Regulation of Human Neural Precursor Cells by Laminin and Integrins (original) (raw)
. Author manuscript; available in PMC: 2008 Jun 3.
Published in final edited form as: J Neurosci Res. 2006 Apr;83(5):845–856. doi: 10.1002/jnr.20778
Abstract
Deciphering the factors that regulate human neural stem cells will greatly aid in their use as models of development and as therapeutic agents. The extracellular matrix (ECM) is a component of stem cell niches in vivo and regulates multiple functions in diverse cell types, yet little is known about its effects on human neural stem/precursor cells (NSPCs). We therefore plated human NSPCs on four different substrates (poly-L-ornithine, fibronectin, laminin, and matrigel) and compared their responses with those of mouse NSPCs. Compared with the other substrates, laminin matrices enhanced NSPC migration, expansion, differentiation into neurons and astrocytes, and elongation of neurites from NSPC-derived neurons. Laminin had a similar spectrum of effects on both human and mouse cells, highlighting the evolutionary conservation of NSPC regulation by this component of the ECM. Flow cytometry revealed that human NSPCs express on their cell surfaces the laminin-binding integrins α3, α6, α7, β1, and β4, and function-blocking antibodies to the α6 subunit confirmed a role for integrins in laminin-dependent migration of human NSPCs. These results define laminin and its integrin receptors as key regulators of human NSPCs.
Keywords: neural stem cell, progenitor, neuron, ECM, laminin, integrin, fibronectin
Neural stem/precursor cells (NSPCs) have garnered much attention, because they can reveal clues about nervous system development (see, e.g., Cayouette et al., 2003; Gabay et al., 2003) and possibly can be used as therapeutic agents. NSPCs have the potential to ameliorate various neurological diseases and injuries, such as Parkinson’s disease, amyotrophic lateral sclerosis, Alzheimer’s disease, stroke, and traumatic brain injury (Shiha-buddin et al., 1999). Endogenous NSPCs in the brain reside in specialized compartments termed “niches,” and recent studies have clarified that cells such as astrocytes and endothelial cells in the niche direct NSPC proliferation and differentiation (Song et al., 2002; Shen et al., 2004). Cells in the niche also secrete an extracellular matrix (ECM) that may regulate NSPC function (Mercier et al., 2002; Alvarez-Buylla and Lim, 2004).
Studies of a wide variety of cells, including postmitotic neurons, have shown that ECM governs myriad cell functions, including mitosis, apoptosis, migration, cytoplasmic signalling, and transcription (Howe et al., 1998; Giancotti and Ruoslahti, 1999; De Arcangelis and Georges-Labouesse, 2000; Bokel and Brown, 2002; Stupack and Cheresh, 2002). Recently, several groups have shown that ECM affects rodent NSPC proliferation, migration, and differentiation. Proliferation of mouse neuroepithelial cells and migration of mouse cerebellar neural precursor cells in vitro (Drago et al., 1991; Kearns et al., 2003), as well as migration of neural precursors through the mouse rostral migratory stream in vivo (Murase and Horwitz, 2002), are influenced by the ECM. ECM effects on mouse neural precursor cells have been linked to activation of β1 integrins on the cell surface, and recent data implicate involvement of the MAP kinase pathway (Jacques et al., 1998; Campos et al., 2004; Tate et al., 2004).
Despite growing evidence that ECM and integrin signalling can regulate rodent NSPCs, almost nothing is known about their effects on human NSPCs. Wu and colleagues have shown that treating human NSPCs with soluble laminin as a priming step can increase the generation of cholinergic neurons (Wu et al., 2002; Tarasenko et al., 2004), but no studies have compared responses of human NSPCs across substrates or defined the integrins involved in ECM-mediated effects.
In this study, we assayed human cortical NSPC behavior on four different substrates: a non-ECM positively charged polymer (poly-L-ornithine; PLO), two purified ECM proteins (fibronectin and laminin), and a secreted ECM that is a complex mixture of proteins (matrigel). By using these substrates, we were able to compare cell–substrate interactions that varied from those based solely on charge to those mediated by binding to single or multiple ligands. We compared the responses of human cortical NSPCs to those of mouse cortical NSPCs and found that they behave similarly. Our results show that the ECM protein laminin stimulated human NSPCs to a greater extent than the other substrates and that the human α6 laminin-binding integrin subunit is involved in these effects. The results of this study show that human NSPCs are regulated by laminin, that specific integrins mediate the laminin effects, and that the effects of ECM on NSPCs are conserved through evolution.
