Stimulation of Inducible Nitric Oxide Synthase Expression by Beta Interferon Increases Necrotic Death of Macrophages upon Listeria monocytogenes Infection (original) (raw)
Abstract
Murine macrophage death upon infection with Listeria monocytogenes was previously shown to be increased by beta interferon, produced by the infected cells. We saw that interferon-upregulated caspase activation or other interferon-inducible, death-associated proteins, including TRAIL, protein kinase R, and p53, were not necessary for cell death. Macrophage death was reduced when inducible nitric oxide synthase (iNOS) was inhibited during infection, and iNOS-deficient macrophages were less susceptible to death upon infection than wild-type cells. The production of nitric oxide correlated with increased death, while no role was seen for iNOS in control of Listeria numbers during infection of resting macrophages. This indicates that the induction of iNOS by beta interferon in cells infected with L. monocytogenes contributes to cell death. Based on morphology, the maintenance of mitochondrial membrane potential, and a lack of dependence on caspase 1, we characterize the type of cell death occurring and show that infected macrophages die by interferon-upregulated necrosis.
The death of host cells is important for pathogenesis following infection with facultative intracellular Listeria monocytogenes. It removes the replication niche of the bacteria at the expense of a potential antibacterial effector, and products or remnants of dead cells influence the development of an antibacterial response. Depending on cell type, death of infected cells occurs by various mechanisms. Hepatocytes die from apoptosis (36), accompanied by the release of neutrophil-attracting cytokines, while the death of dendritic cell lines upon infection is described as either apoptotic or necrotic (13, 22). Extensive death of lymphocytes is observed in the spleen and lymph nodes early in infection (18, 30). Unlike that of other cell types described, lymphocyte death is mediated by soluble listeriolysin O (LLO), the pore-forming toxin secreted by L. monocytogenes, rather than bacterial entry into the cell. Type I interferon (IFN) production during infection contributes to T-cell activation but also sensitizes cells to LLO-mediated apoptotic death (7). This apoptotic cell population is further proposed to trigger the production of anti-inflammatory cytokines by antigen-presenting cells and impair the innate immune response (6).
Macrophages are important for uptake of Listeria into both liver and spleen, but by their antimicrobial action, they are also key effectors of the innate immune response and can present antigens to develop the adaptive response. If not sufficiently activated to destroy the invading bacteria, the infected macrophage will eventually die (3). The control of macrophage death is important, as it may be unwise for the bacteria to destroy this potential replication niche too soon, yet also the host cannot afford for potential effector cells to shrink away upon encountering the pathogen. We have previously reported that beta IFN (IFN-β), a cytokine which macrophages produce in response to intracellular invasion, sensitizes these cells to die upon Listeria infection (41). Type I IFN production can be either beneficial or detrimental to the host during bacterial infections, depending on the bacteria and infection model, with a variety of suggested explanations for the reported effects (9). The response of the host to type I IFN is known to be detrimental in mice during Listeria infection (1, 7, 33). The effect on lymphocyte death described above provides one good explanation for this observation. Whether the effect of IFN-β on macrophage death (or indeed that of other cell types) also plays a role at some stage in infection is not clear.
We could not previously propose a mechanism for this sensitization to a death which results from a combination of a host-produced signal and the intracellular presence of bacteria. We therefore examined the mechanism of death induced, while asking how this could be influenced by the response of the macrophage to IFN-β.
MATERIALS AND METHODS
Reagents.
1-Methyltryptophan (Sigma) was dissolved in phosphate-buffered saline (PBS) and added to cell culture medium to a final concentration of 1 mM. Stocks of z-VAD-fmk (Calbiochem) and gliotoxin (Sigma) were dissolved in dimethyl sulfoxide. Recombinant IFN-β (Calbiochem) was used at a concentration of 500 U/ml. 3-Methyladenine (Sigma) was dissolved in hot water and added to cells to a final concentration of 10 mM. _S_-Methylisourea sulfate (SMT; Sigma) was freshly dissolved in cell culture medium prior to being added to cells.
Bacteria and infection of cells.
The Listeria monocytogenes wild type (strain LO28 [21]) and the isogenic hly (LLO) mutant were cultured in brain heart infusion broth. Bacteria were grown overnight at 37°C with shaking prior to infection. Infection of cells was carried out as described in reference 41: extracellular bacteria were killed after 60 min of infection by the addition of gentamicin-containing medium. Uptake of bacteria was assessed by labeling bacteria with the dye carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) before infection and then harvesting infected macrophages 1 h after infection. Mean fluorescence of the cell population was measured by fluorescence-activated cell sorting (FACS) in the FL-1 channel as described below; extracellular signals were quenched prior to the measurement by dilution of the sample 1:1 in a trypan blue solution (1 mg/ml in citrate buffer; pH 4). Bacteria in infected cells were quantified by lysing infected cells with water and plating serial dilutions of the lysates.
Mice and cells.
