Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds (original) (raw)
Abstract
This study aimed at characterizing the Sonic hedgehog (shh) gene in newt limbs, which encodes a signaling molecule of the zone of polarizing activity (ZPA) responsible for determining the anterior–posterior axis of the embryonic chicken and mouse limbs. The reverse transcription–PCR showed that adult newt regenerating limbs express shh genes. In situ hybridization experiments demonstrated that shh genes were expressed in mesenchymal cells of the posterior region of both embryonic buds and regenerating blastemas of newt limbs, strongly suggesting the presence of ZPA in these tissues. Experiments of the axial reversal graft of blastemas further supported this suggestion. The grafted blastemas regenerated supernumerary limbs, and this has been explained by three models: the polar coordinate model, the boundary model, and the polarizing zone model. In favor of the third model, the shh gene was expressed not only in the original region (new anterior region) of the graft, but also ectopically in the other region (new posterior region) of the same graft. This study implies that the regenerating limb blastema produces ZPA as the signaling center of the AP patterning as in the developing limb bud and, therefore, supports the notion that the limb regeneration recapitulates the limb development.
Keywords: limb regeneration, zone of polarizing activity, supernumerary limbs, recapitulation
The urodele is the only adult vertebrate that can regenerate its limbs (1, 2). Although the process of limb regeneration has remained obscure at the molecular level, substantial progress has recently been made on expressions of morphogenesis-related genes during embryonic limb development (3–10). Some evidence suggests that these two processes share mechanisms of growth regulation and pattern formation (11–14). For example, the expression of homeobox genes during urodele limb regeneration is broadly overlapped with that during limb development (14–18). However, a case was also reported in which some HoxA genes are expressed in the regenerating limbs in a way different from the developing limbs (14).
One of the striking features of limb regeneration is that the regenerate is the correct copy of a missing part, which is formed in the three dimensions, the antero-posterior (AP), dorso-ventral (DV), and proximo-distal axes. The AP patterning has been a target of the most intensive investigation among the three since the discovery of the zone of polarizing activity (ZPA) in a developing chicken limb bud by Saunders and Gasseling (19). ZPA evokes supernumerary digit formation when grafted into the anterior mesenchyme of limb buds, and plays an essential role in their AP patterning.
A group led by Tabin studied the Sonic hedgehog (shh) gene, a vertebrate homologue of the Drosophila hedgehog (hh) gene, and showed that its expression was restricted to ZPA in the chicken limb bud (4). Furthermore, they demonstrated that the implantation of retinoic acid-containing beads into the anterior mesenchyme of limb buds caused duplication of shh expression and resulted in digit duplication (4). When pellets of cells transfected with a retroviral construct containing the shh gene were grafted under the anterior apical ectodermal ridge of a host limb bud, mirror-image duplication digits were induced to develop (4). Therefore, it is likely that shh is a specific molecular marker for ZPA and may play a role in determining the AP patterning of limb buds (2, 4, 7).
Despite its unique position as an experimental animal in the study of development and regeneration of limbs, there have been no reports that investigated at the molecular level whether the urodele forms ZPA when it develops and regenerates its limbs. It should be reasonable to postulate the presence of ZPA and its role for the AP patterning in urodele limb bud as in that of chicken and mouse (20, 21). On the other hand, there is no logical basis to assume the presence of ZPA in regenerating urodele blastema, because the limb of adult chicken and mouse does not have the regeneration capacity and, therefore, no studies have been available that ask whether ZPA is involved in the AP patterning of limb regeneration.
It has been well established that the axial reversal graft of blastema regenerates supernumerary limbs (1, 2). Three models have been proposed to explain the supernumerary limb formation; the polar coordinate model (22, 23), the boundary model (24–26), and the polarizing zone model (27). The supernumerary limb is composed of both graft and stump cells, suggesting the occurrence of mutual and reciprocal cellular interactions between graft and stump cells during its formation (28–32). The first two models postulate an important role of these interactions for the pattern formation and do not assume the presence of polarizing zones like ZPA in blastemas, although the molecular events involved in the interaction have not been clarified. If this is the case, AP patterning in the limb blastema is not a recapitulation of that in the limb buds.
