The Cochaperone Bag-1L Enhances Androgen Receptor Action via Interaction with the NH2-Terminal Region of the Receptor (original) (raw)
Abstract
Members of the Bag-1 family of cochaperones regulate diverse cellular processes including the action of steroid hormone receptors. The largest member of this family, Bag-1L, enhances the transactivation function of the androgen receptor. This occurs primarily through interaction with the NH2 and COOH termini of the receptor. At the NH2 terminus of the receptor, Bag-1L interacts with a region termed τ5. Bag-1M, a naturally occurring variant of Bag-1L that binds to τ5 but is defective in the COOH-terminal interaction, is less efficient in enhancing the transactivation function of the receptor. Surface plasmon resonance and transfection studies showed that the molecular chaperone Hsp70 contributes to the binding of Bag-1L to τ5 and to the regulation of the transactivation function of the androgen receptor. Chromatin immunoprecipitation studies demonstrated that the androgen receptor, Hsp70, and Bag-1L are all targeted to the androgen response elements of the gene that encodes prostate-specific antigen. These studies demonstrate the regulation of transcriptional activity of androgen receptor by a molecular chaperone-cochaperone complex.
Molecular chaperones and cochaperones belong to a group of unrelated proteins that mediate the correct assembly of other proteins but are not themselves components of the final structure (2). In steroid receptor action, they are thought to interact with the receptors to provide the correct conformation for ligand binding (5, 7, 38). This occurs in an ordered fashion, involving an initial binding of Hsp70 and its cochaperones to the receptor, followed by the recruitment of two molecules of Hsp90 to the complex. Later, Hsp70 is reported to dissociate from the complex, allowing p23 and immunophilins to interact to generate an aporeceptor competent for hormone binding (9, 13, 18).
While this contribution of molecular chaperones to hormone binding by steroid receptors is widely accepted, recent studies have reported additional function of molecular chaperones in steroid hormone action at the level of regulation of interaction of the receptor with DNA (16, 32). For example, the molecular chaperone p23 has been shown to act at a late step in nuclear receptor action, promoting disassembly of the transcriptional regulatory complex (14, 16). This enables the receptor to recycle from the nucleus to the cytoplasm to detect and respond to changes in hormone concentration (15). Other studies have reported that Bag-1, a cochaperone of Hsp70, is involved in positive and negative regulation of the transactivation function of steroid receptors (10).
Bag-1 is a family of proteins that associates with the molecular chaperone Hsp70 and serves as a nucleotide exchange factor for this protein (6, 39). In humans, it consists of four proteins, Bag-1L, Bag-1M, Bag-1S, and p29, generated by alternative translation initiation sites on the same mRNA. The Bag-1 proteins therefore have a conserved COOH-terminal domain but differ in the length of their NH2-terminal sequences (41). The Bag-1 proteins have diverse biological functions ranging from inhibition of apoptosis to modulation of the action of steroid receptors (10, 23). Bag-1M and Bag-1L negatively regulate the transactivation function of the glucocorticoid receptor (GR), while Bag-1S has no effect on the action of this receptor (36). On the other hand, Bag-1L but not Bag-1M enhances the transactivation function of the androgen receptor (AR) (17, 26). The mechanism underlying the differences in the regulation of activity of the different steroid receptors is not known.
In this communication we show that Bag-1L enhances the transactivation function of the AR by using its NH2- and COOH-terminal domains to bind to the COOH- and NH2-terminal sequences of the AR. Binding to and regulation of AR action by Bag-1L is assisted by Hsp70. In immunohistochemical studies we demonstrated that Bag-1L is highly expressed in the basal cells of the benign prostate but not in the secretory epithelium where the AR is expressed. The site of expression of Bag-1L is, however, altered in prostate carcinoma, from the basal cells to the secretory epithelium. Hsp70, the molecular chaperone to which Bag-1L binds, is also expressed in a similar pattern to that of Bag-1L. Thus, in tumor cells, Bag-1L and Hsp70 are expressed in the secretory epithelial cells, where they function together to enhance the action of the AR.
MATERIALS AND METHODS
Cell culture.
Human prostate carcinoma PC3 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37°C in an atmosphere of 5% CO2. 22Rv1 derived from prostatic carcinama xenograft CWR22R (40) and LNCaP cells were cultured in RPMI medium 1640 supplemented with 10% fetal bovine serum.
Expression vectors and plasmids.
Bag-1M expression plasmid pT2Rap46 and reporter plasmids pGL3MMTV and pTkRenilla Luc have been described by Schneikert et al. (36). The reporter construct pG5ΔE4-38Luc was previously described by Mink et al. (31). The NLS-Bag1M expression vector was created by PCR amplification of the nuclear localization signal (NLS) of simian virus 40 and fused in frame with the coding sequence of Bag-1M. The fusion construct was then introduced into the _Bam_HI and _Xba_I sites of the vector pT2 (36). The pcDNA3-Bag1L construct (17) was a gift from J. Reed. Plasmids pcDNA3-HA-Bag1L, pcDNA3-HA-ARτ1, and pcDNA3-HA-ARτ5 were created by introducing a cDNA fragment encoding the HA immunotag into _Bam_HI-_Xho_I sites of pcDNA3 (Invitrogen) with subsequent in-frame insertion of the coding sequence for BAG-1L, ARτ1, and ARτ5, respectively, into the resulting vector. The construct encoding the HA-tagged BAG-1LΔC was created by replacing the _Sac_II-_Xba_I fragment of pcDNA3-HA-Bag1L with the _Sac_II-_Xba_I fragment of pGex-3x-Bag1LΔC.
The constructs pSG5-AR, pSG5-ARΔ1-188, pSG5-ARΔ1-280, pSG5-ARΔ1-440, pSG5-ARΔ1-488, and pSG5-ARΔHBD were described previously by Gast et al. (19). pZEM-Hsp70-tag has already been described by Bellmann et al. (4). The constructs pZEM-Hsp70ΔATPase-tag and pZEM-Hsp70ΔSBD-tag contain deletions of amino acids 117 to 314 and 324 to 458 in the ATPase and substrate binding domains, respectively, in the Hsp70 expression vector pZEM-Hsp70-tag. These constructs were supplied by M. Jäättelä. Construct pSG5-ARΔHR was created by replacement of _Hin_dIII-_Tth_111I fragment of the AR cDNA in pSG5-ARwt with a PCR-generated fragment in which the sequence encoding amino acids 633 to 650 had been deleted. The pSG5-AR1-633 construct is a derivative of pSG5-ARΔHR, created by a frameshift mutation resulting in a stop codon at position 633. pM-c/EBPβ plasmid has been described by Mink et al. (30). GAL4-AR τ1 and τ5 constructs (encoding amino acids 101 to 370 and 360 to 548, respectively, of the AR) were generated by PCR amplification. The resulting fragments were inserted in frame into the _Bam_HI and _Xba_I or the _Bam_HI and _Hin_dIII sites of the pM vector (Clontech). Glutathione _S_-transferase (GST)-Rap46 and GST-Rap46ΔC47 have been described by Schneikert et al. (35). pGex-4T-1-BAG1L was provided by J. Reed and contains the full-length Bag-1L sequence cloned in frame with GST in the vector pGex-4T.1 (Pharmacia). GST-Bag-1LΔC expression construct was obtained by cloning the Bag-1L cDNA from pcDNA3Bag-1LΔC (17) in frame in front of GST in the pGex 3X vector. pGex-4T-1-BAG1LNterm, encoding the first 127 amino acids of BAG-1L fused to the GST, was generated by cleaving out the fragment between _Sal_I-_Xho_I with subsequent religation of the vector. The construct pSV-Hsp70-tag containing the entire human Hsp70 cDNA modified by the addition of a sequence encoding the human testis-specific lactate dehydrogenase immunotag at the C terminus has been described previously by Jäättelä et al. (24).
