The small GTP-binding protein Rho links G protein-coupled receptors and Gα12 to the serum response element and to cellular transformation (original) (raw)
Abstract
Receptors coupled to heterotrimeric G proteins can effectively stimulate growth promoting pathways in a large variety of cell types, and if persistently activated, these receptors can also behave as dominant-acting oncoproteins. Consistently, activating mutations for G proteins of the Gαs and Gαi2 families were found in human tumors; and members of the Gαq and Gα12 families are fully transforming when expressed in murine fibroblasts. In an effort aimed to elucidate the molecular events involved in proliferative signaling through heterotrimeric G proteins we have focused recently on gene expression regulation. Using NIH 3T3 fibroblasts expressing m1 muscarinic acetylcholine receptors as a model system, we have observed that activation of this transforming G protein-coupled receptors induces the rapid expression of a variety of early responsive genes, including the c-fos protooncogene. One of the c-fos promoter elements, the serum response element (SRE), plays a central regulatory role, and activation of SRE-dependent transcription has been found to be regulated by several proteins, including the serum response factor and the ternary complex factor. With the aid of reporter plasmids for gene expression, we observed here that stimulation of m1 muscarinic acetylcholine receptors potently induced SRE-driven reporter gene activity in NIH 3T3 cells. In these cells, only the Gα12 family of heterotrimeric G protein α subunits strongly induced the SRE, while Gβ1γ2 dimers activated SRE to a more limited extent. Furthermore, our study provides strong evidence that m1, Gα12 and the small GTP-binding protein RhoA are components of a novel signal transduction pathway that leads to the ternary complex factor-independent transcriptional activation of the SRE and to cellular transformation.
Keywords: G proteins, transcription factors, signal transduction, c_-fos_
Transcription of the early responsive gene c_-fos_ is rapidly and transiently activated by a number of growth stimulating factors acting on tyrosine kinase and G protein-linked receptors. The induction of c_-fos_ transcription is mediated by different promoter elements (1). Among these, the serum response element (SRE) is believed to play a central regulatory role (2, 3), as this sequence has been shown to be necessary and sufficient for the rapid induction of c-fos by most growth-promoting stimuli (4–6). Several proteins reported to bind the c-fos SRE, represent likely candidates to mediate SRE-dependent transcription (2). Among them, a ubiquitously expressed transcription factor of 67 kDa has been shown to bind the SRE in vivo and in vitro as a dimer (2), and has been termed serum response factor. Another protein exhibits the ability to form a ternary complex with SRE and the serum response factor dimer, and has been therefore termed ternary complex factor (TCF) or p62TCF (7–9). The TCF has been shown to regulate SRE activity in response to the activation of the Ras-Raf-extracellular signal-regulated kinase (ERK) mitogen-activating protein kinase (MAPK) pathway (10–12). Recent studies suggest that p62TCF can also be phosphorylated by another MAPK known as NH2-terminal c-Jun kinase (JNK) (13, 14), thus providing a mechanism whereby JNK-activating stimuli, including inflammatory cytokines, can control gene expression through the SRE. Interestingly, SRE can also be regulated in a TCF-independent manner, and certain TCF-independent signaling pathways acting on SRE have been recently shown to be controlled by members of the Rho family of Ras-related small GTPases (15).
Recently, we have used NIH 3T3 fibroblasts engineered to express human muscarinic acetylcholine receptors (mAChRs) as a model system for the study of proliferative signaling through G protein-coupled receptors (16, 17). The mAChR family consists of five distinct but highly related subtypes (m1–m5) (18), which can be divided into two functionally distinct groups: m1, m3 and m5 mAChRs that potently stimulate PIP2 metabolism, and m2 and m4 mAChRs which couple to inhibition of adenylyl cyclase (19). In this biological system, we have shown that the m1 class of mAChRs effectively transduces mitogenic signals and, when persistently activated, behaves as a potent agonist-dependent oncogene (16). We have also shown that α subunits of heterotrimeric G proteins can display oncogenic properties. Whereas constitutively activated mutants of Gαi2 induce alterations in cell growth, analogous Gαq mutants are fully oncogenic when expressed in mouse fibroblasts (20, 21). Furthermore, we (22, 23) and others (40) have shown that genes for activated α subunits of the newly discovered G12/G13 G protein family behave as remarkably potent transforming genes, nearly as potent as v-raf or the human ras oncogene.
