T-cell regulation of peripheral tolerance and immunity: the potential role for Notch signalling (original) (raw)
Abstract
Recognition of antigen by T cells in the periphery may lead either to the generation of productive immunity or the induction of tolerance. These two functional outcomes are a consequence of distinct pathways of T-cell differentiation. T cells are selected to become regulatory cells and their function is to maintain homeostasis with the immune system. In this review we discuss the cell-fate decisions that T cells might make allowing them to promote immunity or induce tolerance in the context of the role that Notch signalling may play in this process.
Introduction
The peripheral immune system must maintain effective control over the effector responses of lymphocytes in order to prevent unwanted immune responses being generated to non-pathogenic antigens. There is increasing evidence that regulatory CD4+ T (Tr) cells help maintain homeostasis of the system through their contribution to the induction and the maintenance of tolerance to self and foreign antigens. Several experimental systems have demonstrated an important function for Tr cells in the periphery. These include the (i) mucosal delivery of immunogenic proteins and peptides, (ii) antibody manipulation of co-receptor and/or co-stimulatory signalling on T cells, and (iii) the analysis of specific thymic and splenic CD4+ T-cell populations which can mediate tolerance in vivo to self and foreign antigens. In this review we shall examine the function of Tr cells in different experimental systems and discuss recent findings, which indicate that Notch signalling on T cells can have profound effects on peripheral immunity especially in enabling the choice between immunity and tolerance.
Activation vs. tolerance
Much is known about the signalling requirements for T cells that to lead to their activation facilitating the generation of a productive immune response. Naïve T cells require the delivery of two separate signals: one through their T-cell antigen receptor (TCR) (signal one), and the second in the form of a co-stimulatory signal delivered by ligation of CD28 (signal two) on the T cell, with the ligands of the B7 family, namely, B7-1 (CD80) and B7-2 (CD86) present on the surface of professional antigen-presenting cells (APCs).1 As T cells are activated by the combination of signals one and two, they undergo clonal expansion and mediate their effector functions, such as the provision of B-cell help or cytotoxicity.
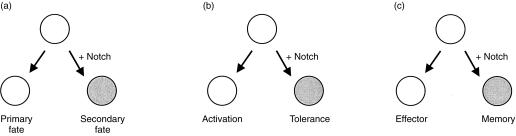
Here we will focus the discussion on the functional role of CD4+ T cells in immune regulation. Recognition of antigen in the periphery by CD4+ T cells can result in productive immunity or the induction of tolerance.2 Therefore, naı¨ve T cells appear to have the ability to choose between two distinct cellular responses in vivo, namely, activation or tolerance, and these events are guided by signals delivered by APCs to naive T cells at the time of initial antigen priming (Fig. 1).
Figure 1.
Notch influences cell-fate decisions. (a) Notch signalling is essential during embryonic development in the process of tissue patterning. Notch signalling can give rise to distinct cell fates during development. (b) We have demonstrated that Notch signalling on naïve T cells at the time of antigen recognition can influence the decision between activation and tolerance. This arises due to Notch inducing the differentiation of naïve CD4+ T cells into a Tr phenotype. (c) We speculate that Notch signalling is likely to have other influences on cell-fate decisions in the peripheral immune system. One such example might be where Notch signalling might influence whether naïve lymphocytes become effector or memory cells following their activation.