This study showed that the expression of newt shh is regulated region- and developmental stage-dependently in both embryonic limb buds and regenerating blastemas in a way similar to that in the chicken and mouse embryonic limb development and, therefore, suggested that the _shh_-expressing region is ZPA. Additional evidence that the newt blastema contains ZPA was obtained by the grafting experiment of AP- or APDV-axes reversed blastemas, which regenerate supernumerary limbs. The grafted blastemas were shown to express shh ectopically in a newly induced ZPA-corresponding region of the extra limb. Therefore, this study strongly supports the notion that the AP patterning in the limb blastema is regulated by the _shh_-expressing ZPA and is a recapitulation of that in limb bud.
MATERIALS AND METHODS
Animals.
Adult newts, Cynops pyrrhogaster, were purchased from a local animal supplier. Female newts were injected with 100 units/day of human gonadotropin (Teikoku Zouki, Tokyo) for 3 days to obtain embryos (33). Embryos, larvae, and adults were reared at 25°C throughout these experiments. Embryos and larvae were staged according to the normal tables described by Okada and Ichikawa (34): stage 38, early limb bud stage; stage 40, medium limb bud stage; stage 42, late limb bud stage; stage 45, two to three digits were formed; stage 55, four digits and two to three toes were formed in forelimbs and hindlimbs, respectively. Larvae at stage 55 and adult newts were anesthetized with 0.025% and 0.1% MS222 (Sigma) for 10 min and 20 min, respectively. Their forelimbs were amputated at the level of the mid-upper arm (stylopodium). Blastemas of regenerating limbs were staged according to Iten and Bryant (35). Regeneration stages of larvae were as follows: 4 days after amputation, early bud to medium bud stage; 6 days after amputation, late bud stage; 8 days after amputaton, late bud to palette stage; and 12 days after amputation, four digits per limb. Regeneration stages of adults were as follows: 14 days, dedifferentiation to early bud stage; 21 days, medium bud stage; and 28 days, palette stage.
Expression of shh Gene.
Total RNA was extracted from adult forelimbs 8 days after amputation (36) and converted to cDNA using a first strand synthesis kit (Pharmacia). PCR was performed using the cDNA and the degenerate primers as described (4) with 35 cycles of denaturation (94°C, 1 min), annealing (47°C, 1 min), and extension (72°C, 2 min). A 220-bp PCR product was isolated from gels, cloned into pCRII vectors by a TA cloning kit (Invitrogen), and sequenced using a Applied Biosystems 373A DNA sequencer.
Whole-Mount in Situ Hybridization.
The protocol of Gardiner et al. (14) was used with slight modifications. Tissues were treated with proteinase K at 37°C for 5 min for limb buds or 15 min for blastemas. After the chromogenic reactions, tissues were rinsed twice with alkaline–phosphatase buffer (14) containing 100 mM Tris (pH 9.5), 100 mM NaCl, and 50 mM MgCl2, and twice with 50% methanol in 80% phosphate buffered saline (PBS) with 0.1% Tween 20 (80% PBS–Tween), stored at 4°C, and then photographed. In the case of both paraffin sections and grafted blastemas, tissues after the chromogenic reactions were rinsed twice with alkaline–phosphate buffer, postfixed overnight in Bouin’s fixative, rinsed in 70% ethanol, and then stored in methanol at −20°C. Grafted tissues were cleared in xylene and photographed. Tissues for paraffin section were cleared in xylene, embedded in paraffin, and sectioned at 10 μm for histological observations. Sections were mounted on glass slides, deparaffinized, and mounted with Marinol (Muto, Japan). The sense and antisense shh probes were prepared by digesting the newt full-length shh cDNA provided by Takabatake (ref. 37; GenBank accession no. D63339) with _Sph_I as an 1,100 bp fragment containing 810 bp of coding region and 290 bp of 3′ untranslated region, and were labeled with digoxigenin according to the manufacturer’s protocol (Boehringer Mannheim).
Grafting of Blastemas.
