Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infection (original) (raw)
Abstract
A genetic defect in a CC-chemokine receptor (CCR)-5, the principal coreceptor for the macrophage-tropic HIV type 1 (HIV-1), recently was found to naturally protect CCR-5-defective, but healthy, individuals from HIV-1 infection. In this study, we mimic the natural resistance of the CCR-5-defective individuals by designing a strategy to phenotypically knock out CCR-5. The inactivation of the CCR-5 coreceptor is accomplished by targeting a modified CC-chemokine to the endoplasmic reticulum to block the surface expression of newly synthesized CCR-5. The lymphocytes transduced to express the intracellular chemokine, termed “intrakine,” were found to be viable and resistant to macrophage-tropic HIV-1 infection. Thus, this gene-based intrakine strategy targeted at the conserved cellular receptor for the prevention of HIV-1 entry should have significant advantages over currently described approaches for HIV-1 therapy.
CC-chemokine receptor (CCR)-5 is a principal coreceptor for HIV type 1 (HIV-1), which is required for macrophage (M)-tropic HIV-1 entry into target cells (1–6). M-tropic viruses represent the most prevalent phenotype isolated in individuals shortly after seroconversion and during the asymptomatic periods of the diseases (7–9). Several recent studies presented compelling evidence that CCR-5 is critical to the infectivity of HIV-1. Liu et al. (10) found that two uninfected individuals, repeatedly exposed to HIV-1, inherited a homozygous CCR-5 defect that contains an internal 32-bp deletion resulting in a frame shift. The lymphocytes from the CCR-5 defective individuals are resistant to M-tropic HIV-1 infection. Samson et al. (11) also identified the same CCR-5 mutation in uninfected individuals, and none of the HIV-infected individuals examined were homozygous for the mutation. Subsequent large population studies confirm the protective role of the CCR-5 homozygous defect (12, 13). Importantly, the individuals with the homozygous CCR-5 defect are not associated with clinical conditions (10–15), suggesting that the biologic function of CCR-5 is compensated by other chemokine receptors due to the redundancy of the chemokine family (16–19). Thus, these findings demonstrate the critical importance of CCR-5 for HIV-1 infection and dispensable nature of its function (10–15), suggesting that inactivation of CCR-5 in lymphocytes or stem cells should have therapeutic implication.
CCR-5 is a seven-transmembrane glycoprotein that is synthesized in the endoplasmic reticulum (ER) and transported to the cell surface, where CCR-5 binds its ligand (16–19) or serves as a HIV-1 coreceptor (2–6). In our previous studies, we developed an intracellular antibody (intrabody) approach to effectively block the cell surface transport of the envelope proteins of HIV-1 by targeting an engineered antibody molecule inside the lumen of the ER (20–24). CC-chemokines, such as macrophage inflammatory protein (MIP)-1α and regulated-upon-activation, normal T cells expressed and secreted (RANTES), bind to their receptor CCR-5 with high affinity (16, 25, 26). In this study, MIP-1α and RANTES were genetically modified and targeted to the ER lumen to intracellularly bind the newly synthesized CCR-5 and prevent its transport to the cell surface. The transduced lymphocytes expressing the intracellular chemokine, termed “intrakines,” were found to resist M-tropic HIV-1 infection.
MATERIALS AND METHODS
Construction of Expression Vectors.
The human MIP-1α and RANTES genes were PCR-amplified from the cDNA of peripheral blood mononuclear cells. The MIP-1α and RANTES genes then were linked with a KDEL sequence by PCR (20). The native MIP-1α and RANTES genes and their derivatives were cloned into expression vectors (Fig. 1A). All of the constructs were confirmed by DNA sequencing (DNA sequencing core facility of Wake Forest University).
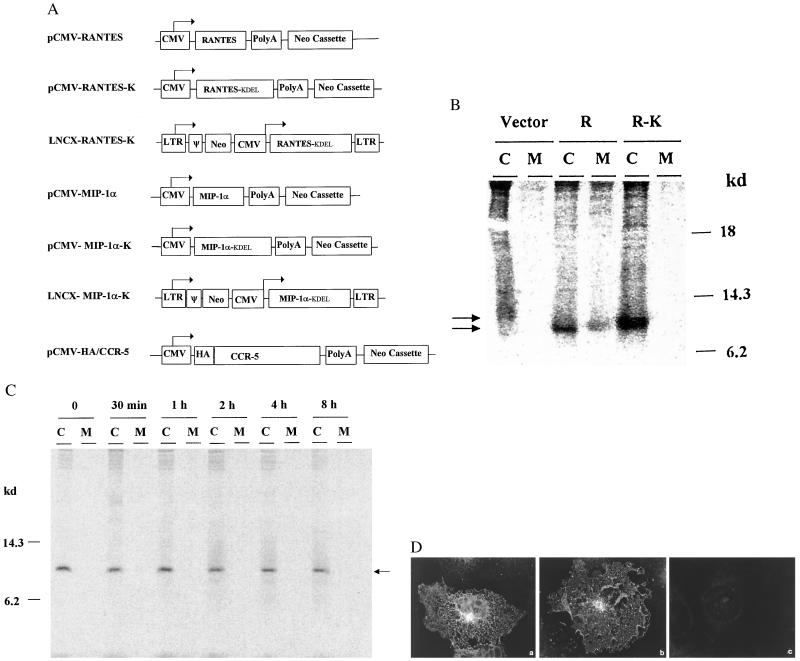
Figure 1.
