Modulation of invariant natural killer T cell cytokine responses by indoleamine 2,3-dioxygenase (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 15.
1. SUMMARY
The intracellular enzyme indoleamine 2,3-dioxygenase (IDO), which degrades the rare and essential aminoacid tryptophan and converts it into a series of biologically active catabolites, has been linked to the regulation of immune tolerance by specific dendritic cell subsets, and to the downmodulation of exacerbated immune responses. Although the immunoregulatory effects of IDO may be in part due to generalized suppression of cell proliferation caused by tryptophan starvation, there is also evidence that tryptophan catabolites could be directly responsible for some of the observed effects. In this report, we investigated the consequences of IDO activity, particularly with regard to the effects of tryptophan-derived catabolites, on the cytokine responses of activated invariant natural killer T (iNKT) cells, a specialized T cell subset known to have immunoregulatory properties. Our results showed that pharmacologic inhibition of IDO skewed cytokine responses of iNKT cells towards a Th1 profile. In contrast, the presence at low micromolar concentrations of the tryptophan catabolites L-kynurenine, 3-hydroxy-kynurenine, or 3-hydroxy-anthranilic acid shifted the cytokine balance towards a Th2 pattern. These findings have implications for our current understanding of immunoregulation, and the mechanisms by which iNKT cells participate in the modulation of immune responses.
Keywords: T cells; iNKT cells; indoleamine 2,3-dioxygenase; Th1/Th2 cells; tolerance
2. INTRODUCTION
The intracellular enzyme indoleamine 2,3-dioxygenase (IDO) mediates the initial, rate-limiting step in the catabolism of tryptophan, a rare and essential aminoacid [1]. IDO catalyzes the oxidative cleavage of the tryptophan indole ring, leading to the production of a series of metabolites, collectively known as kynurenines [2]. Accumulating evidence supports the notion that IDO activity is inducible in specific dendritic cell (DC) subsets, where it plays an essential role in promoting tolerance and in suppressing adaptive immunity [1]. Stimuli known to induce IDO activity in specific DC subsets include B7 ligation by surface CTLA4 expressed on regulatory T cells (Treg) [3] or by the CTLA4-Ig fusion protein [4]. In addition, the regulatory regions of the IDO gene contain sequences that mediate transcriptional control by IFNγ and IFNα and β [5]. Stimuli that trigger IFNγ production can lead to IDO induction, suggesting a role for IDO in feedback suppression of exacerbated immune responses [1,6]. Other stimuli have been reported to inhibit IDO functional activity in tolerogenic DC subsets. These include CD40 engagement on splenic CD8+ DCs [7], which leads to autocrine IL-6 production and blockade of IFNγ-induced IDO activation through down-regulation of cell-surface IFNγ receptors [8]. From these studies, a model of functional plasticity of IDO+ DCs has been proposed, with DC subsets integrating multiple environmental signals and responding with a phenotypically flexible program [1,9]. In this model, upregulation of IDO function is critically involved in promoting tolerance, whereas downregulation of IDO activity allows immune responses to proceed and is linked to pro-inflammatory responses.
Historically, tryptophan depletion was the first mechanism proposed to explain the immunosuppressive activities of IDO [1]. Although some experimental results support this mechanism [10], it has also been shown that several tryptophan catabolites that accumulate as a result of IDO activity can mediate essential immunoregulatory events on their own, even in the absence of tryptophan depletion [11-16]. These catabolites include L-kynurenine, as well as other sequential products of tryptophan metabolism, such as 3-hydroxykynurenine and 3-hydroxyanthranilic acid. Based on current experimental data, it appears that specific tryptophan catabolites do not simply induce a generalized suppression of cell proliferation, but more likely have a variety of specific and highly integrated effects on the immune system. For example, tryptophan catabolites have been reported to induce preferential apoptosis of Th1 versus Th2 clones [16], to inhibit the surface expression of NKp46 and NKG2D-activating NK receptors [14], or, in combination with low tryptophan levels, to downregulate the T cell receptor ζ chains of CD8+ T cells and direct the differentiation of CD4+ CD25+ Foxp3+ T regulatory cells from naïve CD4+ T cells [17]. The multiple effects of IDO and its products on the regulation of the immune response prompted us to examine the relationship between IDO and the immunoregulatory activities of invariant Natural Killer T cells (iNKT cells).
CD1d-restricted iNKT cells constitute a unique autoreactive lymphocyte subpopulation, characterized by the expression of an invariant TCRα chain encoded by a precise rearrangement of a single TCR Vα with one particular Jα gene (Vα14-Jα18 in mice, and Vα24-Jα18 in humans) [18]. These iNKT cells are potently activated by the glycolipid α-galactosylceramide (αGalCer) presented by CD1d, leading to the combined release of both Th1-type cytokines (like IFNγ), and Th2-type cytokines (like IL-4 and IL-13). Paralleling the diverse cytokine response produced by their activation, iNKT cells have been found to modulate a contrasting variety of immune responses [19]. For example, in some model experimental systems iNKT cells can exert effector functions associated with active immunity or inflammation, such as the elimination of tumor cells [20] or infectious agents like bacteria [21, 22] and parasites [23]. Yet, in other situations they display predominantly immunoregulatory or anti-inflammatory functions, such as promoting tolerance of tissues in immune-privileged sites and allografts [24, 25], or modulating autoimmunity [26]. The factors regulating these diverse functions of iNKT cells are still poorly understood.
