Homeostatic expansion and phenotypic conversion of naïve T cells in response to self peptide/MHC ligands (original) (raw)
Abstract
Recent data suggest that survival of resting, naïve T cells requires an interaction with self MHC molecules. From analysis of the class I MHC-restricted T cell receptor transgenic strain OT-I, we report a different response. Rather than merely surviving, these T cells proliferated slowly after transfer into T-depleted syngeneic hosts. This expansion required both T cell “space” and expression of normal levels of self class I MHC molecules. Furthermore, we demonstrate that during homeostatic expansion in a suitable environment, naïve phenotype (CD44low) OT-I T cells converted to memory phenotype (CD44med/high), despite the absence of foreign antigenic stimulation. On the other hand, cells undergoing homeostatic expansion did not acquire cytolytic effector function. The significance of these data for reactivity of T cells with self peptide/MHC ligands and the implications for normal and abnormal T cell homeostasis are discussed.
During thymic development, T cells require an interaction of their clone-specific T cell receptor (TCR) with self peptide/MHC ligands to survive the process of positive selection (1). Once T cell maturation is complete, however, it has been assumed that such reactivity toward self is lost (inherent in the idea of self tolerance) and that the TCR played no significant role in the survival of resting naïve T cells, before encounter with foreign antigen. This image of the TCR playing the role of “Sleeping Beauty,” waiting for the appropriate peptide/MHC ligand to activate the T cell from its rest, has been challenged by recent data suggesting a “Red Queen” analogy is closer to the mark, i.e., that naïve T cells require a constant engagement of the TCR with self ligands simply to persist in an quiescent state (2, 3). Thus, together with a pivotal role for certain cytokines (4), TCR interactions with self may be critical for maintenance of the naïve T cell population.
The most extensive analysis of this phenomena involved adoptive transfer of T cells bearing the anti-H-Y/Db TCR transgene (H-Y TCR) into irradiated hosts (5). These studies showed that naïve CD8 T cells survived for long periods of time in the presence of cognate self MHC molecules (i.e., in that case, H-2Db) and persisted as resting cells in the absence of stimulatory antigen. These same cells disappeared from the secondary lymphoid tissue in the absence of this class I MHC molecule, even if another class I molecule (Kb) was present, suggesting a correlation between survival and TCR recognition of self. Different rules applied to memory H-Y TCR transgenic T cells, which required expression of class I MHC to survive and proliferate but did not distinguish the presence of cognate or noncognate class I (5). Similar conclusions have been drawn by other groups concerning a homeostatic interaction of CD4 and CD8 T cells with self class II and class I MHC molecules, respectively (6–14). Those studies concluded that naïve T cells require TCR interactions with self to survive.
We sought to extend these findings by using the class I MHC-restricted TCR transgenic system, OT-I, specific for ovalbumin residues 257–264 (OVAp) plus Kb (15). Similar to the findings described above, we find that OT-I CD8 T cells differ in their response depending on the presence or absence of self class I MHC molecules in the host. However, in marked contrast to previous reports, we find that naïve OT-1 cells proliferate rather than simply survive in response to self class I MHC ligands. We show that this proliferation depends on both expression of appropriate self peptide/MHC ligands and T cell “space” in the periphery and is accompanied by changes in cell-surface phenotype (conversion from CD44low to CD44med/high) indicative of at least partial activation. These data suggest that, similar to thymic positive selection, recognition of self peptide/MHC ligands can lead to some form of activation of mature T cells. The implications of this expansion in response to self are discussed in the context of normal T cell homeostasis and autoimmunity.
Materials and Methods
Mice.
Six- to 12-week-old recipient mice (C57BL/6, TAP0/0, and RAG0/0) mice were generated and maintained under specific pathogen-free conditions. Donor mice (C57BL/6, OT-I, and OT-I.PL) mice were used at 4–12 weeks of age. Mice used in irradiation experiments were sublethally irradiated (700 rads) 2 days before cell transfer. Thymectomized Thy1.2+ recipients were allowed to recover for several weeks postsurgery before being treated with the anti-Thy1.2 antibody 30-H12 (100 μl ascites) at 4, 2, and 0 days before adoptive transfer of donor cells. Mice were maintained on antibiotic water throughout the experiments.
Adoptive Transfer.
Single-cell suspensions were prepared from lymph nodes of donor mice and CD8+ T cells (from OT-I donor animals) or CD4+ T cells (from normal C57BL/6 donors) were purified by using CD8 or CD4 Cellect columns, respectively (Cytovax Biotechnologies, Edmonton, Alberta, Canada). The purity of the cells ranged from 93% to 95% pure. Cells were stained before or after purification with 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) essentially as described (16, 17). Briefly, pooled lymph node cells were suspended at a concentration of 1–5 × 107/ml in Hanks’ balanced salt solution. After warming to 37°C, CFSE was added at a concentration of 0.5–5 μM for 10 min with occasional mixing, followed by addition of ice-cold RPMI media containing 10% serum and cell recovery by centrifugation. Purified donor cells were resuspended in PBS and injected i.v. into the tail vein of the recipient mice. During multiple experiments 1 million or 3 million cells were injected into irradiated recipients and 1 million to 5 million into unirradiated recipients. Similar results were observed at all these doses of cells. In some cases the mice were primed on day 2 after transfer with ≈100 μg of OVAp emulsified in CFA injected s.c.
