.
It is somewhat surprising that the number of medication review opportunities with the patient did not appear to relate closely to the effectiveness of the intervention. However, this may simply reflect that there are multiple other complex factors at play here, which prevent this analysis from identifying the key ingredients of a ‘successful’ intervention. It should be noted that few studies appeared to be particularly intensive – thus, the CIs on the estimates were broad. Equally, authors did not always provide full details of their interventions, thus making it difficult for us to distinguish successfully between more intense and less intense interventions.
Prescribing data were also infrequently reported by studies, despite drugs being the focus of all interventions. Only a third of studies could be included in the meta-analysis of these data, and, to a greater extent than for admissions, trial results appeared very heterogeneous.
Findings in comparison with other studies
The findings from this review agree with the pooled result of randomized trials included in the earlier review by Royal in primary care [6]. However, other pharmacist interventions with similar components have been effective particularly when pharmacists form part of a team [2, 30, 31]. It is interesting to note that no trial evidence currently exists on the benefit, or otherwise, of general practitioners reviewing older patients' medication, despite extensive guidance recommending its provision [32] and it is now part of their current contract in the UK. Equally, more focused patient review interventions delivered by specialist personnel have been found to be highly effective in conditions such as heart failure [33].
Conclusion
This review emphasizes that pharmacist-led medication review interventions can not be assumed to reduce hospital admissions or mortality rates in older people. These interventions may improve drug knowledge and drug adherence, but insufficient data exist to know whether the latter affects patients' quality of life positively. It should be stressed that no trial has as yet been of a sufficient size to identify a small but important gain in quality of life. The inability to demonstrate consistent benefit may stem from the wide variations in the delivery of care and patient selection in the existing trials, and it may be possible to develop higher quality research into potentially effective interventions. This would ideally involve multicentre, collaborative trials with well-defined medication review components and clearly specified, clinically relevant outcome measures.
Our findings have major implications on the provision of healthcare for older patients. Medication review is a time-consuming process for both the health professional and the patient, but is it worth the time and trouble if there is no clear benefit on hospital admissions and deaths? Delivering a medication review service for an expanding population of older people is also likely to be resource-intensive, Indeed, increasing investment is being made in providing such services (e.g. medication usage reviews in the UK) in spite of the lack of a clear evidence base demonstrating positive effect, and in the face of the potential for increasing health service costs [13]. Although improvements in patient knowledge and adherence are laudable objectives, those who are directly involved in the care of older people may justifiably feel that money is better spent on cost-effective interventions that have a definite effect on reducing hospital admissions and deaths.
Competing interests
Y.K.L. is on the Editorial Board of the British Journal of Clinical Pharmacology, but has had no involvement in the handling and review process of the manuscript while under consideration by the journal. There are no other conflicts of interest to declare.
REFERENCES
- 1.Begley S, Livingstone C, Hodges N, Williamson V. Impact of domiciliary pharmacy visits on medication management in an elderly population. Int J Pharm Pract. 1997;5:111–21. [Google Scholar]
- 2.Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158:1067–72. doi: 10.1001/archinte.158.10.1067. [DOI] [PubMed] [Google Scholar]
- 3.Kennie NR, Schuster BG, Einarson TR. Critical analysis of the pharmaceutical care research literature. Ann Pharmacother. 1998;32:17–26. doi: 10.1177/106002809803200101. [DOI] [PubMed] [Google Scholar]
- 4.Beney J, Bero LA, Bond C. Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes. Cochrane Database Syst Rev. 2000. CD000336. [DOI] [PubMed]
