Inhibition of Hypoxia Inducible Factor Hydroxylases Protects Against Renal Ischemia-Reperfusion Injury (original) (raw)
Abstract
Acute renal failure resulting from hypoperfusion and hypoxia is a significant clinical problem. Hypoxia activates the heterodimeric transcription factor hypoxia inducible factor (HIF), leading to changes in gene expression that promote tissue adaptation and survival. To determine whether HIF may protect the kidney from ischemia-reperfusion injury, we subjected hif1a+/− and hif2a+/− mice to renal ischemia-reperfusion injury. Injury was substantially more severe in hif+/− than in littermate controls, consistent with a protective role for HIF. Because wild-type mice exhibited submaximal HIF accumulation in response to no-flow ischemia, we tested compounds that might augment the protective HIF response following ischemia-reperfusion in these animals. We found that l-mimosine and dimethyloxalylglycine, two small molecules that activate HIF by inhibiting HIF hydroxylases, protected mouse kidneys from ischemia-reperfusion injury. Therefore, pharmacological activation of HIF may offer an effective strategy to protect the kidney from ischemic injury.
Ischemic injury is a major cause of morbidity and mortality. An important therapeutic aim has been to reduce the damage that occurs to ischemic tissues. A potentially attractive way to achieve this is activating the transcription complex hypoxia-inducible factor-1 (HIF).1–3 HIF was originally isolated from studies of an oxygen-responsive enhancer element 3′ to the erythropoietin (EPO) gene, which increases gene expression in hypoxia.4 It is now known that HIF can be activated by low oxygen in all mammalian cells and induces widespread changes in gene expression. Many of the genes whose expression is increased by HIF are expected to increase the capacity of a cell or tissue when oxygen supply is reduced.1,5 Examples include EPO itself, glucose transporters, glycolytic enzymes, angiogenic growth factors, nitric oxide synthases, and heme oxygenase-1.4,6–9 It is therefore plausible that activating HIF may improve the survival of ischemic cells and also promote beneficial adaptive changes such as increased angiogenesis. The concept is supported by observations that exposure to hypoxia protects tissues, including the heart, brain, and kidney, from subsequent ischemic injury.10–12
Recent insights into the functioning of the HIF pathway have provided targets for small molecules that would lead to activation of HIF in the presence of oxygen. HIF is a heterodimeric complex containing an oxygen-regulated α subunit and a constitutive β subunit, both of which are members of multiprotein families.1,2 The dominant mode of regulation of the α subunit is through oxygen-dependent destruction.13 Three genes encode oxygen-responsive HIF-α subunits. HIF-1α and HIF-2α have been extensively characterized and show little redundancy. Their regulation by oxygen is now well understood. Briefly, in the presence of oxygen, specific prolyl residues are hydroxylated by enzyme Prolyl Hydroxylase Domain (PHD)1, 2, or 3, which provides a signal for capture by a specific ubiquitin E3 ligase complex.14–16 The recognition component of the E3 ligase complex is the von Hippel Lindau (VHL) tumor suppressor protein, explaining why cells lacking VHL exhibit constitutive HIF activation.17 HIF-α subunits are also inactivated in the presence of oxygen through hydroxylation of an asparaginyl residue by a different enzyme, Factor Inhibiting HIF (FIH)-1, which prevents recruitment of transcriptional co-activators.18 The HIF hydroxylases, PHD1 through 3 and FIH-1, belong to the superfamily of 2-oxoglutarate (2-OG)-dependent oxygenases, which have a ferrous ion at the active site.19,20 The identification of theses enzymes provides potential targets to activate HIF in the presence of oxygen. This is illustrated by the effects of cobalt chloride and iron chelators, which inactivate the hydroxylases. An attractive idea is that strategies to enhance HIF activation in ischemia might be used to protect organs such as the kidney, brain, and heart from injury and thereby ameliorate acute injury. Evidence is accumulating that HIF plays a role in the response of organs to ischemic injury.21,22 Support for the idea that enhancing HIF activation could protect from ischemic injury comes from studies using gene transfer.23 In addition, cobalt, which inter alia decreases HIF hydroxylase activity, has been shown to be protective in several models.24–26
The kidney is particularly prone to ischemic injury, which leads to necrosis of tubular epithelial cells and acute renal failure. Using genetic and pharmacologic interventions in mice, we investigated whether manipulating the HIF system can alter the outcome of ischemia-reperfusion injury (IRI).
