Cell Polarity Dynamics Controls the Mechanism of Lumen Formation in Epithelial Morphogenesis (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 8.
Published in final edited form as: Curr Biol. 2008 Apr 8;18(7):507–513. doi: 10.1016/j.cub.2008.02.076
Summary
Many organs consist of tubes or spheres of epithelial cells enclosing a central lumen. How the space of this lumen is generated is a key question in morphogenesis. Two predominant mechanisms of de novo lumen formation have been observed: hollowing and cavitation. In hollowing the lumen is formed by exocytosis and membrane separation, whereas in cavitation the lumen is generated by apoptosis of cells in the middle of the structure [1, 2]. Using Madin-Darby canine kidney cells in three dimensional cultures we found an inverse correlation between efficiency of polarization and apoptosis. When cells were grown in collagen, where cells polarized slowly, apoptosis was needed for lumen formation. In contrast in the presence of Matrigel, which allowed rapid polarization, lumens formed without apoptosis. If polarization in Matrigel was perturbed by blocking formation of the apical surface by RNAi of Cdc42, lumens formed by apoptosis. In a complementary approach we plated cells at high density so that aggregates formed with little polarity. These aggregates required apoptosis to form lumens, whereas cells plated at low density formed cysts with rapidly polarizing cells and did not need apoptosis to form lumens. The mechanism of lumen formation in the 3D-MDCK model can shift between hollowing and cavitation, depending on cell polarization.
Results
Reduced apoptosis requirement in lumen formation in the presence of laminin, a strong polarization signal
Madin–Darby canine kidney (MDCK) cells, a commonly used non-tranformed epithelial cell line, form polarized cysts with a hollow lumen when embedded as single cells within collagen gels. To characterize the involvement of apoptosis during lumen formation, MDCK cells forming cysts in collagen were fixed at different times for up to 10 days and then stained for confocal microscopy analysis with polarity markers and an apoptosis marker (Fig. 1a and 1b, Fig. S1, quantification in Fig 1c and 1d). The process of lumen formation during morphogenesis presents two phases, an initial phase (day 1–3) in which the cells present low levels of polarity (indicated by the presence of the apical marker gp135/podocalyxin around the periphery of the cyst and no central lumen) and no staining for the apoptosis marker activated caspase 3 (Fig 1a left panels; Fig S1); and an apoptotic phase (days 4–10) in which the cells acquire polarity, indicated by gp135 staining of the apical surface of the cells facing the central lumen, and the luminal cells are eliminated by apoptosis (Fig. 1a right panels, arrow head indicates apoptotic cell, Fig. S1, quantification in 1c). These results corroborate previous studies showing the requirement for apoptosis to clear the lumen in MDCK cysts in collagen [3].
Fig 1. The presence of Laminin in the ECM reduces apoptosis in lumen clearance.
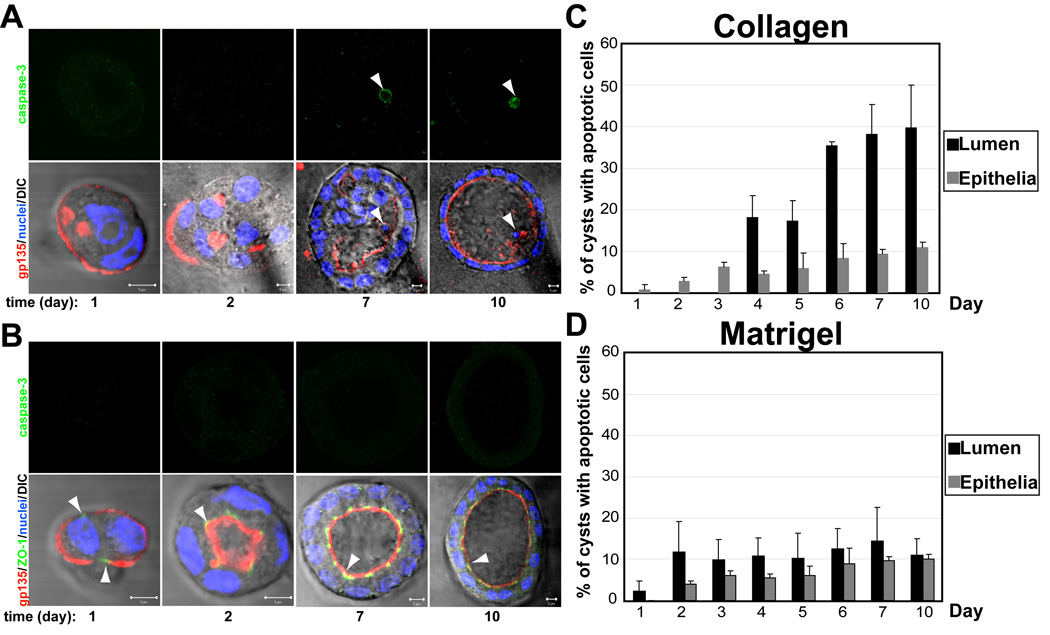
(A) and (B) Effect of laminin on polarity, lumen formation and apoptosis. MDCK cells were plated to form cysts in Collagen (A) or LGF Matrigel (B). Cells were fixed at 1, 2, 3, 4, 5, 6, 7, and 10 days and stained to detect gp135 (red), ZO-1 (green) and nuclei (lower panels merged with DIC); and caspase3 (green, upper panels). Arrowheads indicate apoptotic cells in the lumen of the cysts. Scale bars 5 µm.