Mice were killed for bone marrow at between 7 and 10 weeks of age. All mice (wild type and knockout) were in a C57BL/6 genetic background and were housed under specific-pathogen-free conditions. Fas-deficient (lpr/lpr) mice were a gift of M. Baccarini (University of Vienna, Vienna, Austria). Tumor necrosis factor (TNF)-deficient mice were as described in reference 23, and STAT1-deficient mice were as described in reference 10. TRAIL-deficient mice (38) were a gift of Amgen Inc. (Seattle, WA). p53-deficient mice (16) were a gift of E. Wagner (Institute for Molecular Pathology, Vienna, Austria). Mice conditionally deleted for apoptosis-inducing factor (19) in myeloid cells under the control of the LysMCre system were a gift of J. Penninger (Institute of Molecular Biotechnology of the Austrian Academy of Science, Vienna, Austria). Protein kinase R (PKR)-deficient mice (50) were a gift of H. Unger (Veterinary University of Vienna, Vienna, Austria). p47phox-deficient mice (17) were provided by R. Schulte-Hermann (Medical University of Vienna, Vienna, Austria). Atg5-deficient mice (25) were a gift of L. Klein (Institute for Molecular Pathology, Vienna, Austria). Inducible nitric oxide synthase (iNOS)-deficient mice (28) were a gift of C. Bogdan (Institute of Medical Microbiology and Hygiene, Freiburg, Germany). Caspase 1-deficient mice (40) were gifts of M. Baccarini and A. Zychlinsky (Max Planck Institute for Infection Biology, Berlin, Germany). Bone marrow-derived macrophages (BMDMs) were obtained by culture of bone marrow in L-cell-derived colony-stimulating factor 1 as described previously (2). Macrophages were derived from fetal livers by lysing erythrocytes in a single cell suspension, cultivating the cells for 7 days, and then harvesting adherent cells. RAW 264.7 cell lines expressing Bcl2 were a gift of G. Häcker (Technical University, Munich, Germany) (14) and were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum.
Assays to measure amount and characteristics of cell death.
Crystal violet staining, measurement of lactate dehydrogenase (LDH) release, and analysis of subgenomic DNA were carried out as described in reference 41. Effector caspase activity was measured by luminescence assay using the Caspase-Glo 3/7 Assay System (Promega). For FACS analysis, macrophages were harvested by incubation for 3 to 5 min in citric saline (0.135 M potassium chloride, 0.015 M sodium citrate), collected by centrifugation together with the culture medium to collect cells in suspension, and then washed once in PBS. Cells were stained with propidium iodide (PI) at a final concentration of 5 μg/ml and analyzed immediately. DiOC6(3) (Sigma) was added at a final concentration of 1.5 nM to the cell culture medium 20 min prior to harvesting of the cells.
Measurement of NO production.
NO production was measured indirectly by assaying the concentration of nitrite in the cell culture medium by the Griess method. To 50 μl medium was added 50 μl sulfanyl acid solution (1% [wt/vol] sulfanyl acid in 4 N HCl) and 10 μl 1:1-diluted concentrated HCl, and the reaction mixture was incubated at room temperature for 10 min. Fifty microliters NED solution [1% (wt/vol) (1-naphthyl-)-ethylenediamine in methanol] was then added, and the reaction mixture was incubated for a further 10 min. Absorbance at 530 nm was measured.
Western blot analysis.
Western blot analysis was carried out as described in reference 24, but samples were run on a 15% sodium dodecyl sulfate-polyacrylamide gel. Antibody specific to the cleaved form of caspase 3 (Cell Signaling Technology) was used at a dilution of 1:1,000; antibody to pan-extracellular signal-regulated kinase (BD Transduction Laboratories) was used at 1:2,000. After labeling with a horseradish peroxidase-labeled secondary antibody, these antibodies were detected by enhanced chemiluminescence.
Electron microscopy.
Macrophages were grown on coverslips and infected with L. monocytogenes. Cells were washed once with PBS, fixed with 3% glutaraldehyde in PBS for 45 min, washed three times in PBS, and then stained with Hoechst 33258. Cells were preanalyzed by fluorescence microscopy. Cells were then postfixed in osmium tetroxide, dehydrated, and embedded into epoxy resin. Sections were mounted onto copper grids and contrasted with uranyl acetate and lead citrate.
Statistics.
Results shown are of individual experiments, representative of at least three repeated experiments. Error bars on graphs indicate the standard deviations from measurements of replicates. Results were tested for significance using the Student t test, two-tailed, and assuming the variance of the two groups to be equal.
RESULTS
Analysis of molecules involved in macrophage killing by Listeria.
Death upon Listeria infection is reduced in macrophages deficient for components of the IFN-β production and response pathway—IRF3, IFN-β, IFNAR, and STAT1 (41, 42). With the aim of identifying further proteins necessary for cell death, we screened BMDMs from mice deficient in genes linked to pathogen-mediated cell death, some known to be IFN regulated. Macrophages were infected with Listeria at multiplicities of infection (MOIs) between 2 and 100, and resulting cell death was assessed by microscopic observation, measurement of LDH release after 24 h, and staining of remaining adherent (living) cells after 48 h with crystal violet. Gene products examined and results are summarized in Table 1. None of the candidates examined showed observable reductions in bacterium-induced death, as was seen for the IFN pathway mutants. We also tested the involvement of indoleamine-2,3-dioxygenase, a tryptophan-degrading enzyme induced by IFN-β and involved in both antimicrobial defense (5, 15, 39) and induction of cell death (31). Its specific inhibitor 1-methyltryptophan was added to infected cells, but no difference in cell survival was observed. From these studies, we therefore excluded the involvement of several known mediators of bacterium-induced death. In the case of p47phox and Atg5 deficiency, a more-than-twofold enhancement of cell death upon infection with an MOI of 10 was observed, corresponding to a lack of reactive oxygen species (ROS) production (p47phox) or autophagic response (25). Atg5-deficient macrophages were derived from embryonic livers, as the homozygous knockout is neonatally lethal. A similar result was seen upon addition of the autophagy inhibitor 3-methyladenine to BMDMs during infection. Given that deficiencies in these genes are associated with increased bacterial replication (22, 35), this precludes examination of any role that these genes may play in cell death.
TABLE 1.
Analysis of molecules involved in macrophage killing by Listeria
| Gene product(s) | Method of analysis | Inducibility by type I IFN | Effect on _Listeria_-induced macrophage death |
|---|---|---|---|
| IRF3, IFN-β, IFNAR, and STAT1 | KOa | Pathways of IFN synthesis or response | Decreased (41, 42) |
| Fas | KO | Yes (ligand) | None |
| TNF-α R1 | KO | No | None |
| TRAIL | KO | Yes | None |
| p53 | KO | Yes | None |
| Apoptosis-inducing factor | KO | No | None |
| PKR | KO | Yes | None |
| Indoleamine-2,3-dioxygenase | Pharmacological inhibition | Yes | None |
| p47phox | KO | ?b | Increased |
| Atg5 | KO | No | Increased |
A role for iNOS in cell death.