Results
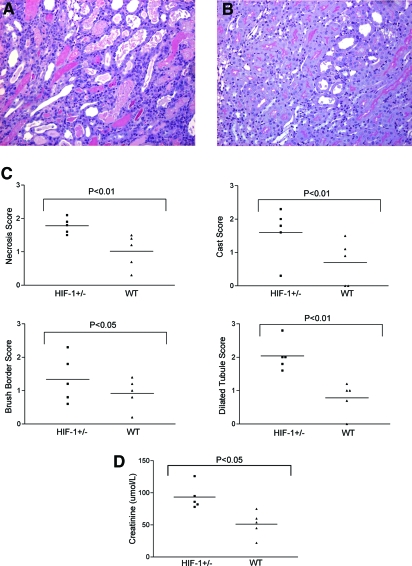
To determine whether HIF-1 may influence the outcome of renal-ischemia reperfusion injury, we first tested the effect of a genetic reduction in HIF-1α or HIF-2α. We could not use mice with homozygous deficiency for hif1a because these die in utero as a result of placental and cardiovascular developmental abnormalities.27 Mice that are heterozygous for hif1a do not have major phenotypic abnormalities but have reduced expression of HIF-1α and reduced HIF-1 responses.1 We performed unilateral IRI for 30 min under isoflurane anesthesia in five pairs of hif1a+/− mice with littermate controls. A global assessment of injury was made by two individuals blinded to the genotype with a forced choice in each pair; in each case, the injury was judged more severe in the hif1a+/− mouse. A previously validated scoring system was also used to quantify injury,28 with separate assessment of the number of necrotic tubular cells, the number of tubules exhibiting loss of the brush border, the number of tubular casts, and the number of tubules appearing dilated. In each case, the injury induced by IRI was more severe in the HIF-1α knockdown mice compared with the controls (Figure 1). For each of these parameters, the extent of injury was significantly more severe in the hif1a+/− mice than in hif1a+/+ littermate controls.
Figure 1.
Hif1a+/− mice show more severe histologic damage after renal IRI. (A) Representative PAS-stained section after renal ischemia reperfusion from a hif1a+/− mouse. (B) Littermate control. (C) Quantitative scores of tubular injury. (D) Serum creatinine values were significantly higher in hif1a+/− mice compared with littermate controls after bilateral injury. Magnification, ×100.
Next, for examination of whether there was a differential effect on renal function, five pairs of hif1a+/− mice and littermate controls were exposed to bilateral renal IRI. Both renal pedicles were clamped for 20 min under general anesthesia. Creatinine values at 72 h were significantly higher in the hif1a+/− group. Taken together, these experiments demonstrate that HIF-1α has a protective effect in IRI of the kidney in mice (Figure 1).
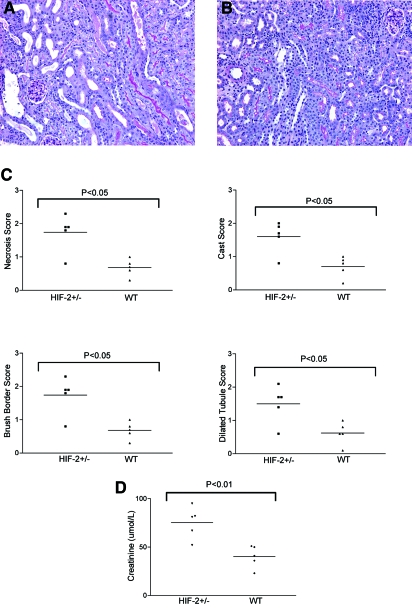
Next, we performed similar experiments in hif2a+/− mice. As with hif1a+/−, homozygosity for HIF-2α is usually lethal. Animals die with abnormal lung and vascular development.29,30 Although with selective breeding some hif2a+/− animals are viable, they have major hematologic and metabolic defects.31 Heterozygote animals for hif2a+/− appear normal. Unilateral renal ischemia for 30 min followed by reperfusion was more severe in hif2a+/− mice compared with littermate controls (Figure 2). In bilateral injury, in which both pedicles were clamped for 20 min, creatinine values were significantly higher compared with those of littermate controls (Figure 2); therefore, both HIF-1α and HIF-2α protect the kidney from IRI.