In the current study, we provide experimental evidence indicating that IDO activity, and selected tryptophan catabolites resulting from the initiation of tryptophan degradation by IDO, can modulate iNKT cell responses. We demonstrated that pharmacologic inhibition of IDO skewed the cytokine response of αGalCer-stimulated splenocytes towards a more Th1 profile, with reduced production of IL-4. Reciprocally, L-kynurenine, 3-hydroxy-kynurenine, or 3-hydroxy-anthranilic acid shifted the cytokine balance towards a more Th2 pattern, with a reduced ratio of IFNγ to IL-4 production. These findings may have significant implications for our understanding of immunoregulation, and provide new insights into how the multiple and often opposing effects of iNKT cells may be controlled.
3. MATERIALS AND METHODS
Reagents
1-Methyl-DL-tryptophan (1-MT) (86,064-6; 97% pure), L-kynurenine (K8625; 99% pure), 3-hydroxy-(D,L)-kynurenine (H1771; 99% pure), anthranilic acid (A89855; ≥98% pure), 3-hydroxy-anthranilic acid (H9391; 97% pure), 2-picolinic acid (P5503; ≥99% pure), and quinolinic acid (P6,320-4; 99% pure) were all purchased from Sigma (St. Louis, MO). A 20 mM solution of 1-MT was prepared in 0.1 N NaOH, and the pH was adjusted to 7.4, as previously described [10]. Tryptophan catabolites were dissolved directly in the media used for splenocyte cultures. α-Galactosylceramide (αGalCer) is [(2S, 3S, 4R)-1-O-(α-D-galactopyranosyl)-N-hexacosanoyl-2-amino-1,3,4-octadecanetriol], and was synthesized and dissolved for use in cell culture or for injection into mice as described previously [27].
Mice
C57BL/6 mice (B6 mice, 6-10 week-old females) were purchased from the Jackson Laboratory. All experiments using mice were in compliance with the standards established and approved by the Institutional Animal Use and Care Committee of the Albert Einstein College of Medicine.
Media and Cell Cultures
RPMI 1640 medium containing L-glutamine (Gibco) was supplemented with 10% heat-inactivated FCS (Gemini Biologic Products; Calabasas, CA), 10 mM Hepes, 0.8 mM L-glutamine, MEM nonessential aminoacid solution (Gibco # 11140-050) diluted 200x (0.05 mM final), MEM aminoacid solution (Gibco # 11130-051) diluted 200x, 55μM 2-mercaptoethanol, 100 U/ml penicillin and 100 ug/ml streptomycin. The final tryptophan concentration of this media was 32 μM. For cell cultures, 3 × 105 C57BL/6 splenocytes per well were cultured in 96-well round-bottom plates in a 37°C humidified incubator with 5% CO2. After 72 or 96 hours (as indicated), supernatants were collected.
Cytokine Assays
Sandwich ELISA was used to determine the IL-4, IL-13, and IFNγ concentrations in culture supernatants. Capture antibodies were clones 11B1 (IL-4), R4-6A2 (IFNγ), and 38213 (IL-13). Biotinylated antibodies were clones BVD6-24G2 (IL-4), XMG1.2 (IFNγ), and goat anti-mouse IL-13, obtained from BD Biosciences (IL-4, IFNγ), and R&D systems (IL-13). Assays were developed using streptavidin-alkaline phosphatase (Zymed) and 4-nitrophenyl phosphate (Sigma-Aldrich) as substrate, and A405 values were determined after incubation for 15-30 minutes using a 96 well ELISA plate reader (Dynex Technologies). Recombinant IL-4 and IFNγ (Peprotech), and IL-13 (R&D systems) were used to generate standard curves. Curve fitting and calculation of cytokine levels in ng/ml were done using Prism 4.0 software (GraphPad). All measurements were performed in duplicate or triplicate. Detection limit was ∼0.02 ng/ml (IL-4), and ∼0.2 ng/ml (IFNγ, IL-13).
In vivo priming with αGalCer
Groups of three C57BL/6 mice were injected with 5 nanomoles each of αGalCer intraperitoneally (i.p.). Seven days later, mice were sacrificed and pooled splenocytes used for in vitro restimulation with αGalCer.