(C) and (D) Quantitation of cysts with apoptotic cells in the lumen (black bars) or in the epithelial wall (grey bars) in cells plated from 1 to 10 days in collagen (C) or LGF Matrigel (D). Values shown are mean ±SD from 4 different experiments. Statistical analysis of the data demonstrates that apoptosis occurs specifically in the lumen (P<0.005)
Our previous studies in MDCK cells have delineated the importance of an external cue from the ECM to create an intracellular signaling that is essential for the initial orientation of apico-basal polarity during cyst formation [4]. According to these studies, the interaction of the integrin β1 with collagen leads to activation of the small GTPase Rac1[5]. Activation of Rac1 induces the assembly of a laminin network, which in turn is required for the orientation of polarity. Importantly, exogenous laminin was sufficient to rescue the inverted polarity phenotype induced by expression of dominant negative Rac1 [4]. Overall, these studies suggested that the presence of laminin in the ECM is essential for the initial orientation of polarity during epithelial morphogenesis. To address the effect of laminin on the requirement for apoptosis during lumen formation, we formed MDCK cysts in the presence of exogenous laminin. To this end, MDCK cells were cultured in Low Growth Factor (LGF) Matrigel, a gel of reconstituted basement membrane derived from Engelbreth–Holm–Swarm (EHS) tumor and comprised primarily of the matrix components laminin, collagen IV, and entactin. Recent studies have indicated that the laminin in the EHS tumor-derived matrix is responsible for driving the morphogenetic process when mammary epithelial cells are cultured three-dimensionally [6]. MDCK cells forming cysts in LGF Matrigel were fixed at different times for up to 10 days and then stained for polarity markers and an apoptosis marker. The confocal images showed that cells polarize and form clear lumens much faster in the presence of laminin (Fig. 1b, Fig S1). Note the presence of apical lumens and tight junctions at very early stages of cysts formation (Fig 1b, arrowheads show the presence of tight junctions at 24h and MDCK cysts with clear lumens at 48h). Importantly, there were a very low number of MDCK cysts stained for caspase3, the apoptosis marker (Fig. 1b, upper panels, quantitation in Fig 1d). The number of cysts with apoptotic cells of the epithelial wall was always very low in comparison with the luminal cells in MDCK cells growing in collagen gels, indicating the specificity of apoptosis to form the lumen during epithelial morphogenesis in collagen (Fig. 1c). These results suggested that apoptosis was not essential for the formation of a central lumen when laminin is present in the ECM.
To further test this hypothesis we prepared movies of MDCK cells forming cysts in Matrigel or collagen for up to 9 days (Fig S2, MovieS1 and MovieS2). MDCK cells in Matrigel formed one single lumen from early time points of cysts formation (36–66h) by the separation of the cell membranes, and without the presence of cells in the lumen (Fig S2, top panels. Arrows indicate the presence of the lumens; MovieS1). However, MDCK cells growing in collagen formed multiple lumens, and at later time points during cysts formation (80–90h, Fig S2, bottom panels. Arrows indicate the presence of the lumens; MovieS2). Additionally, the collagen cysts presented multiple cells in the lumen (Fig S2, bottom panels. Arrowheads)
Apoptosis is essential for lumen formation in MDCK cysts in the presence of laminin when the acquisition of polarity is delayed
Our results suggested that when the acquisition of polarity during epithelial morphogenesis is accelerated by the presence of laminin (a strong ECM signal) the requirement of apoptosis is reduced. To test this hypothesis we delayed the acquisition of polarity in MDCK cysts growing in LGF Matrigel by disrupting a molecular pathway that uses the small GTPases Cdc42 to form the apical membrane and lumen in MDCK cysts [7]. Endogenous Cdc42 was reduced to 10% compared to control using siRNA heteroduplexes (Fig 2c). When control and Cdc42-reduced cells were plated to form cysts, most control cells formed normal lumens at 48–96h (91%) (Fig 2a, left panels), whereas only 34% of MDCK cysts with reduced Cdc42 had normal lumens at this time. Instead, most cysts with reduced Cdc42 of similar size to those of control cysts had multiple small lumens (Fig 2b, left panels). The KD Cdc42 MDCK cysts acquired clear lumens at longer times (Fig 2b, bottom-right panel, 7 days), when the levels of Cdc42 protein were partially recovered (Fig 2c). Importantly, we observed multiple MDCK cysts stained for the apoptosis marker caspase3 at later times of cysts formation (Fig 2b, right panels, days 4–7 arrowheads). By contrast, the control cysts did not show significant number of MDCK cysts stained for the apoptosis marker caspase3 (Fig 2a, upper panels). Quantification in panel D showed 37% of KD Cdc42 cysts had apoptotic cells. These results suggested that apoptosis is involved in the formation of the lumen in MDCK cysts in LGF Matrigel when the acquisition of polarity is delayed.