MATERIALS AND METHODS
Cells and Media
Human NSPCs were isolated from cadaveric postnatal brain cortices by the National Human Neural Stem Cell Resource and grown either as adherent cultures on flasks coated with 10 µg/ml fibronectin (human plasma; BD Biosciences, Bedford, MA) or in suspension as neurospheres (Palmer et al., 2001; Schwartz et al., 2003). Three different sets of human NSPCs were used and are designated SC23 (23 weeks gestation, 1-day-old premature infant), SC27 (23 week gestation, 2-week-old premature infant), and SC30 (25 week gestation, 1-day-old premature infant; Schwartz et al., 2003). Human NSPC base medium comprised DMEM:F12 (Gibco/Invitrogen, Carlsbad, CA), 20% BIT 9500 [bovine serum albumin (BSA), insulin, and transferrin; Stem Cell Technologies, Vancouver, British Columbia, Canada], and 1% antibiotic/antimycotic (penicillin/streptomycin/amphotericin; Gibco/Invitrogen). Growth medium was prepared from base medium by adding 40 ng/ml epidermal growth factor (EGF; BD Biosciences), 40 ng/ml fibroblast growth factor (FGF; BD Biosciences), and 40 ng/ml platelet-derived growth factor (PDGF; Peprotech, Rocky Hill, NJ). Glial conditioned medium was prepared from human glial cells as previously described (Schwartz et al., 2003) and used Neurobasal medium (Gibco/Invitrogen) with B–27 supplement (Gibco/Invitrogen) as a base medium. Differentiation medium was prepared from base medium and glial conditioned medium (1:1) with 20 ng/ml brain-derived neurotrophic factor (BDNF; Peprotech), 20 ng/ml neurotrophin-3 (NT3; Peprotech), 1% fetal bovine serum (FBS; Gibco/Invitrogen), 2.5 ng/ml FGF, and 0.1 µM all-trans-retinoic acid (Sigma, St. Louis, MO; Palmer et al., 2001). Cells were routinely passaged 1:2, and all cells were used at low passage numbers (<10). Human cells were plated as neurospheres or as dissociated cells (50,000–300,000 cells/ml; 12,500–75,000 cells/cm2) onto substrate-coated coverslips for experiments. When plated as dissociated cells, equal numbers of viable cells (determined by trypan blue staining) were plated onto the different substrates.
Mouse NSPCs were isolated from embryonic day 12.5 (E12.5) C57BL/6 (Charles River, Wilmington, MA) mouse cortices and grown in suspension to form neurospheres with medium previously described (Reynolds et al., 1992; Tropepe et al., 1999). Growth medium included 20 ng/ml EGF, 10 ng/ml FGF2, 2 µg/ml heparin (Sigma). In selected experiments, mouse NSPCs were differentiated for 10 days in human NSPC differentiation medium (described above). All experiments were carried out with mouse NSPCs at the secondary sphere stage, and cells were plated onto substrate-coated coverslips either as spheres or as dissociated cells (100,000–300,000 cells/ml; 25,000–75,000 cells/cm2; Reynolds et al., 1992). When plated as dissociated cells, equal numbers of viable cells (determined by trypan blue staining) were plated onto the different substrates.
Substrates
German glass coverslips (Assistant/Carolina Biological Supply, Burlington, NC) were coated with substrate for plating NSPCs. Substrates were used at the following concentrations: poly-L-ornithine (30,000–70,000 MW) 0.001% in DMEM/F12; fibronectin (human plasma; BD Biosciences) 10 µg/ml (2.5 µg/cm2) in EMEM (in some experiments fibronectin was also used at 20 µg/ml and 50 µg/ml with equivalent results); laminin (BD Biosciences) 20 µg/ml (5 µg/cm2) in EMEM; matrigel (BD Biosciences) 167 µg/ml (41.75 µg/cm2) in EMEM (at this concentration, matrigel does not form a gel). For laminin and matrigel coating, coverslips were pretreated for 5 min with poly-D-lysine [10 µg/ml (2.5 µg/cm2) in water; Sigma]. Poly-L-ornithine, fibronectin, and laminin were left on coverslips overnight in a humidified 37°C, 5% CO2 incubator, and then the substrate was removed and coverslips were rinsed with PBS. Matrigel was left on coverslips for 2 hr at room temperature, and coverslips were subsequently rinsed with EMEM, left in EMEM overnight in a humidified 37°C, 5% CO2 incubator, then rinsed in EMEM before plating of cells. Laminin was isolated from the Engle-breth-Holm-Swarm mouse tumor and is laminin 1 (Burgeson et al., 1994). Matrigel is an ECM secreted by the Englebreth-Holm-Swarm mouse tumor cell line and contains laminin, collagen, growth factors, and several other molecules. Based on the percentage of laminin in matrigel (Becton-Dickinson), the concentration of the laminin component is ~94 µg/ml on the matrigel-coated coverslips used here, which is greater than the concentration of purified laminin we used to coat cover-slips (20 µg/ml).
