The Cytokine TWEAK Modulates Renal Tubulointerstitial Inflammation (original) (raw)
Abstract
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF superfamily of cytokines. In addition to binding and activating the fibroblast growth factor–inducible 14 receptor, TWEAK may regulate apoptosis, proliferation, and inflammation; however, the role of this system in kidney injury is unknown. In vitro, it was found that TWEAK induced the sustained activation of NF-κB in a murine tubular epithelial cell line (MCT). NF-κB activation was associated with degradation of IκB-α; translocation of RelA to the nucleus; and increased mRNA and protein expression of monocyte chemoattractant protein-1, RANTES, and IL-6. Similarly, in vivo, the systemic administration of TWEAK induced renal NF-κB activation, chemokine and IL-6 expression, and interstitial inflammation in mice. Parthenolide, which prevents IκB-α degradation, inhibited TWEAK-induced NF-κB activation and prevented the aforementioned changes in vitro and in vivo. After folic acid–induced acute kidney injury, fibroblast growth factor–inducible 14 expression increased in mouse tubular epithelium. Neutralization of TWEAK decreased the expression of chemokines in tubular cells and reduced interstitial inflammation. In conclusion, TWEAK has NF-κB–dependent proinflammatory effects on tubular epithelial cells in vitro and in vivo. Moreover, blockade of TWEAK reduces tubular chemokine expression and macrophage infiltration, suggesting that TWEAK modulates acute kidney injury by regulating the inflammatory response.
Acute kidney injury (AKI) and progressive loss of renal function are associated with interstitial inflammation and tubular injury.1 Infiltration by leukocytes depends on the local expression of inflammatory cytokines. Renal tubular epithelial cells are thought to be a central cell type in renal inflammation.2 Tubular epithelial cells are able to produce an array of cytokines in response to various immune and nonimmune factors,3 contributing to attraction of inflammatory cells to the kidney. Members of the TNF superfamily of cytokines regulate several cell responses, including proliferation, differentiation, cell death, and inflammation.4 Some of these cytokines, such as TNF and Fas ligand, have been extensively studied in kidney diseases and shown to be involved in renal damage.5–8 TNF-like weak inducer of apoptosis (TWEAK; TNFSF12) is a recently characterized member of the TNF superfamily of structurally related cytokines.9 TWEAK may promote cell death in some tumor cell lines and monocytes but also modulates cell proliferation, inflammation, and angiogenesis.10–13 Fibroblast growth factor-inducible 14 (Fn14) has been confirmed as a TWEAK receptor.14–17 TWEAK and its receptor, Fn14, are present in the healthy adult kidney.9,10,18,19 TWEAK does not promote apoptosis in nonstressed tubular cells.19 There is no information on the role of TWEAK and its receptor in tubulointerstitial inflammation. We now report that TWEAK promotes the NF-κB–dependent secretion of proinflammatory cytokines from renal tubular epithelial cells in vitro and whole kidneys in vivo, leading to interstitial inflammation. Consistent with this observation, blockade of TWEAK pathway in an AKI model reduces tubular chemokine expression and macrophage accumulation in the injured kidney.
RESULTS
TWEAK Promotes Sustained NF-κB Activation in Murine Tubular Epithelial Cells
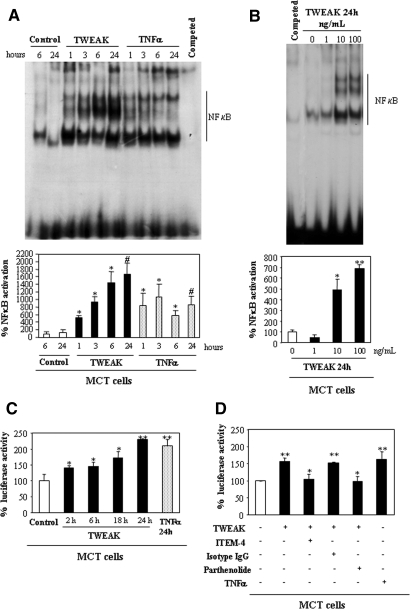
Murine tubular MCT cells, human tubular HK-2 cells, and the murine kidney constitutively express the TWEAK receptor, Fn14 (Supplementary Figure 1).19 In MCT cells, TWEAK increased NF-κB DNA-binding activity in nuclear extracts, as assessed by electrophoretic mobility shift assay (EMSA) in a time- (Figure 1A) and dosage-dependent (Figure 1B) manner. TNF-α, used as a control for NF-κB activation, yielded a different pattern of complexes (Figure 1A). Luciferase gene reporter assays confirmed that TWEAK promotes NF-κB transcriptional activity in MCT cells (Figure 1C). Fn14 is the only characterized TWEAK receptor14,15; however, there is evidence for the existence of additional TWEAK receptors.20 Anti–Fn14-neutralizing antibody prevented TWEAK-induced NF-κB activation (Figure 1D). These results suggest that TWEAK promotes NF-κB activation via Fn14 in the MCT tubular cells. In human HK-2 cells, TWEAK also induced sustained NF-κB activation with a temporal pattern similar to that observed in murine cells, as assessed by EMSA and translocation of RelA to the nucleus (confocal microscopy; data not shown).
Figure 1.