(A) Schematic representation of construction of expression vectors. Human MIP-1α and RANTES genes and the modified RANTES-K and MIP-1α-K genes were cloned into the pRc/CMV vector (Invitrogen). RANTES-K and MIP-1α-K were further cloned into a retroviral vector (pLNCX). A CCR-5 expression vector containing an HA tag sequence (YPYDVPDYA) (34) also was generated. All of the constructs were confirmed by DNA sequencing. ψ, packaging sequence. (B) Expression of native and modified RANTES. COS cells were transfected with 2.5 μg of plasmid DNA/well and, 48 hr later radiolabeled with 60 μCi of [35S]trans (ICN) for 4 hr. The culture media (M) and cell lysates (C) were precipitated with an anti-human RANTES (Sigma), analyzed by SDS/PAGE, and then visualized by a PhosphorImager (Molecular Dynamics). Vector, pRc/CMV vector control; R, RANTES; and R-K, RANTES-K. (C) Intracellular retention of modified chemokines. COS cells were transfected with 2.5 μg of pCMV-RANTES-K, pulse-radiolabeled 48 hr later with 60 μCi of [35S]cysteine for 30 min, and then chased for various times. The culture media (M) and cell lysates (C) were precipitated with the anti-RANTES and analyzed on a 15% polyacrylamide gel. (D) Intracellular localization by immunofluorescent staining. COS cells grown on coverslips were transfected with 1 μg of plasmid DNA, and 48 hr later, stained with the anti-RANTES, followed by an anti-rabbit IgG-fluorescein isothiocyanate conjugate (Sigma). (a) pCMV-RANTES, (b) CMV-RANTES-K, or (c) pCMV control-transfected cells.
Detection of Protein Expression and Immunofluorescent Staining.
To label and precipitate recombinant proteins, cells were radiolabeled with [35S]cysteine or [35S]trans and precipitated with antibodies (27, 28). The protein samples were analyzed by electrophoresis on SDS-polyacrylamide gels and visualized by a PhosphorImager (28). For flow-cytometric assay, 5 × 105 cells were incubated with a primary antibody for 1 hr, followed by incubation with a fluorescein conjugate. The cells then were analyzed on a Becton Dickinson FACScan (23). Indirect immunofluorescent staining was performed as described (28).
Syncytium Formation Assay.
HeLa-T4+ cells (about 50% confluence) were cotransfected with pCMV-CCR-5 and control pRc/CMV (Invitrogen) or various chemokine expression plasmid DNA at different concentrations using a Calcium Phosphate System (Promega). At the same time, HeLa cells were transfected with T cell (T)- or M-tropic HIV-1 envelope protein expression vector (5 μg) and Tat expressor (1 μg) (6, 20). Forty eight hours later, the transfected HeLa cells expressing the envelope proteins (2 × 105) were cocultured with cotransfected HeLa-T4+ cells (1 × 105) on 12-well plates. After 12–24 hr of cocultivation, syncytia in each well were counted after crystal violet staining, and the percentages of inhibition were calculated by syncytia numbers in expressor-cotransfected wells/syncytia numbers in pRc/CMV-cotransfected wells.
Generation of Retroviral Packaging Cell Lines and Gene Transfer.
The MIP-1α-K or RANTES-K genes were cloned into the retroviral pLNCX vector (29, 30) (Fig. 1A). The resultant retroviral shuttle vectors were transfected into the amphotropic packaging cells (PA317), and the culture medium of the transfected cells then was used to infect freshly seeded PA317 cells (31). Forty eight hours later, the infected PA317 cells were selected with a G418 (800 μg/ml)-containing medium for 2 to 3 weeks. The G418-resistant colonies were subcloned and characterized by genetic analysis to ensure the entire incorporation of the genes into the chromosome of the packaging cells. Peripheral blood mononuclear cells from healthy individuals were separated on a Ficoll/Hypaque density gradient, and nonadherent peripheral blood lymphocytes (PBLs) were stimulated in a culture medium [RPMI medium 1640/20% fetal calf serum supplemented with recombinant-interleukin 2 (1,000 units/ml) (Chiron), and anti-CD3 (5 ng/ml) (PharMingen)] for 48 hr (28). The stimulated PBL cells then were transduced by coculture with the recombinant packaging cells in the culture medium containing protamine sulfate (5 μg/ml) for 48 hr. The transduced PBLs were harvested and cultured for 1 day, followed by repeated transduction twice. After final transduction, the PBLs were incubated in the culture containing G418 (600 μg/ml) for 5 days and then expanded in the culture medium for several days.