Immunostaining and Imaging
Antibodies for immunostaining were as follows: anti-glial fibrillary acidic protein (GFAP) polyclonal, 1:1,000 (Chemicon, Temecula, CA); anti-microtubule-associated protein 2 (MAP2; HM2) monoclonal, 1:100 (Sigma, St. Louis, MO); anti-class III beta-tubulin (TuJ1) polyclonal, 1:5,000 (Research Diagnostics, Flanders, NJ); anti-nestin used for mouse cells (rat 401) monoclonal, 1:1,000 (Developmental Studies Hybridoma Bank developed under the auspices of the NICHD, Iowa City, IA); anti-nestin used for human cells, polyclonal, 1:200 (Chemicon); anti-sox2 (Y17) polyclonal, 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA). Antibodies for flow cytometry are described below. Nuclei were stained with Hoechst 33342 (Molecular Probes, Eugene, OR), 2 µg/ml in phosphate-buffered saline (PBS). For immunofluorescence staining, the cells were fixed with paraformaldehyde (4% paraformaldehyde, 5 mM MgCl2, 10 mM EGTA, 4% sucrose in PBS) for 15 min, stained as previously described (Flanagan et al., 2001), and viewed on a Nikon E600 upright epifluorescence microscope. Images were captured with a Spot RT camera and software (Diagnostic Instruments, Sterling Heights, MI). Phase-contrast images of live cells were captured by using a Nikon TS100 inverted microscope and a Nikon CoolPix950 digital camera. Images were processed and compiled in Adobe Photoshop 5.0.
Quantitation
For NSPC migration experiments, spheres within individual experiments were matched for size prior to migration. The diameter of neurospheres was quantified by measuring the diameter of the sphere at its widest point. After NSPC migration out of the spheres, the diameter was taken at the widest point of the halo of migrating cells (with the sphere in the center). Diameters and distances were measured in NIH Image quantitation software. For NSPC expansion experiments, numbers of cells on the different substrates were quantified from randomly selected fields. For NSPC differentiation experiments, neurons and astrocytes were stained with specific antibodies, and all cell nuclei were counterstained with Hoechst. Neurons and astrocytes were manually counted and were expressed as a percentage of the total cells (determined by counting Hoechst-stained nuclei). To maintain strict guidelines for counting of stained cells, we restricted cells in our analyses to those that met the following criteria: 1) cells counted as neurons expressed the neuronal markers MAP2 or TuJ1, showed no reactivity for the astrocyte and precursor marker GFAP, and had at least one neurite that was longer than the cell body; 2) cells counted as astrocytes were GFAP-positive cells that showed no reactivity for MAP2 or nestin (a marker of NSPCs) and exhibited a filamentous pattern of GFAP reactivity in the cytoplasm.
Neurites were quantified from images of neurons stained with antibodies to MAP2 (which detects all isoforms of MAP2 present in neurites, MAP2a, -b, and -c) or TuJ1 (which detects class III beta-tubulin present in newly formed neurites). We used three independent measures: the number of primary neurites, the total neurite length, and the number of neurite branches. Neurites that were fasciculated or could not be accurately assigned to specific cells were omitted from analysis. The number of primary neurites per cell was determined by counting the neurites that extended directly from the cell body. Total neurite length per cell was quantified by measuring the length of all neurites and branches on each cell. The number of neurite branches was quantified by counting the number of points along the neurites where one neurite gave rise to another. The total number of neurite branches for each neuron was divided by the total neurite length of that cell to derive the number of branches per micrometer of neurite. NIH Image was used for quantitation, and all data were graphed in Kaleidagraph. Imaged cells were selected at random, and all visible processes of selected neurons were imaged. All statistical analyses utilized unpaired Student’s _t_-test and were from three or more independent measurements.
Flow Cytometry
Samples from proliferating confluent cultures, passages 3–6, were handled as described previously (Schwartz et al., 2003). Briefly, cells were harvested as a single cell suspension with Cell Dissociation Buffer (Gibco/Invitrogen), incubated with labeled primary antibodies, and analyzed on a FACS Vantage cell sorter equipped with an Enterprise 488 nm argon laser (BD Biosciences). Color compensation was preliminarily set by using Calibrite beads (BD Biosciences), and individual samples were optimized by using single positive antibody labeling for NCAM (CD56), compared with IgG control (clone MOPC), with isotype-matched antibodies as negative controls for each integrin antibody. Two-color live-gating acquisition was used to optimize settings and acquire data. Up to 30,000 events were collected and stored electronically for subsequent analysis in Cytomation Summit 3.1 software. The antibodies used were as follows (all BD Biosciences, and all at 1:10 dilution except as noted): integrin α1, clone SR84; integrin α2, clone AK-7; integrin α3, clone C3II.1; integrin α4, clone 9F10; integrin α5, clone IIa1; integrin α6, clone GoH3; integrin α7, clone H36 (1:100), kind gift of Dr. Stephen J. Kaufman; integrin β1, clone MAR4; integrin β4, clone 439-9B.
