Epstein–Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin (original) (raw)
Abstract
The Epstein–Barr virus-induced gene 3 (EBI3) is a novel soluble hematopoietin component related to the p40 subunit of interleukin 12 (IL-12). When EBI3 was expressed in cells, it accumulated in the endoplasmic reticulum and associated with the molecular chaperone calnexin, indicating that subsequent processing and secretion might be dependent on association with a second subunit. Coimmunoprecipitations from lysates and culture media of cells transfected with expression vectors for EBI3 and/or the p35 subunit of IL-12 now reveal a specific association of EBI3 with p35. Coexpression of EBI3 and p35 mutually facilitates their secretion. Most importantly, a large fraction of p35 in extracts of the trophoblast component of a human full-term normal placenta specifically coimmunoprecipitated with EBI3, indicating that EBI3 is in a heterodimer with p35, in vivo. Because EBI3 is expressed in EBV-transformed B lymphocytes, tonsil, spleen, and placental trophoblasts, the EBI3/p35 heterodimer is likely to be an important immunomodulator.
Interleukin 12 (IL-12) was identified and purified from the cell culture media of Epstein–Barr virus (EBV)-transformed B lymphoblastoid cell lines. In vivo, IL-12 is primarily produced by macrophages and other antigen-presenting cells. IL-12 is a 70-kDa heterodimeric cytokine composed of two disulfide-linked glycoproteins, p40 and p35. The p35 subunit is similar to other α-helix-rich hematopoeitin cytokines, whereas the p40 subunit is similar to the extracellular portion of a hematopoietin α receptor and is most closely related to the IL-6 or ciliary neutrophic factor α receptors. Macrophages and EBV-transformed B cells produce the biologically active p70 heterodimer and an excess of p40. IL-12 p35 appears to be ubiquitously expressed, whereas p40 expression is inducible and is mostly restricted to cell lines expressing the IL-12 heterodimer (refs. 1 and 2; reviewed in ref. 3). The dissociation between p35 and p40 gene regulation has led to speculation that either subunit may associate with other partners (4–6). IL-12 has pleiotropic effects in the development of TH1 responses in natural killer (NK) and T lymphocytes, including induction of interferon (IFN)-γ production, proliferation, and enhancement of cytotoxic activity, and inhibits TH2 responses (refs. 7 and 8; reviewed in ref. 3).
A novel homologue to IL-12 p40 was recently identified through the cloning of the third novel EBV-induced gene (EBI3) (9). EBI3 is expressed at a high level in human B lymphoblast cell lines transformed in vitro by EBV and is also present in vivo in some tonsil and spleen cells and in placental syncitial trophoblasts. EBI3 has 27% amino acid identity to the IL-12 p40 subunit, with conservative substitutions at many other residues. EBI3 further resembles IL-12 p40 in that both proteins are encoded by mRNAs with a 3′ untranslated Alu repeat sequence, lack a membrane anchoring motif, and are predicted to be secreted. However, when overexpressed in B lymphoblasts or COS7 cells, EBI3 tended to accumulate as an immature form in the endoplasmic reticulum associated with the molecular chaperone, calnexin, compatible with the notion that EBI3 associates with another partner that was not sufficiently abundant in these cells to enable its secretion (9). We now report that EBI3 associates noncovalently with the p35 subunit of IL-12 to form a novel secreted heterodimeric hematopoietin, EBI3/p35.
MATERIALS AND METHODS
Cell Culture, Transfection, and Metabolic Labeling.
BJAB is an EBV-negative Burkitt lymphoma cell line (10). COS7 is a simian virus 40-transformed monkey kidney cell line (11). Cell lines were grown in RPMI 1640 medium (BJAB) or DMEM (COS7) supplemented with 10% fetal calf serum. Approximately 107 BJAB cells or 4 × 106 COS7 cells were transfected by electroporation at 210 V and 960 μF in 400 μl of RPMI 1640 medium containing 10% fetal calf serum. For metabolic labeling experiments, cells were incubated 24 h posttransfection for 18 h with 50 μCi/ml (1 Ci = 37 GBq) 35S-labeled met/cys (S35Express, NEN) in methionine and cysteine-free RPMI 1640 medium (ICN), supplemented with 10% dialyzed fetal bovine serum.
Plasmids.