Chemotaxis Assay.
PBLs (5 × 105) were suspended in 100 μl of the RPMI medium 1640 containing 0.25% human serum albumin and added to the top chamber of a 5-μm pore polycarbonate Transwell culture insert (Costar). Six hundred microliters of the RPMI medium 1640/0.25% albumin containing various concentrations of the recombinant human MIP-1α, monocyte chemotactic protein 1, RANTES, or stromal cell-derived factor-1 (R & D Systems) were added to the bottom chamber of the Transwell. After 3-hr incubation at 37°C, the cell numbers in the bottom chamber were counted, and percentages of the transmigration were determined.
HIV-1 Infection and Reverse Transcriptase (RT) Assay.
PBLs in the RPMI medium supplemented with 10% fetal calf serum and recombinant-interleukin 2 (1,000 units/ml) and anti-CD3 (5 ng/ml) were infected with 20,000 cpm RT activities of M- or T-tropic HIV-1 viruses, respectively. After 4-hr incubation at 37°C, the infected PBLs then were washed once and resuspended in the culture medium, and the RT activities in 1 × 105 cells/ml were determined after subtracting the background (27, 32). The transduced and control lymphocyte lines were infected with HIV-1 viruses, and the viral production in the cultures was determined by measuring RT activity as described previously (23, 27).
RESULTS
Expression and Intracellular Localization of Modified RANTES and MIP-1α.
Human RANTES and MIP-1α genes with or without an ER-retention signal (KDEL) (20) were cloned into expression vectors, respectively (Fig. 1A). To determine the expression and localization of the native and modified chemokines, COS cells were transfected with various expression vector DNA and radiolabeled 48 hr later. The cell lysates and culture media then were immunoprecipitated with either an anti-MIP-1 or anti-RANTES antibody. An 8-kDa protein band corresponding to RANTES was found in both the medium and lysates of the pCMV-RANTES-transfected cells, but not in the control cells (Fig. 1B). However, in pCMV-RANTES-K-transfected cells, the modified RANTES-K was predominately found in the cell lysate, not in culture medium. To further determine the cellular retention of RANTES-K, the transfected cells were pulse-radiolabeled for 30 min and chased for various times. The RANTES-K proteins were found to be stably retained intracellularly with a half-life over 4 hr (Fig. 1C). The localization of the native and modified chemokines was examined further by immunofluorescent staining. As shown in Fig. 1D, a cytoplasmic ER staining pattern was observed in the cells transfected with pCMV-RANTES-K, whereas a perinuclear Golgi staining pattern was seen in the cells transfected with pCMV-RANTES. The native and modified MIP-1α also were expressed in a similar manner with the RANTES (data not shown). Thus, these results indicate that the modified CC-chemokines (intrakines) were expressed and stably retained in the ER.
Effects of Intrakine on CCR-5-Mediated Syncytium Formation.
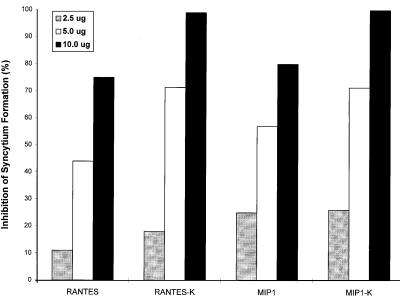
To examine the effects of the intracellular chemokine (intrakine) on the CCR-5 surface expression, a CCR-5/CD4-mediated syncytium formation assay was performed. The transformed cells expressing CD4 (HeLa-T4+) (20) were cotransfected pCMV-CCR5 with different amounts of expression plasmid DNA, and 48 hr later, cocultured with the HeLa cells expressing M (ADA and YU2)-tropic HIV-1 envelope proteins (6) for 12–24 hr. As shown in Fig. 2A, in the cocultures with the cells expressing intrakines, significant inhibition of M-tropic envelope-mediated syncytium formation was observed. When cotransfected with 10 μg of pCMV-MIP-1α-K or pCMV-RANTES-K, M-tropic envelope-mediated syncytium formation was almost completely inhibited. However, the T-tropic envelope-mediated syncytium formation via fusin (33) was not inhibited by the transfection of pCMV-MIP1α-K or pCMV-RANTES-K (data not shown). Inhibitory effects on the syncytium formation of M-tropic envelope proteins also were observed in the cells cotransfected with pCMV-MIP-1α or pCMV-RANTES (Fig. 2). Repeated experiments showed similar inhibitory effects. The partial inhibition by expression of native chemokines may be due to partial saturation of the binding site of CCR5 as well as interference of CCR5 transport to the cell surface. To determine whether the inhibitory effect of intrakines is a result of the intracellular binding, coimmunoprecipitation assays were performed. In the cells cotransfected with pCMV-MIP-1α-K and pCMV-HA-CCR-5 tagged with a hemagglutinin (HA) tag sequence (34), the MIP-1α-K proteins were coprecipitated by using the anti-HA antibody (data not shown). Thus, these results suggest that the intrakines bind newly synthesized CCR-5 and prevent their transport to the cell surface.