Integrin Blocking
Human NSPC spheres (SC27) were allowed to adhere to coverslips that had been coated with substrate, then preblocked with BSA (1% BSA in PBS for 1 hr). Spheres were then incubated with the disintegrin echistatin (2 µM; stock solution 200 µM in DMEM:F12; Sigma) or a function-blocking α6 integrin antibody (clone GoH3, 10 µg/ml; Serotec, Oxford, United Kingdom) in regular growth medium. Control spheres were incubated in regular growth medium for echistatin experiments and in isotype-matched control antibody (rat IgG2a; Chemicon) for α6 integrin experiments. Spheres were imaged before the addition of integrin blocker and again after incubating in blocker for 1, 2, 4, and 24 hr. For experiments with α6 blocking antibody, individual spheres were tracked over time, and NSPC migration was determined by measuring the distance traveled by the front of migrating cells over a period of 24 hr. At the end of 24 hr, all cells were fixed with paraformaldehyde as described above.
RESULTS
Cortical NSPC Motility Is Enhanced on Laminin Matrices
Human or mouse NSPCs were isolated from the developing cerebral cortex and grown by using established protocols to enrich for neural stem cells and NSPCs (Reynolds et al., 1992; Palmer et al., 2001; Schwartz et al., 2003). Immunostaining of cultures with antibodies to markers of undifferentiated NSPCs (nestin and sox2; Cai et al., 2002) confirmed that over 80% of the cells were NSPCs (Fig. 1). We then assessed the motility of human and mouse NSPCs by using a previously described bulk neurosphere migration assay (Jacques et al., 1998; Kearns et al., 2003). We plated human (SC23 or SC27; see Materials and Methods for description of the different human NSPC cultures) or mouse neurospheres in growth medium onto coverslips coated with PLO, fibronectin, laminin, or matrigel. Individual NSPCs migrated radially away from the sphere, resulting in a rim of cells that formed a monolayer around the sphere, and greater migration occurred on the ECM molecules (fibronectin, laminin, and matrigel) than on PLO (Fig. 2A,C). A quantitative measure of migration (see Materials and Methods) confirmed that the ECM proteins encouraged greater migration than PLO and that, within the ECM components, greater migration occurred on laminin and matrigel than on fibronectin (Fig. 2B,D). Matrigel is largely composed of laminin but also contains the cell adhesion molecule collagen IV. The fact that migration was similar on matrigel and purified laminin, coupled with the recent finding that collagen I and collagen IV stimulate little to no migration of mouse NSPCs (Tate et al., 2004), suggests that laminin is the primary migration-inducing factor in matrigel. For the experiments described here, the concentration of laminin on the matrigel-coated coverslips was greater than the concentration of purified laminin coated onto coverslips, so a decreased effect on matrigel compared with laminin is not due to lower amounts of laminin on the matrigel-coated coverslips but may be due to modifying effects of other matrigel components (see Materials and Methods).
Fig. 1.
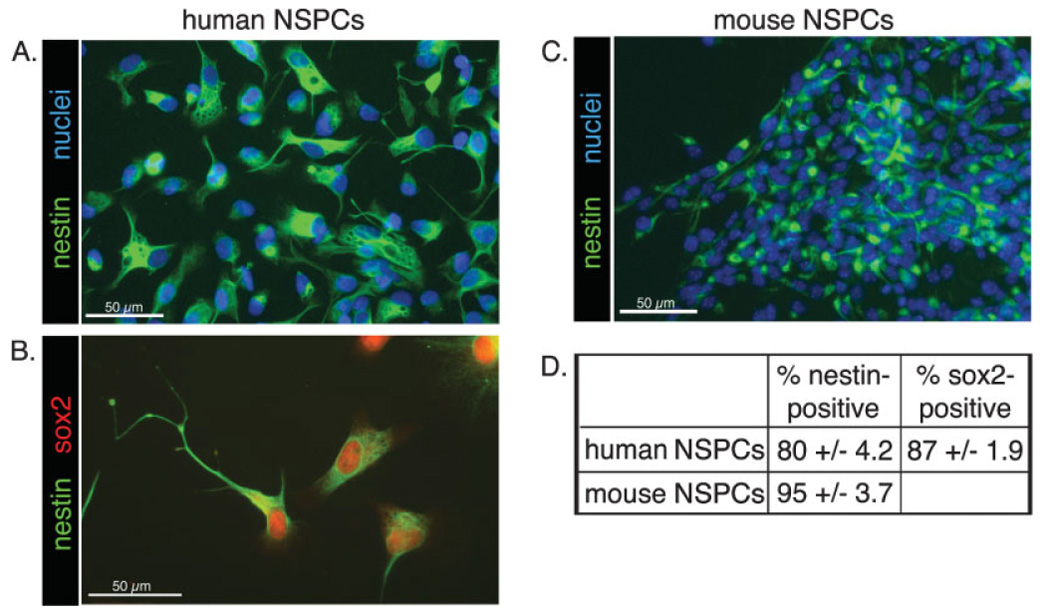
Most cultured human and mouse cortical cells express NSPC markers. A–C: Cultured cortical cells were immunostained to detect the NSPC markers nestin and sox2 (B). Nuclei were counterstained with Hoechst (A,C). D: Positively stained cells were expressed as a percentage of the total number of cells as determined by Hoechst-stained nuclei ± SEM.