Figure 2.
Hif2a+/− mice show more severe histologic damage after renal IRI. (A) Representative PAS-stained section after renal ischemia reperfusion from a hif2a+/− mouse. (B) Littermate control. (C) Quantitative scores of tubular injury. (D) Serum creatinine values were significantly higher in hif2a+/− mice compared with littermate controls after bilateral injury. Magnification, ×100.
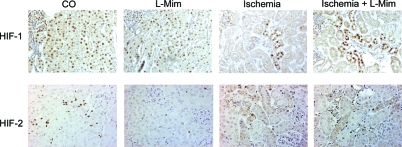
We next investigated the extent to which HIF is activated in IRI to determine whether this might be increased by inhibition of HIF hydroxylases. Immunohistochemistry for HIF-α provides a means of visualizing HIF activation in tissues. We used exposure to carbon monoxide (CO) as a positive control to induce HIF activation in the kidney. CO has a higher affinity for hemoglobin than oxygen and consequently reduces oxygen delivery to the kidney, providing a powerful stimulus for HIF activation. As expected, exposure of mice to 0.1% CO resulted in immunodetectable HIF-1α in epithelial cells throughout the normal mouse kidney (Figure 3). In kidneys from animals exposed to room air (21% oxygen), no HIF-1α subunit accumulation was detected (data not shown). To determine the extent to which acute renal ischemia is able to stimulate HIF-1α, we exposed C57Bl/6 mice to 30 min of unilateral renal ischemia. Decay of HIF-1α protein is very rapid in the presence of oxygen, so animals were killed without reperfusion. HIF-1α was detectable in the ischemic mouse kidney (Figure 3) almost exclusively in tubular epithelial cells, particularly at the corticomedullary junction. However, the amount of HIF-1α detected in acute ischemia was substantially less compared with that seen in animals exposed to CO, indicating that HIF activation in no-flow renal ischemia is submaximal. For HIF-2α, after CO exposure, HIF-2α subunits were widely detected in interstitial cells (Figure 3) in a similar distribution to that described by Bernhardt et al.32 Acute ischemia resulted in less HIF-2α accumulation, predominantly in cells located at the corticomedullary junction (Figure 3).
Figure 3.
(Top) HIF-1α immunohistochemistry. (A) After exposure to 0.1% CO, nuclear HIF-1α is detected in epithelial cells throughout the renal cortex. (B) l-Mimosine–treated animals showed widespread activation of HIF-1α. (C) In ischemic mouse kidney, substantially less HIF-1α is detected. (D) Treatment with l-mimosine before ischemic injury increased expression of HIF-1α. In untreated animals, no nuclear signal for HIF-1α was detected (data not shown). (Bottom) HIF-2α immunohistochemistry. (A) 0.1% CO exposure results in HIF-2α in interstitial cells. (B) l-Mimosine treatment leads to HIF-2α activation but less than CO exposure. (C) In ischemic mouse kidney, substantially less HIF-2α is detected. (D) Treatment with l-mimosine before ischemic injury increased expression of HIF-2α. Magnification, ×200.
Next, we tested a small molecule that has been reported to induce HIF-1 in the kidney in vivo. l-Mimosine is structurally related to 2-OG and is predicted to compete with 2-OG at the catalytic core of PHD enzymes. Previously, it had been reported that l-mimosine can induce HIF in the normal rat kidney.33 Six hours after administration of l-mimosine, HIF-1α was detected throughout the kidney in the normal mouse (Figure 3). HIF-2α was detected in some interstitial cells, but less expression was detected after l-mimosine treatment compared with CO. In control animals treated with vehicle injection, no HIF-1 or HIF-2α subunits were detected (data not shown). We also demonstrated that administration of l-mimosine 6 h before the mice were killed was associated with an increase in expression of several well-characterized HIF target genes by RNase protection assay: Carbonic anhydrase IX, glucose transporter 1, and vascular endothelial growth factor (data not shown). For determination of whether treating a mouse with l-mimosine can augment HIF-α accumulation after ischemia, mice were treated with l-mimosine 6 h before undergoing acute renal ischemia for 30 min. HIF-1α was detected predominantly at the corticomedullary junction and primarily in distal tubular epithelial cells (Figure 3) and was clearly increased compared with kidneys exposed to ischemia without l-mimosine. For HIF-2α, increased expression in interstitial cells was also mainly at the corticomedullary junction. Taken together, these experiments show that both HIF-1 and HIF-2 induction is submaximal in acute ischemia and can be increased by administration of l-mimosine before injury.