Molecules participating in the proliferative and transforming responses to activated G proteins and their coupled receptors are still poorly defined. We have recently observed that activation in NIH 3T3 cells of m1 G protein-coupled receptors induces the rapid expression of a variety of early responsive genes, including the c_-fos_ protooncogene (24). With the aid of reporter plasmids for gene expression we attempt here to elucidate the molecular events involved in the induction of the c-fos by G protein-coupled receptors and oncogenic G proteins, focusing our attention on the SRE. We observed that m1 receptors activated by an agonist potently induced SRE-driven reporter gene activity. Among the α subunits of heterotrimeric G proteins, only the Gα12 family strongly induced the SRE, while β1γ2 heterodimers activated SRE but to a more limited extent. In addition, we provide evidence that the small GTP-binding protein RhoA acts as an integral component of a novel signaling pathway connecting m1 receptors as well as Gα12 to both transcriptional activation of the SRE and to neoplastic transformation.
MATERIALS AND METHODS
Cell Lines and Transfection.
NIH 3T3 fibroblasts expressing ≈20,000 human m1 mAChRs per cell, designated NIH-m1.2 cells (25), were maintained in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% calf bovine serum. Transfections were performed by the calcium-phosphate precipitation technique, adjusting the total amount of plasmid DNA to 5–10 μg per plate with empty vector.
Reporter Gene Assays.
NIH-m1.2 cells were transfected with different expression plasmids together with 1 μg of pCMV–β-gal, a plasmid expressing the enzyme β-galactosidase, and 1 μg of each of the reporter plasmids expressing the chloramphenicol acetyltransferase (CAT) gene [reporter plasmids for wild-type SRE from c-fos (pSREwt) or reporter plasmids for a mutant lacking the TCF binding site (pSREmutL)]. After overnight incubation, the cells were washed with serum-free DMEM, and kept for ≈24 h in DMEM supplemented with 0.5% fetal bovine serum. Cells were then stimulated with agonists for 6 additional h, and lysed using reporter lysis buffer (Promega). Additional DNAs were added to the transfection mixtures as indicated in the figures and total amount of plasmid DNA was adjusted to 5–10 μg per plate with vector DNA (pCDNA3, Invitrogen) when necessary. CAT activity was assayed in the cell extracts by incubation for 6–16 h in the presence of 0.25 μCi [14C]chloramphenicol (100 mCi/mmol; 1 Ci = 37 GBq) and 200 μg/ml butyryl-CoA in 0.25 M Tris⋅HCl, pH 7.4. Labeled butyrylated products were extracted using a mixture of Xylenes (Aldrich) and counted as described (24). β-Gal activity present in each sample was assayed by a colorimetric method, and used to normalize for transfection efficiency.
DNA Constructs.
Reporter plasmids that express the CAT gene under the control of SREwt or a SREmutL, as well as an expression vector for the C3 toxin were kindly provided by Richard Treismann (15). A RhoA mutant that is insensitive to the C3 toxin activity was engineered by PCR-directed mutagenesis of the human RhoA cDNA, replacing asparagine 41 for isoleucine (26). The resulting mutant was designated rhoI41. Sequences of mutagenic oligonucleotides will be made available upon request. Other cDNA expression vectors have been described (27, 28).
Focus Formation Assays.
Transfection of NIH 3T3 cells was performed using the calcium phosphate technique with the DNAs indicated in each figure legend. Cells were cultured in DMEM supplemented with 10% calf bovine serum with or without the addition of 10 μM carbachol as necessary. Transformed foci were scored after 2–3 weeks as described (16, 22).
RESULTS
Stimulation of SRE by G Protein-Coupled m1 Muscarinic Receptors.
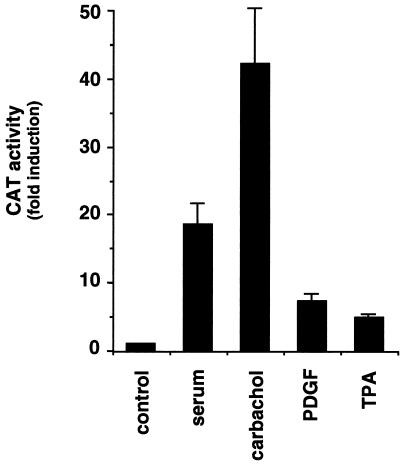
As a first step in understanding the mechanism by which m1 receptors regulate c-fos expression, we focused our attention on the major regulatory element in the c-fos promoter, the SRE (2, 3, 11). We transfected NIH-m1.2 cells with a reporter plasmid that expresses the CAT gene under the control of a minimal promoter and a sequence corresponding to the c-fos SRE (15). Under these experimental conditions, we observed that whereas serum stimulated CAT activity around 20 fold, addition of the cholinergic agonist, carbachol, resulted in an even greater stimulation that ranged between 20- and 50-fold (Fig. 1). Parallel treatment with platelet-derived growth factor, a mitogen acting on endogenous tyrosine kinase receptors, and with phorbol esters (phorbol 12-tetradecanoate 13-acetate, TPA) resulted in a significant but more modest increase of reporter gene activity. These data show that carbachol can stimulate transcriptional activities regulated by SRE in NIH-m1.2 cells to an extent similar to or even greater than that of other growth promoting stimuli.