Figure 2.
Inhibition of CCR5-mediated syncytium formation by intrakines. HeLa-T4+ cells on 6-well plates were transfected with 2.5 μg of pCMV-CCR-5 alone or cotransfected with different amounts (2.5, 5.0, or 10 μg) of expression vectors. Forty eight hours later, the transfected cells (1 × 105) then were cocultured with the HeLa cells expressing M (ADA)-tropic envelope proteins (2 × 105), and the syncytia in each well (duplicate) were examined 12–24 hr later. The percentages of inhibition of syncytium formation are presented.
Blockade of M-tropic HIV-1 Entry into Transduced Lymphocytes.
To further determine the effects of CC-intrakines, a CCR-5/CD4+-human lymphocyte line, PM-1 (35), was transfected with various expression vectors followed by G418 selection. The lymphocytes transduced with the native MIP-1α or RANTES expression vectors failed to grow up, whereas viable transduced lymphocytes expressing the intrakines (PM-RANTES-K and PM-MIP-1α-K) were generated. This result suggests that continuous stimulation by CC-chemokines may have an adverse effect on lymphocytes, and intrakines retain newly synthesized CCR-5 in the ER, probably resulting in their degradation inside the cell. A control PM-1 line transduced with pRc/CMV vector (PM-CMV) also was generated. The expression vector incorporation into the transduced lymphocytes was demonstrated by genomic PCR amplifications. The transcription of the incorporated genes also was demonstrated by RT-PCR (Fig. 3A).
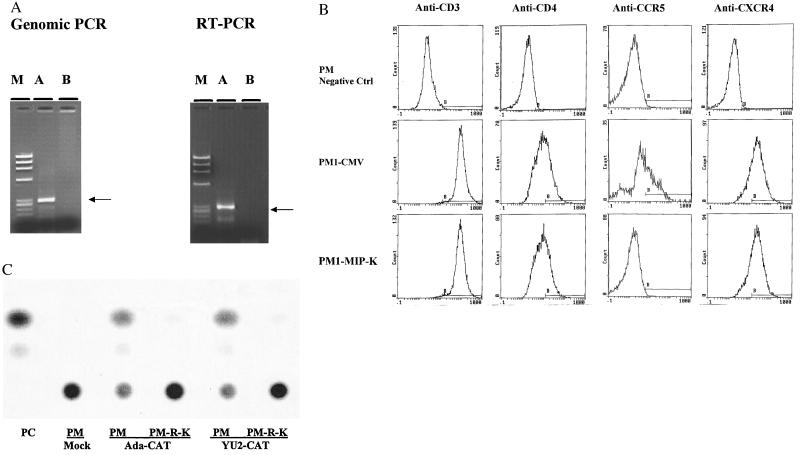
Figure 3.
