PDGF-C Is a Proinflammatory Cytokine that Mediates Renal Interstitial Fibrosis (original) (raw)
Abstract
PDGF-C is a potent mitogen for fibroblasts in vitro. Transgenic PDGF-C overexpression in the heart or liver induces organ fibrosis, and PDGF-C expression is upregulated at sites of interstitial fibrosis in human and rat kidneys; however, the effect of inhibiting PDGF-C on the development of renal fibrosis in vivo is unknown. Renal fibrosis was induced in C57BL/6 mice by unilateral ureteral obstruction (UUO), and then mice were treated with neutralizing anti–PDGF-C antiserum or nonspecific IgG. An increase in PDGF-C expression was observed in fibrotic areas after UUO, contributed in large part by infiltrating macrophages. Treatment with anti–PDGF-C reduced renal fibrosis by 30% at day 5 and reduced interstitial myofibroblast accumulation by 57%. In vitro, PDGF-C was a potent mitogen for renal fibroblasts and induced chemokine expression. In vivo, anti–PDGF-C treatment produced a decrease in the expression of the renal chemokines CCL2 and CCL5 (85 and 67% reductions, respectively), accompanied by a significant decrease in leukocyte infiltration and CCR2 mRNA expression. Further supporting a role of PDGF-C in renal fibrosis, PDGF-C−/− mice demonstrated a reduction in fibrosis and leukocyte infiltration in response to UUO compared with wild-type littermates. In conclusion, specific neutralization or lack of PDGF-C reduces the development of renal inflammation and fibrosis in obstructed mouse kidneys. Leukocyte-derived PDGF-C induces chemokine expression, which may lead to the recruitment of additional leukocytes, creating an amplification loop for renal inflammation and fibrosis.
PDGF-C is a recently identified cytokine that acts via the PDGF-α receptor and is a potent mitogen for human fibroblasts and vascular smooth muscle cells in vitro.1,2 Observations in different organs suggest that PDGF-C plays an important role in the regulation of fibrosis. First evidence was derived from a mouse model in which transgenic overexpression of PDGF-C in the heart induced fibroblast proliferation, resulting in cardiac fibrosis, hypertrophy, and ultimately cardiomyopathy.1,3 Transgenic mice with liver-specific PDGF-C overexpression of the transgene developed liver fibrosis and hepatocellular carcinoma.4 Finally, transgenic mice with a lung-specific PDGF-C overexpression developed massive mesenchymal cell hyperplasia and died from respiratory insufficiency immediately after birth.5 Increased PDGF-C expression has additionally been observed in different experimental models of organ fibrosis in heart and lung tissues.6,7
Additional functions of PDGF-C include the stimulation of angiogenesis8,9 and acceleration of wound-healing processes.2 A dynamic expression of PDGF-C during organogenesis in mice and humans points to a critical role of this growth factor in organ development,10,11 which is further substantiated by studies in PDGF-C−/− mice that die from feeding and respiratory difficulties in the perinatal period when grown on an SV129 background.12 The phenotype of these PDGF-C−/− mice is characterized by defective tube formation (facial clefts, spina bifida occulta), subepidermal blisters, and deficiency of connective tissues in the dorsal midline.12
Little is known about the functional role of PDGF-C within the kidney. We recently identified a constitutive expression of PDGF-C within arterial vascular smooth muscle cells and within collecting duct cells in rat and human kidney.13,14 We detected a de novo expression and/or overexpression of PDGF-C in fibrotic interstitial areas in different rat models of renal disease as well as in diseased human renal biopsy tissues.13,14 We therefore hypothesized that PDGF-C plays a functional role in the pathogenesis of tubulointerstitial fibrosis. We subsequently studied the potential profibrotic role of PDGF-C both by using a neutralizing anti–PDGF-C antiserum in murine renal fibrosis induced by unilateral ureteral obstruction (UUO) and by inducing renal fibrosis in PDGF-C−/− mice.
RESULTS
Affinity-Purified Sheep Anti–PDGF-C Antiserum Specifically Neutralizes PDGF-C–Induced Receptor Phosphorylation In Vitro
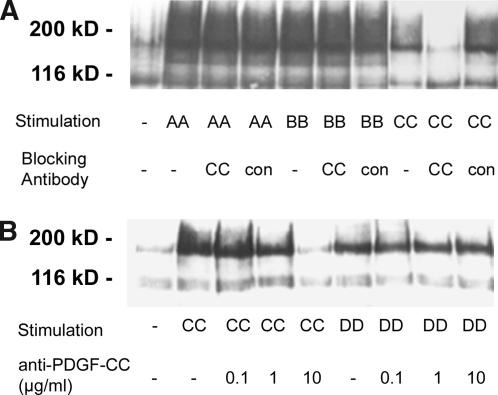
Efficacy and specificity of the anti–PDGF-C IgG was confirmed in an in vitro assay (Figure 1). Addition of anti–PDGF-C IgG specifically inhibited receptor phosphorylation in a dosage-dependent manner in cells that were stimulated with PDGF-C. Receptor phosphorylation induced by the other PDGF isoforms, A, B, or D, remained unaffected by the addition of anti–PDGF-C (Figure 1).