Transient-transfection assays.
Transient transfection was performed with FuGENE 6 transfection reagent (Roche Diagnostics) or by the calcium phosphate coprecipitation method with 2 × 105 PC3 cells in 3.5-cm-diameter dishes. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 0.5% charcoal-treated fetal calf serum (CCS) for 20 h prior to transfection. Unless otherwise stated, all transfection experiments were performed with luciferase firefly indicator genes and a Renilla luciferase construct as an internal control for the efficiency of transfection. The cells were harvested 36 to 44 h after transfection in passive lysis buffer (Promega, Mannheim, Germany) for luciferase activity measurements.
GST pull-down experiments.
Expression of GST fusion proteins and GST pull-down experiments were performed essentially as described previously by Mink et al. (30).
Immunoblotting.
Western blot analyses was performed as described previously by Peterziel et al. (34), using mouse monoclonal AR antibody from BioGenex (clone F39.4.1) or rabbit antiserum raise against amino acids 543 to 558 from the AR. Rabbit polyclonal antibody to BAG-1L has been described previously by Crocoll et al. (12). Antibodies against Bag-1 (C-16) and Hsp70 (K-20) were procured from Santa Cruz Biotechnology, Heidelberg, Germany. Antibody against LDH to detect the LDH tag on Hsp70, Hsp70ΔATPase, and Hsp70ΔPBD was obtained as previously described by Milarski and Morimoto (29).
Immunofluorescence.
For immunofluorescence experiments, the cells were kept in Dulbecco's modified Eagle's medium containing 3% CCS to remove residual steroids. Immunofluorescence experiments were performed as previously described by Herscovics et al. (22), and the photographs were taken with an LSM 510 inverted Zeiss confocal microscope.
Chromatin immunoprecipitation.
22Rv1 cells were grown in phenol red-free RPMI medium 1640 (GIBCO-BRL, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 5% CCS. After 3 days of cultivation the cells were treated either with vehicle alone or with vehicle containing 10−7 M dihydrotestosterone (DHT). Chromatin immunoprecipitaion was performed as described by Shang et al. (37) on the prostate-specific antigen (PSA) gene. Antibodies used in the experiment were αAR, PG-21 (Upstate Biotechnology, Inc., BIOMOL, Hamburg, Germany), αHsp70 (K-20) (Santa Cruz Biotechnology) and Bag-1L-specific rabbit antiserum N15 (12). The primer sequences for amplification of the promoter fragments of the PSA gene were as follows: ARE I/II (−459 to −121), GCCAAGACATCTATTTCAGGAGC (forward) and CCCACACCCAGAGCTGTGGAAGG (reverse); ARE III (−4288 to −3922), GGGGTTTGTGCCACTGGTGAG (forward) and GGGAGGCAATTCTCCATGGTT (reverse); INT (−1997 to −1846), CTGTGCTTGGAGTTTACCTGA (forward) and GCAGAGGTTGCAGTGAGCC (reverse); and β-actin primers: TCCTCCTCTTCCTCAATCTCG (forward) and AAGGCAACTTTCGGAACGG (reverse).
Patients and tissue specimens.
Benign prostate and primary prostate tumor specimens were obtained from patients undergoing radical prostatectomy after prostate cancer had been detected by PSA screening and confirmed by ultrasound-guided biopsies. In addition, a commercially available prostate cancer tissue array comprising 57 specimens from benign prostate, primary cancer, and control tissues, with a diameter of 2 mm each, was used (BioCat, Heidelberg, Germany).
Immunohistochemistry.
Formalin-fixed, paraffin-embedded tissue specimens were analyzed for Bag-1 and Hsp70 expression. Sections (5 μm thick) were cut and subsequently rehydrated through a series of graded alcohol. Pretreatment by wet autoclaving was used for antigen retrieval, and endogenous peroxidase activity was blocked using H2O2 in methanol. Slides were then incubated overnight with the monoclonal anti-Bag-1 antibody (clone 3.9F1E11; NeoMarkers, Fremont, Calif.), or the monoclonal anti-Hsp70 antibody (clone W27; NeoMarkers) diluted 1:100 in Tris-buffered saline solution containing 1% bovine serum albumin. After washing, the color was developed with the PicTrue broad-spectrum DAB kit (Zymed, Carlton Court, Calif.). Negative controls consisting of slides incubated without primary antibody were included in every assay.
Nuclear staining was assessed by a pathologist on the basis of a four-point scale (0, no staining; 1, staining of 0 to 10% of nuclei; 2, staining of 10 to 50% of nuclei; 3, staining of >50% of nuclei). In benign tissue, basal, secretory epithelial, and stromal cells were scored separately. In primary tumor specimens, immunohistochemical staining was correlated to the Gleason pattern. In a subset of specimens, immunostaining was done using a custom-made Bag-1L-specific polyclonal rabbit antiserum (12). The results were similar to those obtained with the monoclonal antibody which recognizes all isoforms of Bag-1. A few specimens were stained with an anti-AR monoclonal antibody (clone AR441; DAKO, Glostrup, Denmark).
Surface plasmon resonance experiments.
The BIAcore 2000 system, sensor chip CM5, and the amine coupling kit containing _N_-hydroxysuccinimide, _N_-ethyl-_N_′-(3-diethylaminopropyl) carbdiimide, ethanol-amine hydrochloride, the recombinant GST, and the anti-GST antibody were all obtained from Biacore AB, Freiburg, Germany, and used as specified by the manufacturer. The buffer used for all CM5 chip experiments was 10 mM HEPES-150 mM NaCl-3.4 mM EDTA-0.05% Tween 20 (pH 7.4) (HBS). Recombinant human Hsp70 was obtained from StressGen Biotechnologies Corp., Victoria, Canada (product SPP-755). The anti-GST antibody was covalently linked to the surface of a CM5 sensor chip used to capture GST-τ5 through antibody-antigen interaction. Routinely, we were able to then capture 1,500 RU of GST-τ5.
RNA preparation and real-time PCR.
Total RNA was extracted for 5 h from 22Rv1 cells treated with vehicle alone (0.1% ethanol) or with vehicle containing 0.1 μM DHT. cDNA was synthesized using SuperScript II (Invitrogen, Karlsruhe, Germany) and DNA amplification with a SYBR Green PCR mastermix and the ABI 7000 sequence detection system (Applied Biosystems, Weiterstadt, Germany). The primer pairs used for human PSA were 487FP (TCACAGCTACCCACTGCATCA), 667RP (AGGTCGTGGCTGGAGTCATC); primers 37FP (TCACCCACACTGTGCCCAT) and 239RP (CTCTTGCTCGAAGTCCAGGG) for human β-actin were used as a normalizing control.
RESULTS
Requirement of the N terminus of AR for Bag-1L action.