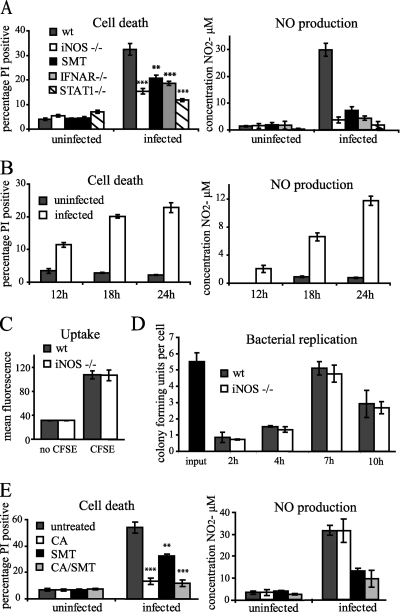
In contrast to the candidates described above, deficiency in iNOS or the iNOS inhibitor SMT reduced death upon Listeria infection (Fig. 1A). Cell death reduction was comparable with that observed for macrophages deficient in components of the IFN-signaling pathway (IFNAR and STAT1), indicating that NO may be the chief effector of the IFN-induced sensitization to death. Both ROS and reactive nitrogen species (RNS) are effectors of both apoptotic and necrotic cell death and of the antimicrobial defense of the macrophage. Transcription of iNOS is upregulated by IFNs in synergy with other signaling pathways during infection with Listeria (42, 49). Production of NO during Listeria infection was observed in wild-type cells but was minimal in iNOS-, STAT1-, and IFNAR-deficient macrophages and upon addition of the inhibitor SMT (Fig. 1A). NO synthesis was undetectable until 12 h after infection and increased thereafter in parallel with the occurrence of cell death (Fig. 1B).
FIG. 1.
Induction of NO production through iNOS by IFN-β is detrimental to cell survival during Listeria infection of macrophages and does not control bacterial number. (A) BMDMs, wild type (wt) or iNOS, IFNAR, or STAT1 deficient, were infected with Listeria at an MOI of 20. Where indicated, SMT was added to wild-type cells at 8 h after infection. At 28 h after infection, cell death was assessed by PI staining, and the concentration of nitrite in the medium was determined. The statistical significance of the difference in cell death of the knockout or inhibitor-treated BMDMs compared to wild-type macrophages is indicated (**, P < 0.01; ***, P < 0.001). (B) Wild-type BMDMs were infected with Listeria at an MOI of 20, and at the time points indicated, cell death and the concentration of nitrite in the medium were assessed. (C) Wild-type or iNOS-deficient BMDMs were infected with CFSE-labeled Listeria at an MOI of 20. After 1 h of infection, bacterial uptake was assessed by measuring CFSE fluorescence of infected cells by FACS, after quenching of extracellular signals. Cells infected with unlabeled bacteria were used as a control. (D) Wild-type or iNOS-deficient BMDMs were infected with Listeria. At the time points shown, CFU in infected cells were assessed. (E) Wild-type BMDMs were infected with Listeria at an MOI of 20. At 8 h after infection, SMT (25 μM) and chloramphenicol (CA) (20 μg/ml) were added as indicated. At 28 h after infection, cell death and the concentration of nitrite in the medium were assessed. The statistical significance of the difference in cell death between treated cells and untreated cells is indicated (see legend to panel A for definition of symbols).
Uptake of bacteria was highly similar between wild-type and iNOS-deficient macrophages (Fig. 1C), and there was little difference in subsequent bacterial growth (Fig. 1D). This shows that NO production by iNOS is not important in controlling bacterial number during the infection of resting macrophages.
To examine whether iNOS induction in macrophages alone could cause the cell death observed, we added the bacterial protein synthesis inhibitor chloramphenicol, and/or SMT, to macrophages after 8 h of infection (Fig. 1E). NO production by macrophages after addition of chloramphenicol was equivalent to that of untreated infected macrophages, as was expected given that cytosolic invasion by Listeria and IFN-β induction have already occurred by this time. However, the addition of this antibiotic dramatically reduced cell death, indicating that the production of NO does not cause cell death in the absence of metabolically active bacteria.
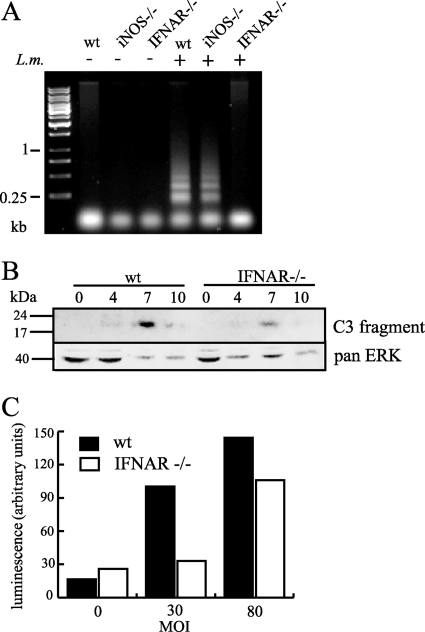
IFN-stimulated caspase activation independent of iNOS.