4. RESULTS
Effects of inhibition of IDO on the cytokine profile of αGalCer-stimulated splenocytes
Activated iNKT cells are capable of rapid secretion of many cytokines, Th1-associated cytokines such as IFNγ and TNF as well as Th2-associated cytokines such as IL-4, IL-5 and IL-13. Under standard culture conditions, splenocyte cultures stimulated with the iNKT cell activator αGalCer leads to the combined release of substantial amounts of both IFNγ and IL-4. To assess the potential role of IDO in regulating cytokine production by iNKT cells, we examined the in vitro effects of 1-MT, a chemical inhibitor of IDO, on IFNγ and IL-4 levels resulting from αGalCer stimulation of B6 splenocytes (figure 1). This revealed a dose dependent inhibition by 1-MT of IL-4 production detected following 72 hours of stimulation with αGalCer (figure 1A). In contrast, IFNγ production in the same cultures was strongly enhanced (figure 1B), resulting in an increase in the IFNγ/IL-4 ratio (figure 1C). Thus, IDO inhibition shifted cytokine production elicited by αGalCer towards a more Th1-associated pattern that favored the predominance of IFNγ..
Figure 1. Skewing of the cytokine profile of αGalCer-stimulated splenocytes by 1-MT to favor a pro-inflammatory response.
B6 splenocytes were cultured for 72 hours with 100 nM αGalCer, or with 100 nM αGalCer combined with graded concentrations of the chemical IDO inhibitor 1-MT. Supernatants were collected. The IL-4 (A) and IFNγ (B) concentrations were determined by sandwich ELISA. Bars represent the mean +/- SEM from duplicate wells. * p<0.05 compared with control (splenocytes + αGalCer). The IFNγ/IL-4 ratios are shown in (C). The results shown are representative of three similar experiments.
Selective suppression by L-kynurenine of the IFNγ production by αGalCer-stimulated splenocytes
IDO introduces two oxygen atoms into the indole ring of tryptophan, cleaving it and resulting in the generation of a series of catabolites, collectively known as kynurenines (figure 2), some of which have been found to exert immunoregulatory functions. We examined the possible effects of these metabolites on αGalCer-induced cytokine secretion. These experiments were performed in RPMI-1640 media supplemented with essential aminoacids, and having a final tryptophan concentration of 32 uM. Thus, the observed effects can be attributed solely to the presence of the added tryptophan catabolites, and not to tryptophan depletion.
Figure 2. Kynurenine pathway of tryptophan degradation.
Metabolites investigated in this report are boxed. Those that were found to skew the cytokine profile of αGalCer-stimulated splenocytes are enclosed in boxes with solid borders. Those with no effect are enclosed in boxes with dashed borders.
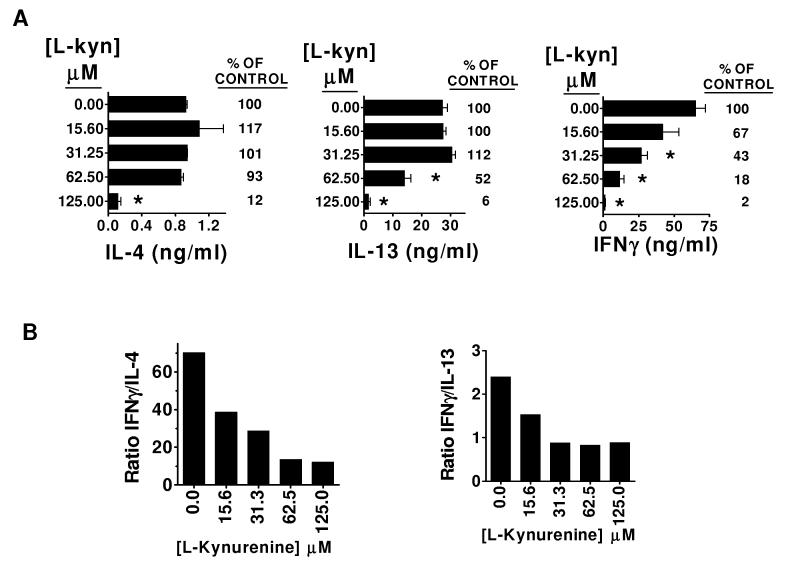
L-kynurenine is the second catabolite in the enzymatic cascade initiated by IDO, constituting the main or end product in most cells [28,29]. Interestingly, this compound had an effect on the cytokine production by αGalCer stimulated splenocytes which was essentially reciprocal to that of 1-MT. At low micromolar concentrations ranging from 15.6 uM to 62.5 μM, the levels of IL-4 released into the supernatant were closely comparable to those produced by splenocytes stimulated with αGalCer alone (figure 3A, left panel), indicating that at these concentrations L-kynurenine had no effect on IL-4 production. A different Th2 cytokine, IL-13, exhibited a similar pattern, with supernatant concentrations unchanged at 15.6 μM L-kynurenine, and slightly increased to 112% in the presence of 31.25 μM L-kynurenine (figure 3A, middle panel) In striking contrast to these Th2 cytokines, IFNγ production was strongly inhibited by L-kynurenine in a dose dependent manner within this lower concentration range (figure 3A, right panel). The net result was a clear reduction of the IFNγ/IL-4 and IFNγ/IL-13 ratios over a range of concentrations (figure 3B). At concentrations above 125 μM, all cytokine production was inhibited (fig 3A), suggesting that this level of L-kynurenine may have been toxic or nonspecifically suppressive of cellular functions.