The development of tolerance to systemic or mucosally delivered antigen is an active process in which transient activation of T cells occurs prior to the development of unresponsiveness.3–6 T cells undergoing tolerance induction exhibit many of the early phenotypic changes observed on T cells that are capable of supporting a productive immune response.7 But there are critical differences in the effector activities of the T cells that emerge from the encounter with antigen that will lead to tolerance. Such T cells become refractory to further stimulation with antigen in vivo, and in many circumstances T cells can develop a regulatory function in that they actively suppress immune responses to antigen. The differentiation of CD4+ cells into Tr cells in vivo appears to be a lengthy process that takes approximately 2 weeks from the first encounter with antigen during the tolerizing condition. However, this differentiation process can be interrupted if animals are given an immunogenic challenge during this initial period of induction.5, 8 Once established, Tr cells remain for long periods in the peripheral circulation. Their persistence is thymic independent but is dependent on antigen.9,10
Evidence for tr cells in vivo
The existence of specific functional subsets of CD4+ T cells in the peripheral immune system have been described that either play a role in active immunity or immune regulation. T cells with a regulatory function were originally found to reside within a specific subset of CD4+ CD45RBlo cells whereas CD4+ CD45RBhi cells, were responsible for inducing immune pathology when adoptively transferred to immunodeficient SCID mice.11, 12 Subsequently, several groups have independently identified equivalent Tr cell populations which can protect animals from a variety of experimentally induced diseases such as diabetes, colitis, and allograft rejection.13–16 Tr cells exhibit a cell-surface phenotype of CD4+ CD45RBlo, CD25+ and CD38+ expression (reviewed in refs 17 and 18). They also express high levels of cytotoxic T lymphocyte antigen-4 (CTLA-4) in vivo, the importance of which will be discussed later. Studies in rodents have established that a subset of mature CD4+ T cells can arise within the thymus which have the capacity to mediate peripheral tolerance.16, 19–21 The emergence of these cells within the mature thymocyte pool suggests that there must be some selection of effector T-cell activity during the differentiation of thymocytes. However, at present, little is known about the mechanism that gives rise to these cells in vivo.
Cytokines involved in tr differentiation
Studies by Powrie and colleagues have begun to identify the conditions that are required for the generation of the naturally occurring Tr cell populations in vivo. These studies have highlighted the importance of interleukin-10 (IL-10) and transforming growth factor (TGF-β1) in the differentiation of CD4+ CD45RBlo cells into Tr cells.22, 23 Mice genetically deficient in expression of TGF-β1 or IL-10, although they contain CD4+ CD45RBlo cells in the peripheral lymphoid tissues, cannot function as Tr cells when used in adoptive transfer experiments. In addition, treating mice with neutralizing antibodies to TGF-β1 or IL-10 can block the differentiation of CD4+ CD45RBlo Tr cells in vivo. It has been reported that CD4+ T cells cultured in the presence of IL-10 together with antibodies to CD3/CD28 can give rise to T-cell clones that exhibit all the functional properties of Tr cells. These clones are poorly proliferative in vitro following ligation of their TCR, but exhibit suppressive properties when used in vivo.24 Studies on intranasal tolerance have also shown an important role for IL-10 in the induction of Tr cells. Neutralizing IL-10 could abrogate tolerance induction to intranasally delivered peptide by preventing the development of peptide-specific CD4+ Tr cells.6 Thus, evidence is accumulating to support the view that IL-10 and TGF-β1 are critical cytokines that can influence the differentiation of naı¨ve CD4+ T cells into Tr cells in vivo.
Failure of peripheral immune regulation in cytokine-gene-deficient mice
Mice that lack expression of the T-cell-derived cytokines IL-2, IL-4 and IL-10, or those deficient in functional T cells (e.g. TCR-β–/– and Rag–/–) all develop spontaneous colitis after 3–4 months of age.17,18 In addition, studies on oral tolerance to the autoantigen myelin basic protein has led to the identification of a unique population of CD4+ T cells that are induced as a result of antigen feeding. These CD4+ T cells, termed Th3, displayed a restricted pattern of cytokine secretion including IL-4, IL-10 and TGF-β1.25, 26 Therefore, the studies on different aspects of mucosal immunity are proving valuable in defining the role of CD4+ T cells in the maintenance of peripheral homeostasis of the immune system.
