Soluble isoforms of CEACAM1 containing the A2 domain: increased serum levels in patients with obstructive jaundice and differences in 3-fucosyl-N-acetyl-lactosamine moiety (original) (raw)
Abstract
CEACAM1 (biliary glycoprotein or CD66a) is a member of the carcinoembryonic antigen (CEA) subgroup of the CEA family. Eleven RNA isoforms derived from the splicing of a single CEACAM1 gene have been described. Some of the CEACAM1 isoforms have been recognized by the CD66 antibodies in T and B lymphocytes, natural killer cells, granulocytes and epithelial cells in several human tissues. Although it is also present in soluble form in bile and serum, and elevated levels have been found in the serum of patients with liver diseases, it is not known which isoforms are primarily involved. In order to learn more about the distribution and properties of particular CEACAM1 isoforms, we have prepared a monoclonal antibody specific for the A2 domain of CEACAM1, designated TEC-11. This antibody does not cross-react with other members of the CEA family. Immunoblotting analysis revealed that the TEC-11 epitope was present in all cell types expressing CEACAM1 containing the A2 domain [CEACAM1(A2)], including granulocytes (160 000 MW isoform) and sperm cells (140 000 MW isoform). A 115 000 MW isoform of CEACAM1(A2) was present in human serum, bile, saliva and seminal fluid. Human bile, saliva and seminal fluid also contained the 160 000 MW CEACAM1(A2) isoform. Significantly higher serum levels of the 115 000 MW CEACAM1(A2) isoform were detected in patients with obstructive jaundice. The 160 000 MW isoform of CEACAM1(A2) in bile, but not a 115 000 MW isoform in serum and bile, carried the 3-fucosyl-_N_-acetyl-lactosamine moiety. The combined data indicate that various isoforms of CEACAM1(A2) are present in different body fluids where they could take part in different CEACAM1-mediated functions.
INTRODUCTION
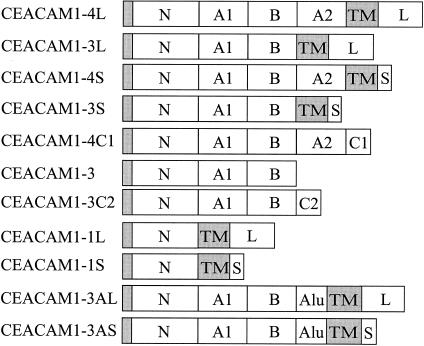
CEACAM1 (biliary glycoprotein or CD66)1 was first identified in human bile through its cross-reactivity with antibodies against the carcinoembryonic antigen (CEA).2 CEACAM1 was later found in T and B lymphocytes, natural killer cells, granulocytes and epithelial cells of several human tissues.3–8 Molecular cloning and sequencing of CEACAM1 cDNA identified this molecule as a member of the immunoglobulin superfamily specifically belonging to the CEA subgroup of the CEA family.9 Other members of the CEA subgroup include CEA itself, CEACAM3, CEACAM6, CEACAM7 and CEACAM8.1 Although only one CEACAM1 gene is present in humans, 11 different mRNA species are generated by alternative splicing (Fig. 1).10–12
Figure 1.
Schematic diagram of CEACAM1 splice variants. The following domains are indicated: the N-terminal domain (N) with the signal polypeptide domain (shaded area), the IgC2-like set domains (A1, B and A2), intron-derived domains containing Alu sequences within the open reading frame (Alu) or different termini generated by splicing (C1, C2), the transmembrane domain (TM) and long (L) or short (S) intracellular domains.
The largest CEACAM1 isoform, CEACAM1-4L, is composed of a 108-amino acid N-terminal immunoglobulin V (IgV)-like domain, two 178-amino acid IgC2 set domains (A1 and B), a 100-amino acid IgC2 set domain (A2), a 32-amino acid transmembrane domain and a 71-amino acid cytoplasmic tail.10,13 As shown in Fig. 1, eight of the CEACAM1 isoforms are anchored to the plasma membrane via the transmembrane domain10,11 whereas three isoforms seem to exist in soluble form.12 An 85 000 to 90 000 MW CEACAM1 isoform has been found in, and isolated from, human bile.2,14 An isoform of CEACAM1 is also found in serum and its levels are increased in patients with liver or biliary tract diseases.15 However, it remains to be established which isoform of CEACAM1 is present in the blood and other body fluids and which is affected by liver/biliary tract disease.
CEACAM1 in granulocytes is a major carrier of the carbohydrate epitope 3-fucosyl-_N_-acetyl-lactosamine (CD15 or Lex).16,17 Lex and its sialylated derivative (sLex) have been shown to be involved in the interaction of granulocytes with E-selectin and P-selectin on endothelial cells and with P-selectin on platelets.18,19 However, it is not known whether soluble isoforms of CEACAM1 also carry Lex and whether they could affect Lex-mediated functions.
In the present study we developed a novel monoclonal antibody (mAb) specific for the A2 domain of CEACAM1, and analysed the CEACAM1 isoforms in body fluids of healthy people and in the serum of patients with obstructive jaundice. The results demonstrate that different isoforms of CEACAM1 containing the A2 domain [CEACAM1(A2)] are present in different body fluids, and that the levels of the 115 000 MW CEACAM1(A2) isoform are increased in patients with obstructive jaundice. Soluble isoforms of CEACAM1(A2) also differ in the presence or absence of the Lex moiety.