Blockade of M-tropic HIV-1 infection into transduced lymphocytes. PM-1, a CD4+-human T lymphocyte line, was transfected with plasmid DNA by electroporation and selected in medium containing G418 (800 μg/ml) for 2 to 3 weeks (28). The G-418 resistant lymphocytes were assessed further. (A) Genomic and RT-PCR of transduced cells. Genomic DNAs and RNAs were extracted from the lymphocytes, and the RNAs then were reverse-transcripted into cDNA, as described (27). The MIP-1α fragments were specifically amplified from the genomic DNA or cDNA of PM-MIP-1α-K (A), not PM-CMV (B), with a pair of primers corresponding to the T7 promoter and 3′-MIP-1α sequence. The DNA molecular weight markers (M) were ØX174-_Hae_III: 1,353, 1,078, 872, 603, 310, and 281 bp. (B) Flow-cytometric assay. PM-MIP-1α-K or PM-CMV (5 × 105) were incubated with the mouse anti-CCR-5 (LeukoSite, Boston) (49), CXCR4 (R & D Systems), CD3, or CD4 antibody (PharMingen), and then stained with an anti-mouse IgG-fluorescein isothiocyanate conjugate (Sigma). The parental PM-1 cells were incubated with an irrelevant anti-rabbit IgG antibody, followed by incubation with the anti-mouse IgG-fluorescein isothiocyanate as negative control. After staining, the cells were analyzed on a Becton Dickinson FACScan. The percentages of positive staining cells are for anti-CD3: PM1/negative control, 1.0%; PM-CMV, 98.6%; and PM-MIP-1α-K, 98%; for anti-CD4: PM1/negative control, 3.3%; PM-CMV, 37.7%; PM-MIP-1α-K, 34%; for anti-CCR5: PM1/negative control, 0.9%; PM-CMV, 36.4%; and PM-MIP-1α-K, 1.0%; and for anti-CXCR4: PM1/negative control, 1.6%; PM-CMV, 74.7%; and PM-MIP-1α-K, 72.8%. (C) Blockade of M-tropic HIV-1 entry by the envelope complementation assay. PM-RANTES-K (R-K) or PM-CMV (PM) (2 × 106) were infected with the recombinant viruses with ADAenv or YU2env (20,000 cpm RT) by overnight incubation. Sixty hours later, the infected cells were lysed and used for determination of CAT activity. PC, positive control for CAT assay.
The effects of the intrakine on the CCR-5 surface expression of the transduced lymphocytes were examined by a flow-cytometric assay. As shown in Fig. 3B, the surface expression of CCR-5 was detected on the PM-CMV, but decreased to a background level on PM-MIP-1α-K. In contrast, comparable levels of the surface expression of CD3, CD4, and CXCR4 were detected on both PM-CMV and PM-MIP-1α-K. The selective inhibition of CCR-5 cell surface expression on the PM-RANTES-K also was observed. These results suggest that RANTES- and MIP-1α-intrakine specifically block the CCR-5 surface expression.
We then examined whether the transduced lymphocytes are resistant to the HIV-1 entry by using an envelope-complementation assay. In this assay, HIV-1 envelope glycoproteins expressed in trans complement a single round of replication of an envelope-deleted provirus encoding the chloramphenicol acetyltransferase (CAT) gene (6, 23). As shown in Fig. 3C, high levels of CAT activities in PM-CMV infected with the recombinant YU2 or ADA viruses were detected, but only low levels of CAT activity in PM-RANTES-K were detected. Dramatic inhibition of M-tropic recombinant HIV-1 entry into PM-MIP-1α-K also was observed. This result indicates that intrakine expression effectively protects the transduced lymphocytes from M-tropic HIV-1 entry.
Inhibition of M-Tropic HIV-1 Infection of Transduced Lymphocytes.
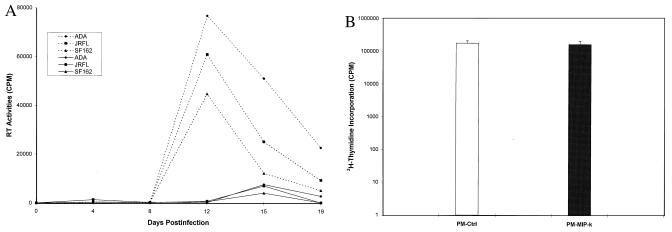
To further determine the intrakine effects, PM-MIP-1α-K and PM-CMV cells in parallel were washed with fresh culture medium, and then infected with each one of several primary HIV-1 isolates (ADA, JRFL, and SF162). The viral particles in the culture medium then were determined by RT assay. As shown in Fig. 4A, a significant increase of RT levels was observed in PM-CMV cell cultures infected with the primary HIV-1 isolates after 8 days of infection, and at day 12 postinfection, the RT levels reached the peak level. In contrast, only transient, very low levels of RT activities were observed in the medium of PM-MIP-1α-K cultures infected with the HIV-1 isolates. Thus, these results indicate that the transduced lymphocytes expressing the MIP-1α-intrakine are resistant to M-tropic HIV-1 infection.
Figure 4.
(A) Resistance to M-tropic HIV-1 infection of transduced lymphocytes. PM-MIP-1α-K or PM-CMV (2 × 105) were equally infected with 20,000 cpm RT of M-tropic HIV-1 viruses for 4 hr, and then replaced with the fresh culture medium. RT activities in duplicate wells after subtracting the background level are presented. Solid lines indicate PM-MIP-1α-K, and dotted lines indicate control PM. (B) DNA synthesis rates of transduced lymphocytes. PM-MIP-1α-K or PM-CMV (0.5 × 106) were incubated with 10 μCi of [3H]thymidine (ICN) for 12 hr at 37°C. After washing, the incorporation of the isotopes into the cells was determined by a scintillation counter and presented after subtracting the background level.