Plasmid pSG5p35Flag was constructed by PCR amplification of the cDNA encoding human IL-12 p35 amino acids 35–252 (Hoffman–La Roche) using the 5′ primer 35A (5′-CGCAGCCATGTGTCCAGCGCGCGCCAGC-3′) and the 3′ primer 35°C (5′-TTCTTATTTGTCATCGTCGTCCTTGTAGTCGGAAGCATTC-3′). The downstream primer places a Flag epitope (DYKDDDDKV) followed by a STOP codon after Ala-252. The p35Flag PCR-derived fragment was blunted and cloned into the blunted _Bgl_II site of pSG5. This plasmid directs IL-12 p35Flag expression from the second methionine (Met-35) that has been shown to be sufficient for expression of functional p35 (5). Plasmid pSG5p40Flag was constructed in a similar manner by PCR amplification of human IL-12 p40 amino acids 1–327 (Hoffmann–La Roche) with the 5′ primer 40A (5′-CGCGGATCCGCAGCCATGGGTCACCAGCAGTTG-3′) and the 3′ primer 40°C (5′-CGCGGATCCCTATTTGTCATCGTCGTCCTTGTAGTCACTGCAGGGCACAGATGC-3′). The PCR product was digested with _Bam_HI and cloned into the _Bam_HI site of pSG5. pSG5p35 contains a 842-bp cDNA fragment of the human IL-12 p35 (nucleotides 204–1046) cloned into the blunted _Bam_HI site of pSG5 and encodes IL-12 p35 amino acids 35–253. The pSG5FlagLMP1 construct used here encodes an N-terminal Flag-tagged fusion of the full-length cytoplasmic carboxyl terminus of the Epstein–Barr virus latent membrane protein 1 (amino acids 187–386) (G. Mosialos, unpublished data). Vent polymerase was used in PCRs, and the sequences of PCR-derived constructs were verified by DNA sequencing of the final clone.
Immunoprecipitation and Immunoblotting.
Twenty to 72 h posttransfection, culture supernatants and cells were harvested. Cells were washed in PBS and lysed for 30 min on ice in ice-cold Nonidet P-40 lysis buffer (0.5% NP40/20 mM Tris, pH 7.4/150 mM NaCl/3% glycerol/1.5 mM EDTA) or digitonin buffer [1% digitonin (Wako)/20 mM Tris, pH 7.4/150 mM NaCl] containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride/1 μg/ml leupeptin/1 μg/ml pepstatin). Cell debris was removed by centrifugation at 14,000 × g for 15 min at 4°C. Cell lysates were precleared with Sepharose beads (Pharmacia) for 1–2 h at 4°C and incubated with M2 anti-Flag antibody (IBI) or affinity-purified rabbit EBI3 antisera (9) for 2 h at 4°C followed by incubation with Protein G- or Protein A-Sepharose beads, respectively, for 1 h at 4°C. Culture supernatants were similarly precleared with Sepharose beads for 1–2 h at 4°C and incubated with M2 anti-Flag affinity gel (IBI) for 2 h at 4°C. Beads were then washed five times with 1 ml of lysis buffer, and protein complexes were recovered by boiling in SDS sample buffer. Placental tissue excised from the maternal face of term placenta was rinsed several times with PBS, mixed with an equal volume of 0.5% Nonidet P-40 lysis buffer containing protease inhibitors, and disrupted on ice using a blender. The placental extract was then further lysed on ice for 1 h and spun at 14,000 × g for 10 min, and the cleared lysate was processed for immunoprecipitation as described above. Precipitated materiel was analyzed by SDS/PAGE followed by autoradiography or immunoblotting. For autoradiography, gels were fixed for 30 min and incubated with Amplify Æ (Amersham) for 30 min before being exposed. In blotting experiments, EBI3 was detected using rabbit polyclonal EBI3 antisera (9) and the p35 subunit of IL-12 was detected using rabbit IL-12 antisera (gift from Genzyme) at a 1:100 to 1:400 dilution. Binding of EBI3 or IL-12 antisera was detected using horseradish peroxidase-conjugated protein A (1:7,500 dilution) and ECL reagents (Amersham).
RESULTS
EBI3 Associates with the p35 Subunit of IL-12 to Form a Secreted Noncovalent Heterodimer.