Figure 1.
Neutralizing activity and specificity of the affinity-purified sheep anti–PDGF-C IgG. Porcine aortic endothelial cells stably expressing transfected relevant PDGF receptor isoforms were stimulated with various PDGF isoforms in the presence or absence of anti–PDGF-C. After lysis of the cells, receptors were enriched, and the receptor phosphorylation level was determined by immunoblotting with phosphotyrosine antibodies. (A) Immunoblot detects significant reduction of phosphotyrosine only in cells treated with anti–PDGF-C. No effects are noted in the case of PDGF-AA–or PDGF-BB–induced receptor phosphorylation. (B) Dosage-dependent effects of PDGF-CC–induced receptor phosphorylation without affecting the PDGF-DD–induced phosphorylation.
PDGF-C Is Overexpressed in Macrophages in Murine Renal Fibrosis
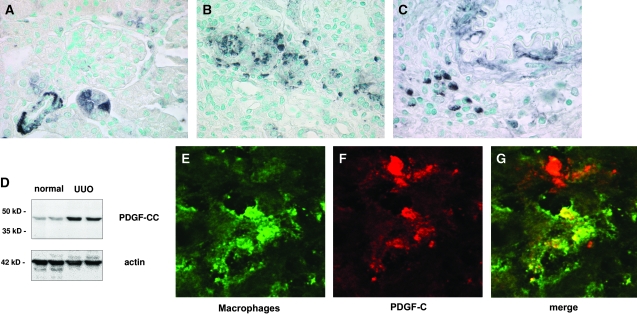
In healthy mouse kidneys, PDGF-C was expressed in vascular smooth muscle cells of small arteries and within individual tubular cells (Figure 2A). After UUO, renal PDGF-C protein was significantly overexpressed as compared with healthy normal control kidneys (Figure 2D). As detected by immunohistochemistry, PDGF-C remained expressed in tubular cells and in the media of arteries (Figure 2, B and C); however, PDGF-C became additionally detectable in infiltrating leukocytes (Figure 2, B and C). Double-label confocal microscopy identified the PDGF-C–expressing infiltrating leukocytes as ER-HR3–positive macrophages (Figure 2, E through G). We were unable to identify PDGF-C expression within CD3-positive T lymphocytes (data not shown).
Figure 2.
PDGF-C is overexpressed in murine renal fibrosis. (A) In healthy normal mice, PDGF-C is localized by immunohistochemistry within arteriolar smooth muscle cells and within individual tubular epithelial cells. (B and C) After UUO, PDGF-C is overexpressed within the injured kidneys. In addition to constitutive arteriolar and tubular expression, PDGF-C is expressed within individual interstitial cells. (D) Western blot analysis confirms the increased renal expression of PDGF-C. Compared with healthy normal mice, PDGF-C expression increases approximately three-fold in renal lysates from mice at day 10 after UUO (top). Labeling of the same blot with an anti-actin antibody confirms equal protein loading in each lane (bottom). (E through G). Confocal microscopy demonstrates that PDGF-C is expressed within infiltrating macrophages. ER-HR3–positive macrophages are labeled with a green color (E) and PDGF-C with a red color (F). The merged image identifies the yellow color of double-positive, PDGF-C–expressing macrophages (G). Note the PDGF-C–expressing cell that is not a macrophage in the top part of the image. This cell is likely a tubular cell or a vascular smooth muscle cell.
PDGF-C Antagonism Significantly Reduces Interstitial Fibrosis and Interstitial Myofibroblast Accumulation
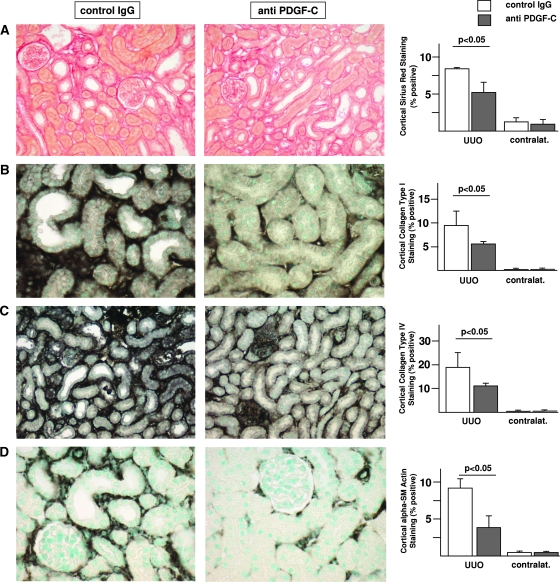
Treatment with neutralizing anti–PDGF-C antiserum resulted in significant reduction of interstitial fibrosis at day 5 as compared with animals receiving irrelevant IgG. There were significant reductions of cortical areas staining positively for Sirius red, collagen I, collagen IV, and α-smooth muscle actin (α-SMA)–positive myofibroblasts at day 5 in anti–PDGF-C–treated mice when compared with IgG-treated controls (Figure 3). In addition, a nonsignificant trend toward reduced renal collagen III deposition was observed in anti–PDGF-C–treated mice (data not shown). Real-time reverse transcriptase–PCR (RT-PCR) analysis of renal collagen IV (3.1 ± 1.3 versus 6.2 ± 2.7, corrected to rRNA expression, anti–PDGF-C versus irrelevant IgG group, respectively; P < 0.05) and fibronectin (6.6 ± 2.2 versus 14.0 ± 6.0; P < 0.05) mRNA expression at day 5 corroborated the results of the immunohistochemistry. At day 10 (i.e., after the washout of specific treatment), no significant differences between the groups persisted for any of the parameters analyzed (data not shown).