Fig 2. Cdc42 siRNA-depletion delays lumen formation in MDCK cyst.
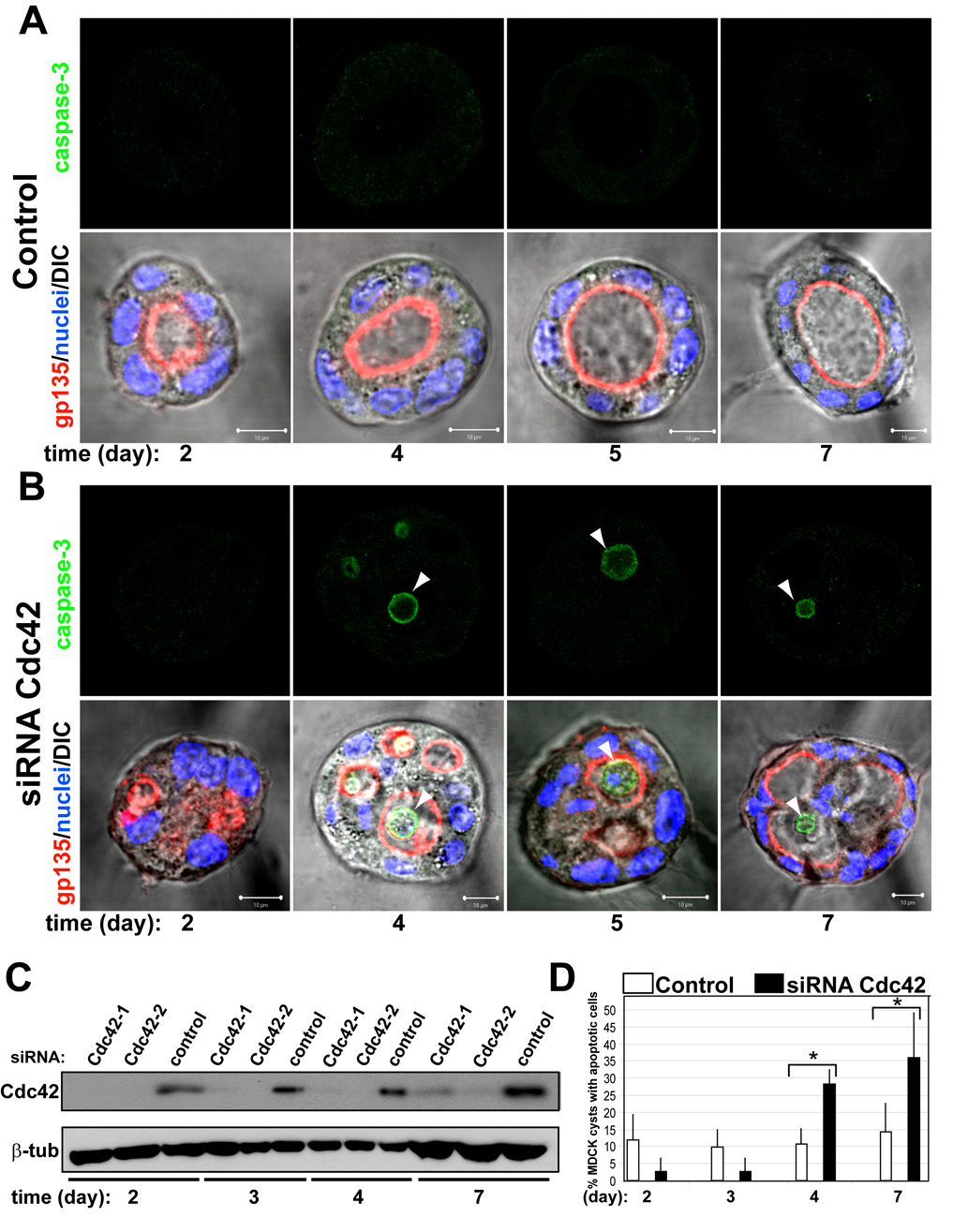
(A) and (B) Effect of Cdc42-1 siRNA on lumen formation and apoptosis. MDCK cells were transfected with Cdc42 siRNA (B) or siRNA control (A) and plated to form cysts. Cells were fixed at 2, 3, 4, 5 and 7 days and stained to detect gp135 (red) and nuclei (lower panels merged with DIC); and caspase3 (green, upper panels). Arrowheads indicate apoptotic cells in the lumen of the cysts. Scale bars 5 µm.