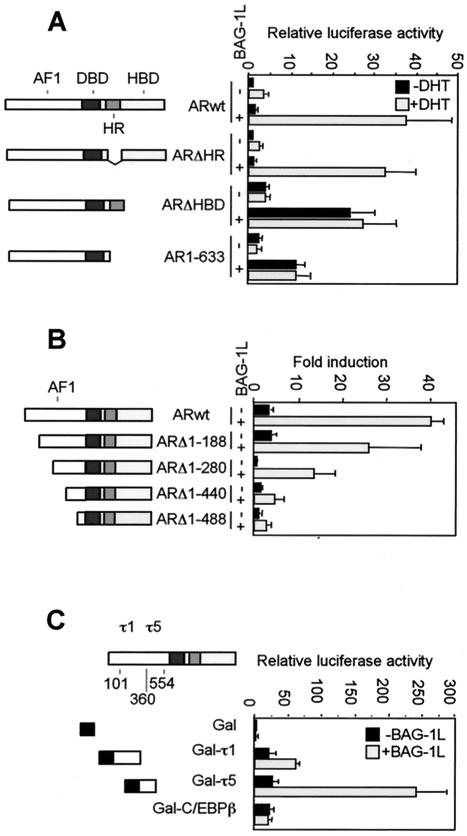
To identify the mechanism by which Bag-1L enhances the transactivation function of the AR, we first determined the domains of the AR necessary for the regulatory action of Bag-1L. Cotransfection experiments with deletion mutants of the AR and a mouse mammary tumor virus (MMTV)-luciferase indicator gene were carried out with an AR-negative PC3 cell clone. These cells were chosen for the deletion mutant analysis because they showed high transfection efficiency and a high androgen response in the presence of Bag-1L.
In the absence of Bag-1L, DHT enhanced the MMTV promoter activity approximately fivefold. Bag-1L expression increased the basal MMTV promoter activity about twofold but robustly enhanced expression at this promoter in the presence of hormone (Fig. 1A). Deletion of the hinge region of the AR did not affect the ability of Bag-1L to enhance the activity of the AR (Fig. 1A) in the presence of hormone. On the other hand, deletion of the hormone binding domain (HBD), either alone or together with the hinge region, generated receptor mutants that transactivated in a hormone-independent manner but whose activity was still upregulated by Bag-1L (Fig. 1A). This identified the NH2 terminus of the AR as a target for the enhancing action of Bag-1L.
FIG. 1.
Effect of BAG-1L on the transactivation function of the AR and its deletion mutants. (A and B) Bag-1L enhances transactivation by the AR through the NH2 terminus of the receptor. PC3 cells were transiently transfected by the calcium phosphate method in 3.5-cm dishes with 0.6 μg of pGL3-MMTV DNA, 0.1 μg of TK-Renilla luciferase construct, 0.2 μg of wild-type AR (ARwt) or AR N-terminal or C-terminal deletion mutants in the presence of 0.5 μg of control vector or BAG-1L-expressing plasmid. At 24 h after transfection, the cells were treated with vehicle (0.1% ethanol) or vehicle containing 10−7 M DHT. The cells were harvested 20 h later for luciferase activity measurements. (C) The regulation of transactivation function of the AR by Bag-1L occurs through the τ5 domain of the receptor. PC3 cells were transiently transfected by the calcium phosphate method in 3.5-cm dishes with 0.5 μg of pG5ΔE4-38Luc reporter plasmid, 0.2 μg of TK-Renilla luciferase construct, 0.2 μg of vector pM, or the vector containing ARτ1 and ARτ5 sequences (pM-ARτ1 or pM-ARτ5) or, as control, pM-C/EBPβ plasmid in the presence of 1 μg of control vector (black bars) or BAG-1L-expressing plasmid (gray bars). At 36 h after transfection, the cells were harvested for luciferase activity measurements. The results are represented as the relative luciferase values (firefly/Renilla) and represent the mean and standard deviation for at least four independent experiments. Schematic diagrams of the AR constructs used in the transfection experiments show the hormone binding (HBD), the DNA binding (DBD), hinge region (HR), and N-terminal transactivation function (AF1) of the receptor. In panel C, the positions of the τ1 and τ5 transactivation domains are depicted in the AF1 region of the AR.
To define the region in the NH2 terminus of the AR for the action of Bag-1L, NH2-terminal deletion mutants with progressive deletions of the AR were analyzed. Bag-1L enhanced the transactivation function of all the mutants, but a clear downregulation in its effect was detected between deletion mutants ARΔ1-280 and ARΔ1-440 (Fig. 1B). This identified the NH2-terminal region proximal to the DNA binding domain (DBD) as most probable target for the enhancing action of Bag-1L. The differences in action of Bag-1L in the analysis of these receptor mutants do not come from differences in the expression levels of these proteins as determined by immunoblot assays (results not shown).
The NH2-terminal region of the AR contains core transactivation domains at amino acid residues 101 to 370 and 360 to 528, termed τ1 and τ5 (25). To determine whether both transactivation domains are used by Bag-1L, we fused them to the DBD of yeast transcription factor Gal4 and cotransfected the resulting constructs together with a Bag-1L expression vector and a luciferase indicator gene controlled by Gal4 binding elements into the PC3 cells. The Gal4 DBD alone is unable to enhance the expression of the indicator gene in the absence and presence of Bag-1L. Fusion of the τ1 or τ5 sequences of the AR to the Gal4 DBD resulted in an efficient transactivation of the indicator gene (Fig. 1C). Transactivation by these NH2-terminal AR sequences was further potentiated by Bag-1L, albeit to a lesser extent through Gal4-τ1 than through Gal4-τ5 (Fig. 1C). In a control experiment, Bag-1L failed to upregulate the transactivation function of a Gal4 DBD-c/EBPβ construct, thus demonstrating the specificity of Bag-1L in enhancing the transactivation function of the AR through τ5.
Requirement of the NH2 and COOH termini of Bag-1L.
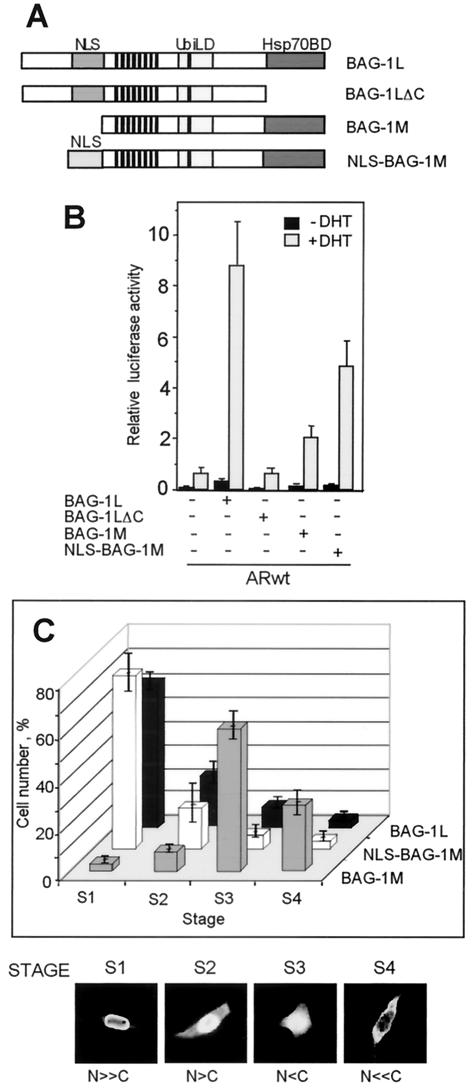
To determine the regions of Bag-1L that contribute to the enhancement of the transactivation function of the AR, we examined the effect of two Bag-1L mutants on the transactivation function of the AR. The two mutants used are Bag-1LΔC, which lacks the carboxyl Hsp70 binding site, and Bag-1M, a naturally occurring mutant which lacks the first 70 amino acids at the NH2 terminus of Bag-1L (Fig. 2A). Both constructs showed significantly reduced ability, compared to the full-length Bag-1L, to enhance the transactivation function of the AR (Fig. 2B).
FIG. 2.