Figure 1.
The cholinergic agonist strongly induces the c-fos SRE in NIH 3T3 cells expressing m1 G protein-coupled receptors. NIH-m1.2 cells were cotransfected with pSREwt and pCMV–β-gal reporter plasmid DNAs (1 μg per plate each). 40 h later, cells were left untreated or exposed for 6–8 h to 10% serum, 100 μM carbachol, 10 ng/ml platelet-derived growth factor, or 50 ng/ml TPA, as indicated. Cells were collected, and the lysates were assayed for CAT and β-gal activity. The data represent CAT activity normalized by the β-gal activity present in each sample expressed as fold induction with respect to control cells, and are the average ± SE of triplicate samples from a typical experiment. Similar results were obtained in four independent experiments.
An Activated Mutant of Gα12 Strongly Stimulates Transcriptional Activity Through the SRE.
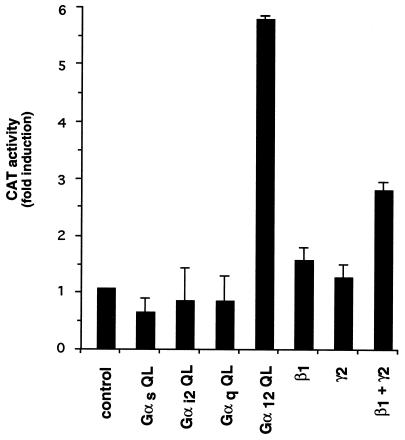
As an approach to investigate which G proteins mediate the activation of c-fos SRE, we took advantage of the observation that expression of GTPase deficient, mutationally activated forms of G protein α subunits can activate effector pathways by obviating the need for receptor stimulation (29). Thus, we cotransfected the reporter gene plasmid pSREwt together with GTPase deficient mutants for Gαs, Gαi2, Gαq, and Gα12, which are representative members for each of the four Gα subunit families (30). When transfected into NIH-m1.2 cells, expression of each G protein α subunit could be demonstrated by Western blot analysis with subtype-specific antibodies (data not shown; refs. 28 and 31). Under our experimental conditions, the activated mutants for Gαs, Gαi2, and Gαq failed to stimulate SRE. In contrast, the GTPase deficient mutant of Gα12 effectively elevated CAT activity (Fig. 2). Similar results were obtained in parental NIH 3T3 cell (data not shown). These data strongly suggest that heterotrimeric G proteins of the G12 family can mediate SRE stimulation.
Figure 2.
Effect of Gβγ dimers and activated mutants of Gα subunits of heterotrimeric G proteins on the activity of the SRE. NIH-m1.2 cells were cotransfected with pSREwt (1 μg per plate) and pCMV–β-gal (1 μg per plate) plasmid DNAs together with expression vectors for the constitutively activated mutants of Gαs Gαi2, Gαq, and Gα12 as well as plasmids expressing β1 and γ2 cDNAs, as indicated. Cells were processed as described in the Methods section. The data represent CAT activity normalized by the β-gal activity present in each cellular lysate, and are the average ± SE of triplicate samples from a typical experiment. Similar results were obtained in three independent repetitions of this experiment.
When activated, receptors linked to G proteins catalyze the replacement of GDP by GTP bound to the α subunit, and induce the dissociation of α-GTP from βγ dimers (30). Although the α subunits were thought to be solely responsible for coupling receptors to second messenger generating systems, recent work (30) has established a critical role for βγ dimers in signal transduction. In particular, we have observed that βγ dimers can activate the ERK2 and JNK1 members of the superfamily of MAPKs (28, 32), which, in turn, have been shown to phosphorylate TCF (10, 11, 13, 14). This prompted us to explore whether βγ dimers participate in signaling to SRE. Following a similar approach to that for the Gα subunits, we observed that, when cotransfected with pSREwt, β1γ2 subunits induce a significant increase in reporter gene activity (Fig. 2). We conclude that both Gα12 and βγ subunits of heterotrimeric G proteins can stimulate SRE activity in NIH 3T3 cells.