Figure 3.
PDGF-C antagonism reduces murine renal fibrosis. Histologic staining for Sirius red (A) and immunohistochemistry for collagen type I (B), collagen type IV (C) and α-SMA (D) reveals a significant decrease of all four parameters of renal fibrosis/fibrogenesis in mice that were treated with anti–PDGF-C as compared with control IgG in the course of UUO. Representative illustrations of left obstructed kidneys of mice treated with control IgG (left) and mice treated with anti–PDGF-C (middle). The results of the computer-assisted morphometry measurements are summarized in the right. □, mice treated with control IgG; ▪, mice treated with anti–PDGF-C. Data are means ± SD.
PDGF-C Is a Potent Mitogen for Renal Fibroblasts In Vitro
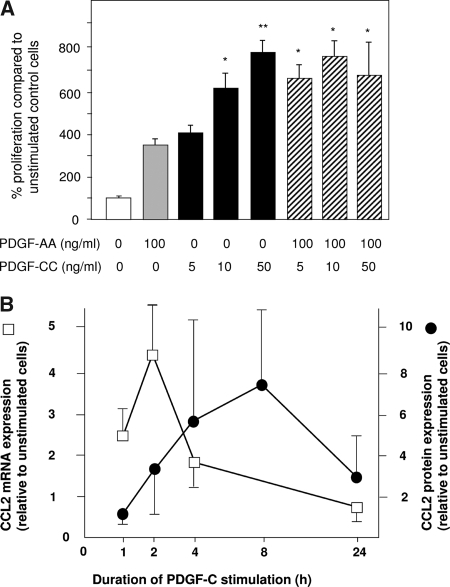
In vitro PDGF-C led to a marked, dosage-dependent proliferative response of renal fibroblasts (Figure 4A). PDGF-C was a more potent mitogen compared with PDGF-A, another ligand of the PDGF-α receptor. At low concentrations, the combination of both cytokines resulted in an additive effect on fibroblast proliferation (Figure 4A). We have not been able to demonstrate an antiapoptotic effect of PDGF-C in renal fibroblasts in vitro that might explain the increased cell numbers of renal fibroblasts after stimulation with PDGF-C (data not shown). These results suggested a direct effect of PDGF-C on intrinsic renal fibroblasts in mediating a profibrotic response; however, given the prominent inflammatory features of renal fibrosis associated with UUO, we hypothesized that additional pathways might play a role in PDGF-C–induced renal fibrosis.
Figure 4.
PDGF-C is a mitogen for renal fibroblasts in vitro and induces chemokine production. (A) Renal fibroblasts were stimulated with different concentrations of PDGF-AA and PDGF-CC. Both isoforms act as mitogens for these cells in vitro. PDGF-C is a more potent pro-proliferative stimulus resulting in significant cell proliferation already at lower concentrations. Combination of both PDGF-A and PDGF-C does have an additive effect on renal fibroblast proliferation. *P < 0.05 versus PDGF-AA (100 ng/ml) and PDGF-CC (5 ng/ml); **P < 0.05 versus PDGF-AA (100 ng/ml) and PDGF-CC (5 and 10 ng/ml). (B) In addition to its mitogenic properties, stimulation of renal fibroblasts with PDGF-C also induces cellular chemokine production. After PDGF-C stimulation, the chemokine CCL2 is increased both on the mRNA level (□) and on the protein level (•) within a few hours.
PDGF-C Stimulation Induces Chemokine CCL2 Expression in Renal Fibroblasts In Vitro
After stimulation with PDGF-C, there was a marked induction of chemokine expression in renal fibroblasts in vitro. Renal fibroblasts responded with a peak 4.5-fold increase of CCL2 (MCP-1) mRNA expression and an eight-fold increase in CCL2 protein expression to stimulation with PDGF-C (Figure 4B). We also observed increases in other chemokine mRNA levels (CCL3, CCL5, and CXCL10; data not shown); however, these increases were less pronounced and more variable compared with the robust and reproducible induction of CCL2.