MATERIALS AND METHODS
Antibodies and cells
The mouse mAb b18.7.7 (IgG1), recognizing an epitope in the N-terminal domain of CEA family, has been described elsewhere.20 The CD66 mAbs CLB-gran/10 (IgG1), F34-187 (IgG1) and F36-54 (IgG1) were obtained from the Vth Leucocyte Culture Conference.6 The TEC-01, IgM specific for Lex, and negative control IgM mAb, TEC-02, have been previously described.21,22 To prepare antibodies specific for CEACAM1, a recombinant fragment containing the A2 domain of CEACAM1 was used. A restriction site for Hin dIII was introduced at nucleotide position 1325 of the cloned CEACAM1-4L cDNA10 using a two-step polymerase chain reaction (PCR) with two internal oligonucleotide primers encompassing the Hin dIII site (underlined; P2: 5′ CCATT TTCTTGTGGTAAAGCTTTATAGTTTACGTTCAG 3′ and P3: 5′ CTGAACGTAAACTATAAAGCTTTACCACAAGAAAATGG 3′) and two upstream and downstream primers, covering Pst I sites at positions 648 and 1677 (underlined; P1: 5′ AGGCTGCAGCTGTCCAATGG 3′ and P4: 5′ ACATCAGCACTGCAGTGAGCA 3′). The primers, P1 and P2, were used in the first step PCR, whereas the P3 and P4 primers were used in the second PCR. PCR-generated fragments were isolated and mixed in the second step of the PCR mutagenesis protocol together with P1 and P4 primers. The resulting PCR-amplified fragment was digested with Pst I and used to replace the Pst I fragment in wild-type CEACAM1-4L. In order to express the fragment containing the A2 domain of CEACAM1 tagged in the N-terminus by six sequential histidine residues, the mutated CEACAM1 was digested with Bam HI and Hin dIII and the fragment covering nucleotides 955–1325 (amino acids 295–416) was subcloned into the His6 expression vector pQE30 (Diagen, GmbH, Hilden, Germany). The vector was introduced into M15 [pREP4]Escherichia coli by electroporation and the recombinant protein was isolated on a Ni-NTA resin (Diagen) according to the manufacturer's instructions. Monoclonal antibodies were prepared after immunization of BALB/c mice with the recombinant protein as described.23 One mAb of the IgG1 subclass, the TEC-11, was found to react in immunoblotting assay (see below) with cells expressing CEACAM1 but not with CEA-positive cells, and was therefore used for further analyses. Polyclonal antibodies were produced by subcutaneous immunization of rabbits with 200 µg of the recombinant protein in complete Freund's adjuvant at 3-week intervals; the third injection was in incomplete Freund's adjuvant.
Stable transfectants of a Chinese hamster ovary (CHO) cell line LR-73 arose from calcium phosphate-mediated transfection of the full-length cDNAs for CEACAM1-4L, CEACAM1-3L, CEA, CEACAM6 or CEACAM8 as described.24 CHO or HeLa cell lines stably transfected with CEACAM7 cDNA or CEACAM3 cDNA,25 respectively, and the corresponding control cells were kindly provided by W. Zimmermann, Institute of Molecular Medicine and Cell Research, University of Freiburg, Freiburg, Germany.
Granulocytes were prepared from human blood fractionated by a double density gradient of Histopaque-1077 and Histopaque-1119 (Sigma, St Louis, MO) according to the manufacturer's instructions.
Samples collection
Peripheral blood serum samples were obtained from patients with obstructive jaundice (n = 17) due to gallstone(s) in the common bile duct (n = 14) or to pancreatic tumour (n = 3). The diagnoses were based on ultrasonography and serum biochemical parameters of ‘liver function tests’ (see below) and were confirmed by laparatomy. As controls we used serum from healthy individuals (n = 32) and from patients with tumours (n = 20) (16 lymphomas and myelomas and four oesophageal carcinomas) having neither clinical nor biochemical signs of liver or biliary tract disease. All sera were collected and examined at the Department of Clinical Chemistry, Faculty Hospital, 1st School of Medicine, Charles University, Prague. The sera were tested for T bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and γ-glutamyltransferase (GMT) using the corresponding kits from Lachema Diagnostica, Brno, Czech Republic. The following values were considered as upper limits of the normal range: T bilirubin, 17·5 µmol/l; ALT, 0·7 µkat/l; AST, 0·6 µkat/l; ALP, 2·2 µkat/l; and GMT, 1·0 µkat/l. Bile, obtained from patients treated surgically for cholelithiasis, and other samples were obtained from the Institute of Clinical and Experimental Medicine, Prague.
Extraction of body fluids and cells, and immunoblotting
Each serum sample (0·15 ml) was mixed with an equal volume of 1·6 m perchloric acid (PA). After 30 min at 4°, the mixture was centrifuged at 14 000 g for 10 min. The supernatant was transferred into another tube and the remaining proteins were precipitated with six volumes of acetone for 16 hr at −20°. The precipitate was dried under vacuum and resuspended in 50 µl sodium dodecyl sulphate (SDS) sample buffer. The same procedure was used for preparation of samples from urine, seminal fluid, saliva and dialysed bile. For immunoprecipitation experiments, the PA-soluble material was neutralized by adding sodium hydroxide solution, concentrated over PM-30 ultrafiltration membranes (Amicon, Danvers, MA), and dialysed against phosphate-buffered saline (PBS). Cell extracts were prepared and electrophoresis and immunoblotting were performed as previously described,24 except that samples presented in Fig. 3(b) were size fractionated by SDS − 10% polyacrylamide gel. Protein bands were quantified by densitometry using the UVP gel documentation system and GelBase/GelBlot software (Ultra-Violet Products Limited, Cambridge, UK). The intensity of the bands on the film is dependent not only on the amount of antigen blotted on the membrane but also on the quality of antibodies used, the time exposure of the film and the conditions of film development. For this reason, an internal control was included into each electrophoretic run, a CEACAM1 band from a lysate of 0·25 × 106 LR-73 CHO cell transfectants expressing CEACAM1-4L (see above). Calibration with CEACAM1 recombinant protein indicated that this band corresponds to approximately 0·4 µg of CEACAM1, as detected by TEC-11 immunoblotting. The intensity of this band was normalized to 100% in order to determine the amount of CEACAM1 in bands under study on the same blot. Preliminary experiments confirmed the linearity of the signal down to 1% of the internal control band.