(C) Down-regulation of Cdc42 by siRNA. Cells were transfected with siRNAs Cdc42-1 and Cdc42-2 against canine Cdc42 or with control siRNA, allowed to form cysts for 2, 3, 4 or 7 days and then total cell lysates western blotted for Cdc42 and β-tubulin (control).
(D) Quantitation of cysts with apoptotic cells in the lumen in cells transfected with control siRNA (white bars), specific siRNA Cdc42-1 (black bars). Values shown are mean ±SD from 4 different experiments. * P≤0.01; P<0.01
To further test this hypothesis we exploited the recently described property of epithelial cells in Matrigel to form cell aggregates when plated at high cellular density [8]. We plated MDCK cells in LGF Matrigel at different cell densities. MDCK cells formed aggregates of cells when they were plated at high density (Fig 3a, bottom panel), and small cell colonies when plated at low density (Fig 3a, upper panel). This strategy allowed us to manipulate the acquisition of polarity, since the cells that form large aggregates lack cell polarity initially, similarly to cells in collagen at early times (compare Fig 3a with Fig S1a). When plated at low density most MDCK cysts form single lumens at 72 h, but they presented multiple lumens when plated at high density (Fig 3b). Quantification showed an inverse relationship between single lumen formation and cell density (Fig 3c). Importantly, when plated at high density 42% of MDCK cysts presented apoptotic cells in the lumen at 72h, but only less than 2% of cysts showed apoptotic cells when plated at low density (Fig 3b, arrowheads). There was a direct, quantitative relationship between apoptosis and cell density (Fig 3d). These results further support the idea that apoptosis is involved in the formation of the lumen in epithelial tissues when the acquisition of polarity is delayed.
Fig 3. Cell density determines apoptosis requirement and single lumen formation.
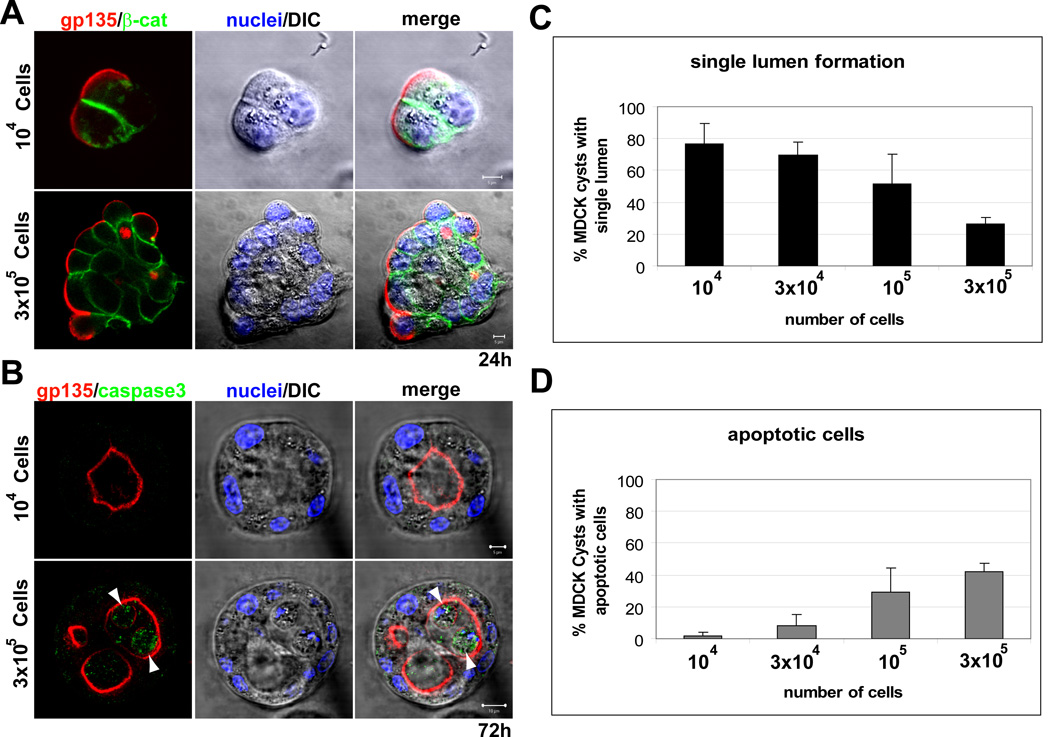
(A) and (B) Effect of cell density on lumen formation and apoptosis. MDCK cells were plated at a cell density of 104 (upper panels) or 3 × 105 cells/ml (lower panels) and allowed to form cysts for 24 (A) or 72h (B). Cells were fixed and stained to detect gp135 (red), β-catenin (green, upper panels) or caspase3 (green, lower panels), and nuclei (middle panels merged with DIC). Arrowheads indicate apoptotic cells in the lumen of the cysts. Scale bars 5 µm.