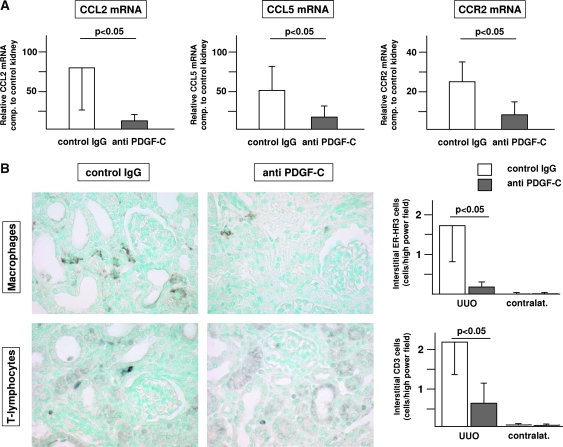
PDGF-C Antagonism Reduces Renal Inflammatory Responses In Vivo
Induction of renal fibrosis by UUO resulted in chemokine mRNA overexpression that was significantly reduced by the treatment with the neutralizing PDGF-C antiserum. At day 5 after UUO, control mice exhibited a mean 78-fold increase of CCL2 mRNA and a mean 51-fold increase of CCL5 mRNA in the obstructed kidneys as compared with the nonobstructed kidneys of the same animal. Treatment with the neutralizing anti–PDGF-C antiserum led to a significant 85% reduction of renal CCL2 mRNA expression as well as a significant 67% reduction of renal CCL5 mRNA expression at day 5 compared with mice treated with irrelevant IgG control (Figure 5A). Consistent with the fibrosis parameters at day 10, no significant differences in chemokine expression levels persisted at day 10 (i.e., after the washout of specific PDGF-C antagonism; data not shown).
Figure 5.
PDGF-C antagonism reduces renal leukocyte infiltration and renal chemokine expression in murine renal fibrosis. (A) Treatment of UUO-induced murine renal fibrosis with neutralizing PDGF-C results in a significant reduction of renal chemokine and chemokine receptor mRNA expression. Graphics illustrate the mean relative chemokine mRNA expression compared with the contralateral control kidney of each mouse. (B) Treatment with neutralizing anti–PDGF-C results in decreased renal infiltration of macrophages (top) and T lymphocytes (bottom). Representative images of left obstructed kidneys of mice treated with control IgG (left) or anti–PDGF-C (middle). The results of the computer-assisted morphometry measurements are summarized in the right. □, mice treated with control IgG; ▪, mice treated with anti–PDGF-C. Data are means ± SD.
PDGF-C Antagonism Reduces Renal Leukocyte Infiltration in Murine UUO
The functional relevance of reduced renal chemokine expression after PDGF-C antagonism was corroborated by reduced renal chemokine receptor CCR2 mRNA expression and leukocyte infiltration at day 5 (Figure 5, A and B). Treatment with the neutralizing anti–PDGF-C antiserum resulted in a significant 90% reduction of ER-HR3–positive macrophages and 67% reduction of CD3-positive T lymphocytes in anti–PDGF-C–versus irrelevant IgG-treated mice (Figure 5B). No significant reductions were detectable for F4/80-positive leukocytes, MAC-2–positive monocytes, or Ly6G-positive neutrophils (data not shown).
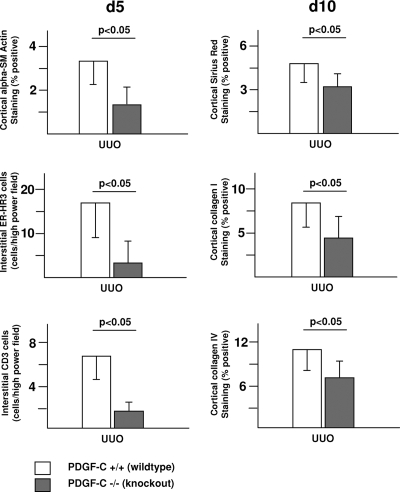
PDGF-C Deficiency Protects from UUO-Induced Renal Fibrosis
PDGF-C−/− mice developed significantly less fibrosing renal injury as compared with their wild-type littermates. Tubulointerstitial accumulation of α-SMA–positive myofibroblasts was reduced by 65% in PDGF-C−/− mice at day 5 after UUO as compared with wild-type littermates (Figure 6). Similar to the findings with the neutralizing antiserum in C57Bl/6 PDGF-C wild-type mice, the PDGF-C–dependent reduction of renal fibrogenesis was associated with a marked reduction of renal leukocyte infiltration. PDGF-C deficiency resulted in a 78% reduction of ER-HR3–positive macrophages and a 72% reduction of CD3-positive T lymphocytes in PDGF-C−/− mice versus control littermates at day 5 after UUO (Figure 6). The reduced myofibroblast and leukocyte accumulation at day 5 was followed by significantly reduced extracellular matrix deposition at day 10 (reduction of interstitial Sirius red by 34%, collagen I by 48%, and collagen IV by 33%; Figure 6).