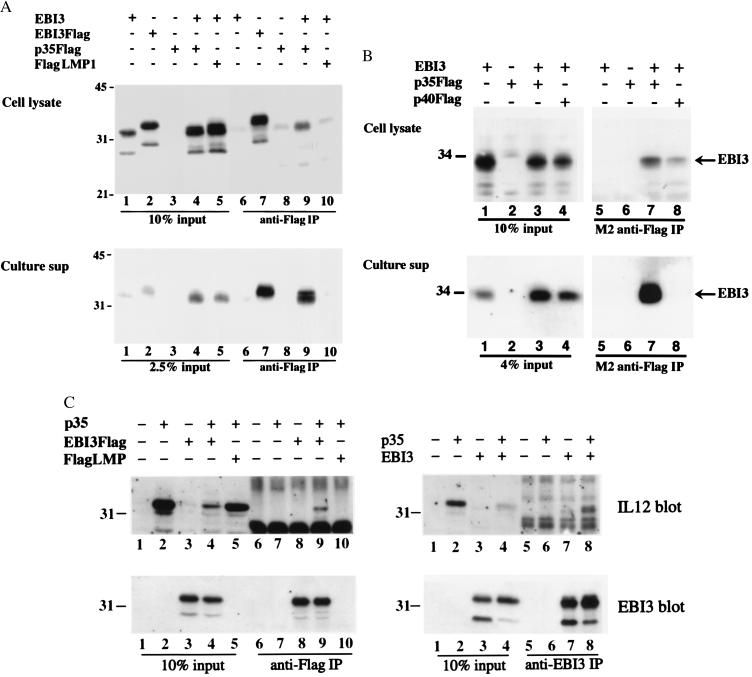
To investigate the hypothesis that IL-12 p35 might be the natural partner for EBI3, we evaluated their association by coimmunoprecipitation from cells overexpressing both proteins. Because of the limited availability and poor reactivity of p35-specific antibodies, we constructed a carboxyl-terminal Flag-tagged p35 expression vector (p35Flag) and transiently transfected BJAB B lymphoma or COS7 cells with EBI3 and p35Flag expression vectors. As controls, EBI3 was coexpressed with the Flag-tagged carboxyl terminal cytoplasmic tail of EBV LMP1 (FlagLMP1) or with a Flag-tagged secreted glycoprotein, IL-12 p40Flag. Flag-tagged proteins were immunoprecipitated with M2 anti-Flag monoclonal antibody, and coprecipitating EBI3 was detected by immunoblotting using rabbit polyclonal EBI3 antisera (Fig. 1 A and B). As previously observed (9), EBI3 accumulated in transfected BJAB cells with a predominant size of 33 kDa; smaller cross-reactive, presumed degradation products, at 24–30 kDa were also evident (Fig. 1A Upper). Approximately 5% of 33-kDa EBI3 coprecipitated with p35Flag from cell lysates (Fig. 1A Upper, compare the amount of EBI3 in the immunoprecipitate in lane 9 with 10% of the lysate in lane 4). Immunoblots with IL-12 antibody indicated that about 20% of p35Flag was immunoprecipitated by Flag antibody (data not shown). Thus, about 25% of EBI3 was associated with p35Flag in the cotransfected cells. EBI3 was not present in anti-Flag immunoprecipitates from BJAB cell lysates after transfection with EBI3 or p35Flag expression vectors alone or after cotransfection with EBI3 and FlagLMP1 expression vectors (Fig. 1A Upper). Approximately 10% of secreted EBI3 also coimmunoprecipitated with p35Flag from the culture medium of BJAB cells, which had been cotransfected with EBI3 and p35Flag expression vectors (Fig. 1A Lower, compare lanes 9 and 4). Because p35Flag could only be detected in the immunoblot of culture medium after immunoprecipitation, the efficiency of p35Flag immunoprecipitation could not be directly determined. In the absence of correction for the efficiency of p35Flag immunoprecipitation, the data indicate that at least 10% of the secreted EBI3 is p35 associated.
Figure 1.