TWEAK promotes NF-κB activation in renal tubular cells by Fn14 activation. (A and B) Representative EMSA (top) and quantification (bottom) of nuclear extracts prepared from MCT cells that were treated with TWEAK. Data are means of five independent experiments. (A) Time response. *P < 0.05 versus control 6 h; #P < 0.02 versus control 24 h. (B) Dosage-response. *P < 0.02 versus control; **P < 0.002 versus control. (C) NF-κB transcriptional activity was measured by the dual luciferase assay system in MCT cells that were co-transfected with pNF-κB-Luc and pRLTK vectors. Luciferase values were normalized to Renilla activity. *P < 0.05 versus control; **P < 0.008 versus control. (D) The anti-Fn14 ITEM-4 antibody or 10 μM parthenolide prevented TWEAK-induced NF-κB activation at 24 h (luciferase gene reporter assay). **P < 0.008 versus control; *P < 0.02 versus TWEAK. Data are means ± SD of four independent experiments (C and D). The concentration of TWEAK was 100 ng/ml unless otherwise specified. TNF-α was used as a control for NF-κB activation.
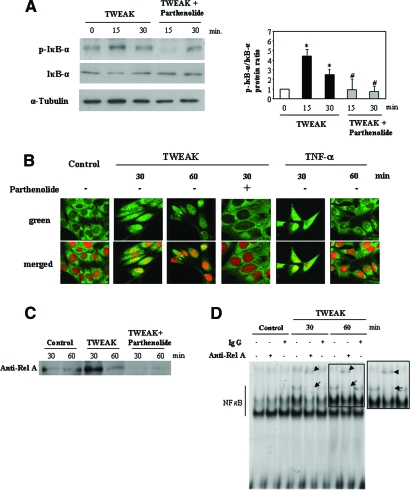
IκB-α Phosphorylation and RelA Nuclear Translocation in TWEAK-Stimulated Renal Tubular Cells
In MCT cells, TWEAK induced phosphorylation of IκB-α within 15 min of stimulation (Figure 2A). This was followed by a reduction in IκB-α, consistent with the degradation of the phosphorylated protein (Figure 2A). IκB-α degradation was temporarily associated with translocation of the RelA subunit of NF-κB from the cytoplasm to the nucleus (Figure 2, B and C). Supershift experiments confirmed the participation of RelA in the DNA-binding complexes (Figure 2D). These findings suggest that TWEAK induces NF-κB activity through the IκB-α phosphorylation pathway.
Figure 2.
Phosphorylation of IκB-α and nuclear translocation of RelA in TWEAK-stimulated MCT cells: Inhibition by parthenolide. (A) Western blot of MCT cells stimulated with 100 ng/ml TWEAK and/or 10 μM parthenolide. *P < 0.007 versus 0 min; #P < 0.02 versus TWEAK alone. Western blot representative of three independent experiments. (B and C) In MCT cells that were treated with 100 ng/ml TWEAK, RelA is translocated to the nucleus. Parthenolide prevented TWEAK-induced RelA translocation as assessed by confocal microscopy (B) and Western blot analyses of nuclear extracts (C). RelA is shown in green; propidium iodide is shown in orange. Cells that were treated with TNF-α were used as a positive control. (D) Representative supershift assay. The nuclear extracts were incubated alone or with control rabbit IgG or anti-RelA. Shifted complexes are marked with arrowheads, and the usual position of the band is marked with an arrow. Magnification, ×320.
Parthenolide Inhibits TWEAK-Induced NF-κB Activation in Renal Tubular Cells
Parthenolide inhibits NF-κB activation by preventing the degradation of IκB-α.21 In MCT cells that were preincubated with parthenolide, IκB-α phosphorylation was inhibited and its degradation was prevented (Figure 2A), and RelA did not translocate to the nucleus (Figure 2, B and C). Parthenolide also inhibited TWEAK-induced NF-κB DNA-binding activity, as assessed by EMSA (data not shown), and NF-κB transcriptional activity (Figure 1D).
TWEAK Increases Inflammatory Cytokine Production in Renal Tubular Cells
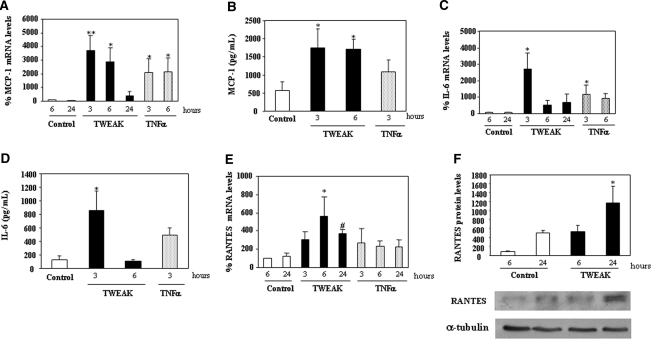
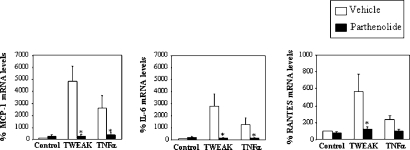
We then addressed the biologic significance of NF-κB activation by TWEAK in MCT cells. TWEAK increased, in a time-dependent manner, the mRNA and protein expression of monocyte chemoattractant protein-1 (MCP-1; Figure 3, A and B), IL-6 (Figure 3, C and D), and RANTES (Figure 3, E and F). TWEAK-induced upregulation of mRNA expression was abrogated by parthenolide (Figure 4), indicating that they are NF-κB activation–dependent responses. In addition, blocking anti-TWEAK antibodies completely prevented the increased expression of MCP-1 and RANTES mRNA at 3 and 6 h (data not shown).
Figure 3.