Adult forelimbs were amputated at a mid-upper arm level. Blastemas at medium bud stage were separated from the stumps and autografted to the original stumps with 180° rotation (reversal of APDV axes) or to the contralateral stumps without rotation (reversal of AP axis). Grafted blastemas were fixed to stumps by tungsten needles. As the control experiments, blastemas were similarly autografted to the original stumps without rotation. Some regenerates from grafted limbs were analyzed for shh expression at 10–14 days after grafting by the whole-mount in situ hybridization as described above. The tissues were cleared in xylene after the hybridization to make the tissues transparent. The remaining experimental animals were reared further for 3 months until supernumerary limbs were fully developed. The regenerates were cut through a middle region of the stump of upper arm, fixed in 10% formalin, and stained with 1% Victoria blue B to observe the cartilage pattern (38, 39).
RESULTS AND DISCUSSION
Expression of shh in Regenerating Limbs.
We asked if regenerating adult newt limbs contained messages of shh gene. A single DNA fragment with the expected size of 220 bp was produced from RNA by RT-PCR of limbs 8 days after amputation. The nucleotide sequence of the fragment was identical to the newt shh reported by Takabatake et al. (37). This result indicates that the shh gene is actually transcribed in regenerating adult newt limbs.
Expression Pattern of shh in Developing Limb Buds.
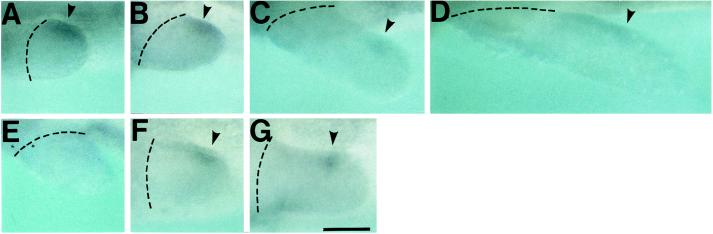
We examined the expression pattern of shh in developing limb buds by whole-mount in situ hybridization. Limb buds at stage 38 (early limb bud stage) showed a clear stain in a posterior region corresponding to ZPA of the chicken limb buds (Fig. 1A). The shh expression reached a maximum level and extended along the almost full length of the posterior border of the limb bud at stage 40 (medium limb bud stage, Fig. 1B). Such a localized expression could not be found when shh sense probes were used (Fig. 1E). When an embryo at stage 40 was observed from the lateral side of the body, the expression was confined to a patch on the posterior side of the limb bud and was shorter than that observed from the dorsal side (Fig. 1F). The shh expression progressively weakened, shifted distally at stage 42 (late limb bud stage) when it was confined to a distal patch (Fig. 1 C and G), and became faint at stage 45 (two to three digits stage, Fig. 1D). It was reported that shh expression in the chicken limb buds begins at stage 18 (early limb bud stage) and lasts until stage 29 (palette to digit stage) (4). Although we cannot compare the development of limb buds between the two species in a strict sense because their shape and the direction of elongation are different, newt limb buds appear to express shh in a fashion basically comparable to chicken limb buds. Therefore, it is strongly suggested that newt limb buds contain ZPA in their posterior margin as originally found in chicken limb buds. The observed residual difference of shh expression pattern between the two might be explainable by the difference in the sensitivity of detection.
Figure 1.
Expression of shh in developing forelimb buds. Hybridization was performed with antisense probes (A_–_D, F, and G) or with sense probes (E). (A) Stage 38, an early limb bud. (B, E, and F) Stage 40, a medium limb bud. (C and G) Stage 42, a late limb bud. (D) Stage 45 when two to three digits were formed. (A_–_E) Views from the dorsal side of the embryos. (F and G) Views from the lateral side of the embryos. (Upper) Posterior region. _shh_-expressing region is shown by arrowheads. Broken lines indicate the base of limb buds. (Scale bar = 200 μm.)
Expression Pattern of shh in Regenerating Blastemas.