EBI3 is secreted in association with IL-12 p35 from cotransfected cells. (A) Immunoblots with affinity-purified rabbit EBI3 antibody detect EBI3 in p35Flag immunoprecipitates from cotransfected BJAB cell lysate (Upper) or cell supernatant (Lower). BJAB cells (107 per transfection) were electroporated with 40 μg of pSG5 vector expressing EBI3 (9), EBI3Flag (9), p35Flag, or FlagLMP1 as indicated by a plus at the top of the figures. Twenty-four to 48 h posttransfection, cells or culture media were harvested and 1% digitonin cell extracts or cell culture media were immunoprecipitated with M2 anti-Flag monoclonal antibody. Fractions of the cell lysates or cell culture media obtained before immunoprecipitation (lanes 1–5) or the immunoprecipitates (lanes 6–10) were analyzed by 10% SDS/PAGE gel and immunoblotting. Molecular mass markers (in kilodaltons) are indicated on the left. In data not shown, immunoblots with M2 anti-Flag monoclonal antibodies or rabbit polyclonal IL-12 antisera indicated that about 20% of the total Flag-tagged proteins were immunoprecipitated from the cell. The efficiency of p35Flag immunoprecipitation from the culture media could not be determined because secreted p35Flag was only detected after immunoprecipitation. (B) EBI3 immunoblots detect EBI3 in anti-Flag immunoprecipitates from the culture media of COS7 cells coexpressing EBI3 with p35Flag, but not in that of COS7 cells coexpressing EBI3 with p40Flag. COS7 cells (4 × 106 per transfection) were electroporated with 12 μg of pSG5 vector expressing EBI3, p35Flag, or p40Flag as indicated, and 3.5 days posttransfection, cell lysates and culture media were coimmunoprecipitated as described in A, except that cells were lysed in 0.5% NP40. Immunoblot using M2 anti-Flag monoclonal antibodies or rabbit polyclonal IL-12 antisera indicated that about 20% of the cell-associated Flag-tagged proteins and most of the secreted Flag-tagged proteins were immunoprecipitated (not shown). (C) Immunoblot analysis of coimmunoprecipitation experiments performed from the lysates of transfected COS7 cells. COS7 cells (4 × 106 per transfection) were transfected with 20 μg of pSG5 vector expressing the proteins indicated at the top of the figures and lysed 48 h posttransfection in 0.5% Nonidet P-40 lysis buffer. Lysates were immunoprecipitated with M2 anti-Flag antibody (Left, lanes 6–10) or EBI3 affinity-purified rabbit polyclonal antibodies (Right, lanes 5–8). A fraction of the lysate (10% input) and immunoprecipitates were analyzed by immunoblotting using IL-12 antisera (Upper) or EBI3 anti-sera (Lower). IL-12 p35 migrates at the level of the IL12 blot lettering and just above the 31-kDa marker.
Similar results were obtained in transfected COS7 cells (Fig. 1B). At least 10% of secreted EBI3 coprecipitated specifically with p35Flag from cotransfected COS7 cell media (Fig. 1B Lower, compare lane 7 with lane 3). EBI3 did not coprecipitate with p40Flag from the culture media of cotransfected cells (Fig. 1B Lower, lane 8). EBI3 also coprecipitated somewhat specifically from COS7 cell lysates after cotransfection with EBI3 and p35Flag. A smaller amount of EBI3 consistently coprecipitated with p40Flag from cell lysates (Fig. 1B Upper, lane 8). The low-level association of EBI3 with p40 in cell lysates is likely to be an artifact resulting from the accumulation of both overexpressed proteins in the same cell compartment because EBI3 was not associated with secreted p40 (Fig. 1B Lower, lane 8).
Reciprocal coimmunoprecipitations confirmed the association of IL-12p35 with EBI3 (Fig. 1C). Immunoblotting with IL-12 antisera detected p35 in anti-Flag or anti-EBI3 immunoprecipitates from lysates of COS7 cells cotransfected with p35 and EBI3Flag or EBI3 expression vectors, respectively, but not in control immunoprecipitates (Fig. 1C Upper). Anti-EBI3 immunoblot indicated that about 10% of EBI3Flag and 20–30% of EBI3 were precipitated with anti-Flag or anti-EBI3 antisera, respectively, (Fig. 1C Lower); approximately 5 and 20% of p35 was detected in the respective EBI3 immunoprecipitates. These data indicate that 50% or more of p35 is associated with EBI3 in cells expressing both proteins. A small amount of p35 is expressed in all cells, and a trace of endogenous p35 may be evident in some immunoprecipitations with EBI3 antibody from cells transfected with the EBI3 expression vector alone (Fig. 1C Upper, lane 7 on the right). Coimmunoprecipitations in parallel from culture media confirmed a high-level association of p35 with secreted EBI3Flag (data not shown).
EBI3 contains only the two pairs of conserved cysteines that are predicted to mediate intramolecular disulfide linkages in hematopoietin receptors (12). As expected from this model, EBI3 was not disulfide-linked to p35 in coimmunoprecipitates from cell lysates or culture medium. The mobilities of p35 and EBI3 in SDS/PAGE were not affected much by whether coimmunoprecipitates were analyzed under reducing or nonreducing conditions (data not shown).
EBI3 and p35 Can Associate in Solution.