Fig. 2.
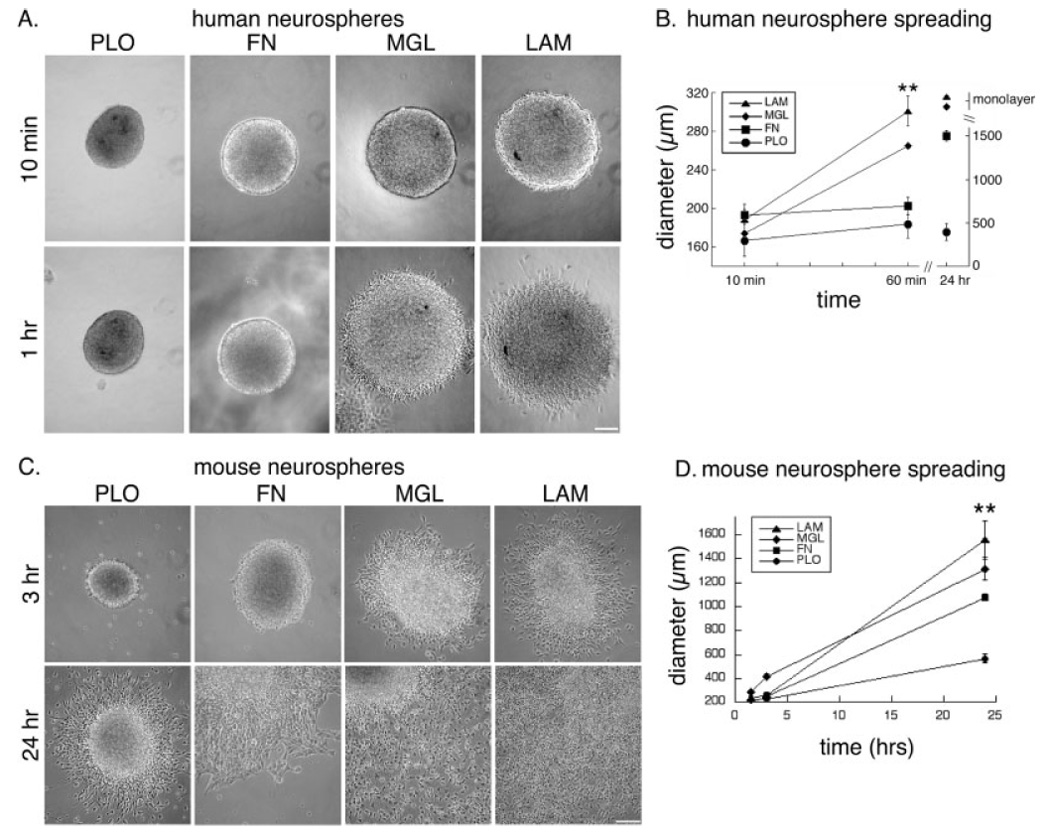
Migration of human and mouse cortical NSPCs out of neurospheres is enhanced on laminin-containing matrices. A: Human neurospheres (SC23) were imaged at 10 min and 1 hr after plating onto the different substrates (PLO, poly-L-ornithine; FN, fibronectin; MGL, matrigel; LAM, laminin). B: Migration of human NSPCs (SC23 and SC27) was quantified by measuring the diameter of the sphere and surrounding rim of migrating cells over time. Human NSPCs on matrigel and laminin formed a dense monolayer by 24 hr that prevented accurate quantitation of migration (**P < 0.01 for diameter on LAM vs. FN or PLO; error bars represent SEM). C: Mouse neurospheres were imaged at 3 hr and 24 hr after plating (neurospheres are located in the upper left corners of the 24-hr images of cells on FN, MGL, and LAM to show the extent of cellular migration from the sphere). D: Quantitation of mouse NSPC migration over a 24-hr period was performed as described for human NSPCs (**P < 0.01 for diameter on LAM or MGL vs. FN or PLO and for FN vs. PLO; error bars represent SEM). Scale bars = 100 µm.