- 5.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166:955–64. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 6.Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care. 2006;15:23–31. doi: 10.1136/qshc.2004.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Davey Smith G, Altman D, editors. Systematic, Reviews in Health Care. Meta-analysis in Context. London: BMJ Books; 2001. pp. 87–106. [Google Scholar]
- 8.Centre for Reviews & Dissemination. Undertaking Systematic Reviews of Research on Effectiveness. York: University of York; 2001. [Google Scholar]
- 9.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, Cowper PA, Landsman PB, Cohen HJ, Feussner JR. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 10.Gourley GA, Portner TS, Gourley DR, Rigolosi EL, Holt JM, Solomon DK, Bass GE, Wicke WR, Braden RL. Humanistic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:586–97. doi: 10.1016/s1086-5802(16)30372-2. [DOI] [PubMed] [Google Scholar]
- 11.Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, Goeree R, Cosby R, Trim K, Sebaldt R, Howard M, Hardcastle L, Poston J. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ. 2003;169:17–22. [PMC free article] [PubMed] [Google Scholar]
- 12.Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. Pharmacy discharge plan for hospitalized elderly patients – a randomized controlled trial. Age Ageing. 2001;30:33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, Christou M, Evans D, Hand C. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330:293. doi: 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granas AG, Bates I. The effect of pharmaceutical review of repeat prescriptions in general practice. Int J Pharm Pract. 1999;7:264–75. [Google Scholar]
- 15.Jameson J, Van Noord G, Vanderwoud K. The impact of pharmacotherapy consultation on the cost and outcome of medical therapy. J Fam Pract. 1995;41:469–72. [PubMed] [Google Scholar]
- 16.Lim WS, Low HN, Chan SP, Chen HN, Ding YY, Tan TL. Impact of a pharmacist consult clinic on a hospital-based geriatric outpatient clinic in Singapore. Ann Acad Med Singapore. 2004;33:220–7. [PubMed] [Google Scholar]
- 17.Bond C, Matheson C, Williams S, Williams P, Donnan P. Repeat prescribing: a role for community pharmacists in controlling and monitoring repeat prescriptions. Br J Gen Pract. 2000;50:271–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Bernsten C, Björkman I, Caramona M, Crealey G, Frøkjaer B, Grundberger E, Gustafsson T, Henman M, Herborg H, Hughes C, McElnay J, Magner M, van Mil F, Schaeffer M, Silva S, Søndergaard B, Sturgess I, Tromp D, Vivero L, Winterstein A. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs Aging. 2001;18:63–77. doi: 10.2165/00002512-200118010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Naunton M, Peterson GM. Evaluation of home-based follow-up of high-risk elderly patients discharged from hospital. J Pharm Prac Res. 2003;33:176–82. [Google Scholar]
- 20.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60:1123–9. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 21.Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ. 2001;323:1340–3. doi: 10.1136/bmj.323.7325.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists' consultations on physicians' geriatric drug prescribing. A randomized controlled trial. Med Care. 1992;30:646–58. doi: 10.1097/00005650-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Williams ME, Pulliam CC, Hunter R, Johnson TM, Owens JE, Kincaid J, Porter C, Koch G. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc. 2004;52:93–8. doi: 10.1111/j.1532-5415.2004.52016.x. [DOI] [PubMed] [Google Scholar]
- 24.Lenaghan E. Randomised Controlled Trial of Home-Based Medication Review by a Community Pharmacist in a High Risk Elderly Population in Primary Care. Norwich: University of East Anglia; 2004. The Polymed Trial. [Google Scholar]
- 25.Volume CI, Farris KB, Kassam R, Cox CE, Cave A. Pharmaceutical care research and education project: patient outcomes. J Am Pharm Assoc (Wash) 2001;41:411–20. doi: 10.1016/s1086-5802(16)31255-4. [DOI] [PubMed] [Google Scholar]
- 26.Cowper PA, Weinberger M, Hanlon JT, Landsman PB, Samsa GP, Uttech KM, Schmader KE, Lewis IK, Cohen HJ, Feussner JR. The cost-effectiveness of a clinical pharmacist intervention among elderly outpatients. Pharmacotherapy. 1998;18:327–32. [PubMed] [Google Scholar]
- 27.Sorensen L, Stokes JA, Purdie DM, Woodward M, Elliott R, Roberts MS. Medication reviews in the community: results of a randomized, controlled effectiveness trial. Br J Clin Pharmacol. 2004;58:648–64. doi: 10.1111/j.1365-2125.2004.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacini M, Wilson E, Smith R, Holland R. Home based medication review in older people: is it cost effective? Pharmacoeconomics. 2007;25:171–80. doi: 10.2165/00019053-200725020-00008. [DOI] [PubMed] [Google Scholar]