Next, the biological features of the transformed lymphocytes were examined to assess the effect of MIP-1α-intrakine on lymphocytes. PM-CMV and PM-MIP-1α-K cells were found to have a similar cell viability determined by trypan blue exclusion. PM-CMV and PM-MIP-1α-K had comparable proliferation rates (data not shown). [3H]thymidine incorporation, which represents the cellular DNA synthesis rate, was examined and found to be similar to parental lymphocytes (Fig. 4B). Thus, the lymphocytes expressing intrakine remain viable.
Anti-HIV-1 Effects of Intrakine in Primary PBLs.
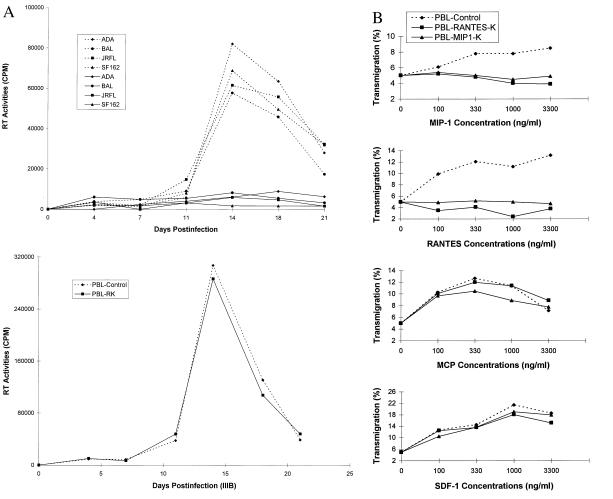
To further evaluate potential therapeutic application, whether intrakine expression would protect primary PBLs from M-tropic HIV-1 infection was assessed. The intrakine gene (RANTES-K or MIP-1α-K) was cloned into a retroviral shuttle vector, pLNCX (Fig. 1A). The resultant vector (LNCX-RANTES-K and LNCX-MIP-1α-K) was transfected into a packaging cell line (PA317), and the recombinant retroviral packaging cell lines were generated after G418 selection. Primary human PBLs were stimulated and then transduced with the recombinant vector by coculture with the recombinant packaging cells, followed by Neo selection. The vector incorporation into the genome of the transduced PBLs was demonstrated by genomic PCR (data not shown). The transduced or mock-transduced PBLs were infected with M- or T-tropic isolates, and the viral production in the cultures was examined by measuring the viral RT activity. As shown in Fig. 5A, only low levels of RT activity were detected in the RANTES-K-transduced PBLs infected with M-tropic viruses (ADA, BAL, JRFL, and SF162), but high levels of RT activity were detected in the mock-transduced PBL culture infected with these HIV-1 viruses. The specificity of the intrakine effect was demonstrated, because T-tropic virus IIIB equally infected the transduced and mock-transduced PBLs (Fig. 5A). The viability of PBLs transduced with RANTES-K was about 90%, comparable to that of mock-transduced PBLs. The majority of PBLs transduced with RANTES-K remained viable during the experiment infected with M-tropic viruses, whereas the mock-transduced PBLs gradually died after M-tropic virus infection, indicating the survival advantage of the transduced PBLs in vitro after HIV-1 infection. Similar anti-HIV-1 activities also were observed in the PBLs transduced with MIP-1α-K (data not shown). Thus, these results demonstrate that transduced primary PBLs expressing intrakines were resistant to M-tropic HIV-1 infection.
Figure 5.
(A) Resistance to M-tropic HIV-1 infection of transduced PBLs. Human primary PBLs were transduced by cocultivation with the recombinant retroviral packaging cells (PA317-RANTES-K). The transduced or mock-transduced PBLs (2 × 105) were equally infected with 20,000 cpm RT of several M-tropic (ADA, BAL, JRFL, and SF162) or T-tropic (IIIB) HIV-1 for 4 hr, and then replaced with the fresh culture medium. Every 3 to 4 days, cell numbers in each well were counted, and RT activities (1 × 105 cells/ml) in duplicate wells are presented after subtracting the background. Solid lines indicate PBLs transduced with RANTES-K, and dotted lines indicate mock-transduced PBLs. (B) Chemotaxis assay. The PBLs (5 × 105) transduced with MIP-1α-K or RANTES-K after Neo selection or mock-transduced PBLs were added to the top chamber of a 5-μm pore size insert (Costar). Six hundred microliters of various concentrations of the recombinant MIP-1α, monocyte chemotactic protein 1, RANTES, or stromal cell-derived factor 1 (R & D Systems) in the culture medium were added to the bottom chamber. After 3-hr incubation at 37°C, the cell numbers in the bottom chamber were counted, and percentages of the transmigration are presented.