(C) and (D) Quantitation of cysts with normal lumens (C) and apoptotic cells in the lumen (D) in cells plated at different cell density. Values shown are mean ±SD from 4 different experiments. Statistical analysis of the data demonstrates a direct relationship between cell density and apoptosis (P<0.005); and an inverse relationship between single lumen formation and cell density (P<0.005)
Next we characterized the effect of disrupting Cdc42 on lumen formation and apoptosis in MDCK cells plated in LGF Matrigel at different cell densities (Fig. S3). Importantly, the quantification of this experiment showed that in MDCK cysts plated at low density there was an specific increase of apoptosis in luminal cells of Cdc42 KD cells at later times of cysts formation (Fig S3a, day 5), indicating that cell death is specifically localized in the luminal region rather than being widely distributed. When the MDCK were plated at high density, we confirmed reduction of normal lumen formation of MDCK cysts that were plated forming aggregates described in fig 3 (compare 5d-control in Fig. S3a and b). Moreover, we observed that the formation of the central lumen was further reduced in MDCK cells KD for Cdc42 (Fig. S3b, day5). This result indicates that polarization is required for lumen formation not only in cells plated a low density but also in large aggregates, since the delay in the acquisition of polarity induced by the reduction of Cdc42 protein levels is accompanied by a delay in the formation of the central lumen.
Inhibition of apoptosis prevents lumen formation in MDCK cysts in the absence of a strong polarization signal
Our previous results suggested that when the acquisition of polarity is efficient, apoptosis is not essential for the formation of the lumen. However, even when polarization is efficient in MDCK cysts at low density in the presence of laminin, we observed that a small percentage of cells die by apoptosis (Fig 1c). To better characterize the role of apoptosis in epithelial morphogenesis, we inhibited apoptosis by the expression of the anti-apoptotic protein Bcl-2 in MDCK cells. For that, we prepared MDCK cells stably expressing Bcl-2 at different levels (Fig 4a). First, we used the clone that expressed the highest level of Blc-2 (clone #6, Fig 4a lane 1). Expression of Bcl-2 was previously reported to inhibit lumen formation in MDCK cells in collagen [3], which we confirmed (Fig S4). Next, we found that expression of Bcl-2 was sufficient to inhibit lumen formation in MDCK cells plated at high density in LGF Matrigel (Fig 4b, d). However, expression of Bcl-2 did not affect the formation of lumens in MDCK plated at low density (Fig 4c, e). We obtained consistent results using stable clones that express moderate-to-low levels of Bcl-2 (Fig S5, clone #14 and #16; Bcl2 expression levels in Fig 4a lanes 2 and 3). To further confirm the role of apoptosis in epithelial morphogenesis, we inhibited apoptosis by treatment with the general caspase inhibitor Q-VD-OPh in MDCK cells growing at low and high density (Fig. 4f, g). This general caspase inhibitor has been described to prevent apoptosis in in vitro and in vivo models [9]. Treatment with Q-VD-OPh inhibited apoptosis in MDCK cells forming cysts at high and low density in a dose-dependent manner (Fig. 4f and 4g). Confirming our previous results with the expression of Bcl-2, we observed that apoptosis inhibition mediated by Q-VD-OPh did not affect normal lumen formation in MDCK cells plated at low density (Fig. 4g), but it was sufficient to inhibit lumen formation in MDCK cells plated at high density (Fig. 4f). These data support our hypothesis that apoptosis is essential for the formation of the lumen in epithelial tissues when the acquisition of polarity is delayed, and clearly demonstrate that when cells polarize efficiently the requirement of apoptosis for lumen formation during epithelial morphogenesis is reduced.
Fig 4. Blc2 expression, prevents apoptosis in MDCK cysts at high density, but has no effect at low density.
