Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias (original) (raw)
Abstract
Mounting evidence indicates that alterations of AKT signalling play important roles in cancer development. An earlier study discovered an oncogenic AKT1 gene mutation (AKT1 E17K) in breast, colorectal and ovarian cancers. The aim of this study was to see whether the AKT1 E17K mutation is common in breast, colorectal, lung, gastric and hepatocellular carcinomas and acute leukaemias. We analysed the presence of the AKT1 E17K mutation in 731 cancer tissues by a single-strand conformation polymorphism assay. In addition, we analysed the corresponding sequences of AKT1 E17K in AKT2 and AKT3 genes. Overall, we detected the four AKT1 E17K mutations in the breast cancers (4/93; 4.3%), but none in other cancers. There was no AKT2 or AKT3 mutation in the cancers. This study demonstrated that the AKT1 E17K mutation occurs in breast cancers at a low frequency, and that it is rare in other common cancers, including colorectal, lung, gastric and hepatocellular carcinomas and acute leukaemias. Despite the confirmed oncogenic function of the AKT1 E17K, the rare incidences of the mutation suggest that it may not play a crucial role in the development of the most common types of human cancers.
Keywords: AKT1 E17K, mutation, oncogene
Protein kinases regulate cell signalling pathways mediating a number of processes in cell proliferation, differentiation and survival (Gschwind et al, 2004). AKT1 (also known as protein kinase B) is a subfamily of serine/threonine protein kinases, and the AKT genes are the mammalian equivalent of murine viral oncogene v-akt (Testa and Bellacosa, 2001). There are three isoforms of the AKT (AKT1, AKT2 and AKT3), and each AKT member contains an N-terminal pleckstrin homology (PH) domain, a short _α_-helical linker and a C-terminal kinase domain (Testa and Bellacosa, 2001). AKTs are major downstream targets of growth factor receptors that signal through phosphatidylinositol 3-kinase (Testa and Bellacosa, 2001). Mounting evidence exists that activation of AKT proteins is important in cancer development (Muise-Helmericks et al, 1998; Dimmeler et al, 1999; Khwaja, 1999; Ozes et al, 1999; Mende et al, 2001; Wei et al, 2001). PTEN inhibits AKT activation and works as a tumour suppressor (Testa and Bellacosa, 2001). Inactivating mutation of PTEN and activating mutation of PIK3CA have been detected in many human cancers and have been known as major activating mechanisms of AKT-signalling pathway in cancers (Testa and Bellacosa, 2001; Samuels et al, 2004). By contrast, activating mutation of AKT genes has not been widely reported in human cancers.
Recently, a research group discovered a recurrent somatic mutation of AKT1 gene in human breast, colorectal and ovarian cancers (Carpten et al, 2007). The AKT1 mutation was a missense mutation that substituted an amino acid (E17K) in the PH domain of AKT1. The AKT1 E17K mutation was found in 5 out of 61 (8.2%) breast cancers, 3 out of 51 (5.9%) colorectal cancers and 1 out of 50 (2.0%) ovarian cancers (Carpten et al, 2007). The E17K mutation was mutually exclusive with respect to PIK3CA mutation and loss of PTEN expression (Carpten et al, 2007). Functionally, the AKT1 E17K mutation stimulates AKT signalling, induces cellular transformation and produces leukaemia in mice, strongly suggesting that this mutation may play a crucial role in cancer development (Carpten et al, 2007).
To further characterise the AKT1 E17K mutation in human cancers, the following questions were investigated in this study: (a) whether human cancer tissues from various histologic origins have the AKT1 E17K mutation; (b) whether there is any ethnic difference of the AKT1 E17K mutation incidence in breast and colorectal cancers; and (c) whether any corresponding mutation of AKT1 E17K occurs in AKT2 or AKT3 genes.
MATERIALS AND METHODS
We analysed AKT1 for the detection of the AKT1 E17K mutation in methacarn-fixed tissues of 93 breast ductal carcinomas, 104 colorectal adenocarcinomas, 180 gastric adenocarcinomas, 68 hepatocellular carcinomas and 157 non-small cell lung cancers (NSCLCs), and fresh non-fixed fresh tissues of 129 adulthood acute leukaemias by polymerase chain reaction (PCR)-single strand conformation polymorphism (SSCP) analysis. All of the cancer patients were Korean. Approval for this study was obtained from the Institutional Review Board of the Catholic University of Korea, College of Medicine. The gastric carcinomas consisted of 40 early and 140 advanced gastric carcinomas. The breast carcinomas consisted of 15 ductal carcinomas in situ and 78 invasive ductal carcinomas. The NSCLCs consisted of 74 adenocarcinomas, 70 squamous cell carcinomas, 3 adenosquamous carcinomas and 10 large-cell carcinomas. The acute leukaemias consisted of 95 acute myelogenous leukaemia, 33 acute lymphoblastic leukaemia (ALL) (29 B-ALL and 4 T-ALL) and 1 undifferentiated acute leukaemia. For the solid tumours, malignant cells and normal cells were selectively procured from haematoxylin and eosin-stained slides by a microdissection as described previously (Lee et al, 1999), whereas for the leukaemia non-fixed fresh bone marrows were directly used. Because the AKT1 E17K mutation was detected in the exon 3, genomic DNA each from tumour cells and corresponding normal cells were amplified with one primer pair covering the exon 3. Radioisotope ([32P]dCTP) was incorporated into the PCR products for detection by SSCP autoradiogram. After SSCP, bands showing mobility shifts were re-amplified and sequenced by a capillary automatic sequencer.