Cortical NSPC Expansion Is Greater on Laminin Matrices
To assess the effect of substrates on the overall growth of NSPC cultures, we plated identical numbers of viable cells on substrate-coated coverslips and assayed the total number of cells on the different substrates over time. Because cell–substrate interactions affect cell attachment, division, and death, all of which contribute to the total number of cells on a substrate, we refer to the increase in the number of cells on the substrates as “cell expansion.” Images and quantitation of human NSPCs (SC23) grown on the different substrates for 5 days showed that greater numbers of cells were present on the laminin-containing matrices than on fibronectin or PLO (Fig. 3A,B). Human NSPCs on laminin and matrigel were predominantly elongated, with occasional rounded, dividing cells present. In contrast, human NSPCs grown on PLO for 5 days were less well spread and were surrounded by cell debris; a comparison of the day 1 and day 5 PLO results (Fig. 3B) suggests that cells on this substrate die over time. Cells on laminin and matrigel increased in number by ~5-fold over the same period, indicative of cell proliferation (and a doubling time of less than 48 hr). Similar findings were obtained with two additional human NSPC isolates (SC27 and SC30). Greater numbers of cells were present on laminin-containing matrices compared with other substrates when human NSPCs (SC23) were plated over a 6-fold range of densities (from 50,000–300,000 cells/ml; 12,500–75,000 cells/cm2) and cultured for 5 days or when cells (SC23, SC27, SC30) were cultured for long periods (up to 25 days; data not shown).
Fig. 3.
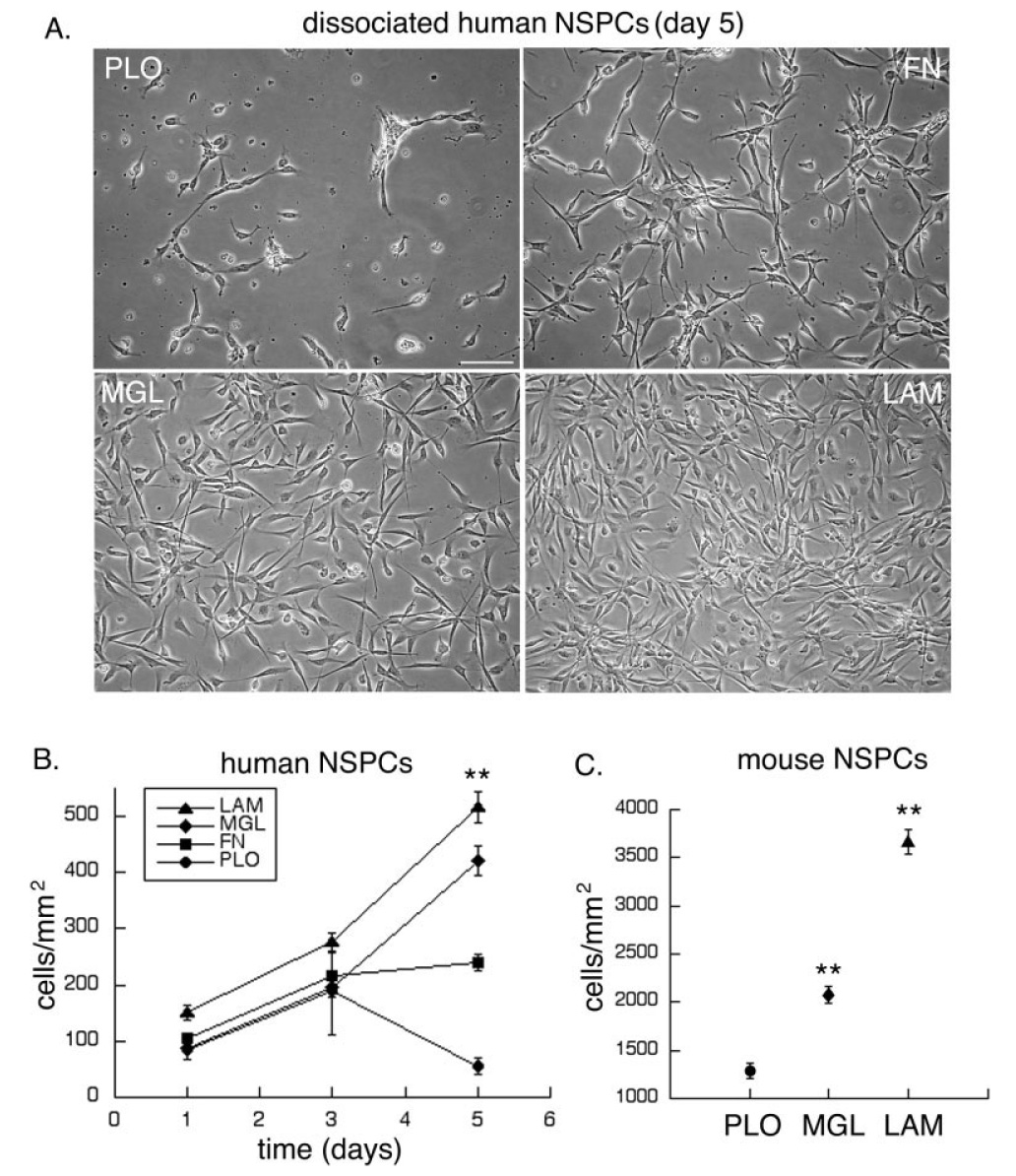
Expansion of human and mouse NSPCs is greater on laminin-containing matrices. Equal numbers of dissociated NSPCs were plated on substrate-coated coverslips, then cultured for varying lengths of time. A: Images of human NSPCs (SC23) cultured for 5 days on the different substrates. B: Total number of human NSPCs per surface area was counted on the different substrates over time (**P < 0.01 