Figure 3.
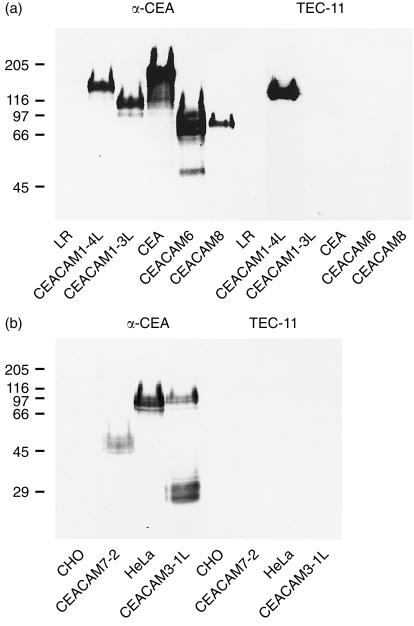
TEC-11 mAb binds to cell transfectants expressing CEACAM1(A2) but not to other members of the CEA family. Mock-transfected LR-73 (LR), CHO, or HeLa cells (negative controls) and transfectants expressing CEACAM1-4L, CEACAM1-3L, CEA, CEACAM6, CEACAM8, CEACAM7-2, or CEACAM3-1L were analysed by immunoblotting with polyclonal rabbit anti-CEA antibody (positive controls) or TEC-11 mAb. Cell extracts were size fractionated by 7·5% (a) or 10% (b) polyacrylamide gel. Molecular-weight markers, shown in kDa on the left, were myosin (205 000), β-galactosidase (116 000), phosphorylase B (97 000), BSA (66 000), ovalbumin (45 000) and carbonic anhydrase (29 000).
Immunoprecipitation
TEC-01 and TEC-02 mAb were purified as described.22 The antibodies were coupled to CNBr-activated Sepharose 4B (Sigma) according to the manufacturer's instructions. Antibody-coated beads were mixed with PA-resistant material from bile or serum or with detergent extract from granulocytes and were incubated for 2 hr at 4°. After washing three times in PBS with 0·1% nonidet P-40, the material bound to the beads was released by boiling in SDS–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer. The samples were size fractionated on SDS–PAGE and analysed by immunoblotting with TEC-11 mAb.
Antibody binding to soluble CEACAM1 constructs
The CEACAM1-Fc soluble domain deletion constructs and the negative control, Muc-18-Fc soluble construct, were prepared as described.26 The constructs were assayed with the YTH71.3.2 rat mAb to CD6627 and the 1B8 mouse antibody to Muc 18,28 using the alkaline phosphatase enzyme-linked immunosorbent assay (ELISA) system from Sigma and appropriate goat anti-rat or anti-mouse antibodies coupled to alkaline phosphatase from Dakopatts (Glostrup, Denmark).26
The specificities of the antibodies for the CEACAM1 domain deletion constructs were assessed in the following way: rabbit F(ab)2 anti-human Fc (Sigma) was dispensed in aliquots (50 µl of 1 : 120 dilution in 10 mm Tris–HCl buffer, pH 8·5) into Immulon III 96-well microtitre plates and incubated overnight at 4°. The plates were washed three times with PBS and 50 µl of the CEACAM1-Fc or Muc-18-Fc construct diluted in PBS (10 µg/ml) was added to each well and left for 6–8 hr at 4°. The wells were washed three times with PBS supplemented with 0·05% (v/v) Tween-20 and 0·25% (w/v) bovine serum albumin (BSA). The CD66 mAbs and TEC-11 mAb were diluted in PBS (1 : 50 for CD66, and 1 : 400 or 1 : 800 for TEC-11), and 50-µl aliquots were added to each construct in the microtitre well in triplicate. The plates were incubated overnight at 4°, washed in PBS/0·05% Tween-20/0·25% BSA three times and the reaction was developed with goat anti-mouse immunoglobulin conjugated to alkaline phosphatase (Dakopatts) at a 1 : 4000 dilution in PBS. After incubation for 1 hr at room temperature, the plates were washed twice with PBS and twice with H2O, and the reaction was developed using the _p_-Nitrophenyl phosphate substrate kit from Sigma for 30–60 min at room temperature. The plates were read at 405 nm on a microplate reader (Bio-Rad, Hercules, CA).
Statistical analysis
The differences between the levels of 115 000 MW CEACAM1(A2) in control and other groups were tested using the Tukey pairwise comparison method.