Figure 3. Selective suppression of IFNγ production by αGalCer-stimulated splenocytes by. L-kynurenine, a major product of tryptophan catabolism in IDO+.
B6 splenocytes were cultured for 96 hours with 100 nM αGalCer, or with 100 nM αGalCer combined with graded concentrations of the tryptophan catabolite L-kynurenine (L-kyn). (A) Concentrations of IL-4, IL-13, and IFNγ in culture supernatants as determined by sandwich ELISA. Bars represent the mean +/- SEM from duplicate wells. * p<0.05 compared with control (splenocytes + αGalCer). (B) Calculated IFNγ/IL-4 and IFNγ/IL-13 ratios are shown. The results are representative of three similar experiments.
Alteration of iNKT cell cytokine responses by downstream metabolites of tryptophan degradation
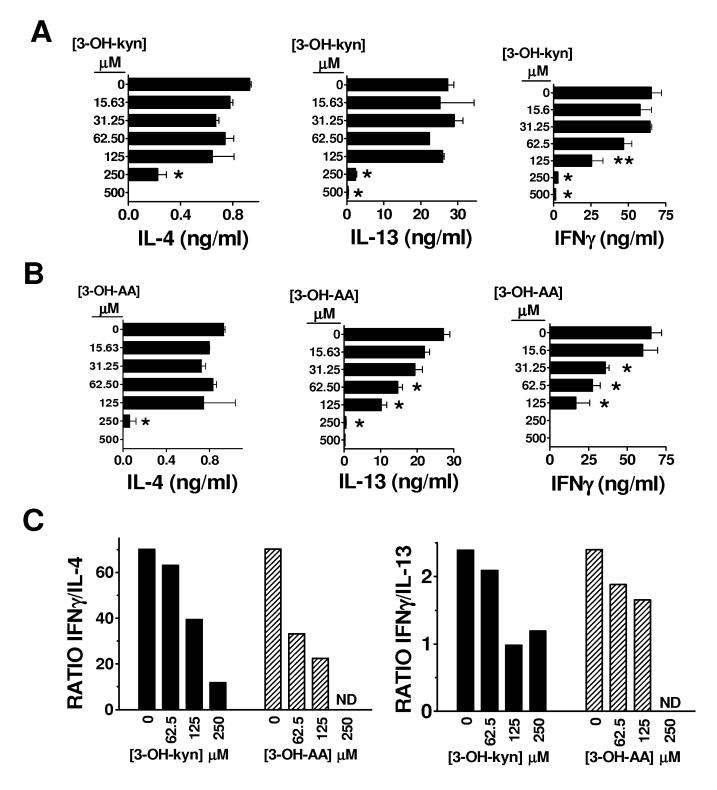
In some cells, the enzyme kynurenine 3-hydroxylase further converts kynurenine to 3-OH-kynurenine (figure 2), which has also been postulated to be immunologically relevant [12, 13, 15]. We tested the effects of 3-OH- (D,L)-kynurenine on the cytokine profile of αGalCer-stimulated splenocytes (figure 4A). We found that 3-OH-(D,L)-kynurenine induced a dose-dependent cytokine skewing effect that was similar to L-kynurenine, preferentially inhibiting IFNγ production. Whereas IFNγ production was significantly reduced by approximately 60% at 125 μM of 3-OH-(D,L)-kynurenine, IL-4 and IL-13 production were not significantly affected at this dose.(figure 4A). At 250 μM of 3-OH-(D,L)-kynurenine, the production of all cytokines was significantly reduced, but IL-4 was still detectable (24% of control) while IFNγ was virtually absent (∼4% of control). The net effect of 3-OH-(D,L)-kynurenine at these concentrations was a reduction in the IFNγ/IL-4 and IFNγ/IL-13 ratios (figure 4C).
Figure 4. Effects of proximal downstream catabolites of tryptophan degradation on the cytokine responses of of αGalCer-stimulated splenocytes.
B6 splenocytes were cultured for 96 hours with 100 nM αGalCer, or with 100 nM αGalCer combined with graded concentrations of the tryptophan catabolites 3-OH-(D,L)-kynurenine (3-OH-kyn) (A), or 3-OH-anthranilic acid (3-OH-AA) (B). Supernatants were collected, and the IL-4, IL-13, and IFNγ concentrations were determined by sandwich ELISA. Bars represent the mean +/- SEM from duplicate wells. * p<0.05, **p=0.06 compared with control (splenocytes + αGalCer). The calculated IFNγ/IL-4 and IFNγ/IL-13 ratios are shown in (C). ND indicates ratios that could not be accurately determined because the level of IFNγ was below the detectable range. The results shown are representative of three similar experiments.