LINKED SUPPRESSION and INFECTIOUS TOLERANCE
Tolerance to a protein antigen can be achieved experimentally either by delivery of the whole protein or by peptides that contain immunogenic T-cell epitopes.27–32 The immune response generated to a protein normally gives rise to a hierarchical response to specific epitopes in a phenomenon referred to as immunodominance.33–35 Immunodominant peptides containing epitopes are far more effective at inducing tolerance to a protein than those that contain only a subdominant epitope.27, 35, 36 We have previously shown that intranasal administration of an immunodominant peptide derived from the major house dust mite antigen, Der p 1, can induce profound tolerance in mice.30 Recognition of the peptide leads to activation of CD4+ T cells that differentiate to become Tr cells that can suppress responses to all epitopes on the antigen, in a phenomenon referred to as ‘linked or bystander suppression’.5, 37 This phenomenon is only observed, however, if tolerant animals are rechallenged with the whole protein.37 Thus, it is possible to uncouple the linked suppression by immunizing tolerant mice with a peptide that contains only a subdominant epitope.37 Similar functional observations on linked or bystander suppression have been obtained in other experimental systems; for example experimental allergic encephalomyelitis (EAE).6, 36
Autoimmune disease can be induced by immunization with a single peptide derived from a suitable target antigen.29, 38, 39 Although the response will be initiated by a single protein, over time the immune response gradually spreads to encompass a variety of different target antigens that are usually expressed within the site of the diseased tissue. This phenomenon has been called ‘determinant spreading’ and can account for the diverse immune responses that occur during the course of a disease 39 and represents the reverse situation to linked suppression. Tolerance to a single peptide epitope on a target antigen could lead to induction of Tr cells, and through their effector activity suppress responses of T cells specific to unrelated antigens that would be presented during the course of the disease.40
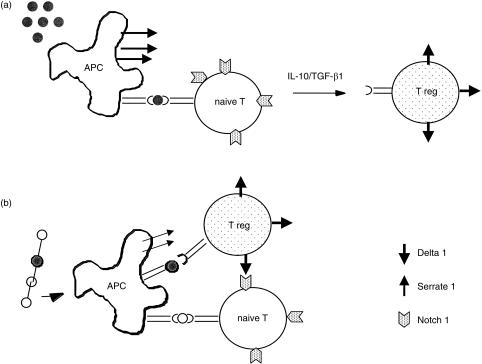
Linked suppression has also been observed in peripheral tolerance to transplantation antigens and in the generation of oral tolerance.41–43 Treating recipient mice with non-depleting antibodies directed to the T-cell co-receptor molecules CD4 and CD8, can allow the animals to accept full allogeneic skin grafts.8 The induction of tolerance in this model is associated with formation of CD4+ Tr cells and tolerance cannot be broken by infusions of non-tolerant T cells.8 Thus it appears the Tr cells can render the immunocompetent cells tolerant over time, in a process referred to as ‘infectious tolerance’. Tolerant animals can also accept F1 grafts, where only one of the major histocompatability complex (MHC) loci on the graft is compatible with the Tr cell MHC restriction specificity.42 These findings are compatible with the concept that Tr cells mediate linked suppression and in this model their effector activity could be partially abrogated by neutralizing IL-4.44 Linked suppression is a feature also observed in oral tolerance, and as discussed above, Tr cells have been shown to be activated as a result of antigen feeding. It is proposed that Tr cells mediate bystander suppression through the secretion of inhibitory cytokines such as IL-4, IL-10 and TGF-β1 (Fig. 2).25
Figure 2.
(a) Recognition of antigen on Serrate 1 + APCs induces naı¨ve T cells to differentiate to become Tr cells and this will be influenced by the cytokines IL-10/TGF-β1. (b) Tr cells are thought to mediate linked suppression when clustered around an APC and they come in contact with naı¨ve T cells. Tr cells are thought to mediate suppression through their capacity to secrete inhibitory cytokines, or alternatively, we have proposed that Tr cells may mediate linked suppression by direct cell–cell contact. The Tr cell, when activated, would begin to express a Notch ligand (e.g. Delta) at the surface and this would engage Notch on the neighbouring naı¨ve T cell. Notch signalling on the naı¨ve T cell would prevent its growth.