DNA laddering is considered a hallmark of caspase activation and apoptosis. It is correlated with _Listeria_-induced macrophage death (41). DNA fragmentation occurred in infected iNOS-deficient macrophages as well as wild-type cells but not in IFNAR-deficient cells (Fig. 2A). As the reduced cell death in iNOS-deficient cells did not correlate with the abolition of DNA fragmentation, we further examined the mechanism of macrophage death and the influence of IFN-β. The cleaved form of caspase 3 could be detected by Western blotting in protein samples from cells infected at a very high MOI (100) (Fig. 2B) but not in cells infected with lower MOIs (data not shown). This suggests that caspase activation during infections at a lower MOI is too weak and/or asynchronous to be detected by this method. Activation of effector caspases could also be detected by luminescence assay (Fig. 2C). Caspase activation was notably weaker in IFNAR-deficient macrophages, corresponding to the reduced DNA laddering in these cells.
FIG. 2.
IFN-β increases caspase activation upon infection independently of iNOS. (A) BMDMs, wild type (wt) or iNOS or IFNAR deficient, were infected with Listeria at an MOI of 20 for 16 h and harvested, and the subgenomic DNA was extracted and analyzed by gel electrophoresis. (B) Wild-type or IFNAR-deficient BMDMs were infected with Listeria at an MOI of 100, and at the time points shown, protein extracts were made and analyzed by Western blotting for the presence of cleaved caspase 3. Blotting for pan-extracellular signal-regulated kinase (pan-ERK) was used to assess the relative loading. (C) Wild-type or IFNAR-deficient BMDMs were infected with Listeria at the MOIs shown. At 10 h postinfection, cells were lysed and effector caspase activity was assessed by luminescence assay.
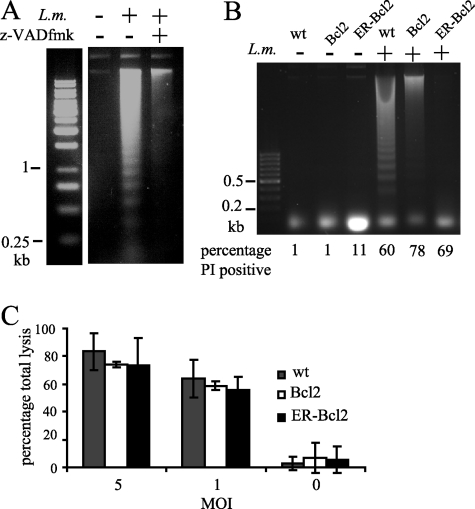
Caspase activity is not necessary for cell death.
Consistent with a previous report (20), the addition of the pan-caspase inhibitor z-VAD-fmk to infected BMDMs caused increased death even after infection with a noninvasive LLO-deficient Listeria mutant (data not shown). To minimize the toxic effect of z-VAD-fmk, we used RAW 264.7 cells (a mouse macrophage cell line) and an earlier time point to examine DNA fragmentation. RAW 264.7 cells show an increased initial bacterial load compared to BMDMs, and so the MOI can be decreased and the time after infection for assay of cell death can be shortened. The signaling triggered upon infection and the morphological appearance of dying infected cells are comparable to those for BMDMs. DNA fragmentation in infected macrophages could be abolished by addition of the pan-caspase inhibitor z-VAD-fmk, indicating that the oligosomal fragmentation observed was downstream of caspase activation (Fig. 3A). In order to observe the effect on cell death of blocking caspase activation, we used RAW 264.7 cells overexpressing the antiapoptotic protein Bcl2, or Bcl2 targeted to the endoplasmic reticulum (ER-Bcl2), and compared these with wild-type cells. Overexpression of Bcl2 reduced the appearance of DNA fragmentation in infected cells, and ER-Bcl2 overexpression had an even stronger effect (Fig. 3B). This suggests that caspase activation upon infection requires the mitochondrial pathway. Comparison of the percentage of PI-positive cells in the population used to assay for DNA fragmentation showed, however, no corresponding decrease in cell death in the absence of caspase activity (Fig. 3B, bottom). This was confirmed at a lower MOI by assay of LDH release from each cell type upon infection (Fig. 3C), where again no difference between the wild-type and Bcl2-overexpressing cells was seen. We conclude from these data that caspase activity is independent of the pathway leading to cell death.
FIG. 3.
Inhibition of caspase activation prevents DNA fragmentation but does not prevent cell death. (A) RAW 264.7 cells were infected with Listeria at an MOI of 10, with or without 90 min of pretreatment with 25 μM z-VAD-fmk. Cells were lysed after 10 h of infection, and subgenomic DNA was extracted and analyzed by gel electrophoresis. (B and C) RAW 264.7 cells, wild type (wt) or overexpressing Bcl2 or ER-Bcl2, were infected with Listeria. At 16 h after infection at an MOI of 10, cells were harvested, cell death was assessed by PI staining, and the subgenomic DNA was extracted and analyzed by gel electrophoresis (B). At 16 h after infection at the MOIs indicated, cell death was assessed by measurement of LDH release (C).
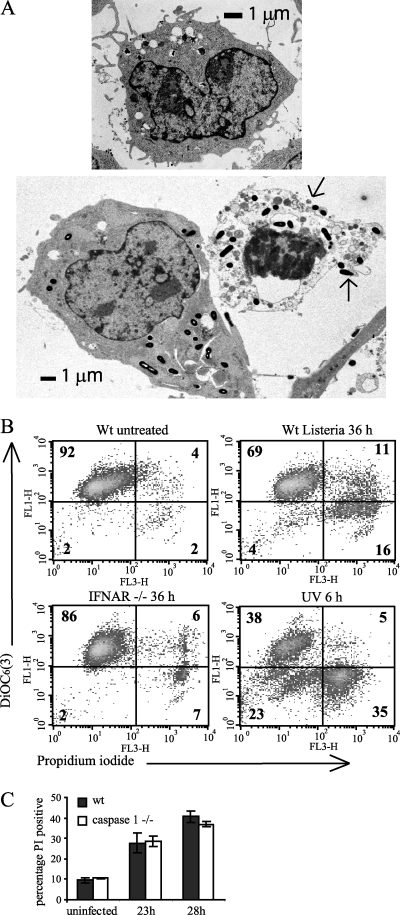
Death upon infection shows characteristics of necrosis.