Bag-1L but not Bag-1M strongly enhances the transactivation function of the AR. (A) Schematic diagram of the Bag-1 constructs used in the transfection experiment showing the Hsp70 binding domain (Hsp70BD), the ubiquitin-like domain (UbiLD), and the NLSs for Bag-1L and NLS-Bag-1M. (B) N-terminal and C-terminal sequences of Bag-1L contribute to the enhancement of transactivation function of the AR by Bag-1L. PC3 cells were transiently transfected with 0.6 μg of pGL3-MMTV firefly luciferase reporter plasmid, 0.1 μg of Renilla luciferase construct, and 0.2 μg of empty expression vector or expression vector containing the coding sequence of the AR with 0.5 μg of control vector or BAG-1L, BAG-1LΔC, BAG-1M, or NLS-BAG-1M expression plasmid. At 24 h after transfection, the cells were treated with vehicle alone (0.1%) or vehicle containing 10−7 M DHT. After incubation for an additional 20 h, the cells were harvested andluciferase activity measurements were made. The results are expressed as the relative luciferase activity (firefly/Renilla) and presented in bar charts as the mean and standard deviation for at least four independent experiments. (C) Bag-1L and NLS-Bag-1M, but not Bag-1M, are localized in the nuclear compartment of the cell. PC3 cells were transiently transfected with 1.5 μg of HA-tagged BAG-1L, BAG-1M, or NLS-BAG-1M expression vector. At 48 h after transfection, the cells were harvested and stained with mouse monoclonal anti-HA antibody 12CA5 followed by an anti-mouse antibody labeled with fluorescent dye Cy2. The cells were visualized with an LSM 510 inverted Zeiss confocal microscope. Subcellular localization of the Bag-1 proteins was determined by the percentage of positive stained cells and by categorizing them into the four indicated stages. The bar chart shows the percentage of cells at each stage, and this was obtained from three independent experiments with more than 3,000 cells counted per experiment.
The NH2 terminus of Bag-1L contains an NLS, which is missing in Bag-1M and could contribute to the different cellular localization and as a result produce differences in the functions of the two proteins. To investigate this, we linked an NLS from simian virus 40 to the NH2 terminus of Bag-1M to target it to the nucleus (Fig. 2A) and then compared its action with that of Bag-1L in enhancing transactivation by the AR. In immunofluorescence studies, about 70% of the cells transfected with Bag-1L showed nuclear localization of this protein, with only a few cells showing both nuclear and cytoplasmic or purely cytoplasmic staining (stages S1 to S4 in Fig. 2C). A similar distribution was also seen with the NLS-Bag-1M protein. In contrast, Bag-1M was mainly cytoplasmic, as shown by the distribution of the proteins mainly at the S3 and S4 stages (Fig. 2C). The cellular localization of these Bag-1 proteins was not altered by the addition of androgen (results not shown). In functional studies, transactivation by the AR was significantly increased by NLS-Bag-1M compared to the effect of Bag-1M, indicating the requirement of nuclear localization of Bag-1M for its effect on the AR. Nonetheless, the effect of NLS-Bag-1M on transactivation by the AR was still less pronounced than that of Bag-1L (Fig. 2B). This shows that additional NH2-terminal sequences of Bag-1L other than the NLS are necessary for enhancing the transactivation function of the AR. This conclusion differs from that of a previous publication of Knee et al. (26), where targeted expression of Bag-1M into the nucleus enhanced transactivation by the AR to the same extent as Bag-1L. The reason for the difference in these results and our present findings is unclear.
Differential binding of Bag-1L and Bag-1M to the AR.
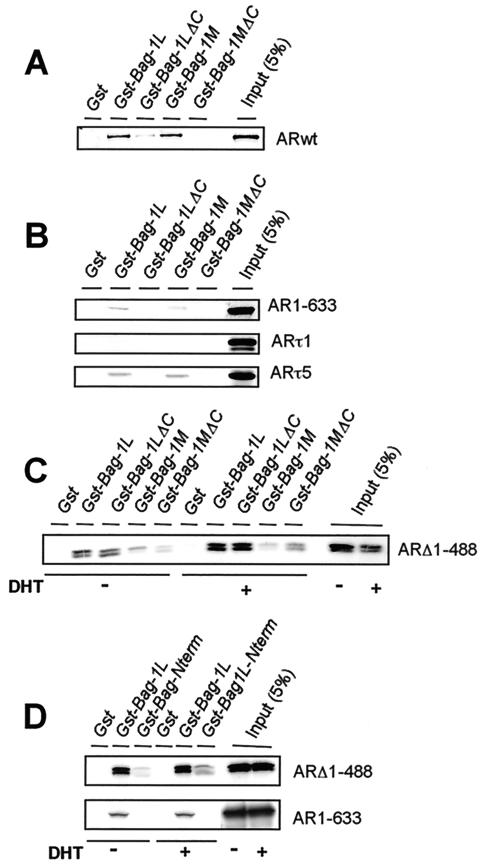
Our finding that structural differences in Bag-1L and Bag-1M may also account for their differences in regulation of AR action prompted us to examine the interaction of the two Bag-1 proteins with the AR. Equal amounts of bacterially purified GST, GST-Bag-1L, GST-Bag-1M, and the GST-Bag-1 proteins lacking the COOH terminus (GST-Bag-1LΔC and GST-Bag-1MΔC) were immobilized onto glutathione-Sepharose and allowed to interact in vitro with radioactively labeled AR treated with or without DHT.
AR interacted strongly with GST-Bag-1L and GST-Bag-1M. It also bound weakly to GST-Bag-1LΔC but not to GST-Bag-1MΔC or GST alone (Fig. 3A). This suggests differences in the way the AR interacts with the two Bag-1 proteins. If, instead of the intact AR, a truncated receptor consisting of the NH2-terminal region of the AR (AR1-633) was used in the binding assay, this truncated receptor bound only to GST-Bag-1L and GST-Bag-1M but not to their COOH-terminal deletion mutants. The interaction of the Bag-1 proteins with the NH2-terminal region of the AR could be further delineated to a defined region by the use of the NH2-terminal τ5 and τ1 domains. The τ1 domain did not show any significant binding to the Bag-1 proteins, but the τ5 domain bound to both GST-Bag-1L and GST-Bag-1M but not to the COOH-terminal deletion mutants of Bag-1L and Bag-1M (Fig. 3B). These data therefore identified the τ5 domain on the AR as the site of interaction by the Bag-1 proteins. At the same time, they revealed that the COOH-terminal domains of the Bag-1 proteins are important for the interaction with τ5 since deletion of these sequences destroyed the binding. However, since Bag-1LΔC but not Bag-1MΔC interacted with the wild-type AR, it shows that the NH2 terminus of Bag-1L must make additional contact with the AR. The interaction of Bag-1L and Bag-1M with the NH2 terminus of the AR was not altered if the AR was ligand bound prior to the interaction studies (results not shown).
FIG. 3.
Bag-1L binds to the C- and N-terminal regions of the AR. Distinct domains of the AR interact with Bag-1L and Bag-1M. The human AR and various fragments of the receptor were translated in vitro, and the [32S]methionine-labeled translation products were incubated with glutathione-agarose beads to which GST, GST-Bag-1L, GST-Bag-1LΔC, GST-Bag-1M, or GST-Bag-1MΔC are bound. The beads were washed, and bound proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by autoradiography. Portions of the 32S-labeled in vitro-translated proteins corresponding to 5% of the protein amounts used in the binding reactions were loaded onto the gel as input. (A to C) Interaction of Bag-1 proteins with the wild-type AR or NH2- or COOH-terminal fragments as well as the τ1 and τ5 fragments of the receptor; (D) binding of the Bag-1L N-terminal fragment to the COOH- but not the NH2-terminal sequence of the AR. In panels C and D, the in vitro-translated mutant AR ARΔ1-488 and AR1-633 were incubated with or without 10−7 M DHT before being used in the binding reaction.