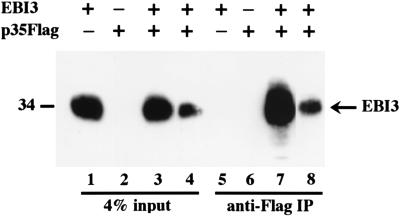
To investigate the extent to which the association of EBI3 with p35 is dependent on coexpression in the same cell as opposed to an ability to associate in solution, COS7 cells were individually transfected with EBI3 or p35Flag expression vectors, cultivated separately for 15 h, and then cocultivated for 3 days. Cells and culture medium were separately harvested, p35Flag was immunoprecipitated with Flag antibody, and EBI3 association was assessed by immunoblot with EBI3 specific rabbit antibodies (Fig. 2). About 4% of the secreted EBI3 coprecipitated with p35Flag (compare the amount of EBI3 in the p35Flag immunoprecipitate in lane 8 with the amount in 4% of the culture medium). EBI3 did not coprecipitate with p35Flag from lysates of the cocultured cells, indicating that the association does not take place rapidly when lysates of cells expressing either protein are mixed at 4°C (data not shown). These data indicate that EBI3 can specifically associate with p35 in culture media at 37°C. The association of EBI3 with p35 was less extensive than when both proteins were expressed in the same COS7 cells (lane 7).
Figure 2.
EBI3 and IL-12p35 can associate in solution. COS7 cells (4 × 106 per transfection) were electroporated with 12 μg of pSG5 plasmids expressing the proteins indicated by a plus at the top of the figure, and culture media were immunoprecipitated with M2 anti-Flag antibody. A fraction of the culture media (lanes 1–4) or immunoprecipitates (lanes 5–8) was analyzed by immunoblotting with affinity-purified EBI3 antibodies. Lanes 3 and 7 correspond to media from cells cotransfected with EBI3 and p35Flag expression vectors, whereas lanes 4 and 8 correspond to media from cells that were independently transfected with EBI3 or p35Flag expression vectors and then cocultured. These later cells were incubated at 37°C for 15 h after transfection, washed twice with PBS, trypsinized, and then cocultivated for 72 h before harvest. Immunoblot with M2 anti-Flag antibody indicated that most Flag-tagged proteins were precipitated (data not shown). As a control for the absence of cotransfected cells, EBI3 was not detected in an anti-Flag immunoprecipitate from the lysate of cocultures of p35Flag transfected cells and EBI3 transfected cells (data not shown).
Coexpression of EBI3 and p35 Facilitates Their Secretion.
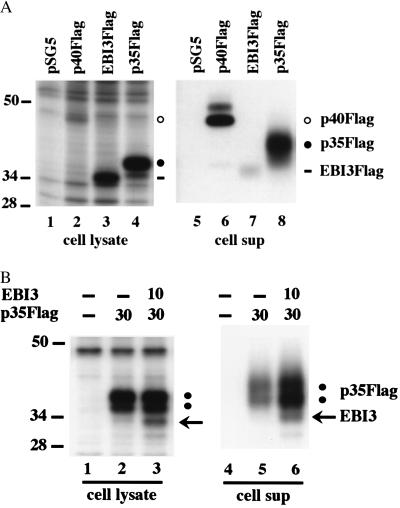
When expressed alone, EBI3 and p35 tend to accumulate in immature forms in the endoplasmic reticulum and are not efficiently secreted, whereas p40 undergoes more efficient Golgi processing and secretion (refs. 5 and 9; Fig. 3A, compare the amount of biosynthetically labeled intracellular and secreted EBI3Flag in lanes 3 and 7, respectively, with the amounts of intracellular and secreted p40Flag in lanes 2 and 6, respectively).
Figure 3.
Effect of EBI3 coexpression on p35 secretion. (A) BJAB cells (107 cells per transfection) were transfected with 30 μg of pSG5 vector control or pSG5 vector expressing EBI3Flag, p35Flag, or p40Flag. Approximately 24 h posttransfection, cells were labeled with [35S]Met/Cys for 18 h, and 0.5% Nonidet P-40 extracts (cell lysate, lanes 1–4) or culture media (cell sup, lanes 5–8) were immunoprecipitated with M2 anti-Flag antibody. Immunoprecipitates were separated on 10% SDS/polyacrylamide gel and analyzed by autoradiography. The positions of EBI3Flag, p35Flag, and p40Flag are indicated by a horizontal line, filled circle, or open circle, respectively. (B) BJAB cells (107 cells) were transfected with the indicated amount (in micrograms) of pSG5 vector expressing p35Flag or EBI3. The total amount of transfected DNA was maintained constant by addition of pSG5 vector. Approximately 20 h posttransfection, cells were labeled with [35S]Met/Cys and processed as in A. Lanes 1–3 are immunoprecipitates from cell lysates, and lanes 4–6 are immunoprecipitates from culture media.