NO production is associated with both apoptotic and necrotic cell death in macrophages through a number of mechanisms (4). To further characterize the NO-enhanced macrophage death, the morphology of infected cells was examined by transmission electron microscopy (Fig. 4A). Comparing an infected but still viable cell (left) with a dead cell (right), features characteristic of necrosis were apparent. Bacteria are distributed throughout the cytoplasm in both cells. Chromatin is clumped within the dead cell, although the nuclear envelope is still visible. The plasma membrane has disintegrated in several places, spilling out the cytoplasmic contents (arrows). The cytosol of the dead cell has lost its homogeneous appearance and contains numerous swollen vesicles and membrane blebs. This is characteristic of the ion flux deregulation that occurs during necrotic cell death (45).
FIG. 4.
Cell death of infected cells has necrotic features and is caspase 1 independent. (A) Electron micrographs of BMDMs not infected (top) or infected at an MOI of 10 for 12 h (bottom) showing a viable cell (left) and a dead cell (right). These are representative of 10 cells studied in detail by electron microscopy. The ruptured plasma membrane is indicated by arrows. (B) Infected cells do not show loss of mitochondrial membrane potential prior to cell membrane permeabilization. Wild-type (Wt) or IFNAR-deficient BMDMs were infected with Listeria at an MOI of 20 or exposed to 15 mJ/cm2 UV. At the time points indicated they were incubated with DiOC6(3) and PI and analyzed by FACS. The percentage of total cells is shown on each quadrant. (C) BMDMs, wild type (wt) or caspase 1 deficient, were infected with Listeria at an MOI of 20. At the time points shown cell death was assessed by PI staining. The difference in cell death between wild-type and caspase 1-deficient macrophages was not statistically significant at either time point after infection.
Apoptosis through the cell-intrinsic pathway is accompanied by a loss of mitochondrial membrane potential due to permeabilization and breakdown of mitochondria (27) prior to the final breakdown of the cell membrane during secondary necrosis. This can be followed by flow cytometry using the cationic dye 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)], whose accumulation in the cell at low concentrations is dependent on the negative mitochondrial membrane potential (37). After induction of apoptosis by UV treatment, a population of cells had lost the mitochondrial membrane potential, but their plasma membrane was still intact (seen in the lower left quadrant of the FACS plot [Fig. 4B]). Such a population was not marked in cells after Listeria infection. Instead the loss of DiOC6(3) accumulation corresponded with the loss of plasma membrane integrity at all time points after infection (24 h and 48 h; data not shown). This was also true for IFNAR-deficient macrophages, where there were fewer PI-positive cells but the pattern of staining did not change. The data suggest that mitochondrial permeabilization is not an obligatory precursor step to death (defined as loss of plasma membrane integrity), as would be seen during classical apoptosis, and that _Listeria_-induced macrophage death is necrotic in character.
A caspase 1-dependent form of cell death displaying both apoptotic and necrotic features, termed pyroptosis, is triggered by infection with several intracellular bacteria, and it has also been recently suggested to occur after Listeria infection of a macrophage cell line (8). We compared occurrence of death in caspase 1-deficient BMDMs (Fig. 4C) and observed no significant difference from the wild type, allowing us to conclude that caspase 1 is not required for cell death in this system.
IFN-β is protective against apoptotic death in macrophages.
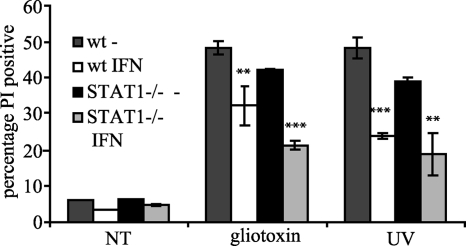
The predominantly necrotic death of _Listeria_-infected macrophages suggests an influence of IFN signaling on the events leading to necrosis and suggests that the increased caspase activation caused by type I IFN (Fig. 2) is an independent event not required for death. Consistent with this assumption we noticed that macrophages pretreated with IFN-β were less sensitive to death in response to typical apoptotic stimuli (UV and gliotoxin) than were untreated cells (Fig. 5). The data point against a general proapoptotic state being induced in macrophages by IFN-β and suggest that extra signals from the infection are required to cause death and caspase activation. Inhibition of apoptosis by IFN-β was independent of STAT1 activation by IFNAR, as this protective effect of IFN-β was also seen in STAT1-deficient macrophages. The effect of IFN-β on both death and caspase activation during Listeria infection is instead notably dependent on STAT1 (41).
FIG. 5.
IFN-β pretreatment of macrophages reduces death upon exposure to apoptotic stimuli in a STAT1-independent manner. Wild-type (wt) or STAT1-deficient BMDMs were pretreated where indicated with 500 U/ml recombinant IFN-β for 16 h and then exposed to 5 μM gliotoxin or 15 mJ/cm2 UV radiation. Cells were harvested after 6 h (gliotoxin) or 8 h (UV), and cell death was analyzed by PI staining. Statistical significance is indicated for each genotype comparing IFN treatment with no IFN treatment (**, P < 0.01; ***, P < 0.001). NT, no treatment.
DISCUSSION
We have demonstrated that death of macrophages upon Listeria infection is increased by the action of IFN-β, and an important cause is the IFN-β-induced induction of iNOS (Fig. 1). Upregulation of iNOS and NO production during infection with Listeria requires STAT1 and is therefore consistent with the STAT1 dependence of the sensitizing IFN-β. Death is not stimulated by IFN-β alone; the cytokine enhances the effect of microbial actions.