RESULTS
TEC-11 mAb reacts specifically with the A2 domain of CEACAM1
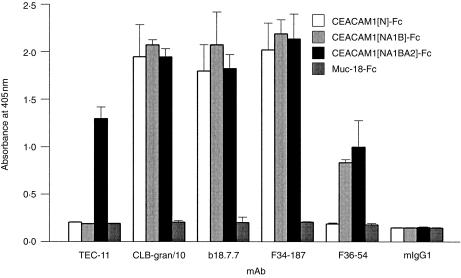
TEC-11 mAb was prepared after immunization of BALB/c mice with a recombinant fragment of CEACAM1 containing the A2 domain. The antigenic specificity of TEC-11 mAb was determined by examining its reactivity with soluble recombinant fragments of CEACAM1 and with transfected cell lines. The following soluble constructs carrying different extracellular domains of CEACAM1 were tested: CEACAM1[N]-Fc carrying only the N-terminal domain of CEACAM1; CEACAM1[NA1B]-Fc with N-terminal and IgC2 domains A1 and B; and CEACAM1[NA1BA2]-Fc with all extracellular domains of CEACAM1-4L, CEACAM1-4S and CEACAM1-4C1 (see Fig. 1). Muc-18-Fc was used as a negative-control soluble protein. The following reagents were used as positive controls: CLB-gran/10, b18.7.7 and F34-187, which bind to the N-terminal domain of CEACAM1 (see below) and react with CD66 subgroup of molecules, CEACAM1, CEACAM3, CEACAM6, CEACAM8 and CEA,6 and F36-54, which binds to A1B domains of CEACAM1 (see below) and reacts with CEACAM1, CEACAM3, CEACAM6 and CEA.6 The data presented in Fig. 2 demonstrate that CLB-gran/10, b18.7.7 and F34-187 bind to all soluble recombinant fragments of CEACAM1, and that F36-54 binds to CEACAM1[NA1B]-Fc and CEACAM1[NA1BA2]-Fc, but not to CEACAM1[N]-Fc. None of these antibodies bound to Muc 18-Fc. TEC-11 bound to CEACAM1[NA1BA2]-Fc, but not to CEACAM1 [NA1B]-Fc, CEACAM1[N]-Fc nor to Muc 18-Fc soluble proteins, indicating that TEC-11 is specific for the A2 domain of CEACAM1.
Figure 2.
TEC-11 mAb binds to CEACAM1(A2) soluble constructs. Soluble recombinant proteins, CEACAM1[N]-Fc, CEACAM1[NA1B]-Fc, CEACAM1[NA1BA2]-Fc and Muc-18-Fc, were attached to 96-well plates via a rabbit F(ab)2 anti-human Fc bridge, and allowed to bind to TEC-11, CLB-gran/10, b18.7.7, F34-187 and F36-54, or an isotype-matched negative control, mIgG1. The binding was detected with goat anti-mouse IgG, conjugated to alkaline phosphatase, and the enzymatic activity was determined as described in the Materials and methods. The data, expressed as absorbance at 405 nm, are means ± SD of triplicate wells in a single experiment. Similar data were obtained in three independent experiments.
To test the reactivity of TEC-11 mAb with transfected cell lines, cell extracts were prepared from mock-transfected LR-73, CHO, or HeLa cells (negative controls) and from LR-73-derived cells stably expressing the CEACAM1-4L, CEACAM1-3L, CEA, CEACAM6, CEACAM8 (Fig. 3a), CHO-derived cells stably expressing CEACAM7-2 and HeLa-derived cells stably expressing CEACAM3-1L (Fig. 3b), and subjected to an analysis by immunoblotting. The polyclonal rabbit anti-CEA antibody served as a positive control. The data presented in Fig. 3 document that all analysed cell extracts from stable transfectants exhibited the corresponding transfected gene products as detected by the polyclonal anti-CEA antibody. The gene products differed in relative molecular weight, being approximately 140 000 for CEACAM1-4L, 115 000 for CEACAM1-3L, 180 000 for CEA, 60 000 for CEACAM6, 60 000 for CEACAM8, 47 000 for CEACAM7-2, and 30 000 for CEACAM3-1L. There was no detectable reactivity with the parental LR-73 cells and CHO cells; polyclonal anti-CEA reacted with a band of about 100 000 in HeLa cells which was not related to the transfected 30 000 CEACAM7 because it was observed in both transfected and mock-transfected cells. TEC-11 mAb reacted with the CEACAM1-4L gene product, but failed to react with the CEACAM1-3L gene product, which lacked the A2 domain (Fig. 1) and with CEA, CEACAM6, CEACAM8, CEACAM7-2 and CEACAM7-2 gene products (Fig. 3). These results indicate that TEC-11 reacts specifically with the A2 domain of CEACAM1 and does not cross-react with other members of the CEA subgroup, nor with membrane or cytoplasmic proteins present in the LR-73, CHO, or HeLa cells.
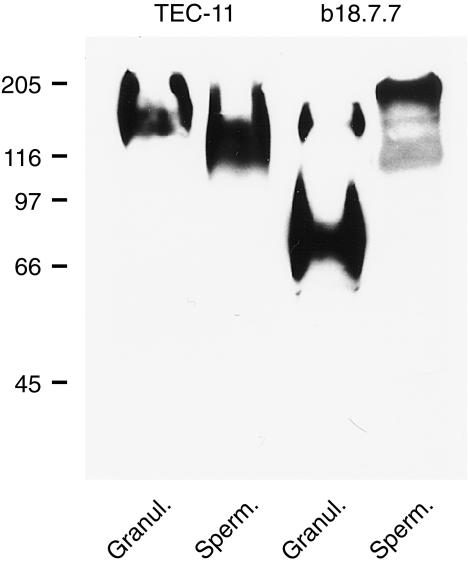
As shown in Fig. 4, the TEC-11 reactive antigen was found in human granulocytes (160 000 isoform) and in human sperm cells (140 000). With the b18.7.7 mAb, which is directed against a conserved region of the N-terminal domain of CEA family members, a 90 000 protein, previously described as CEACAM6,14 was also detected in granulocytes.
Figure 4.
Immunoblotting analysis of CEACAM1(A2) expression in granulocytes and sperm cells using TEC-11 mAb. Immunoblotting with b18.7.7 mAb is also indicated.