TWEAK stimulates chemokine production in proximal tubular MCT cells. (A, C, and E) Quantitative reverse transcription–PCR (RT-PCR) analyses of MCP-1 (A), IL-6 (C) or RANTES (E) mRNA in cells that were treated with 100 ng/ml TWEAK or 30 ng/ml TNF-α. Values for mRNA were normalized to glyceraldehyde-3-phosphate dehydrogenase expression. Data are means ± SD of four independent experiments. **P < 0.005 versus control 6 h; *P < 0.05 versus control 6 h; #P < 0.02 versus control 24 h. Expression level at 6 h control as 100%. (B and D) Secretion of MCP-1 (B) or IL-6 (D). Supernatants from MCT cells were tested by ELISA. Data are means ± SD of three independent experiments. *P < 0.05 versus control. (F) Representative Western blot of RANTES in lysates from MCT cells stimulated with 100 ng/ml TWEAK. Quantification of three independent experiments (arbitrary densitometry units). *P < 0.008 versus control 24 h.
Figure 4.
Parthenolide prevents TWEAK-induced proinflammatory effects in tubular epithelial cells. Quantitative RT-PCR analyses of mRNA for MCP-1 (3 h; A), IL-6 (3 h; B), and RANTES (6 h; C) in cells that were treated with 100 ng/ml TWEAK or TNF-α in the presence or absence of parthenolide. Values for mRNA were normalized to glyceraldehyde-3-phosphate dehydrogenase expression. Data are means ± SD of three independent experiments. *P < 0.05 versus cytokine alone.
Systemic Application of TWEAK Has a Proinflammatory Effect in the Renal Tubulointerstitium
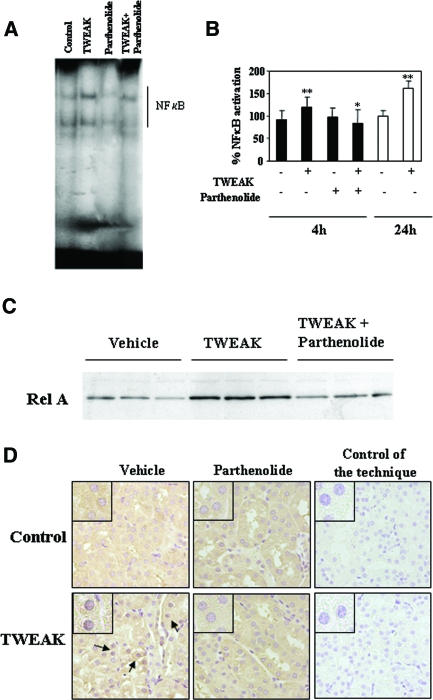
Next, we studied the effects of TWEAK on NF-κB in vivo. TWEAK increased NF-κB DNA-binding activity in nuclear extracts of murine kidneys, as assessed by EMSA (Figure 5, A and B). On the basis of the cell culture observations, we concentrated on early time points. Parthenolide inhibited NF-κB DNA-binding activity (Figure 5, A and B). TWEAK increased nuclear RelA in kidneys, and this was prevented by parthenolide (Figure 5C). Immunohistochemistry showed that RelA translocated to the nucleus of tubular cells (Figure 5D). Parthenolide inhibited the nuclear translocation of RelA (Figure 5, C and D).
Figure 5.
Renal NF-κB activation in mice that were treated with TWEAK: Inhibition by parthenolide. (A) Representative EMSA of nuclear extracts from kidneys that were treated for 4 h with TWEAK, parthenolide, or TWEAK/parthenolide. (B) Quantification of EMSA analyses of kidney nuclear extracts prepared from kidneys that were treated with TWEAK for 4 h (n = 6) and 24 h (n = 7). **P < 0.02 versus control; *P < 0.05 versus TWEAK alone. (C) Western blot analyses of nuclear extracts: RelA translocation to renal nuclei is prevented by parthenolide. (D) RelA immunohistochemistry. Four hours after TWEAK injection, RelA is observed in tubular nuclei from mice that were treated with TWEAK (arrows); this is prevented by parthenolide. Magnifications: ×400 in D; ×1000 in inset.
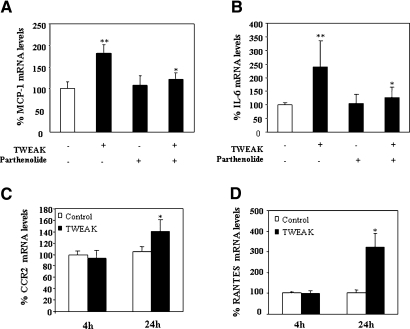
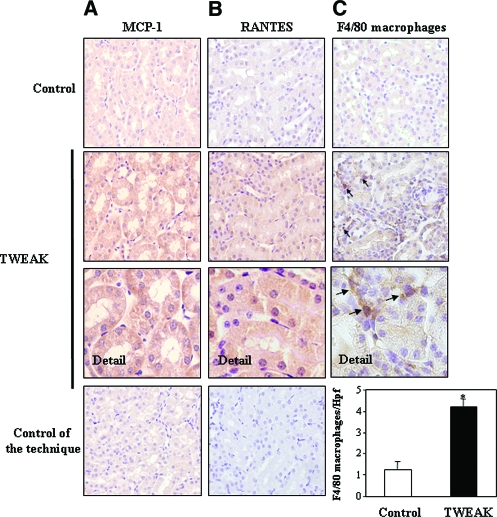
TWEAK-induced upregulation of kidney MCP-1 and IL-6 mRNA was obvious at 4 h and was prevented by parthenolide (Figure 6, A and B). TWEAK-induced upregulation of RANTES mRNA was observed only at 24 h (Figure 6D). Immunohistochemistry confirmed the expression of MCP-1 and RANTES by the tubular epithelium (Figure 7, A and B). The mRNA expression of CCR2 (the MCP-1 receptor) was increased 24 h after TWEAK injection, suggesting that CCR2-expressing inflammatory cells were attracted by MCP-1 (Figure 6C). Macrophages are the main leukocyte type expressing CCR2. TWEAK increased renal interstitial F4/80-positive macrophages at 24 h (Figure 7C). These findings indicate that TWEAK has a proinflammatory effect in the renal tubular epithelium. Although TWEAK did not modify serum creatinine levels (control 0.025 ± 0.013; TWEAK 24h 0.027 ± 0.015 mg/dl; NS), it did cause mild signs of tubular injury, such as loss of brush border and vacuolation (control tubular injury score 1.2 ± 0.3; TWEAK 24 h 2.7 ± 0.9; P < 0.04)
Figure 6.