The difference in the binding of the two Bag-1 proteins to the AR was demonstrated in studies where these proteins were allowed to interact with the COOH terminus of the AR (ARΔ1-488). Both Bag-1L and Bag-1LΔC interacted with ARΔ1-488 with an increased binding in the presence of DHT. However, binding to Bag-1M or Bag-1MΔC was severely compromised and was not influenced by androgen (Fig. 3C). Therefore, it appears that the NH2-terminal sequences of Bag-1L that are missing in Bag-1M may play a role in the interaction with the COOH terminus of the AR.
A further confirmation of the involvement of the NH2-terminal sequence of Bag-1L in interaction with the AR was obtained in experiments where amino acids 1 to 127 of the NH2 terminus of Bag-1L were fused to GST and used in the pull-down study. This NH2-terminal Bag-1L fusion protein was compared with GST-Bag-1L in its ability to interact with the COOH- and NH2-terminal fragments of the AR. GST-Bag-1L interacted with both the COOH- and NH2-terminal fragments of the AR (ARΔ1-488 and AR1-633) (Fig. 3D). In contrast, the 127 N-terminal amino acid fragment of Bag-1L (GST-Bag-1L-Nterm) bound only to the COOH-terminal fragment of the AR (ARΔ1-488) but not to the NH2-terminal fragment (AR1-633) (Fig. 3D). Therefore, the NH2 terminus of Bag-1L binds to the COOH terminus of the AR. Since this region is missing in Bag-1M, this protein interacts primarily with the NH2 terminus of the receptor, and this may partly account for the difference in regulation of the transactivation function of the AR by Bag-1L and Bag-1M. The other factor that also contributes to the difference is the cellular localization of the two proteins.
Hsp70 enhances binding of Bag-1L to the τ5 domain of the androgen receptor.
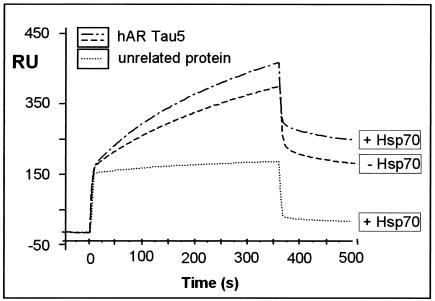
Since the COOH terminus of Bag-1L contains an interaction surface for Hsp70 (8), we investigated whether Hsp70 influences the binding of Bag-1L to the τ5 domain of the AR in surface plasmon resonance experiments.
A GST-τ5 fusion protein captured with an anti-GST antibody covalently linked to a carboxymethylated (CM5) sensor chip was used for interaction studies with bacterially purified Bag-1L cleaved from GST. In the presence of Hsp70, a small but reproducible increase in the interaction of Bag-1L with the τ5 domain of the AR was measured. Injection of an unrelated protein (GST) resulted in a low, nonspecific binding signal that was not affected by the presence or absence of Hsp70 (Fig. 4). Furthermore, Hsp70 did not bind to τ5 of the AR or to an unrelated anti-His antibody (results not shown).
FIG. 4.
Hsp70 enhances the interaction of Bag-1L with the τ5 domain of the AR. A representative sensogram is presented, showing the interaction of Bag-1L (100 ng/μl) with the τ5 domain of the AR (1,500 RU) coupled to the surface of a CM5 sensor chip. The interaction data were obtained in the absence and presence of Hsp70 (100 ng/μl) and compared to the use of an unrelated protein (recombinant GST; 100 ng/μl). The values for the apparent rate constants for the interaction of Bag-1L with τ5 of the AR are as follows: _K_a, 1.41 × 103 M−1s−1; _K_d, 8.54 × 10−4 s−1. From these data, the apparent dissociation equilibrium constant was calculated to be _K_D = 6.07 × 10−7 M. All calculations were based on the assumption of monomolecular 1:1 binding characteristics.
Hsp70 enhances the effect of Bag-1 on transactivation by the AR.
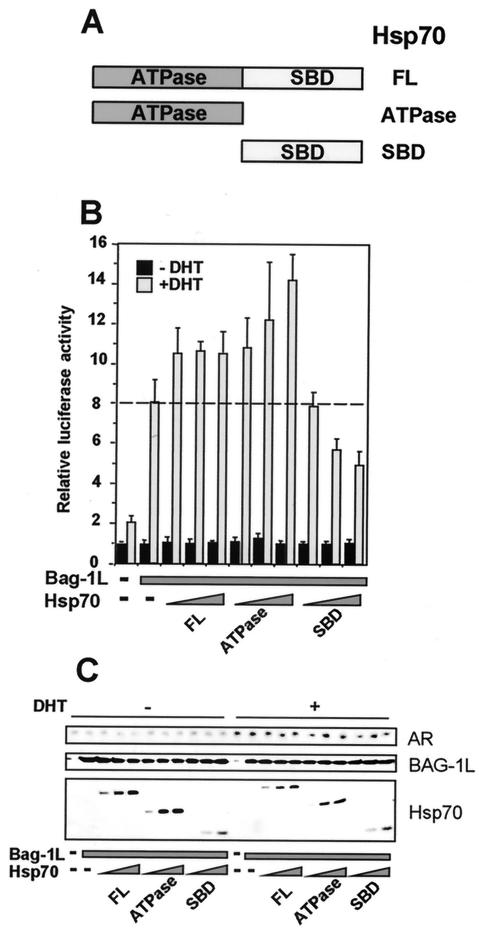
Since Hsp70 affects the binding of Bag-1L to τ5 of the AR, we investigated its effect on Bag-1L-mediated enhancement of transactivation by the AR. We used an expression vector encoding the wild-type Hsp70 or its ATPase domain or substrate binding domain (Fig. 5A). Overexpression of the full-length Hsp70 or its ATPase domain further potentiated the enhancing action of Bag-1L on transactivation by the AR (Fig. 5B). Note that the ATPase domain of Hsp70 is known to interact with the COOH terminus of Bag-1L (42). In contrast, the substrate binding domain of Hsp70, which interacts with the HBD of the AR (38), exerted a dominant negative effect on Bag-1L-mediated activation of the transactivation function of the AR (Fig. 5B). These different effects of the two domains of Hsp70 are not due to differences in the level of AR or Bag-1L, as shown by an immunoblot assay (Fig. 5C).
FIG. 5.
Overexpression of Hsp70 potentiates Bag-1L-mediated enhancement of transactivation by the AR. (A) Schematic diagram of the wild-type, ATPase, and substrate binding (SBD) domains of the Hsp70 constructs used in the transfection experiments. (B) The ATPase domain of Hsp70 potentiates but the substrate binding domain attenuates the effect of Bag-1L on transactivation by the AR. PC3 cells were transiently transfected with FuGENE 6 transfection reagent with 0.6 μg of pGL3-MMTV firefly luciferase indicator gene, 0.1 μg of Renilla luciferase construct 0.2 μgARwt, and 0.5 μg of pcDNA3 vector or BAG-1L expression plasmid in the absence or presence of different amounts (0.25, 0.5, or 1.0 μg) of expression vector encoding LDH-tagged Hsp70, Hsp70ΔATPase, or Hsp70ΔSBD. At 24 h after transfection, the cells were treated with vehicle alone (0.1% ethanol) or vehicle containing 10−7 M DHT and were harvested 20 h later for luciferase activity measurements. The results are expressed as relative luciferase activity (firefly/Renilla) and presented in the form of a bar chart as the mean and standard deviation for at least three different experiments. (C) The level of expression of the transfected AR, Bag-1L, and Hsp70 is shown in an immunoblot assay performed with an anti-AR antibody, an anti-Bag-1L-specific antibody, and an antibody that detects the LDH tag on the Hsp70 proteins.