Flow Cytometry and Functional Assays.
Recipient mice were sacrificed at the time points indicated, and single cell suspensions were prepared separately from the spleen and a pool of major lymph nodes. Lymph node and spleen cells then were stained with combinations of the following antibodies: anti-CD8 conjugated to (phycoerythrin) PE or allophycocyarin, anti-CD4-PE, anti-Thy1.1-bio, and anti-CD44-PE (all from PharMingen). Biotinylated antibodies were revealed by using either streptavidin (SA)-TriColor (Caltag, South San Francisco, CA) or SA-PerCP (PharMingen). The cells were analyzed by using a Becton Dickinson FACSCaliber and analyzed by using both cellquest (Becton Dickinson) and flowjo (TreeStar, San Carlos CA) software.
Cytolytic potential of cells was tested in a 51Cr release assay, essentially as described (18). Briefly, EL4 tumor cells were labeled in 51Cr-sodium chromate with or without addition of 10 μM OVAp. Target cells were washed and incubated with titrated numbers of effector cells in a 4-hr assay. An _in vitro_-generated OT-I cytotoxic T lymphocyte line was used as a positive control. Percentages of OT-I cells in the effector populations were calculated from flow cytometric analysis of a sample of the lymph node preparation, and the data are presented as adjusted effector-to-target (E:T) ratios to reflect this calculation. In the experiment shown, the E:T ratios of both mice in each group were <11% different, hence the average E:T ratio is represented.
Results
OT-I Cells Proliferate in Irradiated, Class I MHC-Expressing Hosts.
The well-characterized OT-I TCR is specific for Kb/OVAp and is positively selected in the thymus by Kb (15). To study the role of the MHC on homeostatic regulation of OT-I cells, we performed adoptive transfers similar to the system described by Tanchot and colleagues (5). OT-I CD8+ cells were purified, labeled with the dye CFSE (19), and transferred into irradiated or normal B6 or transporter associated with antigen processing (TAP)-1-deficient (TAP0/0) mice. CFSE is useful to track the extent of proliferation because it is well retained in nondividing cells, but is diluted roughly 50% upon each cell division (16, 20, 21). TAP0/0 hosts were used because they are impaired in expression of surface class I MHC molecules, which blocks CD8 T cell development, including differentiation of T cells with the OT-I TCR (22, 23). The experimental design involved donor cells that differed from host animals by at least one Thy-1 allele, facilitating identification of donor T cells by using allele-specific antibodies.
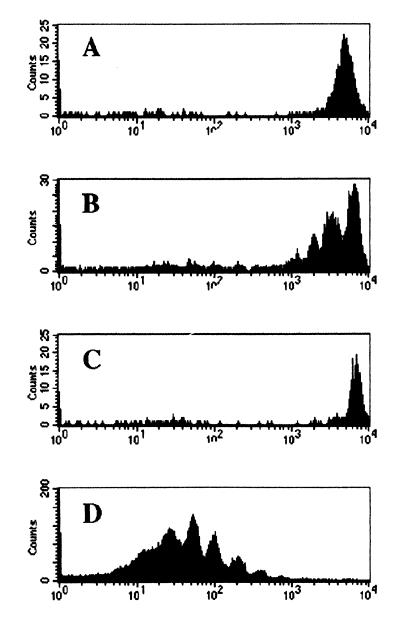
Five days after transfer of CFSE-labeled OT-I cells, we were surprised to observe that the donor cells proliferate after transfer into irradiated B6 mice, as judged by a decrease in CFSE fluorescence (Fig. 1B). In contrast, OT-I cells transferred into unirradiated B6 mice failed to expand (Fig. 1A), in keeping with previous observations from adoptive transfer experiments using this TCR transgenic system (16). Remarkably, this same population of OT-I cells did not expand after transfer into the TAP0/0 hosts, even though these recipients also had been irradiated (Fig. 1C). The proliferation of OT-I cells after transfer into irradiated B6 recipients was slow compared with that in identical hosts immunized with OVAp (Fig. 1D), demonstrating that proliferation in the former group was not maximal.
Figure 1.