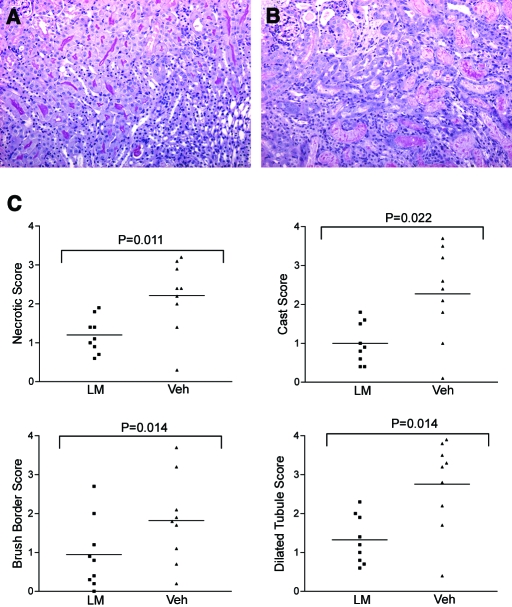
To determine whether l-mimosine could protect mice from renal ischemia, we gave pairs of mice l-mimosine (600 mg/kg) and vehicle control 6 h before the left renal pedicle was clamped for 30 min. Animals were killed 72 h later. A total of nine pairs of animals underwent surgery. All mice receiving l-mimosine before injury exhibited less injury compared with animals treated with vehicle control on the basis of forced-choice analysis by two assessors blinded to treatment. Figure 4, top, shows periodic acid-Schiff (PAS)-labeled sections of the renal cortex from a representative pair of animals. In quantitative assessments, each of four parameters of injury was significantly reduced in animals treated with l-mimosine compared with vehicle controls (Figure 4, bottom). These results show that l-mimosine protects from renal IRI.
Figure 4.
l-Mimosine protects from renal IRI. PAS-stained section from an l-mimosine–treated mouse (A) showing less severe injury compared with section from a vehicle-treated control (B). (C) Tubular injury scores. Magnification, ×100.
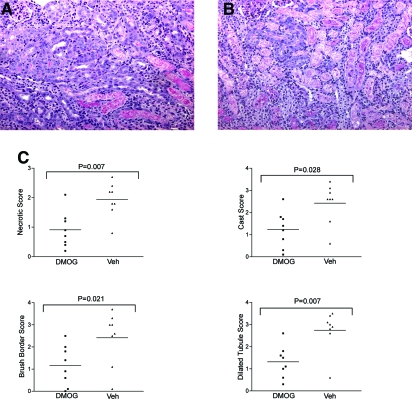
Next, we used a different small molecule, dimethyloxalylglycine (DMOG), which activates HIF in cultured cells.16 DMOG was given as three doses because this has previously been shown to improve outcome in hind-limb ischemia.34 Animals received 8 mg of DMOG 48 h before, 6 h before, and 48 h after ischemic injury. Control animals received vehicle. Animals were killed 72 h after renal injury. Injury was attenuated in animals receiving DMOG. Eight pairs of animals were analyzed. In each pair, the animal treated with DMOG exhibited less severe injury compared with the control animal on a forced-choice analysis.
Figure 5 shows representative PAS staining from the cortex of mouse kidneys. Quantitative analysis showed that the injury score was significantly reduced in DMOG-treated animals compared with paired controls (Figure 5).
Figure 5.
DMOG protects from renal IRI. (A) PAS-stained section from a DMOG-treated mouse. (B) Vehicle-treated control. (C) Tubular injury scores. Magnification, ×100.