Activation of SRE by Members of the Ras and Rho-Family of GTPases.
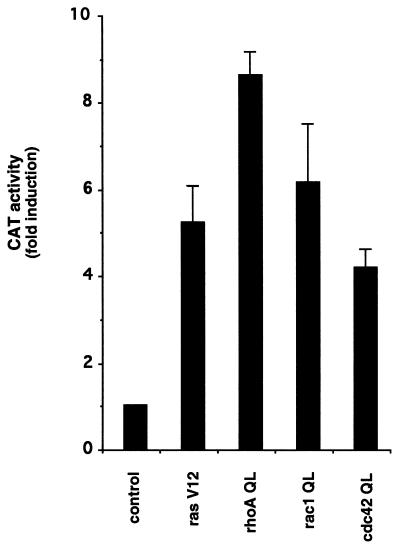
Recently available evidence suggests that small GTP-binding proteins of the Ras superfamily link heterotrimeric G proteins to signaling pathways regulating gene expression. As an approach to study the role of small GTPases in the control of SRE activity in our cellular setting, we made use of expression vectors for the activated forms of Ras (RasV12) and of representative members of each Rho subfamily: RhoA (RhoAQ63L), Rac1 (Rac1Q61L) and Cdc42 (Cdc42Q61L) (QL mutants, ref. 33). All these constructs were detectably expressed when transfected into NIH-m1.2 cells (data not shown) and showed a several fold increase in CAT activity upon cotransfection with the reporter plasmid pSREwt (Fig. 3). Thus, expression of activated forms of representative small GTPases can stimulate SRE transcriptional activity in this cellular system. These data are consistent with those of a recently published report (15).
Figure 3.
Activated mutants of small GTP-binding proteins stimulate the SRE. NIH-m1.2 cells were cotransfected with pSREwt (1 μg per plate) and pCMV–β-gal (1 μg per plate) plasmid DNAs together with an empty expression vector (control) or expressing the Val12 mutant of Ras, the Leu63 mutant of RhoA, or the Leu61 mutants of Rac1 and Cdc42 (rasV12, rhoAQL, rac1QL and cdc42QL, respectively), as indicated. CAT and β-gal activity were assayed as described in Fig. 1. The data represent CAT activity normalized by the β-gal activity present in each cellular lysate, and are the average ± SE of triplicate samples from a typical experiment, expressed as fold induction with respect to control cells. Similar results were obtained in four independent experiments.
Activation of SRE by m1 G Protein-Coupled Receptors and Gα12 Involves a TCF-Independent Pathway.
As discussed above, the SRE binds the transcription factor serum response factor and a group of proteins designated, collectively, as TCF. As the C-terminal domain of TCF is phosphorylated by both ERK and JNK, thereby increasing its transactivating activity, it has been suggested that TCF regulates SRE activity in response to signals acting through the Ras-Raf-ERK or Rac1-JNK biochemical routes (3, 13, 14). On the other hand, it has been recently shown that certain signals act on the serum response factor in an ERK and JNK independent fashion (15). Accordingly, we next set out to investigate whether agonist-stimulated m1 receptors or activated Gα12 regulate SRE activity in a TCF-dependent or independent manner. As an approach, we used a reporter plasmid containing a mutated SRE that cannot bind TCF (pSREmutL, ref. 15).