Proliferation of OT-I T cells in irradiated syngeneic hosts. OT-I CD8+ T cells were purified from Thy1.1 donor mice (OT-I.PL), CFSE labeled and transferred into B6 (A), irradiated B6 (B and D), or irradiated TAP0/0 (C) hosts. (D) The mice also were immunized with OVAp. Five days after transfer, lymph nodes were recovered, and the CFSE expression on donor CD8+ cells was determined. Data are shown for one of the two animals in each group.
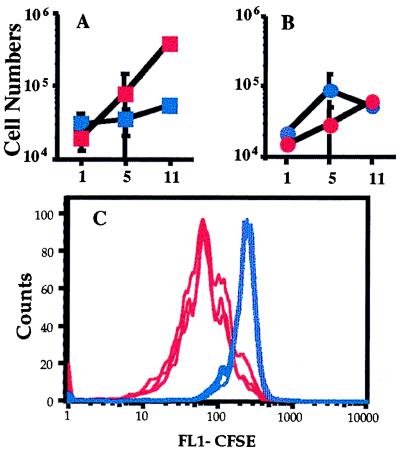
We were concerned that because donor OT-I T cells express class I MHC molecules on the surface they could be targets for cytotoxic T lymphocytes in the TAP0/0 host. Such cells are rare in TAP0/0 mice, but are extremely reactive toward normal levels of self class I MHC molecules (24, 25). Therefore, we cotransferred CD8+ T cells from OT-I animals and CD4+ T cells from normal B6 mice into irradiated hosts, either B6 or TAP0/0. Each population was CFSE-labeled, and survival/expansion was determined 1, 5, and 11 days posttransfer by analysis of total donor cell numbers and CFSE staining (Fig. 2). As before, OT-I T cells expand extensively in the irradiated B6 host but not the TAP0/0 host, as reflected by changes in both total numbers of donor OT-I cells (Fig. 2A) and CFSE expression level (Fig. 2C). On the other hand, the behavior of the CD4+ T cells was identical in both recipient groups; these cells expanded to similar numbers levels in both B6 and TAP0/0 hosts (Fig. 2B), and the level of CFSE staining was similar in both groups (data not shown). These data indicate first that the irradiated TAP0/0 environment is not hostile to TAP+ donor T cell expansion and second that the B6 and TAP0/0 hosts are similarly supportive to CD4 T cell proliferation. This latter result is consistent with CD4+ T cell expansion in response to class II MHC molecules, which are expressed by both B6 and TAP0/0 animals.
Figure 2.
The lack of CD8 expansion in irradiated TAP0/0 recipients is not caused by rejection of transferred T cells. CD8+ T cells from OT-I.PL animals and CD4+ cells from B6.PL animals were cotransferred into irradiated TAP0/0 and B6 recipients. One, 5, or 11 days after transfer, lymph nodes and spleens were harvested from three animals per group. The total cell numbers of donor CD4 and CD8 T cells and the CFSE expression of these populations was determined. Total numbers of donor OT-I.PL CD8+ (A) and B6.PL CD4+ (B) cells are given for irradiated TAP0/0 (blue symbols) and B6 (red symbols) recipients. The average donor cell number (with SD shown as error bars) for the lymph node population is shown. (C) The CFSE expression by the OT-I CD8+ cells in three TAP0/0 (blue lines) and three B6 (red lines) recipients at day 5 after transfer.
The CFSE profile of donor T cells in B6 recipients at day 5 clearly showed “laddering” consistent with 1–3 divisions, whereas the majority of OT-I cells in the TAP0/0 recipient showed no signs of cell division (Fig. 2C). These data suggest that most OT-I donor cells participated in the proliferation in the B6 hosts, because the increase in cell numbers in the B6 hosts between days 1 and 5 (an average of about 6-fold; Fig. 2A) is consistent with a few rounds of proliferation by the majority of input cells. By day 11 the expansion in B6 hosts was even more extensive, reflected in a ≈20-fold increase in cell number (Fig. 2A) and the almost complete loss of CFSE staining (data not shown). In marked contrast, in the TAP0/0 recipients the total cell numbers barely change by day 11 (Fig. 2A). In this experiment, we observed ≈25% OT-I cells had undergone one cell division in the TAP0/0 hosts at day 11, but the rest of the cells maintained high levels of CFSE, indicating that they had not divided (data not shown). In some other experiments, OT-I cell numbers in TAP0/0 recipients decreased slightly by day 11, whereas this population again increased in number in B6 hosts. This finding may reflect an impaired survival of OT-I cells in the absence of class I MHC molecules consistent with reports for other TCR transgenic systems (5, 9) but may simply reflect regeneration of the host T cell compartment, leading to competition for space (26, 27).
Taken together, these experiments suggest that expansion of OT-I cells required at least two elements: empty space induced by the irradiation and expression of self class I MHC molecules by host cells.
Expansion of OT-1 Cells in T Cell and T/B Cell-Deficient Hosts.