Figure 6.
PDGF-C deficiency protects from renal fibrosis. At day 5 after UUO, immunohistologic staining for α-SMA, macrophages (ER-HR3–positive cells), and T lymphocytes (CD3-positive cells) in left obstructed kidneys reveals significant reductions of all three parameters in PDGF-C−/− mice (n = 7) compared with wild-type littermates (n = 7). At day 10 after UUO, labeling of Sirius red and immunohistochemical staining for collagen I and IV in left obstructed kidneys reveal significant reductions of renal fibrosis in PDGF-C−/− mice (n = 6) compared with wild-type littermates (n = 6). Data are means ± SD.
DISCUSSION
The major novel finding of this study is the observation that specific inhibition of PDGF-C markedly reduces renal fibrotic changes in the murine UUO model. Using two independent approaches for PDGF-C depletion in mice (gene knockout and a neutralizing antiserum), we observed virtually identical reductions of parameters of renal fibrosis. Descriptive studies of ours in the past identified PDGF-C expression within intrinsic renal as well as infiltrating cells and noted an association of renal fibrosis with local overexpression of PDGF-C.13,14 In this study, we extend these data obtained in rat and human kidney to the murine kidney. The mechanistic basis for the action of PDGF-C in renal fibrosis is provided by our previous data, showing that the PDGF-C receptor, namely PDGF-α receptor, is constitutively expressed in the renal interstitium and is further upregulated in cases of renal fibrosis.13,14
PDGF-C–induced organ fibrosis is not a unique finding in renal fibrosis. Previous studies in transgenic mice already indicated that local overproduction of PDGF-C is capable of inducing organ fibrosis in heart,3 liver,4 and lung5 experimental models, resulting in severe functional impairment of these different organs. Our study extends these previous findings for the first time to the pathogenesis of renal fibrosis, and it is the first to antagonize specifically PDGF-C in experimental organ fibrosis.
Further support for a critical role of PDGF-C in the development of renal interstitial fibrosis comes from studies in PDGF knockout mice. During embryonic renal development, PDGF-α receptor knockout mice completely lack the metanephric mesenchyme (the cells forming the renal interstitial connective tissue).1 Neither PDGF-A nor PDGF-B or combined PDGF-A/B knockout mice develop a similar phenotype with the lack of these mesenchymal cells.1 In the early and mid-1990s, PDGF-A and -B were the only known ligands for the PDGF-α receptor, and the knockout mice, therefore, provided the first hint to the existence of more ligands of the PDGF-α receptor. The search resulted in the identification of PDGF-C as another ligand of the PDGF-α receptor in 1999.1 Indeed, creating double-knockout mice for PDGF-A and -C, Nagy et al.12 were ultimately able to reproduce exactly the same renal phenotype with the lack of the metanephric mesenchyme as was seen in the PDGF-α receptor knockout mice. These data also suggest that the third ligand of the PDGF-α receptor, namely PDGF-B, has no major role in ontogenesis in driving the development of a normal renal interstitium.
These observations raise the important question on the relative roles of PDGF-A versus -C in renal fibrosis. In the normal kidney, constitutive PDGF-A expression is much wider than that of PDGF-C and includes podocytes, arterial smooth muscle cells, interstitial cells, and distal tubular epithelial cells.15,16 In particular, that PDGF-A and the PDGF-α receptor are constitutively expressed in the renal interstitium in healthy and fibrotic kidneys renders a central role of the PDGF-A/PDGF-α receptor axis for renal fibrosis unlikely. In contrast, PDGF-C is not normally expressed in glomeruli or the renal tubulointerstitium but is induced de novo in renal fibrosis. Such an inducible system seems much more likely to contribute to disease. In addition, using renal fibroblasts, we demonstrate that PDGF-CC is a much more potent mitogen for these cells than PDGF-AA.