Interaction of Bag-1L, Hsp70 and AR with AREs.
The effect of Hsp70 in the modulation of the AR response by Bag-1L suggested that this molecular chaperone may also be involved in the regulation of the transactivation function of the AR.
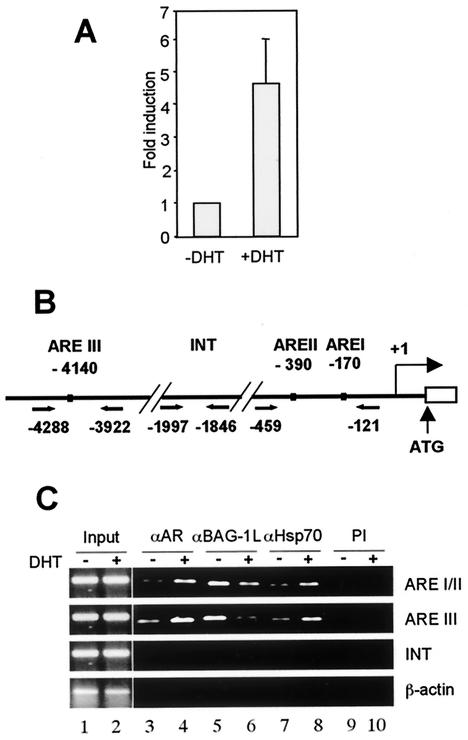
We therefore used the technique of chromatin immunoprecipitation (ChIP) to determine whether Hsp70 and Bag-1L are present together with the AR on the androgen response element (ARE) of an androgen-regulated gene. Although the androgen-responsive LNCaP cell line has been used in ChIP analysis involving binding of the AR to the promoter of the PSA gene (37), these cells contain low levels of Bag-1L. We therefore used the prostate cell line 22Rv1, which had a higher level of Bag-1L expression in an immunoblot assay (results not shown).
In real-time PCR analyses, we could show that treatment of the 22Rv1 cells with DHT for 5 h led to a four- to sixfold increase in expression of the PSA gene (Fig. 6A). These cells were therefore used to determine the DHT-dependent factor occupancy of the AREs at the promoter-proximal region (AREI and AREII) and at the enhancer region (AREIII) of the PSA gene. As a control of the specificity of interaction of the proteins with DNA, a fragment of the PSA promoter lacking AREs (INT) or a β-actin fragment was amplified (Fig. 6B).
FIG. 6.
Androgen-mediated expression of the PSA gene is correlated with binding of the AR, Hsp70, and Bag-1L at the response elements of the gene. (A) Quantitative real-time PCR analysis of the expression of the PSA gene in 22Rv1 cells in the absence and presence of androgen. Total RNA was extracted from 22Rv1 cells treated with 10−7 M DHT for 5 h or left untreated, and the RNA was subjected to real-time reverse transcription-PCR analysis. After amplification, the level of PSA mRNA was determined with reference to a control β-actin reverse transcription-PCR amplification. The expression level was calculated based on the threshold cycle value. The bar chart represents the mean and standard deviation for three independent determinations. (B) Schematic diagram of the PSA gene-regulatory region. Depicted are the putative AREs, along with pairs of arrows indicating the regions specifically amplified in the PCR after the ChIP assay. The numbers are the positions upstream of the PSA gene transcription start site. (C) Occupancy of the PSA gene-regulatory region by AR, Bag-1L, and Hsp70 in a ChIP assay. Soluble chromatin was prepared from 22Rv1 cells treated with DHT for 30 min and immunoprecipitated with antibody to AR (αAR), BAG-1L (αBAG-1L), or Hsp70 (αHsp70), along with preimmune serum (PI) as control. The final DNA extractions were amplified using the primers described for panel B.
In the absence of DHT, low levels of AR and Hsp70 were present in the nucleus since DNA fragments containing the AREs could be immunoprecipitated with antibodies to the AR and Hsp70 (Fig. 6C, lanes 3 and 7). On hormone treatment, the AR and Hsp70 were recruited to the AREs, resulting in an increase in the signal of the PCR products (lanes 4 and 8). Interestingly, Bag-1L was associated with the AREs in the absence of hormone (lane 5). This agrees with a recent report on the localization of Bag-1L in the nucleus and its interaction directly or indirectly with distinct sequences to increase the transcription of specific genes (33). This finding also confirms our results of a weak enhancement of the MMTV promoter activity by Bag-1L in the absence of androgen (Fig. 1A and 2B). Intriguingly, on hormone treatment, the signal representing the level of Bag-1L at the response element was reduced (Fig. 6C, compare lanes 5 and 6). This is perhaps a result of Bag-1L making way for the AR, Hsp70, and other coactivators that are recruited to the nucleus by the hormone to interact with the AREs. Once these factors get into the nucleus, they are bound by Bag-1L for the transcriptional activation of the PSA promoter.
Cellular localization of the AR, Hsp70, and Bag-1L.
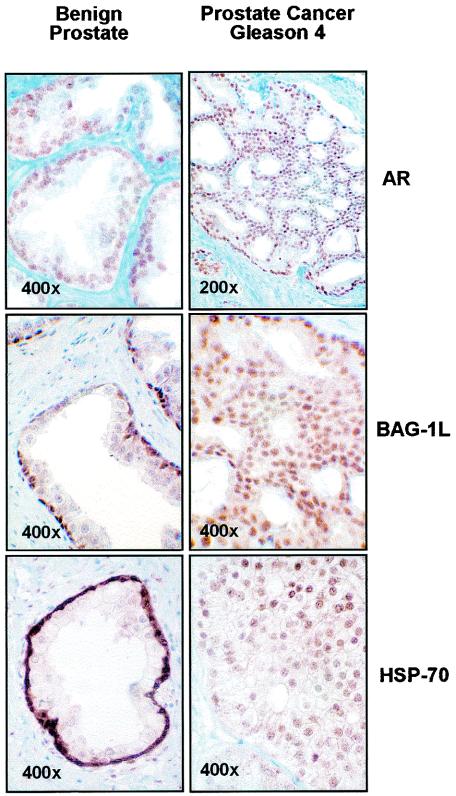
For the AR, Bag-1L, and Hsp70 to have an effect on the regulation of androgen responsive genes, these proteins must be present in the nuclear compartment of the target cells. While this is the case in transfected cells, it may differ in intact cells. We therefore used immunohistochemical staining to determine the cellular localization of endogenous AR, Hsp70, and Bag-1L in the human prostate in the nontumor and tumor situations.
We found that Hsp70 was expressed both in the benign prostate and in prostate carcinoma. In the benign prostate, expression was very strong in the nuclei of the basal cells with almost no staining in the secretory epithelial cells (Fig. 7). The nuclei of the stromal cells were also positive, although not as strongly positive as the basal epithelial cells. In prostate carcinoma, Hsp70 staining was heterogenous. In the majority of tumors, there was moderate to strong nuclear and cytoplasmic staining. Nuclear staining was more evident in high (4 to 5) as compared to low (2 to 3) Gleason patterns (Fig. 7 shows strong staining in a Gleason pattern 4 tumor). Bag-1L was predominantly nuclear in benign and malignant epithelial cells and showed no obvious correlation with the Gleason pattern of the tumors. The AR antibody stained the nuclei of the secretory epithelial cells in the benign tissue as well as in all the tumor samples (Fig. 7). These results show that Bag-1L and the AR are expressed in different cell types in the benign prostate tissue but are expressed together with Hsp70 in the nuclear compartment of the secretory epithelial cells in prostate carcinoma. These results, taken together, demonstrate a nuclear action of the molecular chaperone Hsp70 and cochaperone Bag-1L in the regulation of the action of the AR in prostate carcinoma.