Immunoblot analyses of transfected COS7 or BJAB cell lysates consistently showed that intracellular p35 was less abundant when EBI3 was coexpressed, compatible with the notion that EBI3 coexpression increased the efficiency of p35 secretion (Fig. 1C and data not shown). The low sensitivity of IL-12 or Flag antibodies in detecting p35Flag in immunoblots did not allow direct quantitation of p35 in cell culture media. To further investigate the effect of EBI3 coexpression on p35 secretion, transfected BJAB cell proteins were biosynthetically labeled with [35S]methionine and -cysteine. Labeled EBI3 protein was observed in anti-Flag immunoprecipitates from cell lysates or culture media of BJAB cells coexpressing EBI3 and p35Flag but not in control immunoprecipitates (Fig. 3B, lanes 3 and 6 vs. lanes 2 and 5; FlagLMP1 control data not shown). Note that the extent of EBI3 association with p35Flag is 2- to 3-fold underestimated in the autoradiogram, because EBI3 has only 3 methionine and 4 cysteine residues, whereas p35 has 10 methionine and 7 cysteine residues. Importantly, the relative amount of secreted vs. intracellular p35Flag increased when EBI3 was coexpressed (Fig. 3B and replicate experiments, compare the relative amounts of p35Flag in the lysate and culture medium of cells transfected with p35Flag alone in lanes 2 and 5, respectively, with the relative amounts of p35Flag in the lysate and medium of p35Flag and EBI3 cotransfected cells in lanes 3 and 6, respectively).
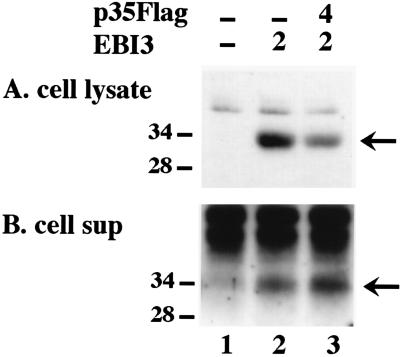
Although the effect was less pronounced, p35 coexpression also increased EBI3 secretion. Immunoblots with cotransfected COS7 cell lysates and culture medium showed a decreased amount of cellular EBI3 and an increased amount of secreted EBI3 when p35 was coexpressed (Fig. 4). These effects of p35 and EBI3 on the secretion of their heterodimeric partner are evidence for the physiologic relevance of their association.
Figure 4.
Effect of p35 coexpression on EBI3 secretion. COS7 cells (4 × 106 per transfection) were transfected with the indicated amount (in micrograms) of pSG5 vector expressing EBI3 or p35Flag. Total transfected DNA was maintained constant by the addition of pSG5 vector. After 20 h, cells were harvested and lysed in SDS buffer. Culture media were immunoprecipitated with EBI3 antisera. Lysates (A) and immunoprecipitates from culture media (B) were analyzed by SDS/PAGE and immunoblotting with EBI3 antisera. The position of EBI3 is indicated by the arrow.
EBI3 Is Associated with p35 in Human Placental Trophoblasts.
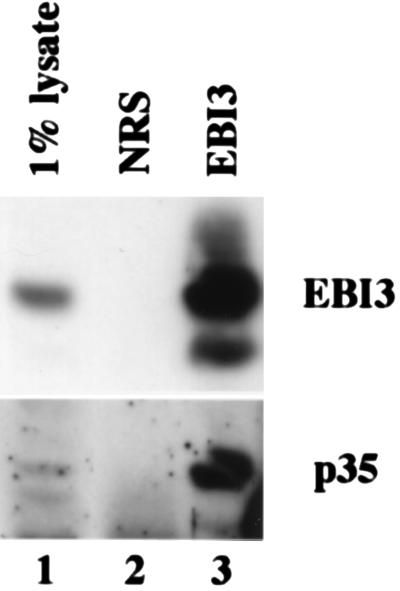
To evaluate whether EBI3 and p35 normally associate in human tissues, extracts were made from the syncitial trophoblast-enriched part of a human full-term placenta. Consistent with previous observations using placental extracts (9), EBI3-specific antibodies immunoprecipitated and identified EBI3 on immunoblots at an apparent size of 33 and 29 kDa (Fig. 5 Upper). IL-12 antibody specifically and reproducibly detected p35 in EBI3 immunoprecipitate and not in immunoprecipitates with normal rabbit serum (Fig. 5 Lower, lanes 3 and 2, respectively) or with irrelevant rabbit antibody (data not shown). The efficiency with which p35 coprecipitated with EBI3 appeared similar to that with which EBI3 precipitated, indicating that a large fraction of the p35 in placental tissue is associated with EBI3. Because p35 could not be immunoprecipitated with our IL-12 antibody, the reciprocal experiment could not be done.