Linked suppression is dependent upon an APC presenting multiple epitopes on the surface and this would occur, for example, when an animal is immunized with a protein antigen to which it has been previously tolerized by the immunodominant epitope.40 The protein would be taken up and processed by host APCs which will present peptide/MHC complexes at their surface (Fig. 2). In this situation, linked suppression will only occur if there is co-expression of peptide epitopes specific for both Tr cells and other naïve cells. Thus, in this situation the Tr cell would be drawn to the surface of an APC, to recognize its epitope, come into the proximity of naïve T cells, and form direct cell–cell contacts. The APC therefore acts as a bridge to provide the antigen-specific link between Tr cells and naı¨ve cells.40
It had been previously postulated that the Tr cells mediate linked suppression through the secretion of inhibitory cytokines.25, 41 However, in our studies on intranasal tolerance to Der p 1, we could not readily detect the presence of inhibitory cytokines by Tr cells, so we considered that Tr cells might directly influence the growth of naı¨ve cells through direct cell–cell interactions.37
NOTCH SIGNALLING and CELL-FATE DECISIONS
The Notch signalling pathway is evolutionarily conserved and plays an important role in regulating cell growth and differentiation.45 Notch was first identified in Drosophila melanogaster as a neurogenic gene, as loss of function mutations in Notch gave rise to excess production of neurons. Notch plays a role in the development of multiple tissues in D. melanogaster.45 Notch is a large transmembrane protein which functions as a receptor that can bind two different ligands, Delta and Serrate. Notch contains 36 epidermal growth factor (EGF)-like repeats on its extracellular domain and has several other structural domains. A Notch/LIN motif is located juxtaposed to the cell membrane, while on the intracellular domain, Notch has eight ankyrin repeats. A number of transcription factors have been found to bind to the intracellular domain of Notch and these include CSL proteins [e.g. Su (H) and CBF-1 and Lag-1]. The CSL proteins bind to a specific site called the RAM23 domain that is located N-terminally to the ankyrin repeats. The cytoplasmic domain of Notch also contains a PEST sequence, a nuclear localization and an OPA sequence.45 When Notch binds its ligand, Delta or Serrate, this induces cleavage of Notch within the intracellular domain in a process that is dependent upon presenilin1 a protein with γ-secretase activity.46–49 The intracellular domain of Notch when released, migrates to the nucleus where it associates with transcription factors (e.g. CSL proteins) and can bind to the promoter of target genes such as the Enhancer of Split [En(spl)] to regulate their expression.45 It is beyond the scope of this review to provide a detailed description of the structure of Notch and its associated biochemical signalling pathways, but this subject has been reviewed extensively elsewhere.50, 51
Vertebrate homologues of the Notch receptor and its ligands have now been identified. Four receptors have been described in vertebrates while multiple ligands for Delta and Serrate (also known as Jagged) are also known.45 It is now clear that the entire signalling pathway from the receptor and its ligands to the downstream signalling components are conserved from D. melanogaster through to humans indicating the fundamental nature of this pathway in regulating cell growth and differentiation. The first human Notch1 gene was isolated from a human T-cell leukaemia and since then there has been growing interest into what function this signalling pathway might have, not only in the development of T cells, but also in haematopoiesis (reviewed in refs 52 and 53).
INDUCTIVE SIGNALLING and LATERAL INHIBITION
Notch signalling has been associated with two different processes, namely inductive signalling and lateral inhibition.45, 54 The receptors and their ligands contain EGF-like repeats on their extracellular domain, which facilitates their role as adhesion molecules to support cell–cell interactions.55 Tissue patterning is an essential process of organogenesis that requires the generation of specific cell types following a specific temporal and spatial plan during embryonic development. The role for Notch in inductive signalling has been observed in at least four different tissues including dorsal ventral (D–V) patterning of D. melanogaster limb and eye as well as the D–V patterning of the tetrapod limb and formation of the vertebrate somite.56 In most of these tissues Notch signalling can give rise to distinct cell types and can influence cell growth within defined boundaries, as observed with the proximo-distal outgrowth of the D. melanogaster leg.
Neurogenesis that occurs during Drosophila development requires the selection of neural precursors from a field of cells that all exhibit the same developmental potential.55, 57 All cells within the field will express the Notch receptor but only one cell will express Delta, and thus will become a neuron through a process termed lateral inhibition. Once the neural precursor begins to express the Delta protein at its surface it can signal to neighbouring cells leading to activation of Notch. The cells receiving the Notch signal are prevented from adopting the neural fate and are maintained as epidermal cells. A similar lateral signalling process has been defined in the nematode worm in the formation of the vulva.54 The establishment of the ventral uterine cell (VU) vs. anchor cell (AC) fates requires the cell–cell interactions between precursor cells expressing the Lin-12 (i.e. Notch receptor) and Lag-2 (i.e. Notch ligand) proteins, respectively. In this latter situation one cell is selected to express higher levels of Notch ligand owing to stochastic fluctuations in the expression of Notch and its ligands. Eventually over time, one cell will begin to express more ligand and as a result will be able to signal to its neighbour. This results in the receiving cell extinguishing expression of the Notch ligand, but increasing expression of the Notch receptor. This situation gives rise to the generation of two different cell fates owing to the cell-autonomous effects of Notch signalling.