Following the enzymatic conversion of kynurenine into 3-OH-kynurenine, the enzyme kynureninase catalyzes the formation of 3-OH-anthranilic acid (figure 2). We tested the effects of this compound on the cytokine profile of αGalCer-stimulated splenocytes. At concentrations ranging from 31.25 to 125 μM, 3-OH-anthranilic acid induced a striking cytokine skewing effect characterized by selective suppression of IFNγ production (figure 4B). At doses of 3-OH-anthranilic acid of 62.5 and 125 μM, the level of IL-4 released into the supernatant was not significantly changed compared to control cultures stimulated in the absence of the compound. However, over the same dose range of 3-OH-anthranilic acid, IFNγ was significantly reduced by as much as 76% compared to control cultures. The net result was again a clear reduction of the IFNγ/IL-4 ratio by 3-OH-anthranilic acid (figure 4C), and a potency in this regard that was only slightly less than that of L-kynurenine. However, the other Th2 cytokine measured, IL-13, exhibited a dose-dependent inhibition by 3-OH-anthranilic acid that was similar to that of IFNγ (figure 4B). Thus, in the case of IL-13, a Th2 skewing effect as reflected in the level of this cytokine relative to IFNγ was not as clearly evident as for IL-4 (figure 4C).
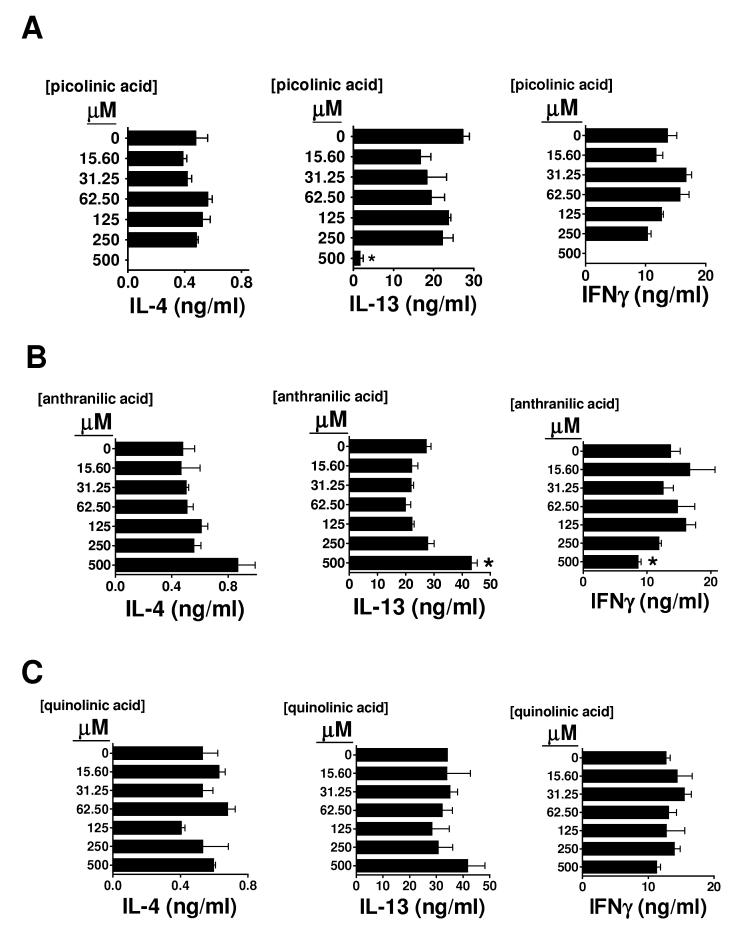
Lack of activity for terminal or side-branch catabolites of tryptophan degradadation on iNKT cell cytokine secretion
Next, we tested the effects on cytokine production by αGalCer-stimulated splenocytes of a terminal catabolite of tryptophan degradation (quinolinic acid), as well as two side-branch enzymatic byproducts of kynurenine catabolism: anthranilic acid and picolinic acid (figure 2). Our results indicated that, in contrast to L-kynurenine, 3-OH-kynurenine, or 3-OH-anthranilic acid, these terminal or alternative byproducts of tryptophan degradation showed little or no skewing effect on the cytokine profiles of αGalCer-stimulated splenocytes (figure 5). Picolinic acid non-specifically inhibited all cytokine production at 500 uM (figure 5A). Anthranilic acid showed very weak preferential IFNγ suppression, but this effect was only visible at the highest concentration tested (500 uM) (figure 5B). Quinolinic acid did not exhibit any effect at all on the cytokine output (figure 5C). These results suggest that the observed skewing effects are only induced by certain specific catabolites, which seem to lie close to kynurenine in the main enzymatic cascade of IDO-inititated tryptophan degradation (figure 2).
Figure 5. Lack of effect of terminal or side-branch catabolites of tryptophan degradation on the cytokine profile of αGalCer-stimulated splenocytes.