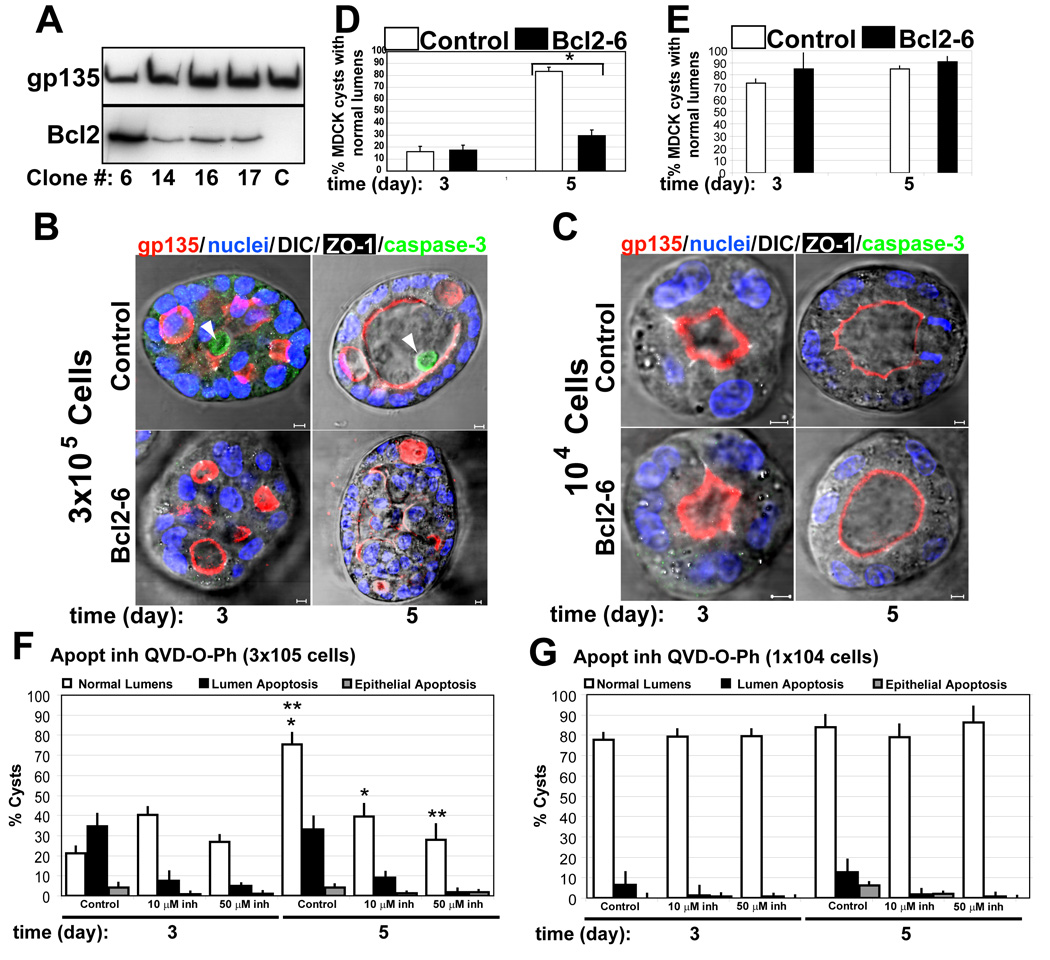
(A) Western blot of stable expression of Bcl2. Extracts from different clones of MDCK Bcl2 were inmunoblotted with gp135 (upper panel) as a control and anti-Bcl2 (lower panel) to detect endogenous and transfected proteins.
(B) Effect of Bcl2 expression on lumen formation and apoptosis in high density MDCK cells. MDCK cells expressing Bcl2 (lower panels) or not (upper panels) were plated at high density (3×105 cell/ml) to form cysts for 3 and 5 days. Cells were fixed and stained to detect gp135 (red), caspase3 (green), ZO-1 (white) and nuclei (merged with DIC). Arrowheads indicate apoptotic cells in the lumen of the cysts. Scale bars 5 µm.
(C) Effect of Bcl2 expression on lumen formation and apoptosis in low density. MDCK cells expressing Bcl2 (lower panels) or not (upper panels) were plated at low density (104 cell/ml) to form cysts for 3 or 5 days. Cells were visualized as in panel B. Scale bars 5 µm.
(D) Quantitation of cysts with normal lumens in high density MDCK cells transfected with Bcl2 (black bars) or control (white bars). Values shown are mean ±SD from 4 different experiments. * P <0.01
(E) Quantitation of cysts with normal lumens in low density MDCK cells transfected with Bcl2 (black bars) or control (white bars). Values shown are mean ±SD from 4 different experiments.