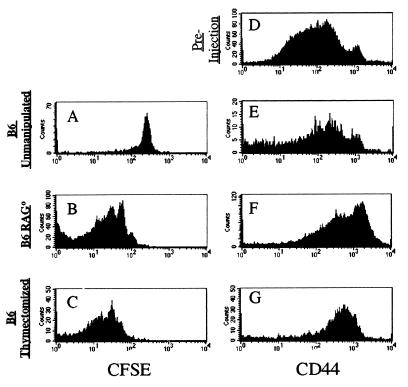
Because irradiation was used to deplete lymphocytes in these experiments we were concerned that other acute effects of irradiation might influence the T cell response. Of special concern was cytokine production induced by irradiation, because some cytokines promote naïve and activate T cell survival (4, 27–31). To avoid these issues, we transferred OT-I cells into RAG-1-deficient (RAG0/0) or thymectomized, T-depleted B6 (TxB6) hosts. As a control, OT-I cells again were transferred into unmanipulated B6 hosts. As shown in Fig. 3, loss of the CFSE dye indicated that the OT-I cells proliferated in both the RAG0/0 (deficient in both B and T cells), as well as the TxB6 mice (deficient only in T cells) but not in the normal B6 hosts. The degree of expansion in these experiments is similar or more extensive than seen in irradiated B6 recipients. These data suggest that the expansion of OT-I cells in an H-2b host is in response to perceived T cell space and is not altered by the presence or absence of B cells. Importantly, the chronic absence of host B and T lymphocytes in RAG0/0 mice indicates that OT-I proliferation is not in response to acute T cell depletion or cytokines produced by host lymphocytes during the experiment.
Figure 3.
OT-I T cell proliferation and CD44 up-regulation occur after transfer into T- and T/B- deficient, nonirradiated hosts. OT-I.PL CD8+ T cells were transferred into unmanipulated B6 (A and E), B6 RAG0/0 (B and F), or thymectomized, T cell-depleted B6 (C and G) recipients for 9 days. Expression levels of CFSE (_A_-C) and staining for CD44 (_E_-G) are shown for the lymph node population gated on donor cells. CD44 expression by the preinjection donor population (D) is shown for comparison.
Phenotypic Conversion Accompanies Expansion of OT-I Cells in Syngeneic Hosts.
The expansion of OT-I cells in appropriate hosts implies that the cells are receiving some sort of signal, and the dependence on class I MHC expression suggests the TCR may be involved in regulating this response. We therefore examined the phenotype of OT-I cells transferred into various hosts for expression of CD44, a marker that is up-regulated upon naïve T cells activation and typically persists into the memory pool (32, 33). OT-I CD8 T cells, like polyclonal CD8 T cells from normal B6 mice, are heterogeneous in CD44 expression, with the majority being CD44low and a small percentage (4–10%) being CD44high (Fig. 3 and data not shown). This phenotype is maintained after transfer into normal B6 hosts or into irradiated TAP0/0 recipients, but after expansion in irradiated, T-deficient, or T-depleted hosts, the cells consistently become CD44med/high (Fig. 3 and data not shown). Typically, the levels of CD44 are not quite as high as the small CD44high population in the input OT-I cells, but the CD44 expression level is clearly above that of naïve T cells, suggesting conversion to memory-like phenotype.
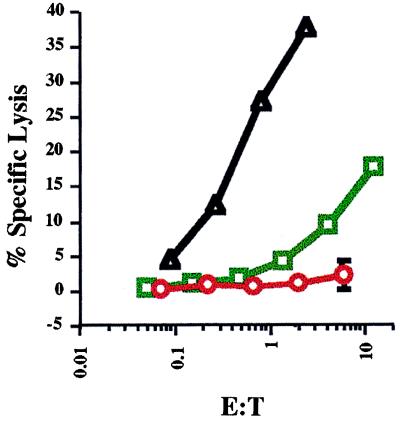
We also studied whether homeostatic expansion of OT-I cells was accompanied by acquisition of effector function. As cytolysis is a highly sensitive effector readout for CD8 T cells (34) we tested the capacity of adoptively transferred OT-I cells to perform in a 51Cr-release assay performed immediately ex vivo. OT-I cells were analyzed 5 days after transfer into irradiated B6 recipients. Some recipients were immunized with OVAp to effectively prime effector cells. As shown in Fig. 4, the cells from immunized mice were effective killer cells, correlating with extensive proliferation and increase in OT-I cell numbers (Fig. 1 and data not shown). In contrast, the cells recovered from unimmunized recipients failed to show any comparable effector function, even though many had undergone cell division (Fig. 1 and data not shown). Hence, the proliferative response toward self MHC ligands plus T-cell “space” was not accompanied by full differentiation of the CD8 T cells to effectors.
Figure 4.