_Listeria_-induced macrophage death is best classified as necrotic, judging by morphology and the lack of requirement for typical apoptotic events required for death (caspase activation and loss of the mitochondrial membrane potential). All experimental evidence presented here argues in favor of caspase activation and other typical apoptotic events being independent of, and not required for, macrophage death. That said, the degree of asynchrony and slow mode of _Listeria_-induced death may preclude detection of a small population of cells progressing rapidly from early apoptosis, i.e., loss of mitochondrial membrane potential, to a very late stage with loss of plasma membrane integrity. In spite of this caveat, equivalent death when Bcl2 is overexpressed (Fig. 3) and the necrotic morphology of dead and dying cells (Fig. 4) clearly argue against apoptosis as the relevant death mechanism.
Although caspase activity was not required for death, it was still notably stimulated by IFN signaling. Type I IFNs are well known for inducing apoptosis in virus-infected cells and tumor cells by a range of mechanisms and signaling pathways, for example, p53 (43) and RNase L (26). However, in our experiments we saw that IFN-β treatment increased the resistance to several well-known apoptotic stimuli (Fig. 5), suggesting that the cytokine alone inhibits apoptosis in uninfected primary macrophages. This activity occurs independently of STAT1, and the prosurvival effect may well be through phosphatidylinositol 3-kinase activation, as suggested by reports in the literature (47) for other cell types. Upon Listeria infection, however, IFN-β increases the activity of proapoptotic as well as pronecrotic pathways. Cell death is enhanced by NO, whereas DNA fragmentation occurs independently of iNOS upregulation and NO production (Fig. 2). This was a reason for the dismissal of iNOS as a death-promoting factor in a previous paper (41), based on the erroneous assumption that DNA fragmentation correlated with cell death. Either DNA fragmentation, which is sustained in iNOS-deficient cells despite decreased death, must be occurring in cells dying through the necrotic pathway, or less likely, necrotic and apoptotic cells are distinct populations and the apoptotic population is too small to produce an observable difference in the total population of dying cells. The lack of reduction in DNA laddering in iNOS-deficient cells clearly shows that the proapoptotic and pronecrotic activities of IFN-β upon Listeria infection are distinct from each other. Both activities require STAT1 (41). The IFN-induced pathway leading to caspase activation in this situation is not known. Several candidates, including caspase 1, TRAIL, and PKR, may be ruled out, as DNA fragmentation was observed upon infection in macrophages deficient for these genes (data not shown).
A recently published study (8) looking at cell death upon Listeria infection in macrophage cell lines reaches some similar conclusions regarding the mechanism of cell death. These include a low level of apoptotic caspase activation and maintenance of mitochondrial membrane integrity in infected cells, although any role for type I IFN is not examined in this study. However, the authors conclude from experiments using an inhibitor of caspase 1 that this molecule is important in eliciting cell death after infection. Our results using caspase 1-deficient BMDMs show no role for this in the death of primary macrophages (Fig. 4). We suggest that the rapid onset of cell death (within 90 min of infection) reported in this study for the IC-21 macrophage cell line is not comparable with the later onset that predominates in our system and that of a previous report (3). Indeed, it was previously shown that death of _Listeria_-infected BMDMs was distinct from the caspase 1-dependent death induced by Shigella flexneri (3). Caution regarding conclusions drawn from use of the caspase 1 inhibitor in macrophages is indicated by our finding that caspase 1 inhibitor, but not the absence of caspase 1, reduces macrophage killing by LLO toxin, suggesting that the inhibitor may have other actions.
Though NO is capable of causing both apoptotic and necrotic death in macrophages through several mechanisms (4), consideration of the situation within the infected macrophage leads to some suggestions of how death is induced. Production of ROS by the phagocyte oxidase complex in macrophages is stimulated immediately upon phagocytosis of bacteria, and this is not type I IFN dependent (our own observations). ROS react with NO to form the particularly cytotoxic species peroxynitrite, which can induce necrotic death, partially through activation of poly(ADP-ribose) polymerase 1 and subsequent energy depletion. NO alone can also inhibit mitochondrial respiration. This is interesting to consider in the context of a cell containing a replicating parasitic microbe and with upregulated antimicrobial defenses. As shown in Fig. 1E, the presence of metabolically active bacteria is required for cell death. Whatever the contribution of NO to death in this system, it requires the context of the bacterial infection. We suggest that energy depletion could be the trigger for cell membrane collapse and necrotic death, but this is difficult to measure given the slow accumulation of dead cells in the population during infection. Upregulation of iNOS has been linked to death during infection of macrophages with both pneumococci (29) and group B streptococci (46), as well as after lipopolysaccharide treatment (48). In all these cases, the death induced is apoptotic in character and by mechanisms that seem quite distinct from what is seen during Listeria infection. To our knowledge, such an iNOS-stimulated necrotic death upon intracellular bacterial infection has not been previously described. It may reflect the intracellular persistence and replication of Listeria, which cause defensive responses and cellular survival to be constantly stimulated by the presence of the bacteria. Strikingly, NO did not exert detectable antimicrobial functions in resting macrophages, as we saw no increase in bacterial number in iNOS-deficient cells. The degree to which RNS contribute to antilisterial defense of activated macrophages varies between different reports (32, 34, 44). iNOS-deficient mice were shown to be more sensitive to Listeria infection (28), but this has been questioned by others (11, 12). Judging from our data, the significance of iNOS in _Listeria_-infected animals may be difficult to assess due to opposing effects of the molecule: an antimicrobial effect on the one hand and prodeath activity against at least some effector cells on the other.
Contrasting with NO, the effect of reducing ROS production (as seen in p47phox-deficient macrophages) was accelerated death. This points to ROS not being detrimental to cell survival or suggests that any detrimental effect is overruled by their antimicrobial function.