Figure 5.
EBI3 and IL-12p35 coprecipitate from placenta extract. Immunoblots with rabbit affinity-purified EBI3 antibodies (Upper) or IL-12 antisera (Lower) of immunoprecipitates from extracts of human placenta. Trophoblast villi were dissected from a normal, full-term human placenta, and 0.5% Nonidet P-40 extracts from the equivalent of about 108 cells were immunoprecipitated with affinity-purified rabbit EBI3 antibodies (EBI3) or preimmune rabbit sera (NRS). Immunoprecipitates (100%) or cell lysate obtained before immunoprecipitation (1%) were analyzed by SDS/PAGE on a 10% gel. No signal was detected in immunoprecipitates with irrelevant rabbit polyclonal antibodies (data not shown). We cannot be certain that the band oberved in the lysate (Lower, lane 1), which is equivalent in mobility to p35 in the EBI3 immunoprecipitate (Lower, lane 3) is entirely p35, because multiple bands were detected in the cell lysate.
DISCUSSION
The findings presented here demonstrate that EBI3 heterodimerizes with IL-12 p35 and that EBI3 and p35 are components of a novel, naturally occurring, heterodimeric hematopoietin. The finding that p35 heterodimerizes with EBI3 as well as p40 is unexpected and highly unusual. Most cytokines are monomeric, homodimeric, or homotrimeric. A few cytokines also form functional heteromers, notably platelet-derived growth factor A and B subunits and lymphotoxin α and β (13, 14), but alternative heteromeric partnering is exceptional.
IL-12 p40 and EBI3 are also atypical hematopoietin components in that they structurally resemble α receptors rather other secreted hematopoietins. Most other hematopoietins signal by forming complexes of the ligand with α, β, and γ receptors. In most cases, the α receptor is restricted in expression and determines the cellular site of action, whereas the β and γ receptor components are responsible for the cytoplasmic signaling effects. Despite its resemblance to α receptors, IL-12 p40 is secreted with p35 as a heterodimeric ligand. In fact, IL-12 p40 expression is the key regulatory event in IL-12 synthesis. Further, IL-12 p40 is the principal determinant in IL-12 binding. However, p35 is essential for the ability of the p35/p40 heterodimer to signal, because p40 homodimers can efficiently bind to IL-12 receptors but do not mediate biological activity and instead act as an antagonist of IL-12 heterodimer (15). Similar to p40, EBI3 expression is also highly restricted. EBI3 is present in interfollicular cells in tonsils, perifollicular cells in spleens, and placental syncytial trophoblasts; its expression is up-regulated by mitogen activation in peripheral blood mononuclear cells or by EBV infection of B lymphocytes. Thus, EBI3 is also likely to be the principal determinant of EBI3/p35 synthesis and site of action.
IL-12 interacts with low- and high-affinity receptors, and these interactions determine the sites of IL-12 effect on NK and T lymphocytes (16–19). Biochemical studies indicate that IL-12 binding to its receptor is largely mediated by epitopes on p40 or by conformational epitopes of the p35/p40 heterodimer (15, 17, 20, 21). Thus, EBI3/p35 could interact with an IL-12 receptor or a unique receptor. However, in preliminary experiments, EBI3/p35 did not inhibit IL-12 binding to human IL-12 β1/β2 receptor cotransfected Ba/F3 cells receptor (David Presky, personal communication). Furthermore, EBI3/p35 did not antagonize or increase IL-12 effects in assays of lymphoblast proliferation or interferon γ secretion from PHA-activated human peripheral blood mononuclear cells (Maurice Gately, personal communication). The EBI3/p35 receptor is therefore likely to differ from IL-12 receptors, and the effects of EBI3/p35 are not likely to be through direct competition with IL-12 for receptor binding.