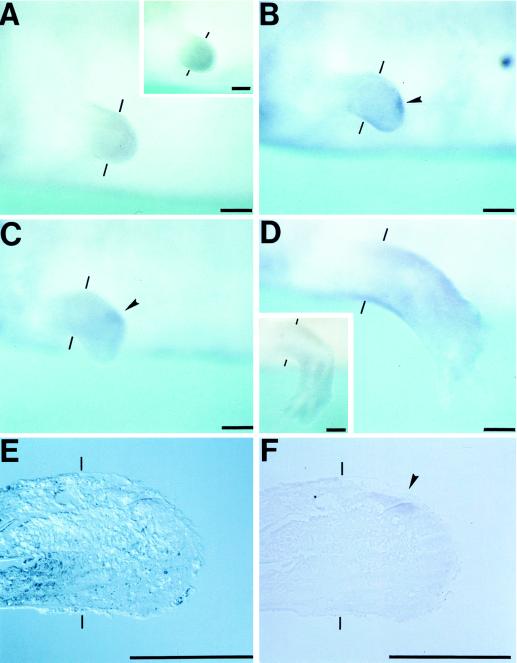
We attempted to identify the _shh_-expressing region in larval blastemas by the whole-mount in situ hybridization. Both forelimbs of larvae at stage 55 when four digits had been formed were amputated at a mid-upper arm (stylopodium) level. Regenerating limbs formed early to medium bud stage-blastemas at 4 days after amputation, which showed no meaningful expression (Fig. 2A). Experiments with shh sense probes showed that regenerating limbs had relatively high background stains (Fig. 2 A and D Insets). Localized stains were first observed at late bud stage in a distal posterior margin of blastemas (Fig. 2B). The shh expression was also detected at late bud to palette stage, which was similar to that in the limb bud at developmental stage 42 (Fig. 2C). Such expression disappeared at day 12 after amputation when four digits had formed (Fig. 2D). The location and the regeneration stage-dependent pattern of shh expression as described above imply that a regenerating limb blastema also contains ZPA in its posterior region.
Figure 2.
Expression pattern of shh in larval regenerating limbs. Amputation planes are shown by thin bars. (A_–_D) Blastemas of larval forelimbs viewed from the lateral side of the body. (Upper) Posterior region. (A) Four days after amputation, early to medium bud stage. (Inset) A case with sense probes. (B) Six days after amputaton, late bud stage. (C) Eight days, late bud to palette stage. (D) Twelve days after amputation, a 4-digit limb. (Inset) A case with sense probes. (E and F) A larval forelimb blastema at 6 days after amputation was treated for the in situ hybridization as above and was sectioned 10 μm thick. The sections were viewed through a microscope with (E) or without (F) Nomarski. (Upper) Posterior region. _shh_-expressing region is shown by arrowheads. (Scale bar = 200 μm.)
To identify _shh_-expressing cells, the regenerates subjected to the whole-mount in situ hybridization as above were sectioned and observed microscopically. The _shh_-expressing cells were in the posterior mesenchyme of blastema, but not in the epidermis (Fig. 2 E and F), as in developing limb buds of other vertebrates (4). This result additionally suggested the presence of ZPA in the blastema.
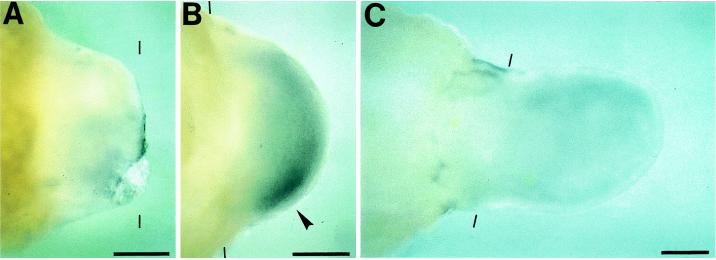
We asked if _shh_-expressing cells are also concentrated in a ZPA-corresponding region of the adult limb blastema. Both forelimbs of adult newts were amputated at a level of the mid-upper arm. There was no detectable stain at 14 days (dedifferentiation to early bud stage, Fig. 3A). The ZPA-corresponding region became intensely positive at 21 days after amputation (medium bud stage, Fig. 3B). The stains faded away at the palette stage (Fig. 3C). This expression pattern of shh was basically similar to that found in larval blastema, suggesting that the AP axis of regenerating adult newt limb is determined by the same molecular mechanism as that of larval regenerates.
Figure 3.
Expression pattern of shh in adult regenerating limbs. Amputation planes are shown by thin bars. Blastemas of adult forelimbs were viewed from the dorsal side. (Lower) Posterior region. (A) Fourteen days after amputation, dedifferentiation to early bud stage. (B) Twenty-one days after amputation, medium bud stage. (C) Twenty-eight days after amputation, palette stage. _shh_-expressing region is shown by an arrowhead. (Scale bar = 500 μm.)