FIG. 7.
Bag-1L and Hsp70 but not the AR are expressed in different cells during prostate cancer progression. The immunostaining shows the cellular localization of Bag-1L, the AR, and Hsp70 in the benign prostate and in Gleason grade 4 prostate carcinoma.
DISCUSSION
Molecular chaperones and cochaperones are known to interact and to keep steroid receptors in an inactive state in the absence of hormone. Following hormone binding, the receptor undergoes a conformational change, dissociates from the molecular chaperones, and gains the ability to interact with discrete nucleotide sequences (7, 38). Recent studies have shown that in addition to their role in providing the correct conformation for hormone binding by steroid receptors, molecular chaperones may modulate the transactivation function of nuclear receptors at the level of their interaction with DNA. For example, they participate in DNA binding by the Drosophila ecdysone receptor-RXR heterodimer (3) and in another case the molecular chaperone p23 promotes the disassembly of the transcriptional regulatory complex containing the GR and is involved in receptor recycling (15, 16). Also, Hsp70 and the cochaperone Bag-1M together inhibit the DNA binding and, as a result, the transactivation function of the GR (35).
In the present communication, we show that both Bag-1M and its isoform Bag-1L bind the AR. We have shown that for Bag-1L, Hsp70 enhances its binding to the AR and that overexpression of Hsp70 potentiates the positive regulatory effect of Bag-1L on transactivation by the AR. We have also shown in chromatin immunoprecipitation studies that Hsp70, the AR, and Bag-1L are all present at the AREs on an androgen-responsive gene in prostate carcinoma cells. It therefore appears that the chaperone-cochaperone-AR interaction may be used for the regulation of gene expression by androgens during prostate carcinogenesis. The prerequisite for this regulation is that all three proteins are expressed in the same cell types as we have shown in immunohistochemical studies.
Studies of the expression of Hsp70 in prostate cancer are controversial. While Alaiya et al. reported an increased expression of this protein in malignant compared to benign tissue of the prostate (1), Cornford et al. did not find any alteration in the Hsp70 level in prostate cancer tissues compared with that in nonneoplastic prostate epithelium (11). In our study, we did not find an increased level of Hsp70 when nontumor and prostate tumor tissues were compared in an immunoblot assay (results not shown). However, we showed qualitative changes in the expression pattern of Hsp70. In the basal epithelial cells of the benign prostate, Hsp70 expression was high and homogenous, but in the neoplastic prostate cells, it was disperse and nonhomogenous with enhanced nuclear localization of the protein in high compared to low-Gleason-pattern tumors in the secretory epithelium. In contrast, Bag-1L was more homogenously expressed in tumors with only a few imunohistochemically negative exceptions. AR was also homogenously expressed in all the secretory epithelial cells of the nonmalignant as well as the malignant cells. It is therefore likely that in many prostate cancers, a portion of tumor cells will have all the necessary requirements (i.e., sufficiently high levels of Bag-1L, the AR, and Hsp70) for the increased expression of androgen-responsive genes, in agreement with the results of this study.
In a search for a mechanism by which Bag-1L enhances the transactivation function of the AR, we have shown that it interacts with the NH2- and COOH-terminal sequences of the AR. Bag-1M, an isoform of Bag-1L that interacts strongly with the NH2 terminus of the AR but less strongly with the COOH terminus, has a rather reduced effect on the transactivation function of the receptor. NH2- and COOH-terminal interactions play an important role in AR action. Normally during transactivation by nuclear receptors, activation function 2 in the ligand binding domains forms a hydrophobic cleft that binds the motif LXXLL of p160 transcriptional coactivators. In the AR, activation function 2 only weakly interacts with p160 coactivators but serves as a contact site for the androgen-dependent NH2-/COOH-terminal interaction (20). This NH2/COOH-terminal interaction creates a new binding surface for the recruitment of coactivators that robustly enhances the transactivation function of the receptor (28). Bag-1L may play a significant role in this process and/or in the recruitment of coactivators. In preliminary experiments, we observed that sequences overlapping the E1A binding site (amino acids 1752 to 1998) of the coactivator p300 bind to the NH2-terminal region of Bag-1L. Furthermore, we could show that sequences encompassing the adenovirus EIA protein binding site of p300 exert a dominant negative effect on Bag-1L-mediated enhancement of transactivation by the AR (S. Mink, and A. C. B. Cato, unpublished observation). These finding suggest the involvement of p300 in the enhancing action of Bag-1L. A ternary complex of AR-Bag-1L and p300, however, remains to be established.
It appears that the Bag-1 proteins use different mechanisms to modulate steroid hormone action. We have previously shown that Bag-1M and Bag-1L negatively regulate the transactivation function of the GR (35). Analysis of the mechanism of action showed that Bag-1M binds to the hinge region of the GR and inhibits DNA binding and the transactivation function of the receptor. In the present studies, we have shown that the hinge region of the AR is not a target for regulation by Bag-1L. Amino acid exchanges in the hinge region of the GR, making it look like the AR, destroyed the interaction of the Bag-1 proteins with the mutant GR and abrogated the negative regulatory action of the Bag-1 proteins on transactivation by the mutant GR (results not shown). This shows that the action of the Bag-1 proteins is dependent primarily on their ability to interact with distinct regions of steroid receptors. The exact sequences that determine where the Bag-1 proteins will bind the steroid receptors remain to be determined.
The Bag-1 proteins are therefore a family of cochaperones that have diverse effects on the transcriptional activity of steroid receptors. Since their levels and cellular location may vary in different physiological states, these cochaperones may turn out to be very important regulators of steroid receptor action in various physiological and pathophysiological situations.
Acknowledgments
We thank Alex Dimerski, Berlin, Germany, for his excellent technical assistance.
This work was supported by the DFG, BMBF grant 0310681B and the Deutsche Krebshilfe. L.S was a recipient of a scholarship from the Schering Research Foundation.