NOTCH and CELL-FATE CHOICES FOR PERIPHERAL T CELLS
Splenic and lymph node DCs, B cells, as well as CD4+ and CD8+ T cells all express transcripts for Notch 1, 2 and the ligands Delta1 and Serrate1.58, 59 We have also observed that the Notch ligands Delta1 and Serrate1 are differentially expressed on CD4+ T cells following during the induction phase of tolerance with intranasally administered peptide. However, the expression of these genes is either unaltered or downregulated on CD4+ T cells following immunization with the same peptide in adjuvant.58 Thus, the expression of Notch and its ligands can be modulated by signals that lead to the induction of tolerance vs. immunity.
We had originally observed expression of Serrate1 transcripts on freshly isolated CD11c+ DCs.59 As DCs are regarded to be the principal APC of the immune system we were prompted to assess the effects of overexpression of the Serrate1 gene in spleen-derived DCs on immune function. These studies revealed that presentation of antigen by Serrate1+ DCs could induce profound peripheral T-cell tolerance which was characterized by a decrease in antigen-specific T-cell proliferation and cytokine production and the development of linked suppression. Furthermore, tolerance could be transferred to naı¨ve recipient mice with CD4+ T cells, suggesting that Serrate1-induced Notch signalling, together with TCR ligation, had induced CD4+ T cells to differentiate into Tr cells in vivo.59
Therefore, consistent with its role in embryonic development, Notch signalling can influence the differentiation of peripheral CD4+ T cells. However, whether Notch signalling functions independently or requires other extracellular signals, such as those delivered by cytokines, is not known. Alternatively, Notch signalling may play a more integrative role in T-cell differentiation and concomitant signalling through TCR, CD28/CTLA-4 and may allow activated T cells to become competent to respond to secreted cytokines (e.g. IL-10 and TGF-β1), thus facilitating their differentiation into Tr cells in vivo.
LINKED SUPPRESSION and NOTCH SIGNALLING
We considered that activation of the Notch pathway may contribute to the phenomenon of linked suppression. It is possible that Tr cells may inhibit the growth of naı¨ve T cells through direct cell–cell contact, akin to Delta–Notch lateral signalling previously described in development. Using retroviral-gene-mediated transfer to overexpress Delta1 on the surface of T cells, we observed that Delta-1+ T cells could inhibit the activation and growth of neighbouring T cells. Furthermore, Delta-1+ T cells could induce antigen-specific tolerance when transferred to naı¨ve mice which was characterized by inhibition of T-cell growth and linked suppression.58
NOTCH and CELL DEATH
It has emerged from the study of T cells that Notch signalling can protect cells from apoptosis.60, 61 During development T cells that react with self are deleted while those that fail to undergo positive selection in the thymus die by neglect. This latter process is probably due to exposure of the thymocytes to endogenous circulating glucocorticoids, which induce apoptosis double-positive thymocytes which do not express a functional T-cell receptor. Studies by two separate groups have revealed that active Notch signalling can protect both thymocytes and mature T cells from apoptosis as a result of either activation-induced cell death (AICD) or glucocorticoid exposure.60, 61 This effect of Notch activity has also been proposed to explain the bias in the CD4:CD8 ratio that is observed in the thymus of mice that express a constitutively active Notch 1 transgene.62 A similar phenotype was also observed in the thymus of bcl-2 transgenic mice.63, 64 It is possible that CD8+ cells, owing to their less stringent signalling requirements, 65 when provided with a constitutively active Notch 1 signal may be preferentially rescued from death during development.
Notch signalling may perform a similar function in the peripheral immune system. Therefore, following the induction of the T-cell response, Notch signalling may operate to protect cells from AICD. Notch signalling may operate at a temporal period during cellular activation, which would protect cells from death but at the same time, enable them to become responsive to cytokine signals present in the local environment and extend their opportunity to mediate effector function. Recently, Scheffold and coworkers 66 have reported that CD4+ T cells can retain a high level of IL-10 on their surface. This observation may help to establish the mechanisms by which IL-10 can influence the differentiation of T cells into a Tr phenotype in vivo, possibly mediated as a result of direct T cell–T cell interactions. Alternatively, T cells that express IL-10 at the cell surface may influence the functional capacity of APCs. It will be relevant to determine if the IL-10hi T cells also express high levels of Notch ligands.