CEACAM1 isoforms in human body fluids
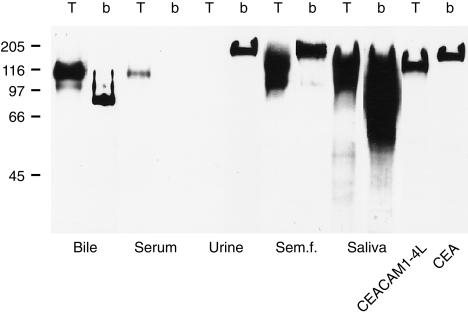
CEACAM1 was initially identified as a CEA cross-reactive antigen in human bile.2 To determine whether the TEC-11 antibody reacts with CEACAM1 in human bile and whether CEACAM1(A2) is present in some other body fluids in normal subjects, we extracted the PA-resistant proteins from bile, serum, urine, seminal fluid and saliva by acetone precipitation, and analysed the proteins by immunoblotting with TEC-11 mAb. The binding of b18.7.7 mAb was also tested as a control reaction. As can be seen in Fig. 5, the TEC-11 mAb reacted in bile predominantly with the 115 000 band. A longer exposure also disclosed a reactivity with the 160 000 band (see below). The b18.7.7 mAb reacted mainly with a band of 85 000, but only weakly with the 115 000 band. In human serum from healthy donors, the TEC-11 mAb reacted weakly with the 115 000 band, while no reactivity was detected under the same immunoblotting conditions with the b18.7.7 mAb. We found no TEC-11-reactive proteins in urine, whereas the b18.7.7 detected a protein of 180 000, presumably CEA. In seminal fluid, TEC-11 detected proteins with apparent molecular weights of 160 000 and 115 000, whereas b18.7.7 reacted predominantly with an antigen of 180 000. Finally in saliva, TEC-11 detected antigenic bands of 160 000 and 115 000, whereas the b18.7.7 bound to antigens of 65 000–160 000. Thus, CEACAM1 molecules containing the A2 domain could be detected in various amounts in all body fluids examined except for urine of healthy subjects.
Figure 5.
Immunoblotting analysis of the presence of CEACAM1(A2) in bile, serum, urine, seminal fluid and saliva using TEC-11 mAb (T). Immunoblotting with b18.7.7 mAb (b) is also indicated. Positive controls (extracts from LR-73 transfectants expressing CEACAM1-4L or CEA, tested with TEC-11 and b18.7.7, respectively) were also included.
Increased levels of the 115 000 CEACAM1(A2) isoform in serum from patients with obstructive jaundice
Increased levels of serum CEACAM1 were previously described in patients with liver or biliary tract disease, and it was suggested that raised levels of CEACAM1 may reflect biliary obstruction.15 In a further experiment we therefore examined whether the 115 000 CEACAM1(A2) isoform was elevated in the serum of patients with obstructive jaundice due to gallstone(s) in the common bile duct or to pancreatic tumour. CEACAM1(A2) levels were evaluated by densitometry of films after immunoblotting and ECL, and related to the internal standard on each gel which was normalized to 100%. Reproducible levels of CEACAM1(A2) (mean 0·3 µg/ml) were found in the sera of healthy donors (control group in Table 1). There was no correlation in this group between CEACAM1(A2) levels and age (from 18 to 76 years), nor was there any sex bias. A significant (P < 0·01) increase in serum CEACAM1(A2) levels (mean 1·5 µg/ml) was found in a group of patients with obstructive jaundice due to gallstone(s) in the common bile duct or to pancreatic tumour. This increase correlated with increased levels of T bilirubin and activity of ALT, AST, ALP and GMT. No significant increase in CEACAM1(A2) was observed in the group of tumour patients having neither clinical nor biochemical signs of liver disease.
Table 1.
Relative levels of 115 000 CEACAM1(A2) isoform and biochemical parameters of liver function tests in blood serum samples from normal donors, patients with obstructive jaundice and patients with tumours
| Group† | |||
|---|---|---|---|
| Parameter | Control | Obstructive jaundice | Tumours |
| n | 32 | 17 | 20 |
| CEACAM1(A2) | 0·3 ± 0·2 | 1·5 ± 0·8* | 0·4 ± 0·3 |
| (µg/ml)‡ | |||
| T Bilirubin (µmol/l) | 9·7 ± 4·8 | 137·0 ± 97·5 | 18·2 ± 10·0 |
| ALT (µkat/l) | 0·4 ± 0·2 | 3·5 ± 4·4 | 1·0 ± 0·9 |
| AST (µkat/l) | 0·4 ± 0·1 | 2·6 ± 2·2 | 0·6 ± 0·2 |
| ALP (µkat/l) | 1·1 ± 0·3 | 7·2 ± 6·2 | 1·4 ± 0·4 |
| GMT (µkat/l) | 0·3 ± 0·2 | 7·7 ± 8·2 | 1·2 ± 0·7 |
Expression of the Lex oligosaccharide on soluble CEACAM1(A2) isoforms
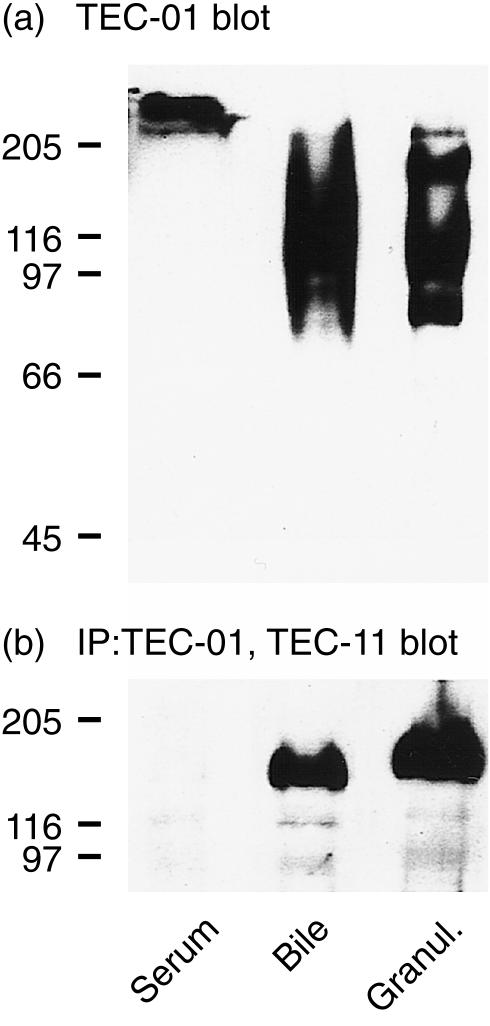