REFERENCES
- 1.Alaiya, A. A., M. Oppermann, J. Langridge, U. Roblick, L. Egevad, S. Brindstedt, M. Hellstrom, S. Linder, T. Bergman, H. Jornvall, and G. Auer. 2001. Identification of proteins in human prostate tumor material by two-dimensional gel electrophoresis and mass spectrometry. Cell Mol. Life Sci. 58**:**307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anfinsen, C. B. 1973. Principles that govern the folding of protein chains. Science 181**:**223-230. [DOI] [PubMed] [Google Scholar]
- 3.Arbeitman, M. N., and D. S. Hogness. 2000. Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell 101**:**67-77. [DOI] [PubMed] [Google Scholar]
- 4.Bellmann, K., M. Jäättelä, D. Wissing, V. Burkart, and H. Kolb. 1996. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 391**:**185-188. [DOI] [PubMed] [Google Scholar]
- 5.Bohen, S. P., A. Kralli, and K. R. Yamamoto. 1995. Hold 'em and fold 'em: chaperones and signal transduction. Science 268**:**1303-1304. [DOI] [PubMed] [Google Scholar]
- 6.Brehmer, D., S. Rudiger, C. S. Gässler, D. Klostermeier, L. Packschies, J. Reinstein, M. P. Mayer, and B. Bukau. 2001. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat. Struct. Biol. 8**:**427-432. [DOI] [PubMed] [Google Scholar]
- 7.Bresnick, E. H., F. C. Dalman, E. R. Sanchez, and W. B. Pratt. 1989. Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem. 264**:**4992-4997. [PubMed] [Google Scholar]
- 8.Briknarova, K., S. Takayama, L. Brive, M. L. Havert, D. A. Knee, J. Velasco, S. Homma, E. Cabezas, J. Stuart, D. W. Hoyt, A. C. Satterthwait, M. Llinas, J. C. Reed, and K. R. Ely. 2001. Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat. Struct. Biol. 8**:**349-352. [DOI] [PubMed] [Google Scholar]
- 9.Buchner, J. 1999. Hsp90 & Co.—a holding for folding. Trends Biochem. Sci. 24**:**136-141. [DOI] [PubMed] [Google Scholar]
- 10.Cato, A. C., and S. Mink. 2001. BAG-1 family of cochaperones in the modulation of nuclear receptor action. J. Steroid Biochem. Mol. Biol. 78**:**379-388. [DOI] [PubMed] [Google Scholar]
- 11.Cornford, P. A., A. R. Dodson, K. F. Parsons, A. D. Desmond, A. Woolfenden, M. Fordham, J. P. Neoptolemos, Y. Ke, and C. S. Foster. 2000. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 60**:**7099-7105. [PubMed] [Google Scholar]
- 12.Crocoll, A., M. Blum, and A. C. Cato. 2000. Isoform-specific expression of BAG-1 in mouse development. Mech. Dev. 91**:**355-359. [DOI] [PubMed] [Google Scholar]
- 13.Dittmar, K. D., and W. B. Pratt. 1997. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J. Biol. Chem. 272**:**13047-13054. [DOI] [PubMed] [Google Scholar]
- 14.Freeman, B. C., S. J. Felts, D. O. Toft, and K. R. Yamamoto. 2000. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 14**:**422-434. [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman, B. C., and K. R. Yamamoto. 2001. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem. Sci. 26**:**285-290. [DOI] [PubMed] [Google Scholar]
- 16.Freeman, B. C., and K. R. Yamamoto. 2002. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296**:**2232-2235. [DOI] [PubMed] [Google Scholar]
- 17.Froesch, B. A., S. Takayama, and J. C. Reed. 1998. BAG-1L protein enhances androgen receptor function. J. Biol. Chem. 273**:**11660-11666. [DOI] [PubMed] [Google Scholar]
- 18.Frydman, J., and J. Höhfeld. 1997. Chaperones get in touch: the Hip-Hop connection. Trends Biochem. Sci. 22**:**87-92. [DOI] [PubMed] [Google Scholar]
- 19.Gast, A., J. Schneikert, and A. C. Cato. 1998. N-terminal sequences of the human androgen receptor in DNA binding and transrepressing functions. J. Steroid Biochem. Mol. Biol. 65**:**117-123. [DOI] [PubMed] [Google Scholar]
- 20.He, B., J. A. Kemppainen, J. J. Voegel, H. Gronemeyer, and E. M. Wilson. 1999. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH(2)-terminal domain. J. Biol. Chem. 274**:**37219-37225. [DOI] [PubMed] [Google Scholar]
- 21.He, B., J. A. Kemppainen, and E. M. Wilson. 2000. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J. Biol. Chem. 275**:**22986-22994. [DOI] [PubMed] [Google Scholar]
- 22.Herscovics, A., J. Schneikert, A. Athanassiadis, and K. W. Moremen. 1994. Isolation of a mouse Golgi mannosidase cDNA, a member of a gene family conserved from yeast to mammals. J. Biol. Chem. 269**:**9864-9871. [PubMed] [Google Scholar]
- 23.Höhfeld, J. 1998. Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol. Chem. 379**:**269-274. [PubMed] [Google Scholar]
- 24.Jäättelä, M., D. Wissing, P. A. Bauer, and G. C. Li. 1992. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 11**:**3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenster, G., H. A. van der Korput, J. Trapman, and A. O. Brinkmann. 1995. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J. Biol. Chem. 270**:**7341-7346. [DOI] [PubMed] [Google Scholar]
- 26.Knee, D. A., B. A. Froesch, U. Nuber, S. Takayama, and J. C. Reed. 2001. Structure-function analysis of Bag1 proteins. Effects on androgen receptor transcriptional activity. J. Biol. Chem. 276**:**12718-12724. [DOI] [PubMed] [Google Scholar]
- 27.Kullmann, M., J. Schneikert, J. Moll, S. Heck, M. Zeiner, U. Gehring, and A. C. Cato. 1998. RAP46 is a negative regulator of glucocorticoid receptor action and hormone-induced apoptosis. J. Biol. Chem. 273**:**14620-14625. [DOI] [PubMed] [Google Scholar]
- 28.Langley, E., Z. X. Zhou, and E. M. Wilson. 1995. Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J. Biol. Chem. 270**:**29983-29990. [DOI] [PubMed] [Google Scholar]
- 29.Milarski, K. L., and R. I. Morimoto. 1989. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J. Cell Biol. 109**:**1947-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mink, S., B. Haenig, and K. H. Klempnauer. 1997. Interaction and functional collaboration of p300 and C/EBPbeta. Mol. Cell. Biol. 17**:**6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mink, S., U. Kerber, and K. H. Klempnauer. 1996. Interaction of C/EBPbeta and v-Myb is required for synergistic activation of the mim-1 gene. Mol. Cell. Biol. 16**:**1316-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto, R. I. 2002. Dynamic remodeling of transcription complexes by molecular chaperones. Cell 110**:**281-284. [DOI] [PubMed] [Google Scholar]
- 33.Niyaz, Y., M. Zeiner, and U. Gehring. 2001. Transcriptional activation by the human Hsp70-associating protein Hap50. J. Cell Sci. 114**:**1839-1845. [DOI] [PubMed] [Google Scholar]
- 34.Peterziel, H., S. Mink, A. Schonert, M. Becker, H. Klocker, and A. C. B. Cato. 1999. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18**:**6322-6329. [DOI] [PubMed] [Google Scholar]
- 35.Schneikert, J., S. Hübner, G. Langer, T. Petri, M. Jäättelä, J. Reed, and A. C. Cato. 2000. Hsp70-RAP46 interaction in downregulation of DNA binding by glucocorticoid receptor. EMBO J. 19**:**6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneikert, J., S. Hübner, E. Martin, and A. C. Cato. 1999. A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J. Cell Biol. 146**:**929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang, Y., M. Myers, and M. Brown. 2002. Formation of the androgen receptor transcription complex. Mol. Cell 9**:**601-610. [DOI] [PubMed] [Google Scholar]
- 38.Smith, D. F., and D. O. Toft. 1993. Steroid receptors and their associated proteins. Mol. Endocrinol. 7**:**4-11. [DOI] [PubMed] [Google Scholar]
- 39.Sondermann, H., C. Scheufler, C. Schneider, J. Höhfeld, F. U. Hartl, and I. Moarefi. 2001. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291**:**1553-1557. [DOI] [PubMed] [Google Scholar]
- 40.Sramkoski, R. M., T. G. Pretlow, Jr., J. M. Giaconia, T. P. Pretlow., S. Schwartz, M. S. Sy, S. R. Marengo, J. S. Rhim, D. Zhang and J. W. Jacobberger. 1999. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev. Biol. Anim. 35**:**403-409. [DOI] [PubMed] [Google Scholar]
- 41.Takayama, S., and J. C. Reed. 2001. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 3**:**E237-E241. [DOI] [PubMed] [Google Scholar]
- 42.Zeiner, M., M. Gebauer, and U. Gehring. 1997. Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 16**:**5483-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]