TWEAK induces kidney chemokine and chemokine receptor mRNA expression in vivo. (A and B) Quantitative RT-PCR analyses of kidney MCP-1 (A) or IL-6 (B) mRNA in mice 4 h after TWEAK, parthenolide, or TWEAK/parthenolide. Data are means ± SD of six mice per group. **P < 0.01 versus control; *P < 0.05 versus TWEAK alone. (C and D) Quantitative RT-PCR analyses of mRNA for CCR2, the MCP-1 receptor (C), or RANTES (D) in kidneys 4 h (n = 6) and 24 h (n = 7) after TWEAK injection. CCR2 expression was increased at 24 h. *P < 0.02 versus control.
Figure 7.
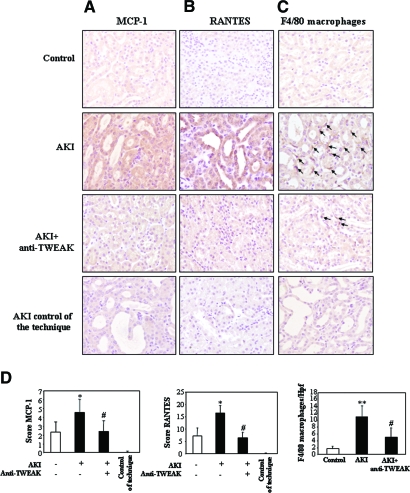
TWEAK induces chemokine protein expression and kidney inflammation in vivo. (A and B) MCP-1 (A) and RANTES (B) proteins are localized to tubular epithelium of kidneys 4 and 24 h after TWEAK injection, respectively. (C) F4/80 antigen immunohistochemistry. An increased number of macrophages stained with F4/80 were noted in the interstitium 24 h after TWEAK (arrows). Controls for the technique are stained with nonspecific Ig. Hpf, high-power field. Quantification as mean ± SD *P < 0.005 versus control. Magnifications: ×400; ×1000 in Detail.
Neutralization of TWEAK Reduces Tubular Chemokine Expression and Interstitial Inflammation in AKI
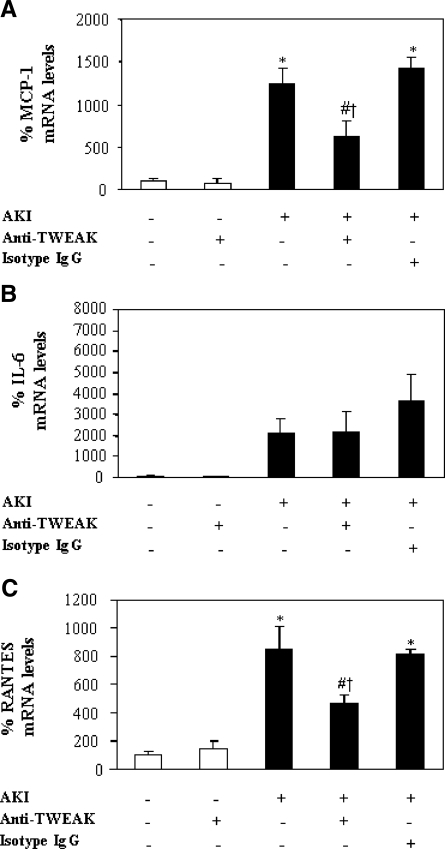
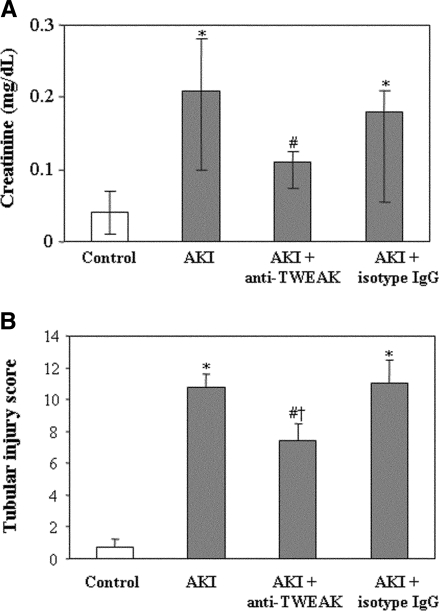
In a model of AKI induced by a folic acid overdose, Fn14 was upregulated in the tubular epithelium (Supplementary Figure 2),19 and MCP-1, RANTES, and IL-6 mRNA were increased at 72 h (Figure 8). The neutralizing anti-TWEAK antibody reduced MCP-1 and RANTES mRNA induction by 60% but not IL-6 mRNA induction (Figure 8). Immunohistochemistry confirmed the reduction in tubular cell MCP-1 and RANTES expression (Figure 9, A and B) and the decrease in macrophage infiltration by 55% (Figure 9, C and D). Neutralization of TWEAK also resulted in significantly milder renal failure and histologic injury (Figure 10).