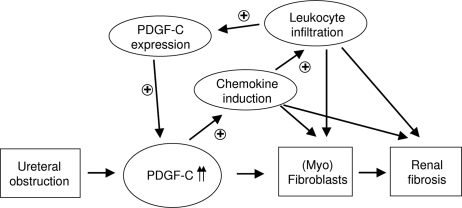
Our second major finding is the identification of mechanisms leading to PDGF-C–induced renal fibrosis (Figure 7). It is well established from recent in vitro studies that PDGF-C acts directly through the PDGF-α receptor. In the context of renal fibrosis, it is widely known that the accumulating interstitial myofibroblasts express high levels of PDGF-α receptor. It is therefore likely that PDGF-C induces proliferation as well as matrix production in interstitial myofibroblasts and possibly also in tubular epithelial cells directly via stimulating their PDGF-α receptor; however, in addition to this direct PDGF-C effect on intrinsic renal cells, we identified a novel indirect role, namely regulating local inflammatory responses (Figure 7). PDGF-C acts as a potent proinflammatory stimulus for resident renal cells, leading to a marked induction of chemokine expression in these cells. Chemokines play a central role in orchestrating renal inflammation, resulting in an infiltration of cells expressing the corresponding chemokine receptors.17 Local chemokine overexpression results in increased leukocyte infiltration. Some of these infiltrating leukocytes (i.e., macrophages) are loaded with PDGF-C that subsequently results in a further increase in renal PDGF-C overexpression. Altogether, these data suggest the existence of an autoamplification loop of PDGF-C–induced renal fibrosis (Figure 7). It has become increasingly clear that renal interstitial fibrosis is not just a passive deposition of extracellular matrix but rather involves active inflammation of the affected renal tissue.17 Consistent with this, a significant reduction of renal fibrosis has been demonstrated in chemokine receptor knockout mice as well as using a chemokine receptor antagonist in the same murine UUO model as was used for our studies.18,19 PDGF-C–induced renal inflammation is not limited to a single chemokine (CCL2); increased expressions of other chemokines were also noted after PDGF-C stimulation. It is unclear whether the induction of chemokines in renal fibroblasts is specific for PDGF-C. Data from other cell culture systems indicate that CCL2 can also be induced after stimulation with PDGF-B20; however, to our knowledge, CCL2 expression has not been studied in renal fibroblasts stimulated with PDGF-B.
Figure 7.
Hypothesis of mechanisms involved in PDGF-C–induced renal fibrosis. Increased PDGF-C expression acts directly on PDGF-α receptor–expressing renal myofibroblasts. In addition, PDGF-C induces local chemokine production, resulting in leukocyte attraction. PDGF-C–containing macrophages result in an autoamplification feedback by locally increasing the amount of renal PDGF-C.
We conclude that PDGF-C–induced renal fibrosis involves direct effects on PDGF-α receptor–expressing cells as well as indirect proinflammatory effects via recruited leukocytes. Renal fibrosis can be successfully treated by PDGF-C antagonism. Our data indicate that PDGF-C should be added as a new member to the growing list of molecular targets for the treatment of renal fibrosis. Our observations also place PDGF-C “upstream” of chemokines and therefore make it a particularly attractive therapeutic target. Finally, our study contributes to a growing list of organ fibroses for which PDGF-C antagonism may represent a common pharmaceutical target.
CONCISE METHODS
Antibodies
A neutralizing anti–PDGF-C antiserum was generated in sheep after immunization with a recombinant form of the core domain of PDGF-CC.1 IgG fractions from immune serum and control serum were purified with Protein-A-Sepharose.
Rabbit anti-human PDGF-CC core domain, affinity-purified against the core domain of PDGF-CC, was used for the specific detection of PDGF-C by immunohistochemistry and Western blotting.13,14 For the specific immunohistochemical detection of collagen subtypes and leukocyte subsets the following antibodies were used: goat anti-human collagen type I, goat anti-human collagen type III, and goat anti-human collagen type IV (all from Southern Biotech, Birmingham, AL); mouse anti–α-SMA (clone 1A4; Dako, Glostrup, Denmark); rat anti-human CD3 (clone CD3–12), cross-reactive with murine CD3 (Serotec, Oxford, UK); rat anti-mouse monocytes/macrophages (clone ER-HR3; BMA Biomedicals, Augst, Switzerland); rat anti-mouse macrophages (clone F4/80; Serotec); rat anti-mouse macrophages (MAC-2, clone 8942AP; Cedarlane Laboratories, Ontario, Canada); and rat anti-mouse granulocytes (Ly6G, clone RB6–8C5; BD Pharmingen, San Diego, CA).
Animals and Animal Models
All animal studies were approved by the local institutional review board. Male C57Bl/6 mice (weighing 20 g) were purchased from Taconic (Ry, Denmark).
Renal Interstitial Fibrosis Induced by UUO.
Ten-week-old male C57Bl/6 mice had their left ureter completely obstructed by bipolar cauterization at the level of the lower renal pole. The ureter was then cut between the two ligations. Mice were treated from day −1 until day 4 with (1) neutralizing affinity-purified sheep anti–PDGF-C IgG or (2) irrelevant affinity-purified sheep control IgG. Treatment consisted of single daily intraperitoneal injections of the compounds. The antiserum was administered in final concentrations of 1 mg/mouse per d. Mice were killed at days 5 (n = 10) and 10 (n = 11). Kidney tissue was obtained from the obstructed as well as from the contralateral nonobstructed kidney. Tissues for morphologic evaluation and immunohistochemistry were fixed in methyl Carnoy solution. Kidney specimens were additionally obtained for protein and RNA isolation and processed as indicated next.
Renal Interstitial Fibrosis Induced in PDGF-C−/− Mice.