Apparently, regenerating blastemas of both larval and adult limbs started to express shh later and ceased its expression earlier than developing limb buds when the stages of both processes were matched according to their morphological resemblance. The stage of shh expression was limited between medium bud stage and late bud to palette stage in the regeneration blastemas. Limb buds have to construct whole limbs, whereas regeneration blastemas reconstruct the only missing part of the limbs and do not have to construct the limb stump proximal from the amputation plane. It is possible that this situation could change the time of shh expression in blastemas and the duration of shh expression, depending on the size and length of the distal missing part.
Correlation Between the Supernumerary Limb Formation and the Duplication of shh Expression in Regenerates of Axial-Reversed Blastemas.
The AP and APDV axial reversal of blastema can be produced by autografting the reversed blastema on the contralateral limb stump without rotation and on the original limb stump with 180° rotation, respectively. The blastema thus operated has been known to regenerate supernumerary limbs (1, 2). The supernumeraries arise at places where the AP axis or the APDV axes between graft and stump are confronted. The regenerate is derived from both grafted blastema cells and host limb stump cells (28–32), indicating the presence of cellular interactions between graft and stump cells during the formation of supernumerary limb. The presence of cellular interaction led researchers to propose a concept that the interaction by “intercalation” or “boundary” apposition is the driving force for the pattern regulation, which does not require the presence of the polarizing zone-like ZPA (28–32). On the other hand, the formation of supernumerary limb has also been interpreted by the polarizing zone model which Tickle et al. (27) first proposed for explaining the formation of supernumerary digits in chicken limbs. This study strongly implies the presence of ZPA in the regeneration blastema of newt limbs. We performed the axial reversal grafting of blastemas on limb stumps to obtain additional supporting evidence for the presence of ZPA in regenerating limb blastema. We predicted that duplication of shh expression could be seen in these grafted blastemas if ZPA is present in the tissues and responsible for the AP patterning.
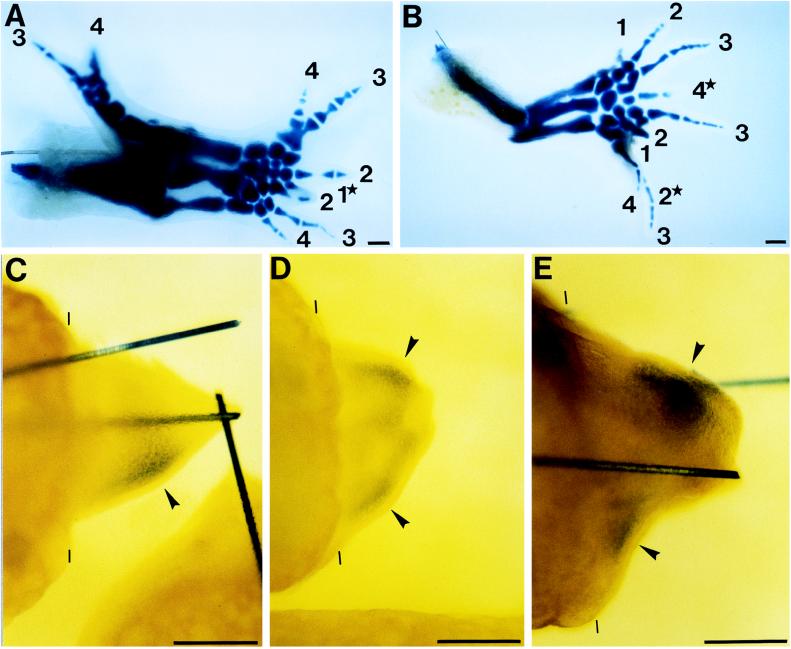
First, we confirmed the supernumerary limb formation in newt axial reversal experiments. When blastemas that had been formed at a mid-upper arm level were rotated 180° and grafted to the original upper arm stumps (APDV axial reversal), 59% of the regenerates formed supernumeraries. The regenerate of a APDV reversal shown in Fig. 4A produced one original regenerate and two (right and left) supernumeraries including 5 radii or ulnae and 9 digits, although the extent of regeneration of each of the three was incomplete. We also performed the experiments of the AP axial reversal graft of blastemas by autografting AP axis-reversed blastemas to the contralateral limb stumps. The rate of supernumeraries was 73%. Such a case, as shown in Fig. 4B, produced one original regenerate and two supernumeraries, which had 3 radii or ulnae and 10 digits.