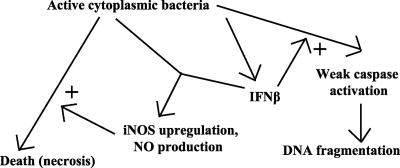
In summary, we provide what is to our knowledge the first description of type I IFN enhancement of necrotic, infection-induced effector cell death. A model summarizing the proposed role of different pathways in death is shown in Fig. 6. Judging from infection experiments in mice this effect of type I IFN may be harmful for the host organism. Future studies must show whether this concept applies to all infections with intracellular bacteria or whether it is a specific Listeria attribute.
FIG. 6.
Model of how cell death and caspase activation are induced upon Listeria infection of macrophages. Intracellular Listeria stimulates production of IFN-β. Together with other signaling pathways, this stimulates induction of iNOS and NO production. The action of the intracellular bacteria alone triggers necrotic death, which is enhanced by NO. IFN-β also enhances the activation of caspases in response to the infecting bacteria, by an unknown mechanism. This caspase activation is independent of the pathways to necrotic cell death.
Acknowledgments
This study was supported by the Austrian Science Foundation (FWF) through grant AP17859 and P20522-B05 (to T.D.), grant SFB 28 (to T.D.), and a Ph.D. fellowship (to H.Z.). Additional funding was provided by the Viennese Foundation for Research and Technology (HOPI initiative, to T.D.).
We gratefully acknowledge critical discussion and helpful suggestions from Emmanuelle Charpentier (Vienna Biocenter) and Wilfried Bursch (Institute of Cancer Research, Vienna, Austria). We also thank Jelena Nedjic (Vienna Biocenter), Tomoki Nakashima (Vienna Biocenter), and Georg Häcker (Technical University, Munich, Germany) for providing mouse organs and cells. Many colleagues listed in the Materials and Methods section are thanked for providing gene-targeted mice. All colleagues in the Decker lab are thanked for help, discussion, and advice.
Footnotes
▿
Published ahead of print on 11 February 2008.
REFERENCES
- 1.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccarini, M., F. Bistoni, and M. L. Lohmann-Matthes. 1985. In vitro natural cell-mediated cytotoxicity against Candida albicans: macrophage precursors as effector cells. J. Immunol. 1342658-2665. [PubMed] [Google Scholar]
- 3.Barsig, J., and S. H. Kaufmann. 1997. The mechanism of cell death in _Listeria monocytogenes_-infected murine macrophages is distinct from apoptosis. Infect. Immun. 654075-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borutaite, V., and G. Brown. 2005. What else has to happen for nitric oxide to induce cell death? Biochem. Soc. Trans. 331394-1396. [DOI] [PubMed] [Google Scholar]
- 5.Carlin, J. M., E. C. Borden, and G. I. Byrne. 1989. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J. Interferon Res. 9329-337. [DOI] [PubMed] [Google Scholar]
- 6.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cervantes, J., T. Nagata, M. Uchijima, K. Shibata, and Y. Koide. 2008. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell. Microbiol. 1041-52. [DOI] [PubMed] [Google Scholar]
- 9.Decker, T., M. Muller, and S. Stockinger. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5675-687. [DOI] [PubMed] [Google Scholar]
- 10.Durbin, J. E., R. Hackenmiller, M. C. Simon, and D. E. Levy. 1996. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84443-450. [DOI] [PubMed] [Google Scholar]
- 11.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47phox-/- and TRp55-/- mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7419-432. [DOI] [PubMed] [Google Scholar]
- 12.Fehr, T., G. Schoedon, B. Odermatt, T. Holtschke, M. Schneemann, M. F. Bachmann, T. W. Mak, I. Horak, and R. M. Zinkernagel. 1997. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J. Exp. Med. 185921-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, C. A., E. Domann, M. Rohde, D. Bruder, A. Darji, S. Weiss, J. Wehland, T. Chakraborty, and K. N. Timmis. 1996. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol. Microbiol. 20119-126. [DOI] [PubMed] [Google Scholar]
- 14.Hacker, H., C. Furmann, H. Wagner, and G. Hacker. 2002. Caspase-9/-3 activation and apoptosis are induced in mouse macrophages upon ingestion and digestion of Escherichia coli bacteria. J. Immunol. 1693172-3179. [DOI] [PubMed] [Google Scholar]
- 15.Hissong, B. D., G. I. Byrne, M. L. Padilla, and J. M. Carlin. 1995. Upregulation of interferon-induced indoleamine 2,3-dioxygenase in human macrophage cultures by lipopolysaccharide, muramyl tripeptide, and interleukin-1. Cell. Immunol. 160264-269. [DOI] [PubMed] [Google Scholar]
- 16.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 41-7. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, J., L. L. Lau, and H. Shen. 2003. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J. Immunol. 1714352-4358. [DOI] [PubMed] [Google Scholar]
- 19.Joza, N., G. Y. Oudit, D. Brown, P. Benit, Z. Kassiri, N. Vahsen, L. Benoit, M. M. Patel, K. Nowikovsky, A. Vassault, P. H. Backx, T. Wada, G. Kroemer, P. Rustin, and J. M. Penninger. 2005. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol. Cell. Biol. 2510261-10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, S. O., K. Ono, and J. Han. 2001. Apoptosis by pan-caspase inhibitors in lipopolysaccharide-activated macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 281L1095-L1105. [DOI] [PubMed] [Google Scholar]
- 21.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68521-531. [DOI] [PubMed] [Google Scholar]
- 22.Kolb-Maurer, A., I. Gentschev, H.-W. Fries, F. Fiedler, E.-B. Brocker, E. Kampgen, and W. Goebel. 2000. _Listeria monocytogenes_-infected human dendritic cells: uptake and host cell response. Infect. Immun. 683680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korner, H., M. Cook, D. S. Riminton, F. A. Lemckert, R. M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St. Groth, and J. D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 272600-2609. [DOI] [PubMed] [Google Scholar]