- 29.Tully MP, Seston EM. Impact of pharmacists providing a prescription review and monitoring service in ambulatory care or community practice. Ann Pharmacother. 2000;34:1320–31. doi: 10.1345/aph.19374. [DOI] [PubMed] [Google Scholar]
- 30.Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, Francis SD, Branch LG, Lindblad CI, Artz M, Weinberger M, Feussner JR, Cohen HJ. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116:394–401. doi: 10.1016/j.amjmed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Meredith S, Feldman P, Frey D, Giammarco L, Hall K, Arnold K, Brown NJ, Ray WA. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. J Am Geriatr Soc. 2002;50:1484–91. doi: 10.1046/j.1532-5415.2002.50402.x. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health. National Service Framework for Older People. London: Department of Health; 2001. [Google Scholar]
- 33.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci. 2004;26:114–20. doi: 10.1023/b:phar.0000018601.11248.89. [DOI] [PubMed] [Google Scholar]
- 35.Ellis SL, Billups SJ, Malone DC, Carter BL, Covey D, Mason B, Jue S, Carmichael J, Guthrie K, Sintek CD, Dombrowski R, Geraets DR, Amato M. Types of interventions made by clinical pharmacists in the IMPROVE study. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20:429–35. doi: 10.1592/phco.20.5.429.35055. [DOI] [PubMed] [Google Scholar]
- 36.Ellis SL, Carter BL, Malone DC, Billups SJ, Okano GJ, Valuck RJ, Barnette DJ, Sintek CD, Covey D, Mason B, Jue S, Carmichael J, Guthrie K, Dombrowski R, Geraets DR, Amato M. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: the IMPROVE study. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy. 2000;20:1508–16. doi: 10.1592/phco.20.19.1508.34852. [DOI] [PubMed] [Google Scholar]
- 37.Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–7. doi: 10.1192/bjp.176.6.563. [DOI] [PubMed] [Google Scholar]
- 38.Graffen M, Kennedy D, Simpson M. Quality use of medicines in the rural ambulant elderly: a pilot study. Rural Remote Health. 2004;4:1–6. (Online) [PubMed] [Google Scholar]
- 39.Grymonpre RE, Williamson DA, Montgomery PR. Impact of a pharmaceutical care model for non-institutionalised elderly: results of a randomised, controlled trial. Int J Pharm Prac. 2001;9:235–41. [Google Scholar]
- 40.Kassam R, Farris KB, Burback L, Volume CI, Cox CE, Cave A. Pharmaceutical care research and education project: pharmacists' interventions. J Am Pharm Assoc (Wash) 2001;41:401–10. doi: 10.1016/s1086-5802(16)31254-2. [DOI] [PubMed] [Google Scholar]
- 41.Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, Downie G, Seymour DG. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30:205–11. doi: 10.1093/ageing/30.3.205. [DOI] [PubMed] [Google Scholar]
- 42.Lipton HL, Bird JA. The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: a randomized controlled trial. Gerontologist. 1994;34:307–15. doi: 10.1093/geront/34.3.307. [DOI] [PubMed] [Google Scholar]
- 43.Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000;50:172–5. doi: 10.1046/j.1365-2125.2000.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackie CA, Lawson DH, Maclaren AG, Kaczorowski J, Sellors J. A randomised controlled trial of medication review in patients receiving polypharmacy in general practice. Pharm J. 1999;263(Suppl.):R7. [Google Scholar]
- 45.McMullin ST, Hennenfent JA, Ritchie DJ, Huey WY, Lonergan TP, Schaiff RA, Tonn ME, Bailey TC. A prospective, randomized trial to assess the cost impact of pharmacist-initiated interventions. Arch Intern Med. 1999;159:2306–9. doi: 10.1001/archinte.159.19.2306. [DOI] [PubMed] [Google Scholar]
- 46.Sellors C, Dalby DM, Howard M, Kaczorowski J, Sellors J. A pharmacist consultation service in community-based family practices: a randomized, controlled trial in seniors. J Pharm Technol. 2001;17:264–9. [Google Scholar]
- 47.Sidel VW, Beizer JL, Lisi-Fazio D, Kleinmann K, Wenston J, Thomas C, Kelman HR. Controlled study of the impact of educational home visits by pharmacists to high-risk older patients. J Comm Health. 1990;15:163–74. doi: 10.1007/BF01350254. [DOI] [PubMed] [Google Scholar]
- 48.Smith L, McGowan L, Moss-Barclay C, Wheater J, Knass D, Chrystyn H. An investigation of hospital generated pharmaceutical care when patients are discharged home from hospital. Br J Clin Pharmacol. 1997;44:163–5. doi: 10.1046/j.1365-2125.1997.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stowasser DA, Collins DM, Stowasser M. A randomised controlled trial of medication liaison services – patient outcomes. J Pharm Prac Res. 2002;32:133–40. [Google Scholar]
- 50.Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freemantle N, Vail A. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technol Assess. 2002;6:1–86. doi: 10.3310/hta6200. [DOI] [PubMed] [Google Scholar]