Nevertheless, EBI3/p35 is likely to antagonize IL-12’s biological effects. EBI3 is made in largest amounts by EBV-transformed B lymphocytes and by syncytial trophoblasts, two cell types that must survive in the presence of potentially highly effective NK and T cell responses. EBV-infected B lymphocytes are readily recognized by NK cells, and their growth is dependent on the expression of at least four nuclear proteins and two integral membrane proteins that have epitopes that can be recognized as foreign in the context of class I major histocompatibility complex proteins (22). The importance of blunting IL-12-induced NK and T cytotoxic effects during EBV infection is highlighted by the expression of a close homologue to human IL-10 from the EBV genome in lytic infection; IL-10 and the EBV IL-10 homologue antagonize the downstream effects of IL-12 on NK and T cytotoxic cell responses (23). Syncytial trophoblasts are at the frontier between the placenta and the potentially immunologically hostile environment of the uterus; very high numbers of NK cells are present in the uterus but are tolerant to the fetus (24). Thus, EBI3/p35 is likely to affect NK or CD8 cytotoxic cell function and thereby block the downstream biological effects of IL-12. By associating with the p35 subunit, EBI3 could also have a direct effect in blocking p40 from associating with p35 in a cell expressing both proteins.
The identification and characterization of the EBI3/p35 hematopoietin reported here will enable ascertainment of the effects of EBI3/p35 on the destruction of autologous EBV-transformed cells by NK and immune cytotoxic T lymphocytes, identification of the EBI3/p35 receptor, and identification of cells bearing the EBI3/p35 receptor.
Acknowledgments
George Mosialos provided the pSG5FlagLMP1 control construct, and Paul Durda from Genzyme provided the IL-12 antibody. This research was supported by Grant CA47006 from the National Cancer Institute of the U.S. Public Health Service. We thank Drs. Maurice Gately and David Presky of the Department of Inflammation and Autoimmune Diseases, Hoffmann–La Roche, Nutley, NJ, for p35 and p40 plasmids and for assays of EBI3/p35 inhibition of IL-12 binding or biological activity.
ABBREVIATIONS
EBV
Epstein–Barr virus
EBI3
EBV-induced gene 3
IL
interleukin
NK
natural killer
References
- 1.DíAndrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DíAndrea A, Aste-Amazaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinchieri G. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 4.Stern A S, Podlasky F J, Hulmes J D, Pan Y C, Quinn P M, Wolitzky A G, Familletti P C, Stremlo D L, Truitt T, Chizzonite R, Gately M K. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick R M, Kelleher K, Herrmann S H, Clark S C, Azzoni L, Chan S H, Trinchieri G, Perussia B. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 6.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 7.Szabo S J, Jacobson N G, Dighe A S, Gubler U, Murphy K M. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 8.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarimiento U, Faherty D A, Gately M K. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 9.Devergne O, Hummel M, Koeppen H, Le Beau M, Nathanson E C, Kieff E, Birkenbach M. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menezes J, Liebold W, Klein G, Clements G. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 11.Gluzman Y. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 12.Bazan J F. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duel T F. Curr Opin Biotechnol. 1991;2:802–806. doi: 10.1016/s0958-1669(05)80110-7. [DOI] [PubMed] [Google Scholar]
- 14.Browning J L, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow E P, Hession C, OíBrine-Grco B, Foley S F, Ware C F. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 15.Ling P, Gately M K, Gubler U, Stern A S, Lin P, Hollfelder K, Su C E, Pan Y C, Hakimi J. J Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 16.Chua A O, Chizzonite R, Desai B B, Truitt T P, Nunes P, Minetti L J, Warrier R R, Presky D H, Levine J F, Gately M K, Gubler U. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- 17.Presky D H, Yang H, Minetti L J, Chua A O, Nabavi N, Wu C Y, Gately M K, Gubler U. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky D H, Gubler U, Sinigaglia F. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Ferrante J, Gately M K, Magram J. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- 20.Chizzonite R, Truitt T, Podlasky F J, Wolitzky A G, Quinn P M, Nunes P, Stern A S, Gately M K. J Immunol. 1991;147:1548–1556. [PubMed] [Google Scholar]
- 21.Podlaski F J, Nanduri V B, Hulmes J D, Pan Y C, Lewin W, Danho W, Chizzonite R, Gately M K, Stern A S. Arch Biochem Biophys. 1992;294:230–237. doi: 10.1016/0003-9861(92)90162-p. [DOI] [PubMed] [Google Scholar]
- 22.Rickinson A B, Moss D J. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 23.Rickinson A B, Kieff E. In: Virology. Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S, editors. Philadelphia: Raven; 1996. pp. 2397–2446. [Google Scholar]
- 24.King A, Loke Y W, Chaouat G. Immunol Today. 1997;18:64–66. doi: 10.1016/s0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]