To further determine the selective inactivation of CCR5 in the primary human PBLs, the PBLs transduced with RANTES-K or MIP-1α-K after Neo selection and mock-transduced PBLs were subjected to chemotaxis assays. As shown in Fig. 5B, the responsiveness of PBLs transduced with RANTES-K or MIP-1α-K to the stimulation of recombinant stromal cell-derived factor 1 or monocyte chemotactic protein 1, which binds the chemokine receptor CXCR4 or CCR2, remained normal, when compared with that of mock-transduced PBLs. In contrast, the PBLs transduced with RANTES-K or MIP-1α-K were insensitive to the stimulation of MIP-1α and RANTES (Fig. 5B). In addition, the PBLs transduced with RANTES-K or MIP-1α-K also were shown to have normal cell proliferation and DNA synthesis rates in the stimulation condition of interleukin 2 and anti-CD3 (data not shown).
DISCUSSION
In this study, we mimic the natural resistance of the CCR-5-defective individuals by designing an intrakine strategy to phenotypically knock out CCR-5. The phenotypic CCR-5 knockout lymphocytes were viable and resistant to M-tropic HIV-1 infection. Therefore, this intrakine strategy should have therapeutic implication to genetically protect HIV-1 permissive cells from HIV-1 infection. This intrakine strategy has several features that may render this strategy superior over currently described anti-HIV approaches. First, this anti-HIV approach is targeted to the conserved cellular receptor, and, therefore, frequent HIV mutations may be not able to evade this strategy (10–15). In contrast, currently described anti-HIV approaches are primarily targeted to the highly variant viral components (36, 37). Second, this intrakine approach is aimed at the prevention of virus entry, rather than interfering with viral replication and maturation after HIV-1 infection targeted by other anti-HIV-1 approaches. Therefore, the intrakine-modified cells should be truly immune to HIV-1 infection.
In addition to these features, this intrakine approach is especially well suited to be translated into clinical practice. Only limited numbers of CCR-5, probably fewer than 700 molecules/cell, are expressed on the surface of lymphocytes (16, 38), and, therefore, achievable expression levels of intrakines by current vectors may be sufficient to inactivate CCR-5. A potential problem of gene therapy is the host immune responses, especially cytotoxic T cells, destroying the transduced cells expressing foreign proteins (39–42). However, transduced cells expressing human chemokines would be not immunogenic.
Moreover, this gene-base approach also would be superior over administration of chemokines for extracellular inhibition of HIV-1 infection. Intrakines expressed by a transduced cell are concentrated in the ER (half-life > 4 hr) and bind and sequester the newly synthesized CCR-5 coreceptors inside the cell. The sequestrated coreceptors likely are degraded inside the cells as demonstrated in previous studies (43–46). Because a genetically modified lymphocyte or stem cell can live over months up to an individual’s lifetime, this gene-based intrakine therapy therefore should have a potent and long-lasting anti-HIV effect. In contrast, chemokines with a short half-life in vivo (<10 min) (16, 47) would require frequent administration into infected individuals throughout their lifetime, which may be ineffective and induce inflammatory reaction (16, 47). The studies to systematically assess the effects of intrakine expression on primary lymphocytes and stem cells in cell culture as well as in animal models are ongoing. Finally, this strategy can be used effectively to inactivate the T-tropic coreceptor (33) as well (48). Thus, this intrakine strategy for genetically immunizing lymphocytes or stem cells from HIV-1 infection can be developed into effective treatment and even prevention of HIV-1 infection.
Acknowledgments
We thank Joel Sodroski for recombinant HIV-1 system and advice, Yanping Cong for technical assistance, L. Kucera and N. Iyer for HIV cultures at the tissue culture core laboratory, Drs. L. Wu and C. R. MacKay for antibodies, N. Landau for the CCR-5 gene, T. Kute for the flow-cytometric assay, National Institutes of Health AIDS Research and Reference Reagent Program for HIV viruses, Drs. J. Chen, D. Lyle, P. Dawson, and F. Torti for helpful suggestions and support, and S. Britt for secretarial assistance. This work was supported by institutional start-up funds, the American Cancer Society, and a North Carolina Baptist Hospital Developmental Technology Grant.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CCR, CC-chemokine receptor; HIV-1, HIV type 1; M, macrophage; T, T cell; ER, endoplasmic reticulum; RANTES, regulated-upon-activation, normal T cells expressed and secreted; MIP, macrophage inflammatory protein; PBL, peripheral blood lymphocyte; RT, reverse transcriptase; HA, hemagglutinin; CAT, chloramphenicol acetyltransferase.