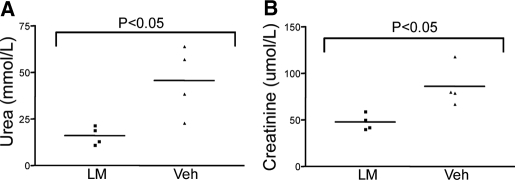
In animals subjected to unilateral renal ischemia reperfusion, biochemical parameters in the blood barely change as the other kidney continues to function. To confirm that activating HIF was associated with improved renal function, we performed a further experiment. Following pilot experiments, we selected a shorter time for clamping the renal pedicles (20 min) to avoid death before 72 h. Four pairs of animals were analyzed, and 72 h after injury, serum urea and creatinine concentrations were assessed and found to be significantly higher in vehicle-treated control animals compared with those treated with l-mimosine (600 mg/kg) administered 6 h before injury (Figure 6).
Figure 6.
Effect of l-mimosine on renal dysfunction after bilateral renal IRI. (A) Serum urea. (B) Serum creatinine. Data are means ± SEM. Significantly lower values were observed in animals that were treated with l-mimosine.
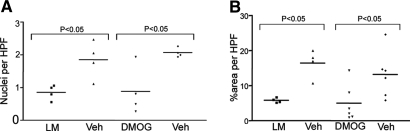
To obtain some insight into the potential mechanisms by which these agents might protect from renal IRI and/or enhanced recovery, we investigated apoptosis, inflammatory cell infiltration, and vascularization in cryostat sections. Apoptosis was assessed using terminal dUTP nick-end labeling (TUNEL). In sections stained for apoptotic bodies, the number of positive nuclei in tubular epithelial cells was significantly lower in sections from the animals receiving l-mimosine or DMOG compared with vehicle-treated controls (Figure 7A). TUNEL-positive cells were particularly located at the corticomedullary junction. Macrophage infiltration was determined using CD68 immunostaining. In kidney sections from animals that received l-mimosine or DMOG, significantly less macrophage infiltration was detected compared with control sections 3 d after IRI (Figure 7B). Vascularity was assessed using antibody to the endothelial marker endomucin. No difference inthe extent of vascularization was detected 3 d after injury in sections from animals treated with l-mimosine, DMOG, or appropriate vehicle controls 3 d after IRI (data not shown).
Figure 7.
Effect of l-mimosine and DMOG on apoptosis and macrophage infiltration after renal IRI. (A) Apoptosis of renal tubular epithelial cells was assessed by TUNEL. Significantly fewer apoptotic nuclei were detected in animals treated with l-mimosine or DMOG. (B) Macrophage infiltration as assessed by CD68 immunohistochemistry was significantly reduced.
Discussion
The main findings of this study are that (1) both HIF-1 and HIF-2 are protective in renal IRI, (2) HIF activation is submaximal in no-flow ischemia, and (3) small molecules that activate HIF offer significant protection. Taken together, these observations support pharmacologic inhibition of HIF hydroxylases as a strategy to protect the kidney from ischemic injury.
Several lines of evidence led us to examine the potential for activating HIF in renal ischemia reperfusion. First, recent advances in understanding regulation of the HIF pathway have provided molecular targets that allow design of small molecules enabling HIF activation.20 Second, pre-exposure of rats to chronic hypoxia was previously shown to protect from renal IRI.35 There are more extensive data to show that pre-exposure to hypoxia prevents the heart from subsequent ischemia, and, in mice, this was recently shown to be mediated by HIF.22 Ischemic preconditioning is extensively documented as a method of protecting organs, including the kidney, from IRI,10 and it seems plausible that this could be mediated in part via HIF. Third, genetic activation of HIF through biallelic loss of VHL function is a key event in most cases of the most common form of renal carcinoma, suggesting that HIF activation has important consequences for survival and proliferation of renal epithelial cells.17,36
Our experiments with mice heterozygous for a defect in either hif1a or hif2a provide direct genetic evidence that both HIF-α subunits have protective roles in the kidney in IRI. Notably, during the course of our studies, another group has shown that a different genetic HIF-2α knockdown strategy also exacerbates renal IRI.37 For potentiation of HIF to be therapeutically relevant, an important question is whether activation in no-flow ischemia was maximal. We observed that HIF activation was submaximal compared with CO exposure despite no blood flow to the kidney for 30 min. Although we did not measure the oxygen tension, we presume that it is very low in this setting; therefore, it might be considered surprising that HIF activation was submaximal, but our findings are consistent with previous observations in the rat.38,39 Explanations for submaximal activation include the possibility that the HIF response may be reduced by changes in the microenvironment (e.g., cytokines) or that an important permissive signal is absent, such as flow along the tubular lumen or through peritubular capillaries. It is also possible that the in vivo response may be diminished under very severe hypoxia. A recent experimental study of radiocontrast medium combined with cyclooxygenase inhibition and Nitro-L-arginine methyl ester (L-NAME) offers support for the latter possibility, because the most severely hypoxic tubules showed reduced HIF activation.40 Furthermore, administration of furosemide, which ameliorates renal hypoxia by reducing oxygen demand in the medullary thick ascending limb,41 was associated not only with protection from injury but also with increased HIF-1α expression. Whatever the explanation for the submaximal activation, our data provide direct support for the possibility that pretreatment with small-molecule HIF hydroxylase inhibitors can increase HIF-α in the kidney in no-flow ischemia.