Efficient cytolytic effector function does not develop in OT-I cells undergoing homeostatic expansion. OT-I T cells were transferred into irradiated B6 mice and analyzed 5 days later. The mice were (green squares) or were not (red circles) immunized with OVAp. Lymph node cells were tested in a 51Cr-release assay against EL4 cells pulsed with or without OVAp. The response toward EL4/OVAp targets is shown versus the effector-to-target (E:T) ratio calculated for OT-I cells (see Materials and Methods). Two mice per group were analyzed and the average response is shown with the range indicated by error bars. Lysis by an _in vitro_-cultured OT-I cytotoxic T lymphocyte (CTL) line was used as a positive control (black triangles). Responses to EL4 with no added peptide were less than 4% for the in vitro CTL line and less than 1% for all other responders.
Expansion Involves Naïve Phenotype OT-I Cells.
The expansion of OT-I cells in irradiated B6 hosts initially was surprising, because previous studies using the anti-HY/Db transgenic model suggested that naïve CD8 T cells persist but do not proliferate after transfer into irradiated MHC syngeneic hosts (5). One difference between our systems is that Tanchot and colleagues (5) used RAG-deficient H-Y TCR transgenic cells, which were shown to be uniformly CD44low, i.e., naïve phenotype. T cells from OT-I mice, however, contain a small, but significant, proportion of CD44high (memory phenotype) cells, as shown above (Fig. 3). It was therefore possible that the proliferating pool we observed after adoptive transfer derived from a small subset of memory OT-I cells and/or cells that possessed a second endogenous TCR. The CD44med/high phenotype of the proliferating donor cells in T-deficient B6 recipients would be consistent with this model.
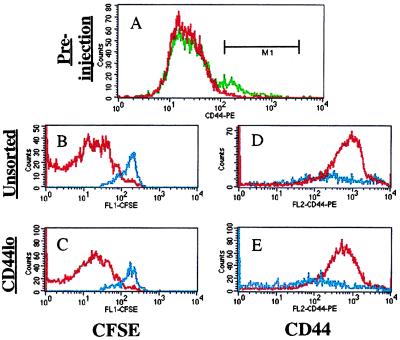
This idea is unlikely based on the data shown in Fig. 2, in which the fold expansion and decrease in CFSE level are consistent with a few rounds of proliferation by many cells, not with many rounds of proliferation by a few cells. Nonetheless, we directly addressed this issue in two ways. First, we transferred RAG0/0 OT-I T cells into irradiated B6.PL hosts and observed extensive proliferation, similar to the results described above using normal OT-I cells (data not shown). Although using RAG0/0 donor cells completely eliminates the possible influence of endogenous TCRs in the response, we notice that there are still some CD44high phenotype cells in OT-I RAG0/0 animals (data not shown). Thus, to test the role of contaminating memory phenotype cells directly, we transferred OT-I T cells either before or after sorting for CD44low cells by using fluorescence-activated cell sorting. The sorted cells were >99.3% CD44low whereas the unsorted population was ≈91% CD44low in this experiment (Fig. 5A). Significantly, the specificity and degree of proliferation was similar in both populations after transfer into irradiated TAP0/0 and B6 hosts (Fig. 5). Furthermore, both the bulk OT-I cells and sorted CD44low cells showed similar CD44 phenotypes after transfer, the cells being transferred into irradiated B6 hosts becoming CD44med/high, with those transferred into TAP0/0 hosts remaining mostly CD44low. Taken together these data indicate that the expansion observed involves naïve phenotype OT-I cells, which convert to a CD44med/high phenotype after proliferation.
Figure 5.
Expansion of sorted CD44low “naïve” OT-I T cells is similar to that of unsorted cells. OT-I CD8+ donor cells were used as a bulk population or after sorting for low CD44 expression. Reanalysis of these populations (A) revealed the unsorted cells (green line) to be 8.7% CD44high, whereas the sorted cells (red line) were <0.7% CD44high, using the marker shown. The unsorted OT-I cells (B and D) and sorted CD44low (C and E) population were transferred into irradiated B6 (red lines) or TAP0/0 (blue lines) and assayed for CFSE expression (B and C) and CD44 staining (D and E) at day 10 after transfer. Data are representative of 2–3 mice per group.
Discussion
The initial impetus for this project was to study how TCR recognition of self peptide/MHC ligands influenced survival versus death of T cells. To our initial surprise, and in stark contrast to the results of others (5, 10), we found that CD8 T cells proliferate rather than persist in a resting state when transferred into a syngeneic, T cell-deficient environment. Our data show that this expansion is mediated by recognition of physiological levels of cognate class I MHC molecules because it does not occur in TAP-deficient hosts. T cell space is required for this homeostatic expansion, because cells transferred into animals that possess a normal complement of T cells do not expand without antigenic stimulation. This response is not caused by side effects of T-depletion methods, however, because we and others (11, 14, 21, 35) show analogous results using irradiated, thymectomized, and RAG0/0 hosts.