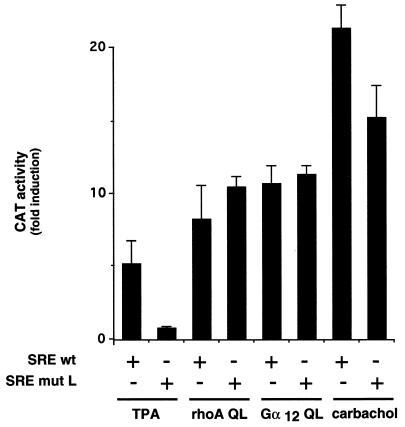
As shown in Fig. 4, NIH-m1.2 cells were transfected with pSREwt or its mutL form, and treated with carbachol or TPA, or cotransfected with activated forms of Gα12 or RhoA. Whereas the SREwt responded to stimulation by TPA with a 5-fold increase in reporter gene activity, no response was observed when we utilized the SREmutL. In contrast, induction of CAT activity by RhoAQL was similar for both the SREwt and the SREmutL reporter plasmids. Thus, these data further support that phorbol esters stimulate SRE through TCF, but that RhoA enhances transcriptional activity of the SRE in a TCF-independent manner (15). Interestingly, Gα12QL potently stimulated CAT activity when cotransfected with either reporter plasmid, resulting in an increase of ≈10-fold for both cases. Stimulation of m1 G protein-coupled receptors produced a slightly different picture. Addition of carbachol to NIH-m1.2 cells transfected with pSREmutL produced a remarkable increase of nearly 20-fold in CAT activity, but this response was slightly lower than that observed with the SREwt reporter plasmid. These observations suggest that Gα12 and m1 receptors signal to the SRE in a TCF-independent manner, although these G protein-coupled receptors can also stimulate a limited TCF-dependent component.
Figure 4.
m1 and constitutively active Gα12 subunit activate the SRE in a TCF-independent fashion. NIH-m1.2 cells were cotransfected with pSRE or pSREmutL (1 μg per plate) and pCMV–β-gal (1 μg per plate) plasmid DNAs. Expression vectors for the constitutively activated mutants of Gα12 and RhoA were included where indicated. Cells were transferred to DMEM supplemented with 0.5% fetal bovine serum, and 24 h later cells were left untreated or stimulated for 6–8 h with 100 μM carbachol or 50 ng/ml TPA, as indicated. The data represent CAT activity normalized by the β-gal activity present in each cellular lysate, expressed as fold induction with respect to control cells, and are the average ± SE of triplicate samples from a typical experiment. This experiment was repeated three times with similar results.
Stimulation of SRE by Both m1 Receptors and Gα12 Subunits Is Dependent on Rho.
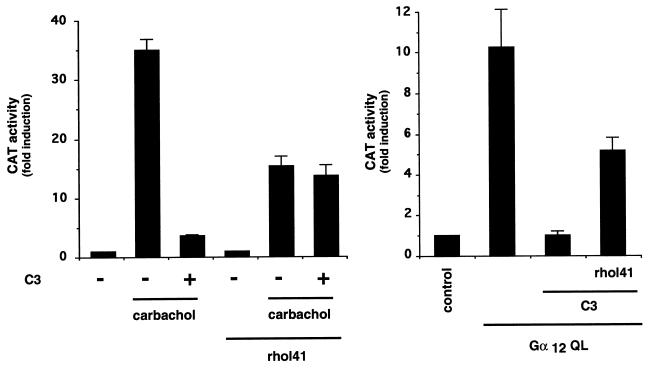
Agonist-stimulated m1 receptors, as well as activated forms of Gα12 subunits, induce TCF-independent activation of the SRE, thus resembling those effects caused by activated forms of RhoA (Fig. 4 and ref. 15). These observations raised the possibility that Rho could be an integral part of the signaling pathway(s) connecting m1 and Gα12 to the SRE. To explore this possibility, we made use of a DNA construct that expresses the botulinum C3 exoenzyme (26). This toxin ADP ribosylates RhoA at asparagine 41, thereby preventing the exchange of GDP by GTP and retaining RhoA in its GDP-bound inactive form (15, 26). As seen in Fig. 5, cotransfection with a plasmid expressing the C3 toxin can reduce stimulation of SRE by carbachol in a range of 85–90%. When replacing asparagine for isoleucine at residue 41 in RhoA, the resulting mutant is no longer a substrate for C3 and becomes insensitive to the action of the C3 toxin (26). As shown in Fig. 5, coexpression of this RhoA mutant (RhoA-I41) with C3 reverted the effect of the toxin on carbachol-induced activation of the SRE. Similarly, while coexpression with C3 abolished the induction of SRE by Gα12, this response was restored to significant levels by coexpression of RhoA-I41 (Fig. 5). Taken together, these data show that both m1 receptors and Gα12 induce SRE activity through a pathway that involves the function of RhoA.
Figure 5.
Constitutively active Gα12 subunits and m1 activate the SRE in a RhoA-dependent manner. NIH-m1.2 cells were cotransfected with pSRECAT (1 μg per plate) and pCMV–β-gal (1 μg per plate) plasmid DNAs and expression vectors for the constitutively activated mutant of Gα12, the C3 toxin and the C3-insensitive mutant of RhoA (rhoAI41), as indicated. Cells were left untreated or stimulated for the last 6–8 h with 100 μM carbachol, as indicated. The data represent CAT activity normalized by the β-gal activity present in each cellular lysate, expressed as fold induction with respect to control cells, and are the average ± SE of triplicate samples from a typical experiment. Similar results were obtained in five independent experiments.