The compounds that we used—l-mimosine and DMOG—are effective inhibitors of HIF hydroxylases.16,33 However, they will have other actions, so it is plausible that the beneficial effect that we demonstrated in renal IRI is not due to activation of HIF. l-Mimosine is an iron chelator, which may be relevant because radicals generated by Fenton chemistry during reperfusion have been implicated in injury.42 It is likely that both agents will inhibit other members of the superfamily of 2-OG-dependent dioxygenases to which the HIF hydroxylases belong. These are a diverse family with important functions, including DNA repair and matrix metabolism.20 Importantly, in a recent expression analysis of the effect of DMOG in cultured cells, there was very close similarity between effects of the HIF pathway (assessed by genetic manipulation) and those of DMOG.43 In addition, the similar effects that we observed with structurally distinct molecules, both of which activate HIF, increases the likelihood that these are mediated by the HIF pathway. Further support for an effect mediated by HIF comes from a previous study showing that cobalt which inhibits HIF hydroxylases and activates HIF protects from IRI.24 In addition, a recently reported study showed that a HIF hydroxylase inhibitor of undisclosed structure, FG-4487, protected the rat from renal IRI and also induced accumulation of both HIF-1α and -2α subunits.32 Taken together, these studies provide cogent evidence that activation of HIF before renal ischemia offers substantial protection. Because both HIF-1 and HIF-2 are activated by these compounds and genetic reduction of either predisposes to injury, it is likely that activation of both HIF-1α and HIF-2α contributes to the protective effect.
HIF operates in all cell types examined to date, is likely to influence directly the expression of more than 100 target genes, and will have further indirect effects. An important implication of this is that the protective effects seen in renal IRI on activating HIF could be mediated by a number of different downstream genes or pathways. Relevant to this, it was recently shown that hypoxia results in extensive changes in gene expression in renal proximal tubular epithelial cells in cell culture, and it is likely that many (but not all) of these changes are mediated by HIF.44 Prime candidates for mediating the effects that we observed are increased expression of heme oxygenase-1 by renal epithelial cells and EPO by the liver or renal fibroblasts. Both have been shown to be direct target genes of HIF in cell culture experiments and to be expressed in vivo when HIF is activated, and both have been shown previously to protect rodents from renal IRI. However, given the widespread operation of the HIF system and the complex pathophysiology of ischemic acute renal failure,42 it is likely that a number of effectors are involved in mediating protection. It is also likely that activating HIF will influence the behavior of a wide range of cell types in the kidney and other organs, for example, circulating cells involved in inflammation and repair.45,46
Our experiments support activating HIF as a therapeutic strategy in ischemic renal injury. Encouraging is that HIF prolyl hydroxylase enzyme inhibitors seem to be well tolerated in initial studies in humans and are effective in terms of increasing hematocrit.47 A particularly attractive aspect of activating HIF to protect the kidneys from acute injury, for example, at the time of cardiac surgery, is that it may simultaneously protect other organs, such as the heart and brain, which are vulnerable to hypoxic/ischemic injury.
Concise Methods
Animals
Male C57BL/6 mice aged 6 to 8 wk were supplied from Harlan UK Ltd (Bicester, UK). Both the hif1a and hif2a defective alleles were maintained on a mixed 129sv:Swiss Webster background with normal littermates used as controls.48 Animals were kept in a specific pathogen-free environment; experiments were performed according to institutional guidelines and with Home Office approval.