(F) Quantitation of cysts with normal lumens (white bars) in high density MDCK cells treated with Q-VD-OPh at different concentrations: control (0) 10µM and 50µM. Apoptosis in luminal (black bars) and epithelial cells (grey bars) were also quantified. Values shown are mean ±SD from 2 different experiments. *P<0.005; **P<0.005
(G) Quantitation of cysts with normal lumens (white bars) in low density MDCK cells treated with Q-VD-OPh at different concentrations control (0), 10µM and 50µM. Apoptosis in luminal (black bars) and epithelial cells (grey bars) were also quantified. Values shown are mean ±SD from 2 different experiments
Discussion
Formation of lumens is a central feature of organogenesis. Cells create luminal space through many different mechanisms, such as invagination and cell migration described for the formation of the vertebrate intestinal tract and neural tube respectively [1]. When the epithelial tubes arise from clusters unpolarized of cells or individual cells that are not epithelial, two major classes of mechanisms predominate: hollowing and cavitation [1, 2]. In hollowing, as occurs in the zebrafish gut tube [10], cells form cell-cell junctions and acquire apical-basal polarity de novo, forming a small lumen that subsequently expands by the generation of additional apical membrane. Alternatively, lumen formation occurs by polarization of cells at the periphery of the cord followed by selective apoptosis of cells in the center, as occurs in the mammalian salivary gland [11]. Apoptosis is a fundamental cellular process that is necessary for embryonic development and for the maintenance of tissue homeostasis in the adult organism. The data presented here demonstrate a functional relationship between cell polarization and apoptosis for the formation of the lumen during epithelial morphogenesis in 3D-MDCK cell culture. We propose that when the acquisition of polarity is efficiently achieved and coordinated with cell proliferation, lumen formation occurs by membrane separation without the requirement of apoptosis. However, when apico-basal polarization is slow or inefficient or when cell polarity is uncoordinated with cell proliferation, apoptosis of cells in the center of the cord becomes essential. Therefore, apoptosis is a control mechanism that ensures the clearance of the lumen during epithelial morphogenesis.
To establish cell polarity epithelial tissues require external cues mediated by the interaction of the cells with the surrounding media, other cells and/or extracellular matrix [1, 12]. In particular, two main events are required in morphogenesis: an initial cue that orients the axis of polarity, mediated by the interaction of the cells with the ECM; and then formation of a lumen, regulated by the generation, transport and exocytosis of membrane carriers containing apical surface components [13]. In the MDCK-3D system, the interaction between integrins and the ECM generates a signaling cascade required for the orientation of polarity [2]. Since laminin is essential for this first event [4, 5], the efficiency of this process hinges on the presence or not of laminin in the ECM. We show that the presence of laminin in the ECM (contributed here by LGF Matrigel) efficiently induced polarization of MDCK cells (Fig 1). Furthermore, inhibition of apoptosis by forced expression of Bcl-2 or a general caspase inhibitor did not block lumen formation. When laminin was not supplied (i.e. when cells where grown in collagen), polarization was slower, resulting in the requirement of apoptosis for the lumen clearance.
After this initial establishment of the axis of polarity, the most important event is the formation of the apical membrane and the lumen. Recent results have suggested that membrane trafficking is needed for the genesis of the apical surface [14]. We have characterized a Cdc42-dependent mechanism needed for formation of the apical membrane and lumen in MDCK cysts [7]. We show that disruption of Cdc42 delayed formation of the apical membrane and central lumen, as described before [7], accompanied by apoptosis specifically in the luminal cells resulting in the clearance of the luminal space(Fig 2).
The mammary-derived cell line MCF-10A represents another widely used 3D model to study epithelial morphogenesis in vitro [15]. MCF-10A cells grown in 3D cultures form acini-like structures with a hollow lumen that clears by apoptosis. Although this in vitro model presents a morphogenetic program similar to the 3D-MDCK in collagen, with slow polarization and apoptosis to clear the lumen, this in vitro model uses Matrigel (laminin rich) as the ECM support. However, it is noteworthy that MCF-10A cells lack tight junctions and therefore never fully polarize [16], which might explain the requirement for apoptosis to clear the lumen and thus the differences observed between the MCF10A model and the 3D-MDCK model described in this paper. When caspase-dependent apoptosis is prevented in the MCF-10A model, cells can eventually die by alternative mechanisms, such as autophagy [17]. We saw no evidence of other types of cell death in our MDCK model in any experiments reported in this paper, even when caspase-dependent apoptosis was inhibited. However we cannot rule out the possibility of alternative mechanisms of cell death at later stages of cysts formation when caspase-dependent apoptosis was inhibited.
In conclusion, we have shown that apoptosis is a control mechanism that ensures the clearance of the lumen in epithelial morphogenesis when the acquisition of apico-basal polarity is disrupted, or delayed, resulting in a mis-coordination of cell polarity with cell proliferation.