In granulocytes CEACAM1 is a major carrier of the carbohydrate epitope Lex.16,17 To determine whether Lex is also expressed on soluble CEACAM1(A2) isoforms, we first analysed by immunoblotting the reactivity of TEC-01 mAb with PA-resistant material from serum and bile. As a control we also used an extract from detergent-solubilized granulocytes. The data listed in Fig. 6(a) indicate that a glycoconjugate of > 200 000 was detectable by TEC-01 in the PA-resistant serum fraction, suggesting that soluble CEACAM1(A2) in serum was not carrying the Lex. In bile extract TEC-01 reacted with several glycoproteins of about 80 000, 90 000, 110 000, 160 000 and > 200 000, whereas in granulocytes it bound to proteins of about 80 000, 115 000 and 160 000. The next step was TEC-11 immunoblotting analysis of TEC-01-immunoprecipitated material derived from PA-resistant fractions of bile and serum, or from detergent-extracted granulocytes. When Lex-positive proteins from bile were used, TEC-11 reacted with a broad band of about 160 000. A glycoconjugate of similar size was identified in TEC-01 immunoprecipitates from granulocytes (Fig. 6b). No binding of TEC-11 was observed with Lex-positive material from blood. The binding of TEC-11 was specific for Lex-positive CEACAM1(A2), because another mAb of IgM class (TEC-02) precipitated no TEC-11 reactive material in any of the tested samples (not shown).
Figure 6.
Presence of the Lex oligosaccharide on soluble CEACAM1(A2) isoform. (a) Reactivity of Lex-specific mAb, TEC-01, with PA-resistant proteins from serum and bile and with cell extract from granulocytes, as detected by immunoblotting. (b) PA-resistant proteins from serum and bile and cell extract from granulocytes were immunoprecipitated (IP) with TEC-01 mAb bound to Sepharose 4B. The immunoprecipitated material was analysed for the presence of CEACAM1(A2) by immunoblotting with TEC-11 mAb.
DISCUSSION
The mAb TEC-11 was prepared after immunization of BALB/c mice with a recombinant protein containing the A2 domain of human CEACAM1. Although the recombinant protein included several amino acids outside the A2 domain, several lines of evidence presented in this paper indicate that the antibody reacts exclusively with the A2 domain. First, in ELISA, TEC-11 mAb reacted with soluble recombinant proteins containing the A2 domain of CEACAM1, but not with those soluble proteins that contain the N-terminal domain plus or minus the A1 and B domains. When other CD66 antibodies were tested in this system, none was found to react specifically with the A2 domain of CEACAM1.26 Second, the TEC-11 mAb reacted in immunoblotting experiments with extracts from transfectants expressing CEACAM1-4L, but not with cells expressing CEACAM1-3L lacking the A2 domain, nor with CEA, CEACAM6, CEACAM8, CEACAM7-2 or CEACAM3-1L. Finally, in granulocytes which are known to express the 160 000 CEACAM1-4L isoform together with the 90 000 CEACAM6, TEC-11 reacted exclusively with the 160 000 band.
A mAb specific for CEACAM1, the 4D1/C2, has been isolated by another research group after immunization of BALB/c mice with CEACAM1 purified from human bile.29 Although its domain specificity has not been reported, the reactivity with the two CEACAM1 isoforms in bile (115 000 and 85 000)14 indicates that it recognizes a domain other than the A2. In fact, our unpublished data indicate that it does not react with the N-terminal domain of CEACAM1 expressed either as the soluble recombinant CEACAM1[N]-Fc construct or as CEACAM1-1S in CHO transfectants, but does react weakly with the soluble recombinant CEACAM1[NA1B]-Fc construct. It should also be noted that the recently prepared mAb against activated intestinal intraepithelial lymphocytes react with the N-terminal domain of CEACAM1.30 The combined data substantiate the notion that TEC-11 is a unique mAb which can detect CEACAM1 isoforms with A2 domain.
An immunoblotting assay was employed to demonstrate the presence of CEACAM1(A2) isoforms in body fluids. In human bile, TEC-11 reacted with the 115 000 isoform but not with the 85 000 isoform. This is in accordance with a previous study in which a polyclonal antibody specific for the A2 domain of CEACAM1 was used.14 Longer exposure times have revealed that human bile also contains small amounts of 160 000 CEACAM1(A2) isoform. In serum, TEC-11 only reacted with a 115 000 protein, whereas in seminal fluid and saliva, both 160 000 and 115 000 CEACAM1(A2) isoforms were detectable.
There are three isoforms of CEACAM1 containing the A2 domain: CEACAM1-4L, CEACAM1-4S, and CEACAM1-4C1. The largest is CEACAM1-4L, a 160 000 molecule found in granulocytes and very likely in seminal fluid, saliva and bile. It has been previously reported that the 115 000 CEACAM1 isoform in bile lacked the long cytoplasmic domain and was therefore predicted to be either CEACAM1-4S or CEACAM1-4C1.14 These are also the likely candidates for the 115 000 CEACAM1 isoform found in serum, seminal fluid and saliva in this study.