B6 splenocytes were cultured for 72 hours with 100 nM αGalCer, or with 100 nM αGalCer combined with graded concentrations of the tryptophan catabolites picolinic acid (A), anthranilic acid (B) or quinolinic acid (C). Supernatants were collected, and the IL-4, IL-13, and IFNγ concentrations were determined by sandwich ELISA. Bars represent the mean +/- SEM from triplicate wells. * p<0.05 compared with control (splenocytes + αGalCer). The results shown are representative of three similar experiments.
Partial reversal of iNKT cell anergy by IDO inhibition in vivo
It is well established that the iNKT cell cytokine response elicited after αGalCer stimulation of splenocytes in vitro changes dramatically if the splenocytes originate from mice previously exposed to αGalCer in vivo. This in vivo priming effect leads to a reduction in subsequent responses to a second stimulation with αGalCer stimulation, which is suggestive of a partially anergic phenotype. Although both IL-4 and IFNγ production are reduced upon restimulation in this setting, there is a much more complete abrogation of IFNγ production compared to IL-4 which results in an apparent Th2 polarization of the response [30]. We sought to examine the effects of inhibition of IDO by 1-MT on the response of previously primed splenocytes to αGalCer to determine whether the mechanism of IFNγ suppression could be associated with activation of IDO (figure 6). Our results indicated that pharmacologic inhibition of IDO during the in vitro restimulation of splenocytes with αGalCer could partially reverse the suppression of IFNγ production induced by previous in vivo priming with αGalCer (figure 6B). IL-4 levels, already low as a consequence of the in vivo priming, further decreased to undetectable levels in the presence of 1-MT (figure 6A). These results were consistent with the view that the initial in vivo priming with αGalCer altered the responses of iNKT cells to subsequent stimulation in part through the activation of IDO. This further supports the hypothesis that activation of IDO in vivo is strongly suppressive of IFNγ production while facilitating IL-4 production, this leading to a polarization of iNKT cell cytokine production that favors downregulation of Th1 inflammatory responses.
Figure 6. Partial reversal of iNKT cell anergy by pharmacologic inhibition of IDO.
B6 splenocytes from three mice injected with αGalCer seven days before (“primed splenocytes”), or from uninjected mice (“naïve splenocytes”), were cultured for 72 hours with 100 nM αGalCer, or with 100 nM αGalCer combined with graded concentrations of 1-MT. Supernatants were collected, and the IL-4 (A) and IFNγ (B) concentrations were determined by sandwich ELISA. Bars represent the mean +/- SEM from duplicate wells.
5. DISCUSSION
In this study, we investigated the modulation of iNKT cell cytokine responses by IDO activity, including an examination of the in vitro effects of a variety of tryptophan-derived catabolites. Our findings indicated that the IDO inhibitor 1-MT and the tryptophan catabolites L-kynurenine, 3-hydroxy-kynurenine, and 3-hydroxy-anthranilic acid, induced inverse cytokine skewing patterns in αGalCer-stimulated splenocytes, pointing to a novel mechanism for the regulation of iNKT cell responses. IDO inhibition by 1-MT skewed αGalCer-stimulated splenocyte cytokine responses towards a more Th1 pattern associated with a predominance of IFNγ. Reciprocally, the presence of specific catabolites, which are a byproduct of functional IDO upregulation, resulted in a cytokine response skewed towards a more Th2 pattern, with reduced IFNγ and preservation of IL-4 or IL-13 production. These findings may have important implications for understanding how the opposing activities of iNKT cells can be selectively controlled.
iNKT cells can exert effector functions associated with a pro-inflammatory response, such as protection against tumor cells [20] or infectious agents [21-23]. Yet, they have also been implicated as immunoregulatory cells that are important for modulating anterior chamber associated immune deviation [24], promoting transplantation tolerance [25], or modulating autoimmunity [26]. The underlying basis for these diverse functions of iNKT cells is unclear. The existence of distinct iNKT cell subsets has been proposed as one possible mechanism to account for this functional diversity [31]. However, the specific organ or tissue from which iNKT cells are isolated seems to be an even more important determinant of their functional characteristics [31]. This tissue-specific modulation of iNKT cell function could be the result of interactions with local antigen-presenting cells (APCs). For example, the function and phenotype of thymic or splenic iNKT cells can be modulated by APCs originating in different organs, and switching the origin of the APC on which αGalCer is presented to the iNKT cells can modify their cytokine response [32]. The precise mechanisms underlying this phenomenon are unclear, although different types or levels of costimulatory molecules could play a role. The findings presented here indicate that IDO activity in local tissues could be a modulatory influence on iNKT cell responses. Basal levels and functional activity of IDO in local tissue microenvironments, as well as temporary inducible surges in IDO activity, could all influence iNKT cell responses.