Focus Formation by m1 and Activated Gα12 in NIH 3T3 Cells Is Dependent on Rho.
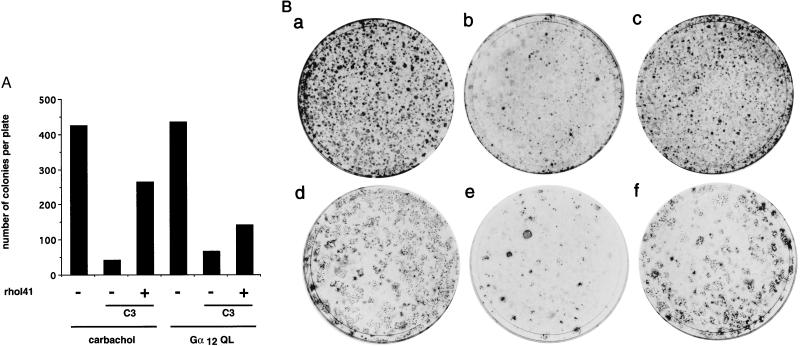
In previous studies, we have reported that carbachol stimulation of NIH 3T3 cells expressing m1 receptors, as well as the expression of activated forms of proteins of the Gα12 family, result in the formation of characteristic foci of transformation (16, 22, 23). In this study, we show that Rho is necessary for m1- and Gα12-mediated induction of the SRE. As many growth-induced genes exhibit SRE in their promoter regions, we speculated that Rho may mediate transformation by G protein-coupled receptors and Gα12. We addressed this issue by assaying the ability to induce focus formation by Gα12QL or m1 receptors upon cotransfection with an expression plasmid for the botulinum C3 exoenzyme. As shown in Fig. 6, the number of foci induced by stimulation of m1 or by Gα12QL was drastically reduced by C3, and the transforming ability of both focus-forming genes was restored by coexpression of a plasmid containing RhoA-I41. These data establish a role for RhoA in focus formation by m1 and Gα12, thus correlating with the necessity of functional RhoA for the induction of SRE activity by signaling from G protein-coupled receptors or heterotrimeric G proteins.
Figure 6.
Focus formation by agonist-stimulated m1 receptors and the constitutively active Gα12 subunit is dependent on functional RhoA. NIH 3T3 cells were transfected with LTR-gpt m1 or pCEV-HA-G12QL (0.1 μg and 0.2 μg, respectively) plasmid DNAs together with expression vectors for the C3 toxin and the C3 insensitive mutant of RhoA (rhoAI41) (0.1 μg and 0.2 μg respectively), as indicated. Cells were cultured for three weeks in the presence or absence of 100 μM carbachol, as indicated. In A, the data shown correspond to the number of foci scored 18 days after transfection in a representative experiment that was repeated twice. In B, representative plates transfected with LTR-gpt m1 (a), LTR-gpt m1 + pC3 (b), LTR-gpt m1 + pC3 + pCEFLrhoAI41(c), pCEV-HA-G12QL (d), pCEV-HA-G12QL + pC3 (e), or pCEV-HA-G12QL + pC3 + pCEFLrhoAI41 (f), and were cultured in presence (a_–_c) or absence (d_–_f) of carbachol (100 mM) and stained 3 weeks after transfection.
DISCUSSION
An emerging body of evidence indicates that heterotrimeric G proteins and their coupled receptors can effectively stimulate proliferative pathways. Furthermore, utilizing transfected NIH 3T3 cells in culture, we and others have reported that certain G protein-coupled receptors (such as m1 mAChRs) and serotonin 1C and α1 adrenergic receptors, can behave as potent agonist-dependent oncogenes (16, 34, 35). Furthermore, somatic mutations in Gαs and Gαi2 genes have been recently found in human tumors (refs. 20 and 36 and references therein), and members of the Gαq and Gα12 subfamilies of heterotrimeric G proteins have been shown to behave as fully transforming oncogenes (21, 22). In an effort to describe the intracellular events associated with cell transformation by these molecules, we focused our attention on the regulation of gene expression.