Our data fit well with a recent report from Viret and colleagues (13). Those authors used an MHC class II-restricted TCR transgenic system and showed that these CD4 T cells survive and slowly expand in response to self class II molecules. Furthermore homeostasis of normal CD4 T cells was impaired in hosts that lack H-2M expression, and which therefore have a much more restricted peptide diversity than wild-type hosts (13). Viret et al. did not report whether CD4 proliferation in their system was accompanied by phenotypic conversion as we show here for CD8 T cells, but the overall similarity of the responses is striking and indicates that similar homeostatic expansion may occur in both T cell subsets.
Recent data indicate the TCR specificity for expansion is similar to that for positive selection (13, 35, 36). Our preliminary results using a system allowing specific peptide presentation in the absence of TAP-1 support this view but also indicate that some peptide/MHC ligands that can drive positive selection appear not to support mature T cell expansion (unpublished data). Furthermore, Marrack’s group (14) showed that while mature CD4 T cell expansion requires host expression of class II molecules, proliferation did not correlate with recognition of the probable positively selecting ligand. Instead, those authors conclude that mature T cells respond to “unfamiliar” MHC-bound peptides (i.e., peptides not present in the thymus), which may be over-represented or newly accessible in T-depleted hosts. Similarly, T cells bearing the HY TCR fail to proliferate in syngeneic T-deficient hosts unless exposed to male antigen (37, 38). These differences between TCR systems are accentuated in recent data from Ernst et al. (35) who show that T cells from some TCR transgenic systems proliferate in response to self peptide/MHC ligands (similar to OT-I), whereas T cells from other systems do not (35).
What can we make of these differences in expansion requirements for different TCR transgenic mice? Freitas and colleagues (26) attributed such differences to contaminating memory cells in the donor population, but our data and that of others (35) using sorted CD44low cells argues against this interpretation. Possibly some TCR transgenes fail to encounter a suitable self peptide/MHC ligand in the periphery. But this cannot explain data from the H-Y system, because absence of Db does indeed affect naïve H-Y TCR transgenic T cells, implying functional recognition of self peptide/MHC ligands but at the level of survival not expansion. TCR recognition of self ligands could induce different responses depending on the nature of the ligand and/or the affinity of the TCR-peptide/MHC interaction, similar to the activity of TCR partial agonists (39–41). A related idea is that fine tuning during or after thymic development might alter T cell reactivity; an interesting candidate is CD5, expression of which varies between TCR transgenic strains and inversely correlates with T cell reactivity (42). In keeping with this idea, H-Y transgenic T cells have very low CD5 levels, suggesting negligible self reactivity (42). An interesting consequence of these differences is that homeostatic expansion will bias the T cell repertoire; proliferation of some T cells (exemplified by OT-I) will occur at the expense of others (exemplified by the H-Y TCR transgenic). Indeed, such a competition has been demonstrated experimentally by Freitas and colleagues (26).
Given this discrepancy in TCR transgenic models, it is important to determine which system best reflects the characteristics of normal T cells. Work from Rocha and Tanchot (43) and Freitas and colleagues (26) suggested that normal, nontransgenic T cells behaved like the H-Y TCR transgenic cells. However, these experiments need to be reevaluated based on the phenotypic conversion we report here: the previous experiments involved adoptive transfer of nontransgenic T cells containing both naïve and memory T cells and the fates of these two subsets was determined by staining for CD44 (43). Because we show that CD44low T cells become CD44med/high upon homeostatic expansion, those data become considerably harder to interpret. More directly, recent data indicate that CD4 and CD8 T cells isolated from normal non-TCR transgenic mice undergo expansion and phenotypic conversion in T-depleted mice, arguing that this property is typical of most normal T cells (14, 35).
The phenotypic conversion of OT-I T cells from CD44low to CD44med/high during expansion in T-depleted, Kb-expressing animals initially was surprising. However, there is precedent for this observation: Bell and Sparshott (44) reported that, in the rat, CD45high (naïve phenotype) cells transferred into nude recipients proliferate and many become CD45low (memory phenotype), similar to the results described here. Those same authors have concluded based on more recent data that there is no role for environmental antigen in this phenotypic conversion, although a role for recognition of self peptide/MHC complexes was not considered (45). Interestingly, Bell and Sparshott report that transferred CD45high cells become CD45low within a few weeks but reacquire the CD45high phenotype after several months (44). It will be interesting to see whether OT-I cells transferred into T-deficient B6 mice convert back into the CD44low phenotype at later time points than reported here.