A unique feature of activated iNKT cells is their rapid release of IL-4 [18], and it is possible that these cells may be the main or only source of this cytokine in bulk αGalCer-stimulated splenocyte cultures. Depletion experiments have shown that the IL-4 recovered from αGalCer-stimulated bulk splenocyte culture supernatants is almost entirely produced by NK1.1+ or DX5+ cells, strongly suggesting its iNKT cell origin [32]. Thus, conserved IL-4 production in the presence of low concentrations of kynurenines (figures 3A, 4) could be explained by the fact that the steps leading to IL-4 release by iNKT cells are refractory to the effects of these catabolites. Reciprocally, the presence of the IDO inhibitor 1-MT resulted in decreased IL-4 production by these cells (figure 1), suggesting that some level of IDO activity is actually needed to maintain their IL-4 production.
In contrast, IFNγ secreted following αGalCer stimulation of mixed cell populations such as splenocytes is known to be released both by iNKT cells, and by downstream activated NK cells [18,19]. Thus, reduced IFNγ production in the presence of specific catabolites could be explained by effects on iNKT cells, on NK cells, or both (figure 7). In this regard, it is of interest that L-kynurenine has been shown to inhibit the surface expression of NK cell activating receptors [14]. Alternatively, kynurenines could induce selective NK cell apoptosis [12]. Tryptophan-derived catabolites could also modulate the transcription or translation of specific cytokine genes in selected cell types or during discrete differentiation stages of these cells.
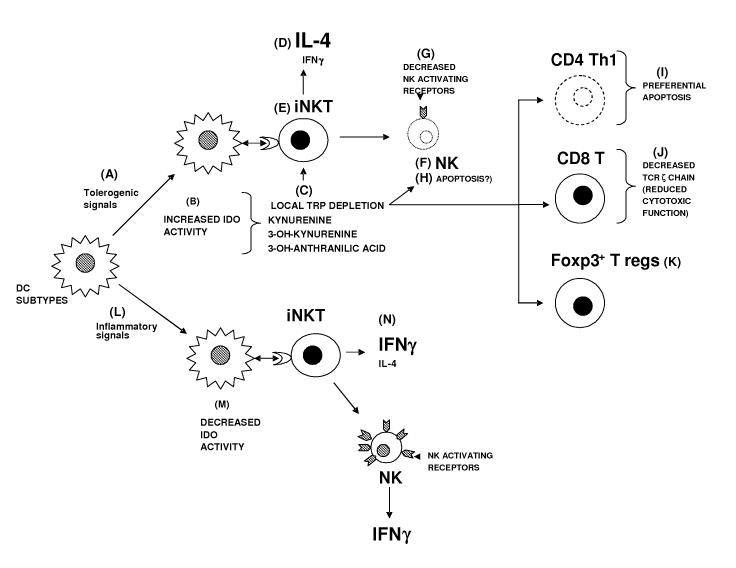
Figure 7. Schematic representation of proposed modulatory effects by IDO on iNKT cell dependent responses.
Tolerogenic signals [A], such as B7 engagement by CTLA4 expressed on T regulatory cells, leads to upregulation of IDO activity in specific DC subsets [B]. Increased IDO activity depletes tryptophan locally and increases the concentration of its catabolites [C]. Kynurenine, 3-OH-kynurenine, and 3-OH anthranilic acid modulate the cytokine response elicited by neighboring iNKT cells towards a Th2 pattern, reducing IFNγ but maintaining production of IL-4 and possibly other Th2 cytokines including IL-13 [D]. This reduced IFNγ could be the result of a specific effect on iNKT cells [E], of effects on downstream activated NK cells [F], or both. Tryptophan-derived catabolites have been reported to inhibit the surface expression of NK cell activating receptors [G]. Alternatively, they may selectively induce NK cell apoptosis [H]. Other effects of tryptophan depletion and/or accumulation of kynurenines include preferential apoptosis of Th1 clones [I], downregulation of CD8+ T cell receptor ζ chains [J], and differentiation of CD4+ CD25+ Foxp3+ T regulatory cells from naïve CD4+ T cells [K]. These effects combine to suppress exacerbated immune responses, and restore tolerance. In contrast, certain early pro-inflammatory signals [L], such as CD40 engagement, can downmodulate IDO activity in specific DC subsets [M]. Neighboring iNKT cells respond with a skewed pro-inflammatory cytokine pattern with reduced IL-4 [N], probably amplifying the inflammatory nature of the response.
The physiologic range of L-kynurenine concentrations encountered by immune cells in local tissue microenvironments exhibiting functional upregulation of IDO is currently not known. However, studies measuring its concentration in the supernatants of DCs exposed to physiologic (10-100 μM) levels of tryptophan suggest a 5-50 μM range [16,17]. This is in accordance with our results, which showed strong selective effects on IFNγ production at concentrations of L-kynurenine in this range (figure 3). Furthermore, it is conceivable that locally higher levels may be achieved in close proximity to IDO+ cells. In addition, previous reports examining other immunologic effects of L-kynurenine reported thresholds of efficacy of ∼200-300 μM for cultured human peripheral blood mononuclear cells (PBMC) [11] or NK cells [14], and of ∼170 QM for rat allogeneic T cell reponses [15]. These values are all significantly higher than the low concentration range of 15.6 to 62.5 uM needed to skew the cytokine profile of αGalCer-stimulated splenocytes.