Experimental Procedures
Antibodies and Reagents
Primary antibodies against: β-tubulin (mouse, Chemicon); β-catenin (rabbit, Sigma); Cdc42 (mouse, BD Transduction Lab); Caspase3 (rabbit, Upstate); ZO-1 (rat R40.76; gift from B Stevenson); gp135 (mouse, gift of George Ojakian). Secondary antibodies were highly cross-absorbed anti-mouse AlexaFluor 546 and anti-rabbit AlexaFluor 488 (Molecular Probes,), goat anti-rabbit or anti-mouse HRP (Jackson). Actin filaments were stained with AlexaFluor 488, 546 or 633 phalloidin (Molecular Probes). Nuclei were stained with Hoescht (Molecular Probes). General caspase inhibitor Q-VD-OPh (R&D Systems)
Biochemistry
Preparation of lysates, immunoblotting and coimmunoprecipitation from cysts were described before [4, 5]
Cells
MDCK cells were grown in 2D or in collagen as described [4, 7]. MDCK stably expressing Bcl2 were made by cotransfection with blasticidin resistant gene. After 3 weeks in selective medium clones were isolated and analyzed by WB.
To prepare cysts in Matrigel, cells were trypsinzed to a single cell suspension and plated at low density (3 × 104/ml) or high density (3 × 105/ml) in 2% Matrigel. 250 µl of cells in Matrigel were plated in 8 well coverglass chambers (Nalge Nunc) covered with Matrigel. Cells were fed every 2d and grown for 2–7 d until cysts with lumen formed.
Microscopy
Immunofluorescence of cysts was previously described [4, 5]. Cysts were analyzed on a Zeiss 510 LSM. Cysts with gp135 staining at the interior surface and β-catenin facing the ECM were identified as normal lumens (interior AP pole). Cysts that had gp135 either absent, in small multiple lumens or at the periphery were considered as abnormal lumens. Cysts with caspase3 positive cells in the lumen were identified as apoptotic-positive in all quantifications. Per condition >100 cysts/experiment were analyzed, SD calculated and statistical significance was by paired Student's _t_- test.
Time-Lapse Images
Cells were grown in Matrigel-coated 24-well plates or collagen plates, and observed using a Zeiss Axiovert S-100 microscope (Carl Zeiss, Thornwood, NY). Time-lapse movies were recorded beginning 8 h after plating and ending after 166 h (collagen) or 212 h (Matrigel) of culture. We used a 10_ A-Plan objective lens on a Cohu high-performance charge-coupled device camera. Light exposure was regulated by a Ludl shutter and controller, which also controlled the Ludl x-y-z–motorized stage. Temperature and carbon dioxide were held at 37°C and 5%, respectively, by using a CTI Controller 3700 and Temperature Control 37.2 combination (Carl Zeiss). Images were acquired every 15 min by using a custom macro implemented in OpenLab 4.0.2 (Improvision, Lexington, MA). Images were recorded using OpenLab LIFF series (Improvision) and compiled into Quick-Time movies 8 frames/sg. Cell movement was analyzed with MetaMorph software (Molecular Devices, Sunnyvale, CA).
RNAi
25-nt siRNA duplexes targeting mRNA sequences of canine Cdc42, were described before [7]. Briefly, siRNA duplexes were purchased from Invitrogen. The specificity of Cdc42 down-regulations was further checked by Western blotting analysis. MDCK cells plated on 10 cm plates were transfected with 20 nM of Cdc42 siRNA duplex, or scrambled siRNA using Lipofectime 2000™ (Invitrogen) and incubated for 24h after transfection in the same plates. Then the cells were plated to form cyst for 2–7 days as described above, and prepared for confocal and western blot analysis.
Supplementary Material
01
02
03
Acknowledgements
We thank Carmen Martin Ruiz-Jarabo for comments on the manuscript, and members of the Mostov lab for discussion. Supported by HFSP fellowship LT00426/2004-C to FM-B, NIH grants to KM, a grant from the Ministerio de Educación y Ciencia (BFU2006-01925) to MAA. An institutional grant from the Fundación Ramón Areces to CBMSO is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 3.Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ. Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int. 1999;55:168–178. doi: 10.1046/j.1523-1755.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 5.Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andriani F, Margulis A, Lin N, Griffey S, Garlick JA. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Invest Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu W, Fang X, Ewald A, Wong K, Hunt CA, Werb Z, Matthay MA, Mostov K. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell. 2007;18:1693–1700. doi: 10.1091/mbc.E06-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 10.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- 11.Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med. 2000;11:199–215. doi: 10.1177/10454411000110020401. [DOI] [PubMed] [Google Scholar]
- 12.Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 13.Mostov K, Martin-Belmonte F. Developmental biology: the hole picture. Nature. 2006;442:363–364. doi: 10.1038/442363a. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Belmonte F, Mostov K. Phosphoinositides control epithelial development. Cell Cycle. 2007;6:1957–1961. doi: 10.4161/cc.6.16.4583. [DOI] [PubMed] [Google Scholar]
- 15.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 16.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 17.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03