Figure 4.
Supernumerary limb formation and duplication of shh expression in regenerates of axial-reversed blastemas. (A and B) Skeletal patterns of supernumerary limbs. Arabic numerals show the number of digits. (Lower) Posterior region. (A) APDV axial reversal grafts. Light limbs of adult newts were amputated at a mid-upper arm level and the resulting blastemas were amputated at medium bud stage. Blastemas were rotated 180° to make the APDV axes the opposite of the axes of the original site and ipsilaterally grafted to the original stumps. 1★, digit 1, which is partly hidden by the lower digit 2. Only part of its proximal phalanx can be seen. (B) AP axial reversal grafts. Right and left limbs were amputated at a mid-upper arm level and the resulting left blastemas at medium bud stage were contralaterally grafted to the light stumps without rotation. 2★, another digit 2, which is not visible here because it is completely hidden by the lowest digit 3. The tip of 4★ was found to be forked when it was seen through a stereoscopic microscope and, therefore, digit 4★ is digit 4, which was formed by a fusion of two digits. (C_–_E) Whole-mount in situ hybridization. (Lower) Posterior region. Amputation planes are shown by thin bars. (C) Control grafts. Control experiments for axial reversal grafting. Experiments were carried out as in A except that the blastemas were grafted without rotation to make the APDV axes of the graft meet those of the stump. Limbs were analyzed for shh expression 14 days after grafting. (D and E) Axial reversal grafts. (D) APDV axial reversal grafts. Limbs were subjected to the APDV axial reversal graft as described in A and were analyzed for shh expression at 14 days after grafting. (E) AP axial reversal grafts. Limbs were subjected to the AP axial reversal graft as described in B and were analyzed for shh expression 12 days after grafting. _shh_-expressing regions are shown by arrowheads. Solid bars in C and E are tungsten needles inserted to fix the grafts on stumps. (Scale bar = 500 μm.)
The pattern of shh expression in grafted blastemas was determined by the whole-mount in situ hybridization. shh was demonstrated to be expressed not only in the normal original region (new anterior region) of graft, but also in the other additional region (new posterior region) of both APDV and AP axial reversal grafts as we predicted (Fig. 4 D and E). No supernumeraries and no additional shh expressions were induced in control grafts, in which blastemas were grafted to limb stumps, making their axes meet those of the stumps (Fig. 4C). We performed a similar experiment of APDV and AP axial reversal graft four and five times, respectively, and reproducibly obtained identical results in each experiment. We also performed experiments of the APDV axial reversal graft of larval blastemas and obtained similar results; the supernumerary limb formation and the duplication of shh expression in operated blastemas (data not shown). These results support the notion that the regenerating limb blastemas possess ZPA on the posterior region, since shh is a specific molecular marker for ZPA.
We clearly showed that the formation of supernumerary limbs was closely related to the duplication of shh expression on axial reversal grafts of blastema. The digit pattern in Fig. 4A is 34, 4321, and 1234. The pattern of 34, 4321 would be a result of the sharing of the original ZPA, whereas the more posterior 1234 would be formed by a newly induced ZPA. This digit pattern in the regenerating limb might be in agreement with the results of experiments carried out with chicken (40). The AP axis reversal of blastema caused an originally shh nonexpressing anterior region to face to the posterior side of the limb stump and, as a result, would induce there a second shh expression in the cells (Fig. 4 D and E). Thus, the duplication of shh expression could represent the presence of two ZPAs. The boundary model could be operating if shh was induced in the posterior (formerly anterior) tissue as a result of the reorientation juxtaposing new borders. But the duplication of shh expression was not observed until 10–14 days after axial reversal grafting. Healing of the grafts requires 2–3 days, indicating that the new shh induction took place 7–11 days after the establishment of new borders. It is unlikely that the posterior (formerly anterior) cells require such a long time to respond to stimuli from the reoriented new borders. Alternatively, we deduce that the posterior cells of the original blastema are competent to express shh. Rotating the stump places these cells in the anterior of the graft. Cells from the posterior of the host stump are also competent to activate shh. As the graft heals and the blastema recovers these two populations of posterior origin both activate shh expression and induce the duplications.