There is evidence that signalling through CTLA-4 on T cells can induce TGF-β1 secretion 67 which could have important consequences on the selection of Tr cells. Potentially, T-cell recognition of antigen delivered under conditions that induce tolerance may activate the Notch pathway, which in the presence of IL-10 and/or TGF-β1, allows the cell to adopt a regulatory phenotype. The Tr cells through ligation of Notch on naı¨ve T cells may enhance CTLA-4 expression and so become tolerant.
Conclusions
The expression of Notch receptors and their ligands is widespread on cells of the immune system. Most studies of Notch function to date have focused on its role in precursor cell differentiation, such as haematopoietic stem cells, granulocytes and T cells.68–71 Although these analyses have revealed some novel insights into the function of Notch signalling in these different processes, the majority have used constitutively active forms of Notch 1 or Notch 2. There are four different Notch receptors and four different ligands that can be expressed by cells of the immune system 45 and it is therefore necessary to determine how the different Notch ligands and receptors are temporally expressed during productive immunity and the induction of tolerance. From studies on haematopoietic stem cells, some effector functions have been demonstrated for the Notch ligands. The expression of the Serrate 1 (Jagged 1) protein is restricted to stromal cells while CD34+ cells express both Notch1 and 2.72–74 Culturing CD34+ cells with Serrate1+ stroma prevented the cells from differentiating, which suggests that Serrate1 may be an important signal in the maintenance of the stem-cell fate in early haematopoietic precursor cells 2.72–74
It is possible that the Notch signalling pathway is used repeatedly and in different situations during immune effector function. We have focused on the functional role of the Notch ligands, Delta1 and Serrate1 in the peripheral immune system and demonstrated that expression of Notch ligands on T cells and APCs can lead to the development of T-cell tolerance. At present we do not know if all the ligands will behave in the same way when expressed by cells of the immune system or if there is differential usage of specific Notch ligands and/or receptors by different cell types. Preliminary data in our laboratory with conditional targeted transgene expression for Delta1 and Serrate1 to T cells, suggest that these two Notch ligands have distinct functions on thymocytes and mature T cells. Furthermore, there may be preferential pairing of particular Notch ligands with receptors within the immune system. It appears that Serrate1 has the capacity to interact with all four Notch receptors in vitro, 75 and these studies also revealed a potential hierarchy of binding affinity. The effect this may have on functional activity or differentiation of lymphocytes is unknown.
A common cell-fate decision faced by peripheral lymphocytes is the decision between immune effector activity and memory. Notch signalling may play some role in this process considering the critical role that this pathway has in influencing binary cell-fate decisions.45, 52 So could Notch signalling influence lymphocyte survival during memory? Notch signalling can protect cells from apoptosis, 60, 61 which together with other signals may permit long-term survival of memory cells in the peripheral circulation. Notch signalling may have an analogous role in biasing the generation of B-cell tolerance rather than activation as we have demonstrated for T cells. Cognate interaction between Th cells and B cells induces productive immune responses, which are essential in order for B cells to undergo clonal expansion and isotype switching. Activation of Th cells enables them to navigate from the cortical region of the lymph node to interact with B cells within the follicle.76 The correct positioning of CD4+ T cells within the lymph node facilitates their interaction with B cells. Thus, through their ability to signal through Notch, T cells may influence the effector activity of B cells.
The function of Notch signalling in the peripheral immune system is attracting interest and as we have discussed there are many possible ways in which Notch signalling could influence peripheral immunity. The therapeutic applications of manipulating Notch signalling are diverse and exciting and their potential in transplantation, allergy and autoimmune disease remains to be explored.
We are at an early stage in understanding the full importance of Notch signalling in peripheral immunity. However, based on its powerful influences during embryogenesis it is likely that the Notch pathway will have an important role in regulating the immune system.