Figure 8.
TWEAK neutralization decreases chemokine mRNA expression in an acute kidney injury model. (A through C). TWEAK neutralization decreased kidney MCP-1 (A) and RANTES (C) but not IL-6 (B) mRNA (quantitative RT-PCR) at 72 h after injury. Data are means ± SD of six mice per group. *P < 0.008 versus control; #P < 0.04 versus AKI at 72 h; †P < 0.008 versus AKI+isotype IgG for MCP-1; †P < 0.03 versus AKI+isotype IgG for RANTES.
Figure 9.
TWEAK neutralization decreases chemokine protein expression and tubulointerstitial inflammation in vivo as assessed by immunohistochemistry. (A) MCP-1. (B) RANTES. Note epithelial cell localization of increased MCP-1 and RANTES expression in mice with AKI when compared with healthy control or in mice that had AKI and were treated with anti-TWEAK. Note interstitial infiltration by cells that are identified as macrophages in C. (C) The increased number of interstitial macrophages stained with anti-F4/80 (arrows) in AKI kidneys was reduced by anti-TWEAK. Controls for the technique are stained with nonspecific Ig. (D) Quantification as means ± SD *P < 0.03 versus control; **P < 0.002 versus control; #P < 0.02 versus AKI. Magnification, ×200.
Figure 10.
TWEAK neutralization decreased the degree of renal dysfunction and histologic tubular injury in AKI. (A) Renal function was assessed by serum creatinine levels. Data are means (25th to 75th percentile) of six mice per group. *P < 0.04 versus control; #P < 0.05 versus AKI. (B) Histologic tubular injury was assessed by a tubular injury score which had a maximum value of 12. Data are means ± SD of six mice per group. *P < 0.0001 versus control; #P < 0.04 versus AKI; †P < 0.03 versus AKI+isotype IgG.
DISCUSSION
Using renal tubular epithelial cells, we showed that TWEAK binding to Fn14 activates the NF-κB transcription factor by promoting IκB-α phosphorylation and degradation, leading to expression of chemokines. TWEAK also activated NF-κB and inflammation in murine kidneys in vivo. Furthermore, TWEAK neutralization decreased chemokine expression and inflammation in a model of acute tubulointerstitial injury.
In the course of renal injury, tubular epithelial cells become activated and acquire a proinflammatory phenotype that contributes to inflammatory cell recruitment.2 TWEAK is a novel regulator of the activation of tubular epithelial cells. Increased TWEAK and Fn14 expression are found in experimental models of AKI, such as folate overdose nephropathy, in which they localized to tubular epithelium.19 Potential sources of TWEAK in the kidney include infiltrating monocytes and T lymphocytes and mesangial and tubular cells.19,22–24 TWEAK is a multifunctional cytokine that may promote cell death, cell proliferation, inflammation, and angiogenesis.12,17 Because of the potential cell-type specificity of TWEAK actions and the influence of the cell microenvironment, it is important to characterize the biologic effects of TWEAK in renal cells.9,11,19,24 Contrary to renal mesangial cells,24 TWEAK alone does not induce apoptosis in tubular epithelium19; therefore, we explored the effect of TWEAK on NF-κB activation and inflammation.
The transcription factor NF-κB is a key player in the inflammatory response of tubular cells. The classical form of NF-κB is a heterodimer of a p50 and a p65 (RelA) subunit.25 In resting cells, NF-κB proteins are associated with IκB proteins and retained in the cytoplasm, where they are inactive. TWEAK induced phosphorylation and degradation of IκB-α in tubular epithelium, resulting in nuclear translocation of RelA, where it activated transcription of target genes encoding inflammatory cytokines that are known mediators of renal injury.26,27 This is consistent with the canonical pathway of NF-κB activation.28 Inhibition of NF-κB activation by anti-Fn14 antibodies implicates this receptor in signal transduction in tubular epithelium and does not support an essential participation of the postulated but not yet characterized additional TWEAK receptor(s) or NF-κB activation as a consequence of direct nuclear translocation of TWEAK.20,29 Furthermore, TWEAK administration in vivo induces sustained renal NF-κB activation, RelA nuclear translocation in tubular epithelium, and tubular expression of chemokines. A delayed increase in RANTES mRNA with respect to MCP-1 mRNA was noted both in cell culture and in vivo, emphasizing the physiologic relevance of the cell culture observation. TWEAK was recently found to increase MCP-1 expression in cultured glomerular mesangial cells and to upregulate whole-kidney MCP-1 mRNA24; however, that report did not address the following questions: (1) The molecular pathway leading to gene expression, (2) whether there was a concomitant increase at the chemokine protein level, (3) which are the cells responsible for mRNA expression in the kidney, (4) whether TWEAK elicits renal inflammation, and (5) whether it has a role in renal injury. We now show that TWEAK also increases the renal expression of CCR2, the MCP-1 receptor, which is mainly expressed by monocytes/macrophages.30 This was associated with renal interstitial macrophage infiltration, suggesting that, in addition to potential glomerular effects, TWEAK induces renal interstitial inflammation. By contrast to kidney cells, TWEAK did not promote MCP-1 expression in bronchial epithelium or keratinocytes.31,32
Finally, we addressed the relevance of TWEAK in an experimental model of AKI induced by a folic acid overdose. This model is characterized by tubular injury and inflammatory cell infiltration, which was detected from 48 h and increased thereafter.33 Tubular cells are a source of inflammatory chemokines, such as MCP-1 and RANTES, and we now show that this is TWEAK dependent. As a consequence, TWEAK blockade reduced infiltration by macrophages. By contrast, the increase in kidney IL-6 expression was independent of TWEAK in this model, suggesting preferential regulation by other mediators.