PDGF-C+/− mice were provided by Andras Nagy, Hao Ding, and Christer Betsholtz.12 When bred in a mixed genetic background (between SV129 and C57Bl/6), PDGF-C deficiency did not cause perinatal lethality. None of the PDGF-C−/− mice used in this study showed any obvious pathology. PDGF-C deficiency or wild-type status was confirmed by PCR.12 Renal fibrosis was induced by UUO in 10-wk-old male PDGF-C−/− mice (n = 13) and their wild-type littermates (n = 13). Mice were killed at day 5 (n = 14) and day 10 (n = 12).
Renal Morphology and Immunohistochemistry
Tissue for light microscopy and immunoperoxidase staining was fixed in methyl Carnoy solution and embedded in paraffin. Four-micron sections were stained with periodic acid-Schiff reagent and counterstained with hematoxylin. All kidneys were additionally stained with the Sirius red reagent. Immunohistochemistry (including negative controls) was performed in sections of methyl Carnoy–fixed kidneys using previously published indirect immunoperoxidase techniques.13,14 Double-labeling immunohistochemistry and confocal microscopy were performed in acetone-fixed, frozen kidney sections.
The morphologic changes in the mouse UUO model were quantified by computer-assisted image analysis. Images were taken on an Olympus BX50 microscope (Olympus Optical, Hamburg, Germany), using a SIS ColorView II digital camera (Soft Imaging Systems, Münster, Germany). Morphometric image analysis was performed using the SIS analySIS pro Software from Soft Imaging Systems. All murine tissue evaluations were performed in a blinded manner by a single investigator (E.B.). For the quantification of Sirius red, collagen, and α-SMA positivity 20 random, consecutive pictures were taken of the renal cortex of each animal. The percentage of positively stained area was extracted for intensity in each picture. A mean area was calculated from the 20 pictures of each animal. The numbers of individual leukocytes were counted in cortical pictures taken at high-power magnifications.
Western Blotting
Specific inhibitory activity of sheep anti–PDGF-CC IgG was analyzed by co-incubation of PDGF ligands with anti–PDGF-CC or control IgG, followed by analyses of the ability of the preparations to stimulate PDGF receptor phosphorylation, as described previously.21 PDGF-C expression was analyzed in protein lysates from kidney tissues from healthy mice and from UUO mice following published protocols.13 After the staining of PDGF-CC, the blot was stripped and subsequently labeled for actin (affinity isolated, rabbit anti- actin, A2066; Sigma) as a loading control.
Real-Time Quantitative RT-PCR
RNA isolation, RNA purity determination, cDNA synthesis, and RT-PCR were performed as described previously.22 The primer sequences are listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase cDNA amplification was used as an internal standard.
Table 1.
Primers for real-time RT-PCR
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Primers for the detection with SybrGreen probes (qPCR Core Kit for SYBR Green I) | |||
| mGAPDH | GGCAAATTCAACGGCACAGT | AGATGGTGATGGGCTTCCC | |
| mCCL2 | TGGCTCAGCCAGATGCAGT | ATTGGGATCATCTTGCTGGTG | |
| mCCL5 | AGTGCTCCAATCTTGCAGTCG | CACTTCTTCTCTGGGTTGGCA | |
| mCCR-2 | TGTGATTGACAAGCACTTAGACCA | ATGACAGGATTAATGCAGCAGTGT | |
| rGAPDH | ACAAGATGGTGAAGGTCGGTG | AGAAGGCAGCCCTGGTAACC | |
| rCCL2 | CCAGATGCAGTTAATGCCCC | TCTCCAGCCGACTCATTGG | |
| rCCL3 | TGAGACCAGCAGCCTTTGG | GATCTGCCGGTTTCTCTTGG | |
| rCCL5 | ACTCCCTGCTGCTTTGCCT | GTGTAAAAATACTCCTTCACGTGGG | |
| rCXCL10 | AGAACGGTGCGCTGCAC | TCCTATGGCCCTGGGTCTC | |
| Primers for the detection with Taqman probes (qPCR Core Kit) | |||
| mGAPDH | GGCAAATTCAACGGCACAGT | AGATGGTGATGGGCTTCCC | AAGGCCGAGAATGGGAAGCTTGTCATC |
| mCol4a1 | GGCGGTACACAGTCAGACCAT | TGGTGTGCATCACGAAGGA | TCCGCAGTGCCCTAACGGTTGGT |
| mFibronectin | GATGGAATCCGGGAGCTTTT | TGCAAGGCAACCACACTGAC | CCGGCCTGAGGCCCTGCAG |
Cell Cultures/Cell Proliferation Assay
A cell line of rat renal fibroblasts (NRK-49F) was purchased from DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and propagated according to the manufacturer's suggestion. Serum-starved cells were stimulated with purified PDGF-CC protein1,13 and purified PDGF-AA protein (provided by J. Hoppe, University of Würzburg, Würzburg, Germany). Fibroblast proliferation was assessed as described previously.13,22 Cell proliferation experiments were independently performed four times with duplicate measurements. Chemokine CCL2 protein was measured in cell culture supernatants at 1, 2, 4, 8, and 24 h after stimulation with PDGF-C and controls using a CCL2 ELISA kit from R&D Systems (Wiesbaden, Germany). Chemokine CCL2, CCL3, CCL5, and CXCL10 mRNA expressions were measured by real-time RT-PCR in isolated cells at 1, 2, 4, and 24 h after stimulation with PDGF-C and controls.