It is noticeable that the T cell expansion we observed is slow to start, with only one or two rounds of division apparent by day 5 posttransfer. In contrast, OT-I responses toward OVA/Kb initiate rapidly, leading to massive expansion within 2–3 days after antigen exposure (Fig. 1), even in situations where there is no overt adjuvant or danger effect (16) (M. McGargill, E. Parke, and K. Hogquist, personal communication). Similar slow expansion in syngeneic hosts has been reported (11, 13). Furthermore, we notice that the CD44 levels on OT-I cells that expand by homeostatic proliferation are typically not quite as high as seen on true memory T cells (Figs. 3 and 5 and data not shown). Thus, it appears that the response of OT-I T cells toward the self ligands is qualitatively and/or quantitatively different from response to antigenic ligands. This view is reinforced by our demonstration that the OT-I cells that proliferate in response to self MHC plus space did not efficiently differentiate into effector cells, in contrast to those primed by antigen. This result is unlikely to be caused by differences in the number of cell divisions because cytolytic effector function is acquired within one cell division (46). Hence we propose that the activation that drives T cell expansion in response to self ligands is insufficient to drive full and/or sustained commitment to effector function.
If mature T cells have the capacity to respond (at least by proliferation) to self peptide/MHC ligands, why is this not manifested as runaway autoimmunity? Based on the requirement for T cell space, we speculate that the “brake” that prevents this overt self-reactivity depends on the maintenance of a normally sized T cell pool. Indeed, reconstitution experiments described by Ernst et al. (35) clearly make this point, although the mechanism by which T cells perceive T cell space is unclear.
The systems used in this and other reports involved experimentally T-depleted hosts, hence an important issue is the practical relevance of these findings to clinical settings. Certain disease states (e.g., AIDS) and certain therapeutic approaches (e.g., chemo- or radio-therapy) deplete the majority of T cells. In keeping with our observations, it has been observed that in such T-depleted humans, the remaining T cells often are in cycle and typically express activation and/or memory markers (47, 48). Furthermore, certain gene-targeted mouse strains show phenotypic patterns similar to those predicted from this model. For example, in invariant chain-deficient mice (_Ii_0/0), CD4 development in the thymus is reduced, yet peripheral CD4 cells display up-regulation of some (but not all) activation markers (49–51). This reactivity, especially expression of high levels of CD44, is more marked when there is Ii expression in the bone marrow-derived cells (51). Furthermore, an Ii transgene that only partially rescues class II MHC molecule expression simultaneously restores CD4 positive selection and prevents the appearance of such activated-phenotype peripheral CD4 cells (49, 50). Similarly, in mice lacking the transcription factor Tcf-1, the poor maturation of thymocytes coincides with proliferation and expression of activation markers by mature T cells in the periphery (52). In our model, these effects all may result from peripheral homeostatic expansion and phenotypic conversion of the few T cells that mature in the thymus of these mutant mice.
Does such homeostatic expansion occur in the normal T cell population? An interesting candidate would be the first waves of mature T cells exiting the thymus in a newborn mouse. However, mature T cells in the neonatal mouse are not in cycle nor do they express memory markers (53, 54), which appears to hold true for neonatal OT-I animals (unpublished data). On the other hand, a population of such memory phenotype T cells have been detected in human fetuses, but not in the newborn, suggesting the population appears early in human T cell colonization but changes or is replaced later in development (55). More refined studies will be required to determine whether such expansion occurs physiologically in the mouse.
The unexpected proliferative response described here raises the interesting possibility that the TCR interaction with self peptide/MHC complexes not only dictates basic T cell survival (2, 3) but also may shape the size and diversity of T cell repertoire, because of competition between T cells for available space.
Acknowledgments
We thank Charlie Surh for a timely gift of anti-Thy1.1 antibody and Charlie Surh, Ananda Goldrath, and Mike Bevan for communicating their data before publication. We also thank Kris Hogquist for critical review of the manuscript and members of the Jameson and Hogquist labs for many helpful discussions. This work was supported by grants from the National Institutes of Health (AI38903) and the American Cancer Society (JFRA-639 and RPG-99-264) (to S.C.J.). W.C.K is a recipient of a predoctoral fellowship from National Institutes of Health Immunology Training Grant T32 AI-07313.