Thus, the supernumerary limb regeneration from the AP or APDV axial reversal graft could be interpreted as a result of duplicated expression of shh, which had been predicted when we assumed the presence of ZPA in the regenerating newt blastema. The correlation between supernumerary limb regeneration and duplicated shh expression apparently supports the polarizing zone model for the supernumerary regeneration. However, this correlation does not necessarily exclude the other two models, the polar coordinate model and the boundary model. At present, it might be said that all these models can be merged into a local cell interaction model, with the ZPA as a posterior organizing center in both the embryonic limb bud and regenerating blastema. The cells along the urodele posterior limb border are probably competent to express shh through the lifetime of the organism with the right stimulus, which contrasts with that of chicken (41) and probably other vertebrates.
Muneoka and Bryant attempted to compare patterning mechanisms between the developing and regenerating axolotl limb (11, 12). Their results showed that transplanted developing limb buds induce limb duplications on an amputated blastema stump and vice versa, and that cells of both limb buds and blastemas are similarly incorporated in the supernumerary limb. From this observation, the authors suggested that the same signaling mechanisms were at work in both cases. This study also strongly suggests that the shh gene expression in regenerating newt limbs is regulated in a similar manner as that in their embryonic limb buds and, therefore, supports the idea at the molecular level that the AP patterning in limb regeneration is a recapitulation of the embryonic limb development.
Acknowledgments
We thank Dr. Takabatake and Dr. Takeshima for providing the full-length cDNA of Japanese newt shh gene, Dr. Gardiner and Dr. Bryant for sending their protocol of whole-mount in situ hybridization of axolotl, and Dr. Koshiba for giving her protocol of cartilage staining with Victoria blue. We also thank Ms. Keiko Sekiguchi for technical assistance and our laboratory colleagues for helpful discussions.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AP, antero-posterior; DV, dorso-ventral; ZPA, zone of polarizing activity.
References
- 1.Wallace H. Vertebrate Limb Regeneration. Toronto: Wiley; 1981. [Google Scholar]
- 2.Stocum D L. Molecular Biology Intelligence Unit: Wound Repair, Regeneration and Artificial Tissues. New York: Springer; 1995. [Google Scholar]
- 3.Tabin C J. Cell. 1991;66:199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- 4.Riddle R D, Johnson R L, Laufer E, Tabin C. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 5.Tickle C, Eichele G A. Rev Cell Biol. 1994;10:121–152. doi: 10.1146/annurev.cb.10.110194.001005. [DOI] [PubMed] [Google Scholar]
- 6.Chang D T, López A, von Kessler D P, Chiang C, Simandl B K, Seldin M F, Fallon J F, Beachy P A. Development (Cambridge, UK) 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- 7.Laufer E, Nelson C E, Johnson R L, Morgan B A, Tabin C. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 8.Niswander L, Jeffrey S, Martin G R, Tickle C. Nature (London) 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 9.López-Martínez A, Chang D T, Chiang C, Porter J A, Ros M A, Simandl B K, Beachy P A, Fallon J F. Curr Biol. 1995;5:791–796. doi: 10.1016/s0960-9822(95)00156-4. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Niswander L. Cell. 1995;80:939–947. doi: 10.1016/0092-8674(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 11.Muneoka K, Bryant S V. Nature (London) 1982;298:369–371. doi: 10.1038/298369a0. [DOI] [PubMed] [Google Scholar]