Acknowledgments
We thank the Medical Research Council, the Wellcome Trust and the British Lung Foundation for supporting this work.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Mondino A, Khoruts A, Jenkins MK. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA. 1996;93:2245–52. doi: 10.1073/pnas.93.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–56. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 4.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–8. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 5.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Int Immunol. 1996;8:335–42. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol. 1999;11:625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 7.Metzler B, Burkhart C, Wraith DC. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int Immunol. 1999;11:667–75. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- 8.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Infectious transplantation tolerance. Science. 1993;259:974–7. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 9.Metzler B, Wraith DC. Inhibition of T-cell responsiveness by nasal peptide administration: influence of the thymus and differential recovery of T-cell-dependent functions. Immunology. 1999;97:257–163. doi: 10.1046/j.1365-2567.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann H, Cobbold S. How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annu Rev Immunol. 1998;16:619–44. doi: 10.1146/annurev.immunol.16.1.619. [DOI] [PubMed] [Google Scholar]
- 11.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990;172:1701–8. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 13.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 15.Suri-Payer E, Amar AZ, McHugh R, Natarajan K, Margulies DH, Shevach EM. Post-thymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. Eur J Immunol. 1999;29:669–77. doi: 10.1002/(SICI)1521-4141(199902)29:02<669::AID-IMMU669>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaün J. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 17.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–55. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 18.Groux H, Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol Today. 1999;20:442–5. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- 19.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184:2393–8. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4 (+) CD45RC- cells and CD4 (+) CD8 (-) thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutinho A, Salaün J, Corbel C, Bandeira A, Le Douarin N. The role of thymic epithelium in the establishment of transplantation tolerance. Immunol Rev. 1993;133:225–40. doi: 10.1111/j.1600-065x.1993.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 22.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB (low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groux H, O'garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 26.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 27.Clayton JP, Gammon GM, Ando DG, Kono DH, Hood L, Sercarz EE. Peptide-specific prevention of experimental allergic encephalomyelitis. Neonatal tolerance induced to the dominant T cell determinant of myelin basic protein. J Exp Med. 1989;169:1681–91. doi: 10.1084/jem.169.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wraith DC, McDevitt HO, Steinman L, Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989;57:709–15. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- 29.Gaur A, Wiers B, Liu A, Rothbard J, Fathman CG. Amelioration of autoimmune encephalomyelitis by myelin basic protein synthetic peptide-induced anergy. Science. 1992;258:491–1494. doi: 10.1126/science.1279812. [DOI] [PubMed] [Google Scholar]
- 30.Hoyne GF, O'hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993;178:1783–8. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–65. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 32.Dick AD, Cheng YF, McKinnon A, Liversidge J, Forrester JV. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Opthamol. 1993;77:171–9. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–61. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 34.Gammon G, Geysen HM, Apple RJ, Pickett E, Palmer M, Ametani A, Sercarz EE. T cell determinant structure: cores and determinant envelopes in three mouse major histocompatibility complex haplotypes. J Exp Med. 1991;173:609–17. doi: 10.1084/jem.173.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ria F, Chan BM, Scherer MT, Smith JA, Gefter ML. Immunological activity of covalently linked T-cell epitopes. Nature. 1990;343:381–3. doi: 10.1038/343381a0. [DOI] [PubMed] [Google Scholar]
- 36.Anderton SM, Wraith DC. Hierarchy in the ability of T cell epitopes to induce peripheral tolerance to antigens from myelin. Eur J Immunol. 1998;28:1251–61. doi: 10.1002/(SICI)1521-4141(199804)28:04<1251::AID-IMMU1251>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Hoyne GF, Jarnicki AG, Thomas WR, Lamb JR. Characterization of the specificity and duration of T cell tolerance to intranasally administered peptides in mice: a role for intramolecular epitope suppression. Int Immunol. 1997;9:1165–73. doi: 10.1093/intimm/9.8.1165. [DOI] [PubMed] [Google Scholar]