for cells/mm2 on LAM, MGL, or FN vs. PLO and for LAM or MGL vs. FN; P < 0.05 for LAM vs. MGL; error bars represent SEM). C: Mouse NSPC numbers were determined after culturing the cells on the different substrates for 3 days (**P < 0.01 for cells/mm2 on LAM or MGL vs. PLO and for LAM vs. MGL; error bars represent SEM). Scale bar = 100 µm.
To determine whether laminin also encourages expansion of mouse NSPCs, equal numbers of cells were plated in growth medium on the different substrates and the number of cells was quantified after 3 days. Greater numbers of mouse NSPCs were present on laminin than on the other substrates (Fig. 3C). Therefore, human and mouse cortical NSPC expansion is stimulated by laminin.
More Neurons and Astrocytes Derived From Human Cortical NSPCs Are Present on Laminin Matrices, Independent of the Effects of Laminin on Cell Density
We next tested whether substrate might affect the differentiation of NSPCs into neurons and glia. Equivalent numbers of viable human NSPCs (SC27) were plated onto substrate-coated coverslips and grown under differentiation conditions (see Materials and Methods). After 20 days, many cells on laminin stained with the neuronal marker MAP2, whereas fewer MAP2-positive neurons were generated on fibronectin or matrigel (Fig. 4A,C, red diamonds). Likewise, greater absolute numbers of neurons formed on laminin compared with fibronectin or matrigel, and similar results were obtained with all three human NSPC isolates. Because neurons are considered postmitotic cells, the effect of laminin on neuron numbers is not likely to be due to enhanced proliferation of neurons once formed, but instead is likely to be related to the generation of new neurons from human NSPCs or their increased survival on laminin once differentiated. We differentiated mouse NSPCs using the same conditions employed for human NSPCs and found that greater numbers of neurons were present on laminin than on fibronectin (Fig. 4C). Therefore, both human and mouse NSPCs derive greater numbers of neurons on laminin compared with the other substrates.
Fig. 4.