- 12.Muneoka K, Bryant S V. Dev Biol. 1984;105:179–187. doi: 10.1016/0012-1606(84)90273-2. [DOI] [PubMed] [Google Scholar]
- 13.Bryant S V, Gardiner D M. Dev Biol. 1992;152:1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- 14.Gardiner D M, Blumberg B, Komine Y, Bryant S V. Development (Cambridge, UK) 1995;121:1731–1741. doi: 10.1242/dev.121.6.1731. [DOI] [PubMed] [Google Scholar]
- 15.Savard P, Gates P B, Brockes J P. EMBO J. 1988;7:4275–4282. doi: 10.1002/j.1460-2075.1988.tb03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown R, Brockes J P. Development (Cambridge, UK) 1991;111:489–496. doi: 10.1242/dev.111.2.489. [DOI] [PubMed] [Google Scholar]
- 17.Beauchemin M, Savard P. In: Limb Development and Regeneration. Fallon J F, Goetinck P F, Kelly R O, Stocum D L, editors. New York: Wiley–Liss; 1993. pp. 41–50. [Google Scholar]
- 18.Simon H-G, Tabin C J. Development (Cambridge, UK) 1993;117:1397–1407. doi: 10.1242/dev.117.4.1397. [DOI] [PubMed] [Google Scholar]
- 19.Saunders J W, Gasseling M T. In: Epithelial–Mesenchymal Interactions: Ectodermal–Mesenchymal Interactions in the Origin of Limb Symmetry. Fleischmajer R, Billingham R E, editors. Baltimore: Williams & Wilkins; 1968. pp. 78–97. [Google Scholar]
- 20.Cameron J, Fallon J F. Dev Biol. 1977;55:320–330. doi: 10.1016/0012-1606(77)90175-0. [DOI] [PubMed] [Google Scholar]
- 21.Thoms S D, Fallon J F. J Embryol Exp Morphol. 1980;60:33–55. [PubMed] [Google Scholar]
- 22.French V, Bryant P J, Bryant S V. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 23.Bryant S V, French V, Bryant P J. Science. 1981;212:993–1002. doi: 10.1126/science.212.4498.993. [DOI] [PubMed] [Google Scholar]
- 24.Meinhardt T. Developmental Order: Its Origin and Regulation. New York: Liss; 1982. pp. 439–461. [Google Scholar]
- 25.Meinhardt T. J Embryol Exp Morphol. 1983;76:115–137. [PubMed] [Google Scholar]
- 26.Campbell G, Tomlinson A. Development (Cambridge, UK) 1995;121:619–628. doi: 10.1242/dev.121.3.619. [DOI] [PubMed] [Google Scholar]
- 27.Tickle C, Summerbell D, Wolpert L. Nature (London) 1975;254:199–202. doi: 10.1038/254199a0. [DOI] [PubMed] [Google Scholar]
- 28.Stocum D L. J Embryol Exp Morphol. 1982;71:193–214. [PubMed] [Google Scholar]
- 29.Muneoka K, Bryant S V. Dev Biol. 1984;105:166–178. doi: 10.1016/0012-1606(84)90272-0. [DOI] [PubMed] [Google Scholar]
- 30.Papageorgiou S, Holder N. J Embryol Exp Morphol. 1983;74:143–158. [PubMed] [Google Scholar]
- 31.Maden M, Mustafa K. J Embryol Exp Morphol. 1984;84:233–253. [PubMed] [Google Scholar]
- 32.Holder N, Weekes C. J Embryol Exp Morphol. 1984;82:217–239. [PubMed] [Google Scholar]
- 33.Imokawa Y, Eguchi G. Int J Dev Biol. 1992;36:407–412. [PubMed] [Google Scholar]
- 34.Okada K, Ichikawa M. Annu Rep Exp Morphol. 1947;3:1–16. [Google Scholar]
- 35.Iten L E, Bryant S V. Wilhelm Roux’ Archiv. 1973;173:263–282. doi: 10.1007/BF00575834. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 37.Takabatake T, Takahashi T C, Inoue K, Ogawa M, Takeshima K. Biochem Biophys Res Commun. 1996;218:395–401. doi: 10.1006/bbrc.1996.0069. [DOI] [PubMed] [Google Scholar]
- 38.Iten L E, Bryant S V. Dev Biol. 1975;44:119–147. doi: 10.1016/0012-1606(75)90381-4. [DOI] [PubMed] [Google Scholar]
- 39.Koshiba K, Tamura K, Ide H. Dev Growth Differ. 1994;36:357–364. doi: 10.1111/j.1440-169X.1994.00357.x. [DOI] [PubMed] [Google Scholar]
- 40.Fallon J F, Crosby G M. Dev Biol. 1975;42:24–34. [Google Scholar]
- 41.Ros M A, López-Martínez A, Simandl K, Rodriguez C, Belmonte J C I, Dahn R, Fallon J F. Development (Cambridge, UK) 1996;122:2319–2330. doi: 10.1242/dev.122.8.2319. [DOI] [PubMed] [Google Scholar]