Statistical Analyses
All values are expressed as means ± SD. Statistical significance (defined as P < 0.05) was evaluated using ANOVA and Bonferroni t test.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft OS 196/1-1 (T.O. and F.E.) and SFB 542, project C7 (T.O. and J.F.).
The technical help of Andrea Cosler, Gabi Dietzel, Regina Lanzmich, Gerti Minartz, and Lydia Zimmermanns is gratefully acknowledged. We thank Elisa Liehn and Christian Weber for help with the CCL2 ELISA and Gerhard Müller-Newen for help with the confocal microscopy.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U: PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol 2: 302–309, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, Gao Z, Shoemaker K, Bukowski TR, Moore M, Feldhaus AL, Humes JM, Palmer TE, Hart CE: Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem 276: 27406–27414, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Ponten A, Li X, Thoren P, Aase K, Sjoblom T, Ostman A, Eriksson U: Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol 163: 673–682, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS, Yeh MM, Fausto N: Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A 102: 3389–3394, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuo Y, Hoyle GW, Shan B, Levy DR, Lasky JA: Over-expression of PDGF-C using a lung specific promoter results in abnormal lung development. Transgenic Res 15: 543–555, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Grun K, Markova B, Bohmer FD, Berndt A, Kosmehl H, Leipner C: Elevated expression of PDGF-C in coxsackievirus B3-induced chronic myocarditis. Eur Heart J 26: 728–739, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Zhuo Y, Zhang J, Laboy M, Lasky JA: Modulation of PDGF-C and PDGF-D expression during bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 286: L182–L188, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y: Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J 16: 1575–1583, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Li X, Tjwa M, Moons L, Fons P, Noel A, Ny A, Zhou JM, Lennartsson J, Li H, Luttun A, Ponten A, Devy L, Bouche A, Oh H, Manderveld A, Blacher S, Communi D, Savi P, Bono F, Dewerchin M, Foidart JM, Autiero M, Herbert JM, Collen D, Heldin CH, Eriksson U, Carmeliet P: Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest 115: 118–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H, Wu X, Kim I, Tam PP, Koh GY, Nagy A: The mouse Pdgfc gene: Dynamic expression in embryonic tissues during organogenesis. Mech Dev 96: 209–213, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Aase K, Abramsson A, Karlsson L, Betsholtz C, Eriksson U: Expression analysis of PDGF-C in adult and developing mouse tissues. Mech Dev 110: 187–191, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Ding H, Wu X, Bostrom H, Kim I, Wong N, Tsoi B, O'Rourke M, Koh GY, Soriano P, Betsholtz C, Hart TC, Marazita ML, Field LL, Tam PP, Nagy A: A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet 36: 1111–1116, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Eitner F, Ostendorf T, Van Roeyen C, Kitahara M, Li X, Aase K, Grone HJ, Eriksson U, Floege J: Expression of a novel PDGF isoform, PDGF-C, in normal and diseased rat kidney. J Am Soc Nephrol 13: 910–917, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Eitner F, Ostendorf T, Kretzler M, Cohen CD, Eriksson U, Grone HJ, Floege J: PDGF-C expression in the developing and normal adult human kidney and in glomerular diseases. J Am Soc Nephrol 14: 1145–1153, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Alpers CE, Hudkins KL, Ferguson M, Johnson RJ, Rutledge JC: Platelet-derived growth factor A-chain expression in developing and mature human kidneys and in Wilms’ tumor. Kidney Int 48: 146–154, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Taneda S, Hudkins KL, Topouzis S, Gilbertson DG, Ophascharoensuk V, Truong L, Johnson RJ, Alpers CE: Obstructive uropathy in mice and humans: Potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol 14: 2544–2555, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Segerer S, Alpers CE: Chemokines and chemokine receptors in renal pathology. Curr Opin Nephrol Hypertens 12: 243–249, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D: A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eis V, Luckow B, Vielhauer V, Siveke JT, Linde Y, Segerer S, De Lema GP, Cohen CD, Kretzler M, Mack M, Horuk R, Murphy PM, Gao JL, Hudkins KL, Alpers CE, Grone HJ, Schlondorff D, Anders HJ: Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol 15: 337–347, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Freter RR, Alberta JA, Lam KK, Stiles CD: A new platelet-derived growth factor-regulated genomic element which binds a serine/threonine phosphoprotein mediates induction of the slow immediate-early gene MCP-1. Mol Cell Biol 15: 315–325, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K: Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res 61: 2929–2934, 2001 [PubMed] [Google Scholar]
- 22.van Roeyen CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J: Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int 69: 1393–1402, 2006 [DOI] [PubMed] [Google Scholar]