A possible discrepancy is observed between the more modest effect of the in vivo administration of TWEAK on proinflammatory gene expression in healthy kidneys and the more marked effects in cultured cells or the protection offered by anti-TWEAK strategies in damaged kidneys. Possible explanations include that the microenvironment of the healthy kidney may be less permissive for inflammatory responses than that found in culture or in an injured kidney. In the latter, the expression of the receptor in target cells is increased, and cells may be already primed by other cytokines.19 We cannot exclude additional actions of a pleiotropic cytokine in the injurious microenvironment of AKI that lead to further inflammation.
It is conceivable that TWEAK elicits other biologic responses that also depend on NF-κB and differ from those elicited by other cytokines. In this regard, the persistent NF-κB activation by TWEAK in tubular cells is consistent with the recently identified noncanonical NF-κB activation, which is not activated by TNF.34 Indeed, different patterns of DNA-binding complexes were obtained in MCT cells that were treated with TWEAK or TNF, which was used as a positive control for NF-κB activation, suggesting that TNF and TWEAK are not redundant cytokines in renal injury.
In summary, TWEAK promotes sustained NF-κB activation in tubular epithelium and whole kidney. One of the consequences of tubular cell NF-κB activation by TWEAK is the secretion of proinflammatory cytokines, leading to renal interstitial inflammation. This action may be relevant for the interstitial inflammation that accompanies AKI. Additional consequences of NF-κB activation by this cytokine in tubular cells should be further characterized, because they might allow definition of specific pathways that are amendable to therapeutic manipulations.
CONCISE METHODS
Cells and Reagents
MCT murine proximal tubular epithelial cells and human proximal tubular epithelial (HK-2) cells (ATCC, Rockville, MD) were studied.35 Recombinant TWEAK was from Alexis (Läufelfingen, Switzerland). Murine TNF-α (Immugenex, Los Angeles, CA) was used at a concentration of 30 ng/ml. Parthenolide (Sigma, St. Louis, MO) at 10 μM inhibits NF-κB in MCT cells without decreasing cell viability. Blocking anti-TWEAK mAb (clone P2D10) was used at 10 μg/ml.
Western Blot
Samples that were homogenized in lysis buffer were separated by 10 to 12% SDS-PAGE under reducing conditions.36 Primary antibodies were rabbit polyclonal anti–IκB-α (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Fn14 (1:500),18 anti-RANTES (1:250; Chemicon, Temecula, CA), mouse monoclonal anti–p-IκB-α (1:250; Santa Cruz Biotechnology), and anti-Fn14 ITEM-4 (eBioscience, San Diego, CA). Blots were then probed with anti–α-tubulin (1:2000; Sigma), and levels of expression were corrected for minor differences in loading.37 Cytosolic and membrane proteins were obtained by lysing the cells in 2% Triton X-114 precondensed in PBS and processing both phases.6
Quantitative Reverse Transcription–PCR
One microgram of RNA isolated by Trizol (Invitrogen, Paisley, UK) was reverse-transcribed with High Capacity cDNA Archive Kit, and real-time PCR was performed on an ABI Prism 7500 PCR system (Applied Biosystems, Foster City, CA) using the DeltaDelta Ct method.38 Expression levels are given as ratios to glyceraldehyde-3-phosphate dehydrogenase. Predeveloped primer and probe assays were from Applied Biosystems.
EMSA
Cells were resuspended in buffer A and homogenized.39 Nuclei and cytosolic fractions were separated by centrifugation at 1000 × g 10 min. Nuclei (pellet) were washed twice in buffer A and resuspended in the same buffer, with a final concentration of 0.39 mol/L KCl. Nuclei were extracted for 1 h at 4°C and centrifuged at 100,000 × g 30 min. Supernatants were dialyzed in buffer C and then cleared by centrifugation and stored at −80°C. The protein concentration was determined by the bicinchoninic acid method. Frozen kidneys were pulverized in a metallic chamber and resuspended in cold extraction buffer.40,41 The homogenate was vigorously shaken for 30 min, and the insoluble materials were precipitated by centrifugation at 40,000 × g for 30 min at 4°C.
EMSA was carried out as described previously.39 For supershift assays, nuclear extracts were incubated with 1 μg anti-RelA antibody (Santa Cruz Biotechnology) or 1 μg rabbit IgG for 1 h at 37°C before incubation with the labeled probe.
NF-κB Luciferase Reporter Gene Assay
MCT cells were plated at a density of 8 × 104 cells in six-well plates 24 h before transfection with FuGENE 6 (Roche, Indianapolis, IN), according to the manufacturer's instructions. pNF-κB-Luc (Stratagene, La Jolla, CA) and pRLTK vectors, which contain the luciferase gene Renilla (Promega, Madison, WI), were used in a ratio of 10:1. The medium was replaced with RPMI without serum 4 h after transfection, and cells were treated with 100 ng/ml TWEAK. In some experiments, 10 μM parthenolide or 2.5 μg/ml anti-Fn14 antibody, ITEM-4, was added to the cell culture 1 h before TWEAK. As a control, cells were treated with TNF-α for 24 h. Luciferase activity was determined by a luciferase assay system (Promega) and a luminometer (Berthold, Nashua, NH) and normalized to Renilla activity to control for differences in transfection efficiency.