We also analysed AKT2 exon 3 and AKT3 exon 2 by the same PCR-SSCP method. In this approach, we used 45 breast ductal carcinomas, 45 colorectal adenocarcinomas, 45 gastric adenocarcinomas, 45 hepatocellular carcinomas, and 45 NSCLCs and 45 adulthood acute leukaemias.
RESULTS AND DISCUSSION
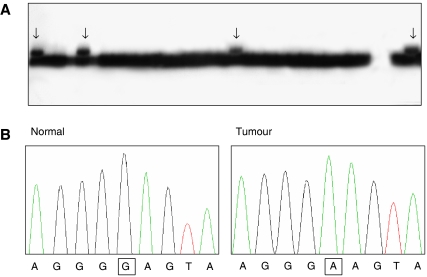
The PCR-SSCP analysis of the AKT1 exon 3 in the 731 cancers identified aberrantly migrating bands in four breast cancers (4/93; 4.3%), but not in other cancers (Figure 1A). All of the four breast cancers were invasive ductal carcinomas. DNA sequence analysis revealed that the aberrant bands represented an identical mutation (c.49G>A (p.E17K)) (Figure 1B). None of the normal samples from the patients with the AKT1 E17K mutation showed evidence of mutation by the SSCP, indicating that the mutations had risen somatically. We also confirm the mutation by a direct DNA sequencing in the four breast cancers (data not shown). In addition, we performed direct DNA sequencing analyses for the AKT1 E17K mutation in 120 cases of the cancers and found no additional AKT1 E17K mutation in the cancers (data not shown).
Figure 1.
Representative data on AKT1 E17K mutation in the cancers. (A) The PCR products of the exon 3 from breast cancers were visualised on SSCP. The arrows indicate aberrant bands as compared with the wild-type bands. (B) One of the aberrant bands on the SSCP was sequenced. The boxes indicate nucleotide changes in the tumour DNA as compared with normal tissue DNA.
Next, we attempted to find out the corresponding mutation of AKT1 E17K in both AKT2 and AKT3 genes. However, the SSCP from the tumours did not reveal any aberrantly migrating band compared with the wild-type bands from the normal tissues (data not shown). We repeated the experiments twice, including tissue microdissection, PCR, SSCP and DNA sequencing analysis to ensure the specificity of the results and found that the data were consistent.
Because the previous study showed modest incidences of AKT1 E17K mutation (up to 8.2%) in a wide range of the cancers (Carpten et al, 2007), we expected to detect comparable AKT1 E17K mutations in our cancer specimens. However, we detected the AKT1 E17K mutation in the breast cancers (4.3%), but not in other cancers. Statistically, there is no difference of the AKT1 E17K mutation frequency in the breast cancers between the previous study and our study (Fisher's exact test, _P_=0.253). By contrast, there is a significant difference of the AKT1 E17K mutation frequency in the colorectal cancers between the previous study and our study (Fisher's exact test, _P_=0.034), suggesting a possibility that a racial difference might exist. However, because the previous reports did not describe the ethnicity of the patients (Carpten et al, 2007), it is not possible to conclude whether the difference arose from a racial difference. Together, these data suggest that the AKT1 E17K mutation may be rare in common human cancers besides breast cancers. Also we found neither AKT2 nor AKT3 mutation in the corresponding sequences to the AKT1 E17K, indicating that mutational alteration of neither AKT2 nor AKT3 contributes to cancer development.
Because the most impressive examples of recent cancer therapies target activated kinases by genetic alterations, cancer researches have focused on evaluating kinases as promising molecular targets for cancer treatment (Druker et al, 2001; Fukuoka et al, 2003; Gschwind et al, 2004; Paez et al, 2004). The discovery of the AKT1 E17K mutation in cancers raised a possibility to treat cancers by targeting the mutated AKT1. However, our data confirm the earlier data only in breast cancers, but not in other types of common cancers. Therefore, the present study suggested that the AKT1 E17K mutation should further be analysed in a wider range of cancers.
Acknowledgments
This work was supported by Korea Research Foundation (2007-313-E00105).
References
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448: 439–444 [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605 [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 344: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 21: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A (2004) The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer 4: 361–370 [DOI] [PubMed] [Google Scholar]
- Khwaja A (1999) Akt is more than just a Bad kinase. Nature 401: 33–34 [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Park WS, Kim SY, Kim HS, Han JY, Park GS, Dong SM, Pi JH, Kim CS, Kim SH, Lee JY, Yoo NJ (1999) Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene 18: 3754–3760 [DOI] [PubMed] [Google Scholar]
- Mende I, Malstrom S, Tsichlis PN, Vogt PK, Aoki M (2001) Oncogenic transformation induced by membrane-targeted Akt2 and Akt3. Oncogene 20: 4419–4423 [DOI] [PubMed] [Google Scholar]
- Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N (1998) Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem 273: 29864–29872 [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401: 82–85 [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500 [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304: 554. [DOI] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A (2001) AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA 98: 10983–10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Yang Y, Yu Q (2001) Tyrosine kinase-dependent, phosphatidylinositol 3′-kinase, and mitogen-activated protein kinase-independent signaling pathways prevent lung adenocarcinoma cells from anoikis. Cancer Res 61: 2439–2444 [PubMed] [Google Scholar]