Induction of Tissue Hypoxia Using CO
A cylinder of 0.1% CO mixed with air (BOC UK, Manchester, UK) was connected to a modified individually ventilated cage (IVC). Animals were placed in the IVC with bedding material and free access to food and water. The IVC was flushed with the gas mixture before a maintenance input of 1 L/min was supplied. Animals were exposed to CO for either 30 min or 8 h before being killed by cervical dislocation. Both exposures resulted in similar HIF-1α and HIF-2α stabilization as assessed by immunohistochemistry.
HIF Hydroxylase Inhibitors
Animals were experimented on in pairs, one receiving the active compound and the other receiving vehicle control. Dimethyloxalylglycine (Axxora Ltd, Nottingham, UK) was dissolved in normal saline (16 mg/ml) and administered by intraperitoneal injection. Animals received three injections of 8 mg at 48 h before, 6 h before, and 48 h after surgery.
l-Mimosine (Sigma, Dorset, UK) was suspended in 0.1 M hydrochloric acid to give a concentration of 30 mg/ml. Mice received 600 mg/kg l-mimosine by oral gavage 6 h before renal IRI surgery.
IRI
Mice were anesthetized with isoflurane (Abbott Laboratories Ltd, Maidenhead, UK). Each pair of animals underwent surgery together, using a twin-headed anesthetic device with two masks. Body temperature was maintained by performing surgery on a heat pad. After midline abdominal incision, the renal pedicles were exposed by blunt dissection, and a microvascular clamp (Fine Science Tools GmbH, Heidelberg, Germany) was applied for the indicated period of time. The kidneys were observed for 1 min after removal of the clamp(s) to assess reperfusion. After reperfusion, 0.8 ml of prewarmed saline was placed in the peritoneal cavity and the abdomen was closed in layers. Mice were killed 72 h later unless otherwise stated. Tissues were fixed by immersion in neutral buffered formalin (Sigma) for 24 h before being transferred to 70% alcohol and processing for paraffin embedding. Three-micrometer sections were cut onto Superfrost (BDH Laboratory Supplies, Poole, UK) slides.
Histologic Examination
Formalin-fixed and paraffin-embedded kidney sections were stained with both hematoxylin-eosin and PAS. Histologic changes were mainly evaluated by quantitative measurement of tubular injury29 by assessment of specific variables in 10 individual high-power fields (magnification ×400). A percentage of the area affected was estimated for the number of necrotic cells, loss of brush border, cast formation, and tubule dilation as follows: 0, 0 to 5%; 1, 5 to 10%; 2, 11 to 25%; 3, 26 to 45%; 4, 46 to 75%, and 5, >76%. The 10 fields analyzed in each section were selected at random. All evaluations were made on coded sections without knowledge of the experimental group to which the mice belonged. Two individuals blinded to the sample identity made histologic assessments of injury.
Immunohistochemistry
Sections were first dewaxed and rehydrated. For HIF-1α detection, sections were heated in a pressure cooker for 25 min in target retrieval solution (Dako, Ely, UK). Sections were then incubated with polyclonal rabbit anti-HIF antibodies (HIF-1α and HIF-2α; Novus Biologicals, Littleton, CO) at a 1:10000 dilution for 1 h at room temperature followed by detection with a Catalyzed Signal Amplification kit (Dako), used according to the manufacturer's instructions. Sections were counterstained with hematoxylin, dehydrated, and mounted in DPX.
Macrophage staining was performed on frozen sections using monoclonal rat anti-mouse CD68 antibody (Serotec, Oxford, UK) diluted 1:50. Endogenous peroxidase activity was blocked with a 1% solution of hydrogen peroxide in 50% methanol. Visualization was with peroxidase-conjugated mouse anti-rat antibody diluted 1:200 (Jackson Immunoresearch, West Grove, PA) followed by rat peroxidase anti-peroxidase complexes diluted 1:100 (Jackson Immunoresearch) and diaminobenzidine substrate (Dako). The area labeled was quantified using Image-Pro Plus software. Apoptosis was assessed by TUNEL staining using the In Situ Cell Death Detection kit, POD (Roche Applied Science, East Sussex, UK) following the manufacturer's instructions and quantified by counting the number of apoptotic nuclei in 20 high-power fields in each section. Sections were examined using an Olympus (Watford, UK) BX4 microscope and CoolSnap digital camera (RS Photometrics, Marlow, UK).