ELISA
Cells were stimulated with 100 ng/ml TWEAK, and murine MCP-1 and IL-6 in the supernatants were determined by ELISA (BD Pharmingen, San Diego, CA).
Immunostaining
Cells plated onto Labtek slides were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100/PBS, washed in PBS, and incubated with rabbit polyclonal anti-RelA (1:75), followed by FITC secondary antibody (1:200; Sigma). Nuclei were counterstained with propidium iodide.
Animal Model
Studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Balb/c mice (12 to 14 wk of age; IFFA-CREDO, Barcelona, Spain) were treated with 70 μg of parthenolide or its vehicle (0.05% DMSO) and 0.75 μg of TWEAK or saline. Treatment and control groups (n = 6) were as follows: (1) Parthenolide followed by TWEAK, (2) vehicle (DMSO) followed by TWEAK, (3) parthenolide followed by saline, and (4) vehicle (DMSO) followed by saline. Parthenolide was injected 2 4h and TWEAK 4 h before the mice were killed. In a second set of experiments, mice were killed 24 h after injection of either 0.75 μg of TWEAK (n = 7) or vehicle (200 μl 0.9% NaCl; n = 7). The dosage of TWEAK was calculated on the basis of in vitro experiments for an extracellular volume of 7.5 ml/mouse. The dosage of parthenolide was established on the basis of previous experience.42
Folic acid nephropathy is a classical model of AKI.19 C57/BL6 mice (12 to 14 wk of age) received a single intraperitoneal injection of folic acid (Sigma) 250 mg/kg in 0.3 mol/L sodium bicarbonate or vehicle, and mice were killed 72 h later. Mice were dosed intraperitoneally with either 200 μg of blocking anti-TWEAK mAb (clone P2D10) or 200 μg of isotype IgG (mouseIgG2a, clone P1.17; n = 6 per group). Mice received the mAb treatments 1 d before the folic acid injection and 2 d thereafter. The kidneys were perfused in situ with cold saline before removal. One kidney from each mouse was fixed in buffered formalin, embedded in paraffin, and used for immunohistochemistry. The other kidney was snap-frozen in liquid nitrogen for RNA and protein studies.
Immunohistochemistry
Immunohistochemistry was carried out as described previously in paraffin-embedded tissue sections 5 μm thick.42 Primary antibodies were rabbit polyclonal anti-RelA (1:60; Santa Cruz Biotechnology), goat polyclonal anti–MCP-1 (1:100, Santa Cruz Biotechnology), rabbit polyclonal anti-RANTES (1:10; Chemicon), and rat polyclonal anti-F4/80 antigen (1:50; Serotec, Oxford, UK). Sections were counterstained with Carazzi's hematoxylin. Negative controls included incubation with a nonspecific Ig of the same isotype as the primary antibody. MCP-1 and RANTES staining was evaluated by a quantitative scoring system, Image-Pro Plus software (Media Cybernetics, Bethesda, MD) in 10 randomly selected fields (×20) per kidney. The total number of F4/80-positive macrophages was quantified in 20 randomly chosen fields (×40) using Image-Pro Plus software. Samples were examined in a blinded manner.
Tubular injury was evaluated in hematoxylin-eosin section by an outside pathologist (J.B.) who was blinded to the nature of the samples. Evidence of cell injury (loss of brush border, vacuolization), cell desquamation, and tubular dilation and signs of regeneration were scored on a semiquantitative scale from 0 to 3, and results from each item were added to yield the tubular injury score, which had a maximal value of 12.
Statistical Analyses
Statistical analysis was performed using SPSS 11.0 (SPSS, Chicago, IL). Results are expressed as means ± SD. Significance at the P < 0.05 level was assessed by t test for two groups of data and ANOVA for three or more groups.
DISCLOSURES
None.
Supplementary Material
[Supplemental Data Files]
Acknowledgments
This study was supported by FIS CP04/00060, 06/0046, SAF03/884, SAF2005-03378, SAF2007/60896 EU QLG1-CT-2002-01215, Sociedad Española de Nefrologia, National Institutes of Health (HL39727 to J.A.W.), ISCIII-RETIC REDinREN/RD06/0016, REDinREN/RD06/0004, and Comunidad de Madrid/FRACM/S-BIO0283/2006. Authors’ salaries were supported by FIS (P.J. and A.S.), MEC (M.D.S.N.), and Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM; A.O.).
Mar Gonzalez García-Parreño helped with confocal microscopy.
Published online ahead of print. Publication date available at www.jasn.org.