Using a radioimmunoassay technique with polyclonal antibodies, Svenberg et al. found that serum levels of CEACAM1 were elevated in patients with liver or biliary tract diseases.15 However, these results did not distinguish among the different CEACAM1 isoforms. The quantitative immunoblotting analysis with TEC-11 mAb that we have described here indicates for the first time that it is the 115 000 CEACAM1(A2) isoform that is significantly increased in patients with a hepatobiliary system disease. Importantly, there was no significant enhancement of this CEACAM1 isoform in a group of patients suffering from liver-unrelated diseases. Previous studies have established that in granulocytes CEACAM1 is a major carrier of the carbohydrate epitope Lex.16,17 Immunoblotting analyses showed that Lex was also present on glycoconjugates in the PA-resistant fraction of serum and bile. Interestingly, the 115 000 CEACAM1(A2) isoform is Lex-negative and only a minor 160 000 isoform present in bile (but not in blood) is Lex-positive. The origin of the 160 000 Lex-positive CEACAM1(A2) in bile ought to be determined.
The function of CEACAM1 in body fluids as well as in granulocytes, lymphocytes and some other normal and tumour cells is not completely understood. Localization on cell surfaces suggests that it is involved in surface recognition phenomena. It has been shown that some CEACAM1 isoforms function in vitro as homotypic and heterotypic cell adhesion molecules.31 CEACAM1 expressed on granulocytes could be important for their homing to the areas of inflammation.17 Aggregation of surface CEACAM1 with antibodies has been found to mediate signal transduction.32 In this connection it is interesting that mouse CEACAM1 has been recognized as the receptor for mouse hepatitis viruses,33 and that human CEACAM1 can serve as a receptor for gonococcal34 and meningococcal35 opacity proteins. The carbohydrate moiety of CEACAM1 can also function as a ligand for some strains of bacteria and allow bacterial colonization.36 Furthermore, it has also been shown that CEACAM1 has a tumour-suppressive role37 and that rat CEACAM1 can function as an ecto-ATPase and bile transporter.38 Although it is not known which of the above mentioned properties represents the real function of CEACAM1 in vivo, some of these could be affected by soluble CEACAM1 in body fluids. Increased serum levels of CEACAM1 in patients with obstructive jaundice could have an impact on CEACAM1 function and thus contribute to the secondary symptomatology of this disease. It should be noted that increased levels of CEACAM1 were also found in serum and urine of rats with experimentally induced liver diseases.39 These data corroborate our present conclusions and indicate that increased levels of CEACAM1 in body fluids during liver diseases may be a rather general phenomenon common to various species.
Acknowledgments
We thank Professor Sir D. J. Weatherall for his support, Dr J. Vorlíček for help with statistical analysis, Dr M. Jirsa for valuable discussion on the manuscript, M. Aulická and H. Mrázová for technical assistance and donors of antibodies, cell lines and plasmids. This work was supported by grants 600-3, 3755-3 and M18-3 from the Ministry of Health of the Czech Republic, by grants 310/00/0205 and 204/00/0204 from the Grant Agency of the Czech Republic and by grants from the National Cancer Institute of Canada and the Medical Research Council of Canada. The research of P.D. was supported in part by an International Research Scholar's award from the Howard Hughes Medical Institute. S.M.W. was supported by grants from the Medical Research Council and Leukaemia Research Fund, UK, and by European Union (E. U. Biotech., PECO and INTAS/RFBR).
Abbreviations
ALP
alkaline phosphatase
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CEA
carcinoembryonic antigen
GMT
γ-glutamyltransferase
mAb
monoclonal antibody
PA
perchloric acid
REFERENCES
- 1.Beauchemin N, Draber P, Dveksler G, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–9. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 2.Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976;17:588–96. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- 3.Svenberg T, Hammarström S, Zeromski J. Immunofluorescence studies on the occurrence and localization of the CEA-related biliary glycoprotein I (BGP I) in normal human gastrointestinal tissues. Clin Exp Immunol. 1979;36:436–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroki M, Matsuo Y, Ohtani T, et al. A novel CEA-cross-reacting antigen of molecular weight 140,000 expressed on human lymphoid cell lines. Mol Immunol. 1990;27:689–96. doi: 10.1016/0161-5890(90)90012-o. [DOI] [PubMed] [Google Scholar]
- 5.Coutelier JP, Godfraind C, Dveksler GS, et al. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–90. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skubitz KM, Micklem K, Van Der Schoot E. CD66 and CD67 cluster workshop report. In: Schlossman SF, Boumsell L, Gilks W, et al., editors. Leucocyte Typing V. New York: Oxford University Press; 1995. pp. 889–99. [Google Scholar]
- 7.Möller MJ, Kammerer R, Grunert F, Von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–5. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Kammerer R, Hahn S, Singer BB, Luo JS, Von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–74. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Hammarström S, Olsen A, Teglund S, Baranov V. The nature and expression of the human CEA family. In: Stanners CP, editor. Cell Adhesion and Communication Mediated by the CEA Family. Amsterdam: Harwood Academic Publishers; 1998. pp. 1–30. [Google Scholar]