Measurement of Biochemical Parameters
Blood samples were collected when animals were killed. Samples were centrifuged to separate plasma. Plasma urea and creatinine concentrations were measured at Department of Chemical Pathology, Hammersmith Hospital. Levels of these compounds were measured using an Olympus AU2700 analyzer (Olympus Diagnostics). Urea was measured by the urease and glutamate dehydrogenase enzymatic method, and creatinine was measured by a kinetic Jaffe method.
Statistical Analyses
All values described in the text and figures are expressed as means ± SEM. Data were analyzed using Mann-Whitney U test for comparisons, and P < 0.05 was considered statistically significant. Statistical analysis was carried out using GraphPad Prism 4.0 (GraphPad, San Diego, CA)
Disclosures
P.M. is a scientific founder, director, and consultant for ReOX Ltd., which is developing HIF Hydroxylase inhibitors.
Acknowledgments
This work was funded by a Wellcome Trust Clinical Training Fellowship to P.H. and by a British Heart Foundation programme grant to P.H.M.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Maxwell PH: Hypoxia-inducible factor as a physiological regulator. Exp Physiol 90: 791–797, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza, GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza GL: Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 106: 809–812, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL, Wang GL: A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza, GL: Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ: Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci U S A 91: 6496–6500, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM: Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997 [PubMed] [Google Scholar]
- 8.Levy AP, Levy NS, Wegner S, Goldberg MA: Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270: 13333–13340, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L: A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 182: 1683–1693, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonventre JV: Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gidday JM: Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7: 437–448, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Yellon DM, Hausenloy DJ: Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Clin Pract Cardiovasc Med 2: 568–575, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ: Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem 272: 11205–11214, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe, PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 3999: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16: 1466–1471, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schofield CJ, Zhang Z: Structural and mechanistic studies on 2-oxoglutarate-dependent oxygenases and related enzymes. Curr Opin Struct Biol 9: 722–731, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow, C: Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci 25: 4099–4107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza, GL: Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Manotham K, Tanaka T, Ohse T, Kojima I, Miyata T, Inagi R, Tanaka H, Sassa R, Fujita T, Nangaku M. A biologic role of HIF-1 in the renal medulla. Kidney International 67: 1428–1439, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ran R, Xu H, Lu A, Bernaudin M, Sharp FR: Hypoxia preconditioning in the brain. Dev Neurosci 27: 87–92, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Xi L, Taher M, Yin C, Salloum F, Kukreja RC: Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol 287: H2369–H2375, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL: Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling H, Edelstein C, Gengaro P, Meng X, Lucia S, Knotek M, Wangsiripaisan A, Shi Y, Schrier R: Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol 277: F383–F390, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL: The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12: 3320–3324, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng J, Zhang L, Drysdale L, Fong GH: The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A 97: 8386–8391, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA: Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Warnecke C, Griethe W, Weidemann A, Jurgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt, KU: Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. Faseb J 17: 1186–1188, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Milkiewicz M, Pugh CW, Egginton S: Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol 560: 21–26, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien CT, Chen CF, Hsu SM, Lee PH, Lai MK: Protective mechanism of preconditioning hypoxia attenuates apoptosis formation during renal ischemia/reperfusion phase. Transplant Proc 31: 2012–2013, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG Jr: Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 1: 237–246, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M: Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 18: 1218–1226, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Rosenberger C, Griethe W, Gruber G, Wiesener M, Frei U, Bachmann S, Eckardt KU: Cellular responses to hypoxia after renal segmental infarction. Kidney Int 64: 874–886, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Rosenberger C, Heyman SN, Rosen S, Shina A, Goldfarb M, Griethe W, Frei U, Reinke P, Bachmann S, Eckardt KU: Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int 67: 531–542, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML: Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int 45: 981–985, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM: Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J Biol Chem 281: 15215–15226, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT: The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem 278: 40296–40304, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC: Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Günzler V, Muthukrishnan E, Neumayer HH, Sacherer K, Schmidt R, Mitzner A, Wiecek A, Piecha G, Ignacy W, Scigalla P: FG-2216 increases hemoglobin concentration in anemic patients with chronic kidney disease. J Am Soc Nephrol 16: 758A, 2005 [Google Scholar]
- 48.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P: Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 111: 1519–1527, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]