Abbreviations
TCR
T cell receptor
OVAp
ovalbumin residues 257–264
CFSE
5-(and 6-)carboxyfluorescein diacetate succinimidyl ester
TAP
transporter associated with antigen processing
References
- 1.Fink P J, Bevan M J. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 2.Freitas A A, Rocha B. Science. 1997;277:1950. doi: 10.1126/science.277.5334.1950. [DOI] [PubMed] [Google Scholar]
- 3.Benoist C, Mathis D. Science. 1997;276:2000–2001. doi: 10.1126/science.276.5321.2000. [DOI] [PubMed] [Google Scholar]
- 4.Marrack P, Mitchell T, Bender J, Hildeman D, Kedl R, Teague K, Kappler J. Immunol Rev. 1998;165:279–285. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanchot C, Lemmonnier F A, Perarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 6.Takeda S, Rodewald H R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 7.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 8.Brocker T. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markiewicz M A, Girao C, Opferman J T, Sun J, Hu Q, Agulnik A A, Bishop C E, Thompson C B, Ashton-Rickardt P G. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruno L, von Boehmer H, Kirberg J. Eur J Immunol. 1996;26:3179–3184. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 12.Nesic D, Vukmanovic S. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 13.Viret C, Wong F S, Janeway C A J. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 14.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 16.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulcher D A, Lyons A B, Korn S L, Cook M C, Koleda C, Parish C, Fazekas de St. Groth B, Basten A. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jameson S C, Bevan M J. Eur J Immunol. 1992;22:2663–2667. doi: 10.1002/eji.1830221028. [DOI] [PubMed] [Google Scholar]
- 19.Weston S A, Parish C R. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 20.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 21.Oehen S, Brduscha-Riem K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Van Kaer L, Ashton Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 23.Hogquist K A, Tomlinson A J, Kieper W C, McGargill M A, Hart M C, Naylor S, Jameson S C. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 24.Ljunggren H-G, Van Kaer L, Ashton-Rickardt P G, Tonegawa S, Ploegh H L. Eur J Immunol. 1995;25:174–178. doi: 10.1002/eji.1830250129. [DOI] [PubMed] [Google Scholar]
- 25.Aldrich C J, Ljunggren H-G, Van Kaer L, Ashton-Rickardt P G, Tonegawa S, Forman J. Proc Natl Acad Sci USA. 1994;91:6525–6528. doi: 10.1073/pnas.91.14.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freitas A A, Agenes F, Coutinho G C. Eur J Immunol. 1996;26:2640–2649. doi: 10.1002/eji.1830261115. [DOI] [PubMed] [Google Scholar]
- 27.Tanchot C, Rocha B. J Exp Med. 1997;186:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C M, Limanni A, Baker W H, Dobson M E, Kalinich J F, Patchen M L. J Interferon Cytokine Res. 1997;17:567–572. doi: 10.1089/jir.1997.17.567. [DOI] [PubMed] [Google Scholar]
- 29.Kundig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vella A T, Dow S, Potter T A, Kappler J, Marrack P. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vella A, Teague T K, Ihle J, Kappler J, Marrack P. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pihlgren M, Lightstone L, Mamalaki C, Rimon G, Kioussis D, Marvel J. Eur J Immunol. 1995;25:1755–1759. doi: 10.1002/eji.1830250640. [DOI] [PubMed] [Google Scholar]
- 33.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valitutti S, Muller S, Dessing M, Lanzavecchia A. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 36.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha B, von Boehmer H. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 38.von Boehmer H, Hafen K. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabinowitz J D, Beeson C, Wulfing C, Tate K, Allen P M, Davis M M, McConnell H M. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 40.Kersh G J, Allen P M. Nature (London) 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 41.Jameson S C, Bevan M J. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 42.Azzam H S, Grinberg A, Lui K, Shen H, Shores E W, Love P E. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanchot C, Rocha B. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 44.Bell E B, Sparshott S M. Nature (London) 1990;348:163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 45.Bell E B, Sparshott S M. Semin Immunol. 1997;9:347–353. doi: 10.1006/smim.1997.0092. [DOI] [PubMed] [Google Scholar]
- 46.Oehen S, Brduscha-Riem K. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 47.Roederer M, De Rosa S C, Watanabe N, Herzenberg L A. Semin Immunol. 1997;9:389–396. doi: 10.1006/smim.1997.0097. [DOI] [PubMed] [Google Scholar]
- 48.Margolick J B, Donnenberg A D. Semin Immunol. 1997;9:381–388. doi: 10.1006/smim.1997.0096. [DOI] [PubMed] [Google Scholar]
- 49.Naujokas M F, Arneson L S, Fineschi B, Peterson M E, Sitterding S, Hammond A T, Reilly C, Lo D, Miller J. Immunity. 1995;3:359–372. doi: 10.1016/1074-7613(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 50.Shachar I, Elliott E A, Chasnoff B, Grewal I S, Flavell R A. Immunity. 1995;3:373–383. doi: 10.1016/1074-7613(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 51.Wong P, Rudensky A Y. J Immunol. 1996;156:2133–2142. [PubMed] [Google Scholar]
- 52.Schilham M W, Wilson A, Moerer P, Benaissa-Trouw B J, Cumano A, Clevers H C. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 53.Modigliani Y, Coutinho G, Burlen-Defranoux O, Coutinho A, Bandeira A. Eur J Immunol. 1994;24:1223–1227. doi: 10.1002/eji.1830240533. [DOI] [PubMed] [Google Scholar]
- 54.Gabor M J, Godfrey D I, Scollay R. Eur J Immunol. 1997;27:2010–2015. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 55.Byrne J A, Stankovic A K, Cooper M D. J Immunol. 1994;152:3098–3106. [PubMed] [Google Scholar]