P.J. and M.D.S.-N. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Remuzzi G, Ruggenenti P, Benigni A: Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997 [DOI] [PubMed] [Google Scholar]
- 2.de Haij S, Daha MR, van Kooten C: Mechanism of steroid action in renal epithelial cells. Kidney Int 65: 1577–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 3.van Kooten, Daha MR, van Es LA: Tubular epithelial cells: A critical cell type in the regulation of renal inflammatory processes. Exp Nephrol 7: 429–437, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Locksley RM, Killeen N, Lenardo MJ: The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 104: 487–501, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Justo P, Sanz A, Lorz C, Gomez-Garre D, Mezzano S, Egido J, Ortiz A: Expression of Smac/Diablo in tubular epithelial cells and during acute renal failure. Kidney Int 86[Suppl 52]: S52–S56, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Lorz C, Ortiz A, Justo P, Gonzalez-Cuadrado S, Duque N, Gomez-Guerrero C, Egido J: Proapoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol 11: 1266–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ortiz A, Gonzalez-Cuadrado S, Bustos C, Alonso J, Gómez-Guerrero C, López-Armada MJ, González E, Plaza JJ, Egido J: Tumor necrosis factor and glomerular damage. J Nephrol 8: 27–34, 1995 [Google Scholar]
- 8.Boonstra JG, van der Woude FJ, Wever PC, Laterveer JC, Daha MR, van Kooten C: Expression and function of Fas (CD95) on human renal tubular epithelial cells. J Am Soc Nephrol 8: 1517–1524, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401–32410, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, Michaelson J, Burkly L, Putterman C: The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front Biosci 9: 2273–2284, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Harada N, Nakayama M, Nakano H, Fukuchi Y, Yagita H, Okumura K: Pro-inflammatory effect of TWEAK/Fn14 interaction on human umbilical vein endothelial cells. Biochem Biophys Res Commun 299: 488–493, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, Dayer JM: Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: Blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res 4: 126–133, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran NL, McDonough WS, Savitch BA, Sawyer TF, Winkles JA, Berens ME: The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem 280: 3483–3492, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Nakayama M, Harada N, Okumura K, Yagita H: Characterization of murine TWEAK and its receptor (Fn14) by monoclonal antibodies. Biochem Biophys Res Commun 306: 819–825, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Wiley SR, Winkles JA: TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor 14: 241–249, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA: The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J 371: 395–403, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Yoon K, Lee K, Kim K, Jang H, Lee NK, Hwang K, Young Lee S: TNF-related weak inducer of apoptosis receptor, a TNF receptor superfamily member, activates NF-kappa B through TNF receptor-associated factors. Biochem Biophys Res Commun 13; 305: 789–796, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA: The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274: 33166–33176, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Justo P, Sanz AB, Sanchez-Niño MD, Winkles JA, Lorz C, Egido J, Ortiz A: Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T: TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TWEAKR: Evidence for a second TWEAK receptor. J Biol Chem 278: 32317–32323, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hehner SP, Hofmann TG, Droge W, Schmitz ML: The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol 163: 5617–5623, 1999 [PubMed] [Google Scholar]
- 22.Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, Richardson BC: The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol 169: 6020–6029, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Nakayama M, Kayagaki N, Yamaguchi N, Okumura K, Yagita H: Involvement of TWEAK in interferon gamma-stimulated monocyte cytotoxicity. J Exp Med 192: 1373–1380, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C: Proinflammatory effects of tweak/fn14 interactions in glomerular mesangial cells. J Immunol 176: 1889–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Barnes PJ, Karin M: Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Ping D, Boekhoudt GH, Rogers EM, Boss JM: Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol 162: 727–734, 1999 [PubMed] [Google Scholar]
- 27.Segerer S, Nelson PJ, Schlöndorff D: Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11: 152–176, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz JL, Baltimore D: Two pathways to NF-kappaB. Mol Cell 10: 693–695, 2002 [DOI] [PubMed] [Google Scholar]
- 29.De Ketelaere A, Vermeulen L, Vialard J, Van De Weyer I, Van Wauwe J, Haegeman G, Moelans I: Involvement of GSK-3beta in TWEAK-mediated NF-kappaB activation. FEBS Lett 566: 60–64, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D: Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol 166: 4697–4704, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Okamoto A, Ichikawa J, Ando T, Tasaka K, Masuyama K, Ogawa H, Yagita H, Okumura K, Nakao A: TWEAK/Fn14 interaction stimulates human bronchial epithelial cells to produce IL-8 and GM-CSF. Biochem Biophys Res Commun 318: 422–427, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Nakao A, Nakayama M, Yamaguchi N, Kojima Y, Nakano N, Tsuboi R, Okumura K, Yagita H, Ogawa H: Induction of RANTES by TWEAK/Fn14 interaction in human keratinocytes. J Invest Dermatol 122: 1175–1179, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Doi K, Okamoto K, Negishi K, Suzuki Y, Nakao A, Fujita T, Toda A, Yokomizo T, Kita Y, Kihara Y, Ishii S, Shimizu T, Noiri E: Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am J Pathol 168: 1413–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beinke S, Ley SC: Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J 382: 393–409, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justo P, Lorz C, Sanz A, Egido J, Ortiz A: Intracellular mechanisms of cyclosporin A-induced tubular cell apoptosis. J Am Soc Nephrol 14: 3072–3080, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ortiz A, Lorz C, Catalan MP, Danoff TM, Yamasaki Y, Egido J, Neilson EG: Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int 57: 969–981, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Cohen CD, Frach K, Schlondorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J: Angiotensin II participates in mononuclear cells recruitment in the kidney through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 gene expression. J Immunol 161: 430–439, 1998 [PubMed] [Google Scholar]
- 40.Hernandez-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, Egido J: Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 95: 1532–1541, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Negoro N, Kanayama Y, Haraguchi M, Umetani N, Nishimura M, Konishi Y, Iwai J, Okamura M, Inoue T, Takeda T: Blood pressure regulates platelet-derived growth factor A-chain gene expression in vascular smooth muscle cells in vivo: An autocrine mechanism promoting hypertensive vascular hypertrophy. J Clin Invest 95: 1140–1150, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C: Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol 161: 1497–1505, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data Files]