The mechanisms by which G proteins and G protein-coupled receptors control cell proliferation remain poorly understood. One of the most characteristic responses to growth-promoting stimuli is the rapid induction of the expression of certain genes, known collectively as early responsive genes (4, 24). In previous studies, we have shown that agonist activation of m1 receptors can trigger the expression of a variety of such early responsive genes belonging to the jun and fos families (24). In this study, we show that m1 receptor stimulation, as well as expression of an activated mutant of Gα12, but not of any other Gα subtype, can strongly activate a critical regulatory element present in the c-fos promoter, the SRE.
The regulation of SRE activity can be mediated by a variety of signaling pathways converging on the TCF, or through a TCF-independent biochemical route (2). Regarding the former pathway, both ERK-2 and JNK1 MAPKs have been shown to phosphorylate the transactivation domain of TCF (11, 13, 14). In previous studies, we have shown that coexpression of β1γ2 subunits results in ERK-2 and JNK1 activation, and that activation of these MAPKs by m1 receptors occurs in a βγ-dependent manner (28, 32). In line with these observations, we show here that β1γ2 subunits can induce transactivation of the SREwt, although to an extent more limited than that by agonist-stimulated m1 or constitutively active Gα12. Preliminary experiments indicate that pSREmutL (15) does not respond to β1γ2 (data not shown). Therefore, we can postulate that β1γ2 activation of the SRE is performed in a TCF-dependent manner that is likely to account for the limited TCF-dependent component observed in the m1-mediated induction of the SRE.
Using the pSREmutL, we demonstrate here that both m1 and Gα12 can exert their effects on the SRE through a pathway that is independent of the TCF, similar to that described for RhoA (15). This observation suggests that RhoA may be part of the regulatory mechanisms downstream of m1 receptors and Gα12. The availability of a plasmid expressing the botulinum C3 exoenzyme, which inactivates Rho by ADP ribosylation of asparagine 41, afforded the possibility of exploring the role for RhoA in signaling from m1 receptors and Gα12 to the SRE. We showed that coexpression of C3 blocks SRE activation by both m1 and Gα12, and that this blockade is reversed by coexpression of a C3-insensitive mutant of Rho. Together, these data indicate that Rho participates in both m1 and Gα12-mediated activation of SRE.
Using a similar approach, we found that the focus-forming abilities of m1 and Gα12 expressed in NIH 3T3 cells require a functional Rho. In line with this observation, NIH 3T3 cells transformed by activated G12 proteins or by agonist-stimulated G protein-coupled receptors were found to form foci with a morphology nearly identical to that caused by expression of activated RhoA (refs. 16, 32, 37; N.X. and J.S.G., unpublished observations). However, the transforming efficiency of activated RhoA is much lower than that of G12 (16, 32, 37). These findings raise the possibility that Gα12 might activate pathways in addition to those elicited through Rho, that acting in a cooperating fashion, could help achieve a greater transforming activity. In this regard, others have recently observed that dominant negative mutants of Rac1 can reduce the focus-forming ability of Gα12 (A. Chan, personal communication). Thus, Gα12-induced transformation appears to involve Rho-dependent and Rho-independent biochemical routes, and our data strongly suggest that the Rho-dependent pathway is strictly required for transformation. In addition, others have recently shown a functional linkage between Gα12 and RhoA in the regulation of stress fiber formation and focal adhesion assembly (38). However, the biochemical route(s) connecting G protein-coupled receptors and G12 to Rho, and Rho to the SRE remain largely unknown. Regarding the latter, evidence presented by Hill et al. (15) suggests that Rho signals to the SRE through a pathway independent of any MAPKs described to date. Furthermore, recent work (39) has demonstrated that Rho regulates a number of signaling molecules, including critical enzymes involved in phospholipid metabolism, and several novel kinases such as Rho-kinase and protein kinase N. Whether these or additional downstream targets for Rho signal to SRE is under current investigation.
In summary, our findings provide strong evidence that m1, Gα12 and RhoA are components of a novel signal transduction pathway that leads to transcriptional activation and cellular transformation. As discussed above, the molecular mechanisms linking m1 receptors to Gα12, RhoA, and SRE remain elusive. Current studies exploring the mechanism(s) whereby G12 activates Rho, as well as the nature of those molecules linking Rho to transcriptional factors, including those acting on SRE are expected to help define the molecular components of this novel, biologically relevant pathway.
Acknowledgments
We thank R. Treismann for the gift of the SRE reporter plasmids, and A. Chan for sharing unpublished results. C.F. was supported by the Howard Hughes Medical Institute as a National Institutes of Health Research Scholar.