- 24.Kovarik, P., D. Stoiber, M. Novy, and T. Decker. 1998. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 173660-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuma, A., M. Hatano, M. Matsui, A. Yamamoto, H. Nakaya, T. Yoshimori, Y. Ohsumi, T. Tokuhisa, and N. Mizushima. 2004. The role of autophagy during the early neonatal starvation period. Nature 4321032-1036. [DOI] [PubMed] [Google Scholar]
- 26.Li, G., Y. Xiang, K. Sabapathy, and R. H. Silverman. 2004. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. J. Biol. Chem. 2791123-1131. [DOI] [PubMed] [Google Scholar]
- 27.Ly, J. D., D. R. Grubb, and A. Lawen. 2003. The mitochondrial membrane potential (deltapsi[m]) in apoptosis; an update. Apoptosis 8115-128. [DOI] [PubMed] [Google Scholar]
- 28.MacMicking, J. D., C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q. W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81641-650. [DOI] [PubMed] [Google Scholar]
- 29.Marriott, H. M., F. Ali, R. C. Read, T. J. Mitchell, M. K. B. Whyte, and D. H. Dockrell. 2004. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 181126-1128. [DOI] [PubMed] [Google Scholar]
- 30.Merrick, J. C., B. T. Edelson, V. Bhardwaj, P. E. Swanson, and E. R. Unanue. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151785-792. [PMC free article] [PubMed] [Google Scholar]
- 31.Morita, T., K. Saito, M. Takemura, N. Maekawa, S. Fujigaki, H. Fujii, H. Wada, S. Takeuchi, A. Noma, and M. Seishima. 1999. L-tryptophan-kynurenine pathway metabolite 3-hydroxyanthranilic acid induces apoptosis in macrophage-derived cells under pathophysiological conditions. Adv. Exp. Med. Biol. 467559-563. [DOI] [PubMed] [Google Scholar]
- 32.Myers, J. T., A. W. Tsang, and J. A. Swanson. 2003. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J. Immunol. 1715447-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell, R. M., S. K. Saha, S. A. Vaidya, K. W. Bruhn, G. A. Miranda, B. Zarnegar, A. K. Perry, B. O. Nguyen, T. F. Lane, T. Taniguchi, J. F. Miller, and G. Cheng. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohya, S., Y. Tanabe, M. Makino, T. Nomura, H. Xiong, M. Arakawa, and M. Mitsuyama. 1998. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect. Immun. 664043-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Py, B. F., M. M. Lipinski, and J. Yuan. 2007. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 3117-125. [DOI] [PubMed] [Google Scholar]
- 36.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156679-684. [PubMed] [Google Scholar]
- 37.Rottenberg, H., and S. Wu. 1998. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim. Biophys. Acta 1404393-404. [DOI] [PubMed] [Google Scholar]
- 38.Sedger, L. M., M. B. Glaccum, J. C. Schuh, S. T. Kanaly, E. Williamson, N. Kayagaki, T. Yun, P. Smolak, T. Le, R. Goodwin, and B. Gliniak. 2002. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 322246-2254. [DOI] [PubMed] [Google Scholar]
- 39.Shemer-Avni, Y., D. Wallach, and I. Sarov. 1989. Reversion of the antichlamydial effect of tumor necrosis factor by tryptophan and antibodies to beta interferon. Infect. Immun. 573484-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, D. J., M. J. McGuire, M. J. Tocci, and D. L. Thiele. 1997. IL-1 beta convertase (ICE) does not play a requisite role in apoptosis induced in T lymphoblasts by Fas-dependent or Fas-independent CTL effector mechanisms. J. Immunol. 158163-170. [PubMed] [Google Scholar]
- 41.Stockinger, S., T. Materna, D. Stoiber, L. Bayr, R. Steinborn, T. Kolbe, H. Unger, T. Chakraborty, D. E. Levy, M. Muller, and T. Decker. 2002. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 1696522-6529. [DOI] [PubMed] [Google Scholar]
- 42.Stockinger, S., B. Reutterer, B. Schaljo, C. Schellack, S. Brunner, T. Materna, M. Yamamoto, S. Akira, T. Taniguchi, P. J. Murray, M. Muller, and T. Decker. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 1737416-7425. [DOI] [PubMed] [Google Scholar]
- 43.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 424516-523. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka, T., S. Akira, K. Yoshida, M. Umemoto, Y. Yoneda, N. Shirafuji, H. Fujiwara, S. Suematsu, N. Yoshida, and T. Kishimoto. 1995. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 80353-361. [DOI] [PubMed] [Google Scholar]
- 45.Trump, B., and I. Berezesky. 1998. The reaction of cells to lethal injury: oncosis, necrosis and the role of calcium, p. 57-96. In R. A. Lockshin, Z. Zakeri, and J. L. Tilly (ed.), When cells die: a comprehensive evaluation of apoptosis and programmed cell death. Wiley-Liss, New York, NY.
- 46.Ulett, G. C., and E. E. Adderson. 2005. Nitric oxide is a key determinant of group B streptococcus-induced murine macrophage apoptosis. J. Infect. Dis. 1911761-1770. [DOI] [PubMed] [Google Scholar]
- 47.Wang, K., D. Scheel-Toellner, S. H. Wong, R. Craddock, J. Caamano, A. N. Akbar, M. Salmon, and J. M. Lord. 2003. Inhibition of neutrophil apoptosis by type 1 IFN depends on cross-talk between phosphoinositol 3-kinase, protein kinase C-δ, and NF-κB signaling pathways. J. Immunol. 1711035-1041. [DOI] [PubMed] [Google Scholar]
- 48.Xaus, J., M. Comalada, A. F. Valledor, J. Lloberas, F. Lopez-Soriano, J. M. Argiles, C. Bogdan, and A. Celada. 2000. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood 953823-3831. [PubMed] [Google Scholar]
- 49.Xie, Q. W., R. Whisnant, and C. Nathan. 1993. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. 1771779-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 146095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]