- 10.Barnett TR, Kretschmer A, Austen DA, et al. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989;108:267–76. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett TR, Drake L, Pickle Ii W. Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed protein. Mol Cell Biol. 1993;13:1273–82. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroki M, Arakawa F, Matsuo Y, et al. Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library. Biochem Biophys Res Commun. 1991;176:578–85. doi: 10.1016/s0006-291x(05)80223-2. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspective. J Clin Lab Anal. 1991;5:344–66. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 14.Stoffel A, Neumaier M, Gaida F-J, et al. Monoclonal, anti-domain and anti-peptide antibodies assign the molecular weight 160,000 granulocyte membrane antigen of the CD66 cluster to a mRNA species encoded by the biliary glycoprotein gene, a member of the carcinoembryonic antigen gene family. J Immunol. 1993;150:4978–84. [PubMed] [Google Scholar]
- 15.Svenberg T, Wahren B, Hammarström S. Elevated serum levels of a biliary glycoprotein (BGP I) in patients with liver or biliary tract disease. Clin Exp Immunol. 1979;36:317–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Stocks SC, Albrechtsen M, Kerr MA. Expression of the CD15 differentiation antigen (3-fucosyl-N-acetyl-lactosamine, Lex) on putative neutrophil adhesion molecules CR3 and NCA-160. Biochem J. 1990;268:275–80. doi: 10.1042/bj2680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuijpers TW, Hoogerwerf M, Van Der Laan LJ, et al. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992;118:457–66. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips ML, Nudelman E, Gaeta FC, et al. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–2. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 19.Larsen E, Palabrica T, Sajer S, et al. PADGEM-dependent adhesion of platelets to monocytes and neutrophils is mediated by a lineage-specific carbohydrate, LNF III (CD15) Cell. 1990;63:467–74. doi: 10.1016/0092-8674(90)90443-i. [DOI] [PubMed] [Google Scholar]
- 20.Haggarty A, Legler C, Krantz MJ, Fuks A. Epitopes of carcinoembryonic antigen defined by monoclonal antibodies prepared from mice immunized with purified carcinoembryonic antigen or HCT-8R cells. Cancer Res. 1986;46:300–9. [PubMed] [Google Scholar]
- 21.Dráber P, Pokorná Z. Differentiation antigens of mouse teratocarcinoma stem cells defined by monoclonal antibodies. Cell Differentiation. 1984;15:109–113. doi: 10.1016/0045-6039(84)90060-5. [DOI] [PubMed] [Google Scholar]
- 22.Dráber P. The epitope of mouse embryonic antigen(s) recognized by monoclonal antibody TEC-02 is a carbohydrate carried by high-molecular-weight glycoconjugate. Cell Differentiation. 1987;21:119–30. doi: 10.1016/0045-6039(87)90419-2. [DOI] [PubMed] [Google Scholar]
- 23.Dráber P, Zikán J, Vojtísková M. Establishment and characterization of permanent murine hybridomas secreting monoclonal anti-Thy-1 antibodies. J Immunogenet. 1980;7:455–74. doi: 10.1111/j.1744-313x.1980.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 24.Rojas M, Fuks A, Stanners CP. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2+-dependent intercellular adhesion molecule. Cell Growth Differ. 1990;1:527–33. [PubMed] [Google Scholar]
- 25.Schölzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595–605. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira AM, Fawcett J, Simmons DL, Watt SM. The N-domain of the biliary glycoprotein (BGP) adhesion molecule mediates homotypic binding: domain interactions and epitope analysis of BGPc. Blood. 1994;84:211–9. [PubMed] [Google Scholar]
- 27.Watt SM, Sala-Newby G, Hoang T, et al. CD66 identifies a neutrophil-specific epitope within the hematopoietic system that is expressed by members of the carcinoembryonic antigen family of adhesion molecules. Blood. 1991;78:63–74. [PubMed] [Google Scholar]
- 28.Fawcett J, Holness CL, Needham LA, et al. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocyte. Nature. 1992;360:481–4. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- 29.Drzeniek Z, Lamerz R, Fenger U, et al. Identification of membrane antigens in granulocytes and colonic carcinoma cells by a monoclonal antibody specific for biliary glycoprotein, a member of the carcinoembryonic antigen family. Cancer Lett. 1991;56:173–9. doi: 10.1016/0304-3835(91)90093-w. [DOI] [PubMed] [Google Scholar]
- 30.Morales VM, Christ A, Watt SM, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–70. [PubMed] [Google Scholar]
- 31.Stanners CP, Fuks A. Properties of adhesion mediated by the human CEA family. In: Stanners CP, editor. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. Amsterdam: Harwood Academic Publishers; 1998. pp. 57–71. [Google Scholar]
- 32.Draber P, Skubitz KM. Signal transduction mediated by the CEA family. In: Stanners CP, editor. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. Amsterdam: Harwood Academic Publishers; 1998. pp. 121–40. [Google Scholar]
- 33.Dveksler GS, Dieffenbach CW, Cardellichio CB, et al. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus A-59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray-Owen SD, Dehio C, Haude A, et al. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–45. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virji M, Watt SM, Barker S, et al. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–39. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 36.Leusch HG, Drzeniek Z, Hefta SA, et al. The putative role of members of the CEA-gene family (CEA, NCA an BGP) as ligands for the bacterial colonization of different human epithelial tissues. Zentralbl Bakteriol. 1991;275:118–22. doi: 10.1016/s0934-8840(11)80775-9. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh JT, Luo W, Song W, et al. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–7. [PubMed] [Google Scholar]
- 38.Lin SH, Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989;264:14408–14. [PubMed] [Google Scholar]
- 39.Lucka L, Sel S, Danker K, et al. Carcinoembryonic antigen-related cell-cell adhesion molecule C-CAM is greatly increased in serum and urine of rats with liver diseases. FEBS Lett. 1998;438:37–40. doi: 10.1016/s0014-5793(98)01265-4. [DOI] [PubMed] [Google Scholar]