References
- 1.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 2.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 5.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerad C, Sodroski J. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 8.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 9.Roos M T, Lange J M, de Goede R E, Coutinho R A, Schellekens P T, Miedema F, Tersmette M. J Infect Dis. 1992;165:427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 11.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 12.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 14.Fauci A S. Nat Med. 1996;2:966–967. doi: 10.1038/nm0996-966. [DOI] [PubMed] [Google Scholar]
- 15.Littman D R, Hill C M. Nature (London) 1996;382:668–669. doi: 10.1038/382668a0. [DOI] [PubMed] [Google Scholar]
- 16.Baggiolini M, Dewald B, Moser B. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 17.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 18.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 19.Strader C D, Fong T M, Tota M R, Underwood D, Dixon R A. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 20.Marasco W A, Haseltine W A, Chen S-Y. Proc Natl Acad Sci USA. 1993;90:7889–7893. doi: 10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S-Y, Mhashilkar A, Marasco W A. Exp Opin Invest Drugs. 1995;4:823–832. [Google Scholar]
- 22.Chen S-Y, Khouri Y, Bagley J, Marasco W A. Proc Natl Acad Sci USA. 1994;91:5932–5936. doi: 10.1073/pnas.91.13.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S-Y, Bagley J, Marasco W A. Hum Gene Ther. 1994;5:595–601. doi: 10.1089/hum.1994.5.5-595. [DOI] [PubMed] [Google Scholar]
- 24.Buonocore L, Rose J K. Proc Natl Acad Sci USA. 1993;90:2695–2699. doi: 10.1073/pnas.90.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schall T J, Jongstra J, Dyer B J, Jorgensen J, Clayberger C, Divis M M, Krensky A M. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 26.Oppenheim J J, Zachariae C O, Mukaida N, Matsushima K. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 27.Yang A-G, Chen S-Y. Nat Biotech. 1997;15:46–51. doi: 10.1038/nbt0197-46. [DOI] [PubMed] [Google Scholar]
- 28.Chen S-Y, Yang A-G, Chen J-D, Kute T, King C R, Collier J, Cong Y, Yao C, Huang X F. Nature (London) 1997;385:78–80. doi: 10.1038/385078a0. [DOI] [PubMed] [Google Scholar]
- 29.Miller A D. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- 30.Miller A D, Rosman G J. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A D, Trauber D R, Buttimore C. Somat Cell Mol Genet. 1986;12:175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- 32.Chen J-D, Yang Q, Yang A-G, Marasco W, Chen S-Y. Hum Gene Ther. 1996;7:1515–1525. doi: 10.1089/hum.1996.7.13-1515. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 34.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu M, Poeschla E, Wong-Staal F. Gene Ther. 1994;1:13–26. [PubMed] [Google Scholar]
- 37.Woffendin C, Ranga U, Yang Z, Xu L, Nabel G J. Proc Natl Acad Sci USA. 1996;93:2889–2894. doi: 10.1073/pnas.93.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J M, McVicar D W, Oppenheim J J, Kelvin D J. J Exp Med. 1993;177:699–705. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddell S R, Greenberg P D, Overell R W, Loughran T P, Gilbert M J, Lupton S D, Agosti J, Scheeler S, Coombs R W, Corey L. Hum Gene Ther. 1992;3:319–338. doi: 10.1089/hum.1992.3.3-319. [DOI] [PubMed] [Google Scholar]
- 41.Riddell S R, Elliott M, Lewinsohn D A, Gilbert M J, Wilson L, Manley S A, Lupton S D, Overell R W, Reynolds T C, Corey L, Greenberg P D. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 42.Koenig S. Nat Med. 1996;2:165–167. doi: 10.1038/nm0296-165. [DOI] [PubMed] [Google Scholar]
- 43.Lippincott-Schwartz J, Bonifacino J S, Yuan L C, Klausner R D. Cell. 1988;54:209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- 44.Rose J K, Doms R W. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- 45.Hurtley S M, Helenius A. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- 46.Fra A M, Fagioli C, Finazzi D, Sitia R, Alberini C M. EMBO J. 1993;12:4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Zee K J, Fischer E, Hawes A S, Hebert C A, Terrell T G, Baker J B, Lowry S F, Moldawer L L. J Immunol. 1992;148:1746–1752. [PubMed] [Google Scholar]
- 48.Chen, J-D., Bai, X., Yang, A-G., Cong, Y. & Chen, S.-Y. (1997) Nat. Med., in press. [DOI] [PubMed]
- 49.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]