The kynurenine pathway can be conceptually divided into three sequential segments, and three or four distinct side branches [33]. The initial segment involving the breakdown of tryptophan to kynurenine can occur in many cell types upon IFNγ stimulation [28,29,33]. A second segment includes 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and quinolinic acid (figure 2). This second segment appears to occur in a more restricted cellular compartment that includes DCs [34]. Interestingly, while the two proximal catabolites in this second segment consistently exhibit immunomodulatory properties [12,13,15,16], the terminal catabolite quinolinic acid, as well as a side-branch byproduct (anthranilic acid), have not shown any effect on human or rat T cell proliferation induced by anti-CD3 [12], allogeneic [15], or PHA [11] stimulation. These terminal or side-branch catabolites which have generally been found not to be immunologically active, also did not show any cytokine-skewing effects on αGalCer-stimulated splenocytes in our experiments (figure 5B and C). It is possible that different experimental systems are required to show effects specific to these terminal or side-branch catabolites. Quinolinic acid, for example, has been reported to induce preferential apoptosis of Th1 clones and of thymocytes [16]. Picolinic acid, another side-branch byproduct of kynurenine metabolism, has been shown to suppress proliferation of PHA-activated peripheral blood lymphocytes in a dose-dependent manner [11].
Finally, it is worth mentioning that the effects reported in the current study could have therapeutic applications. Although several glycolipid analogs have been described that skew the iNKT cell cytokine response towards a Th2 profile [18,27,35], and at least one has the opposite effect [36], the effects of combining 1-MT or kynurenines with αGalCer could extend beyond cytokine skewing and offer novel strategies for therapeutic potentiation (figure 7). IDO activity may help promote acquired tolerance to tumor antigens, and 1-MT has shown marked synergy with several clinically relevant anti-cancer agents [37]. It is possible that combining αGalCer with 1-MT could potentiate its effects in tumor models as well. Alternatively, therapies that combine αGalCer with kynurenines could prove beneficial in selected autoimmune diseases where iNKT cells are known to modulate disease outcome, and where the additional effects of the catabolites could synergize to suppress an exacerbated autoimmune response and restore tolerance.
ACKNOWLEDGEMENTS
We thank Ms. Helen Hu for technical assistance. This work was supported by grants from the National Institutes of Health (AI45889, AI064424, AI51392, and DK20541). G.S.B. is a former Lister Institute-Jenner Research Fellow and acknowledges support from the Medical Research Council (UK), the Wellcome Trust, the Royal Society Wolfson Research Merit Award, and from the James Bardrick Research Chair. Flow cytometry studies were supported by the FACS Core Facilities of the AECOM Center for AIDS Research (NIH/NIAID AI51519) and the AECOM Cancer Center (NIH/NCI P30 CA13330).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 2.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs, and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 3.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nature Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 4.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA4-Ig regulates tryptophan catabolism in vivo. Nature Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 5.Hassanain HH, Chon SY, Gupta SL. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-γ and α. J Biol Chem. 1993;268:5077–5084. [PubMed] [Google Scholar]
- 6.Silva NM, Rodrigues CV, Santoro MM, Reis LFL, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous γ interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grohmann U, Fallarino F, Silla S, Bianchi R, Belladona ML, Vacca C, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2001;166:277–283. doi: 10.4049/jimmunol.166.1.277. [DOI] [PubMed] [Google Scholar]
- 8.Grohmann U, Fallarino F, Bianchi R, Belladona ML, Vacca C, Orabona C, et al. IL-6 inhibits the tolerogenic function of CD8α+ dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 9.Grohmann U, Bianchi R, Orabona C, Fallarino F, Vacca C, Micheletti A, et al. Functional Plasticity of Dendritic Cell Subsets as Mediated by CD40 Versus B7 Activation. J Immunol. 2003;171:2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 10.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 11.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Battista Ferrara G. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 14.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R, et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK cell function. Blood. 2006;108:4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 15.Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transp Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 16.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death and Diff. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 17.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ chain and induce a regulatory phenotype in naïve T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Ann Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Ann Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 20.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. α-galactosylceramide activated Vα14 natural killer T cells mediate protection against murine malaria. PNAS-USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonoda K-H, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seino KI, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. PNAS-USA. 2001;98:2577–2581. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, et al. Activation of natural killer T cells by α-galactosylceramide treatment prevents the onset and recurrence of autoimmune type I diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 27.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. PNAS-USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261:3648–3653. [PubMed] [Google Scholar]
- 29.Werner-Felmeyer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim Biophys Acta. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 30.Burdin N, Brossay L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NKT cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, et al. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol. 2003;171:5913–5920. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 33.Moffett JR, Namboodiri MAA. Tryptophan and the immune response. Immunol Cell Bio. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 34.Belladona ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. PNAS-USA. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou D-Y, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]