- 38.Smilek DE, Wraith DC, Hodgkinson S, Dwivedy S, Steinman L, McDevitt HO. A single amino acid change in a myelin basic protein peptide confers the capacity to prevent rather than induce experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1991;88:9633–7. doi: 10.1073/pnas.88.21.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 40.Hoyne GF, Lamb JR. Regulation of T cell function in mucosal tolerance. Immunol Cell Biol. 1997;75:197–201. doi: 10.1038/icb.1997.29. [DOI] [PubMed] [Google Scholar]
- 41.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–7. [PubMed] [Google Scholar]
- 43.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–6. [PubMed] [Google Scholar]
- 44.Davies JD, Martin G, Phillips J, Marshall SE, Cobbold SP, Waldmann H. T cell regulation in adult transplantation tolerance. J Immunol. 1996;157:529–33. [PubMed] [Google Scholar]
- 45.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 46.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain [see comments] Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 47.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–5. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 48.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A Ligand-Induced Extracellular Cleavage Regulates γ-Secretase-like Proteolytic Activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 49.Brou C, Logeat F, Gupta N, et al. A Novel Proteolytic Cleavage Involved in Notch Signaling: The Role of the Disintegrin-Metalloprotease TACE. Mol Cell. 2000;5:207–16. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 50.Kopan R, Cagan R. Notch on the cutting edge. Trends Genet. 1997;13:465–7. doi: 10.1016/s0168-9525(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 51.Osborne B, Miele L. Notch and the immune system. Immunity. 1999;11:653–63. doi: 10.1016/s1074-7613(00)80140-5. [DOI] [PubMed] [Google Scholar]
- 52.Robey E. Regulation of T cell fate by Notch. Annu Rev Immunol. 1999;17:283–95. doi: 10.1146/annurev.immunol.17.1.283. [DOI] [PubMed] [Google Scholar]
- 53.Milner LA, Bigas A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood. 1999;93:2431–48. [PubMed] [Google Scholar]
- 54.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–62. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 55.Irvine KD. Fringe. Notch, and making developmental boundaries. Curr Opin Genet Dev. 1999;9:434–41. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 56.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–32. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 57.Chitinis AB. The role of Notch in lateral inhibition and cell fate specification. Mol Cell Neurosci. 1995;6:311–21. [PubMed] [Google Scholar]
- 58.Hoyne GF, Dallman MJ, Lamb JR. Linked suppression in peripheral T cell tolerance to the house dust mite derived allergen Der, p. 1. Int Arch Allergy Immunol. 1999;118:122–4. doi: 10.1159/000024046. [DOI] [PubMed] [Google Scholar]
- 59.Hoyne GF, Le Roux I, Corsin-Jimenez M, et al. Serrate1-induced Notch signalling regulates the decision between immunity and tolerance made by peripheral CD4+ T cells. Int Immunol. 2000;12:177–85. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 60.Deftos ML, He YW, Ojala EW, Bevan MJ. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–86. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jehn BM, Bielke W, Pear WS, Osborne BA. Protective effects of notch-1 on TCR-induced apoptosis. J Immunol. 1999;162:635–8. [PubMed] [Google Scholar]
- 62.Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–92. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 63.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–99. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 64.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–88. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 65.Lovatt M, Yang TH, Stauss HJ, Fisher AG, Merkenschlager M. Different doses of agonistic ligand drive the maturation of functional CD4 and CD8 T cells from immature precursors. Eur J Immunol. 2000;30:371–81. doi: 10.1002/1521-4141(200002)30:2<371::AID-IMMU371>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 66.Scheffold A, Assenmacher M, Reiners-Schramm L, Lauster R, Radbruch A. High-sensitivity immunofluorescence for detection of the pro- and anti-inflammatory cytokines gamma interferon and interleukin-10 on the surface of cytokine-secreting cells. Nature Med. 2000;6:107–10. doi: 10.1038/71441. [DOI] [PubMed] [Google Scholar]
- 67.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4 (+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milner LA, Bigas A, Kopan R, Brashem-Stein C, Bernstein ID, Martin DI. Inhibition of granulocytic differentiation by mNotch1. Proc Natl Acad Sci USA. 1996;93:13014–9. doi: 10.1073/pnas.93.23.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 1998;18:2324–33. doi: 10.1128/mcb.18.4.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–91. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Milner LA, Deng Y, et al. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8:43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- 73.Jones P, May G, Healy L, Brown J, Hoyne G, Delassus S, Enver T. Stromal expression of Jagged 1 promotes colony formation by fetal hematopoietic progenitor cells. Blood. 1998;92:1505–11. [PubMed] [Google Scholar]
- 74.Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–91. [PubMed] [Google Scholar]
- 75.Shimizu K, Chiba S, Kumano K, et al. Mouse Jagged1 physically interacts with Notch2 other Notch receptors. Assessment of quantitative methodsJ Biol Chem. 1999;274:32961–9. doi: 10.1074/jbc.274.46.32961. [DOI] [PubMed] [Google Scholar]
- 76.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]