ABBREVIATIONS
SRE
serum response element
TCF
ternary complex factor
SREwt
wild-type SRE from c-fos
SREmutL
mutant SRE lacking TCF
mAChR
muscarinic acetylcholine receptor
CAT
chloramphenicol acetyltransferase
TPA
phorbol 12-tetradecanoate 13-acetate
ERK
extracellular signal-regulated kinase
MAPK
mitogen-activating protein kinase
JNK
c-Jun kinase
β-gal
β-galactosidase
References
- 1.Hipskind R A, Nordheim A. J Biol Chem. 1991;266:19583–19592. [PubMed] [Google Scholar]
- 2.Treisman R. Trends Biochem Sci. 1992;17:423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 3.Treisman R. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen F E, Prywes R. Mol Cell Biol. 1994;14:5920–5928. doi: 10.1128/mcb.14.9.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treisman R. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg M E, Siegfried Z, Ziff E B. Mol Cell Biol. 1987;7:1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw P E, Schroter H, Nordheim A. Cell. 1989;56:563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- 8.Hipskind R A, Rao V N, Mueller C G, Reddy E S, Nordheim A. Nature (London) 1991;354:531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- 9.Dalton S, Treisman R. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- 10.Gille H, Sharrocks A D, Shaw P E. Nature (London) 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 11.Treisman R. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 12.Marais R, Wynne J, Treisman R. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 13.Gille H, Strahl T, Shaw P E. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 14.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 15.Hill C S, Wynne J, Treisman R. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 16.Gutkind J S, Novotny E A, Brann M R, Robbins K C. Proc Natl Acad Sci USA. 1991;88:4703–4707. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens E V, Kalinec G, Brann M R, Gutkind J S. Oncogene. 1993;8:19–26. [PubMed] [Google Scholar]
- 18.Bonner T I. Trends Neurosci. 1989;12:148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- 19.Jones S V, Barker J L, Buckley N J, Bonner T I, Collins R M, Brann M R. Mol Pharmacol. 1988;34:421–426. [PubMed] [Google Scholar]
- 20.Hermouet S, Merendino J J, Jr, Gutkind J S, Spiegel A M. Proc Natl Acad Sci USA. 1991;88:10455–10459. doi: 10.1073/pnas.88.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinec G, Nazarali A J, Hermouet S, Xu N, Gutkind J S. Mol Cell Biol. 1992;12:4687–4693. doi: 10.1128/mcb.12.10.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu N, Bradley L, Ambdukar I, Gutkind J S. Proc Natl Acad Sci USA. 1993;90:6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu N, Voyno-Yasenetskaya T, Gutkind J S. Biochem Biophys Res Commun. 1994;201:603–609. doi: 10.1006/bbrc.1994.1744. [DOI] [PubMed] [Google Scholar]
- 24.Coso O A, Chiariello M, Kalinec G, Kyriakis J M, Woodgett J, Gutkind J S. J Biol Chem. 1995;270:5620–5624. doi: 10.1074/jbc.270.10.5620. [DOI] [PubMed] [Google Scholar]
- 25.Crespo P, Xu N, Daniotti J L, Troppmair J, Rapp U R, Gutkind J S. J Biol Chem. 1994;269:21103–21109. [PubMed] [Google Scholar]
- 26.Paterson H F, Self A J, Garrett M D, Just I, Aktories K, Hall A. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 28.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 29.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 30.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 31.Crespo P, Cachero T G, Xu N, Gutkind J S. J Biol Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 32.Coso O A, Teramoto H, Simonds W F, Gutkind J S. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 33.Bourne H R, Sanders D A, McCormick F. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 34.Allen L F, Lefkowitz R J, Caron M G, Cotecchia S. Proc Natl Acad Sci USA. 1991;88:11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julius D, Livelli T J, Jessell T M, Axel R. Science. 1989;244:1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- 36.Gaiddon C, Boutillier A L, Monnier D, Mercken L, Loeffler J P. J Biol Chem. 1994;269:22663–22671. [PubMed] [Google Scholar]
- 37.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buhl A M, Johnson N L, Dhanasekaran N, Johnson G L. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- 39.Ridley A J. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 40.Chan A C M, Fleming T P, McGovern E S, Chedid M, Miki T, Aaronson A. Mol Cell Biol. 1993;13:762–768. doi: 10.1128/mcb.13.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]