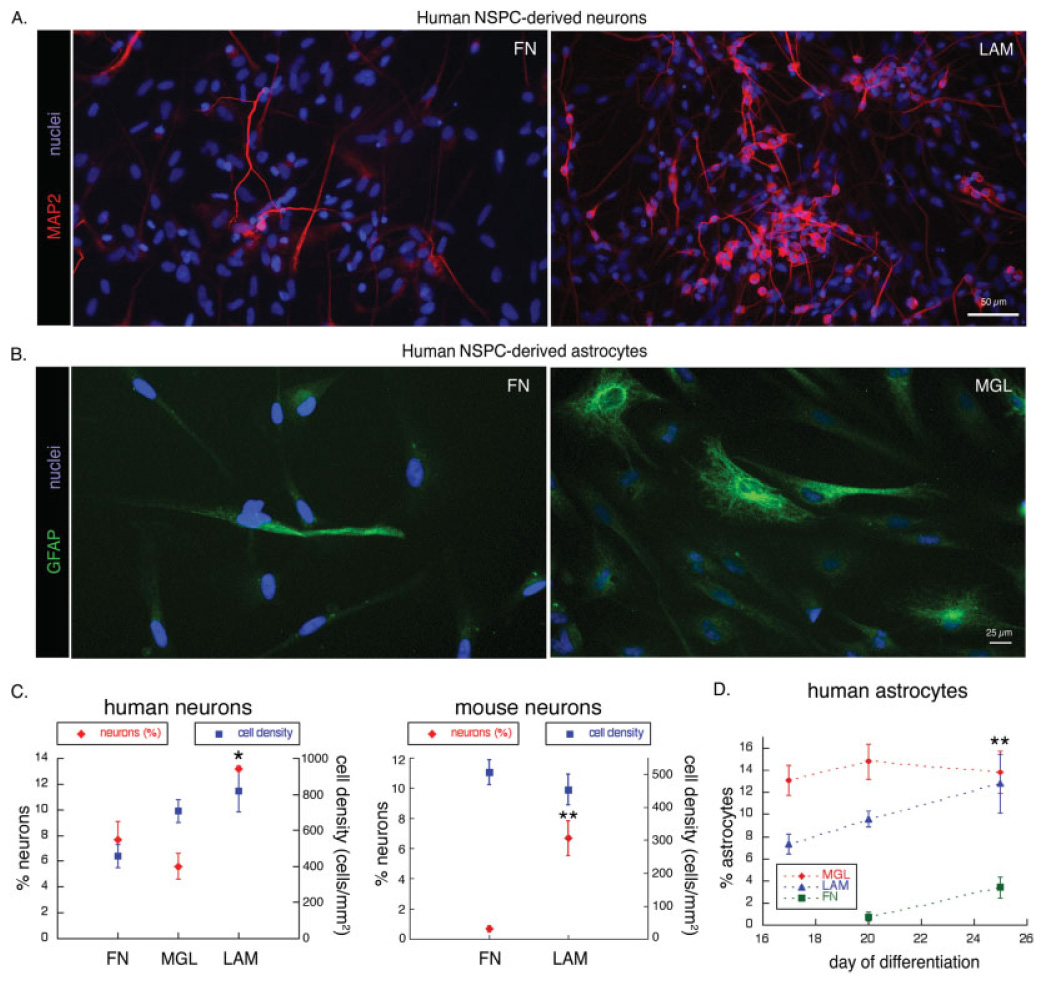
Greater numbers of neurons and astrocytes are derived from human NSPCs on laminin. A: Human NSPCs (SC27) were differentiated for 20 days on FN or LAM and immunostained to detect expression of the neuronal marker MAP2. All nuclei were stained by Hoechst. Similar patterns of expression of neuronal markers were obtained with two other human NSPC isolates (SC23 and SC30; data not shown). B: Human NSPCs (SC23) were differentiated for 20 days on FN or MGL and astrocytes visualized by immunostaining for GFAP. C: Human NSPCs were differentiated for 20 days on the various substrates, and cells expressing the neuronal marker MAP2 were counted and expressed as a percentage of the total number of cells (determined by Hoechst stained nuclei; *P < 0.05 for percent neurons on LAM vs. FN and P < 0.01 for percentage neurons on LAM vs. MGL; error bars represent SEM). The percentage of neurons (red diamonds) and total cell density (blue squares) are graphed together to illustrate that the effect of substrate on neuronal number is independent of cell density. Mouse NSPCs were differentiated for 10 days on the various substrates, and cells expressing neuronal markers (either MAP2 or TuJ1) were counted and expressed as a percentage of the total number of cells (red diamonds; **P < 0.01 for percent neurons on LAM vs. FN; error bars represent SEM). The density of mouse NSPCs did not differ on LAM vs. FN (blue squares). D: Numbers of GFAP-positive astrocytes were counted after 17–25 days of differentiation on the various substrates and expressed as a percentage of the total number of cells (**P < 0.01 for percent astrocytes on LAM or MGL vs. FN; error bars represent SEM).
Cell density can influence the generation of neurons from NSPCs. We therefore assessed this parameter in our experiments and found that cell density could not account for the differences in neuronal numbers on the substrates. The density of human cells (Fig. 4C, blue squares) on matrigel was similar to that on laminin (laminin 900 ± 127 cells/mm2, matrigel 702 ± 68 cells/mm2, P > 0.1), but ~2.4-fold more neurons formed on laminin than matrigel (Fig. 4C, red diamonds; P < 0.01). In additional experiments, we plated human NSPCs at high density on fibronectin, but after the time required for differentiation the cell density was always lower than that on laminin, making this approach not instructive. Differentiated mouse NSPCs had similar densities of cells on laminin and fibronectin (Fig. 4C, blue squares; laminin 454 ± 47 cells/mm2, fibronectin 507 ± 38 cells/mm2; _P_ > 0.1), whereas ~10-fold more neurons formed on laminin than on fibronectin (Fig. 4C, red diamonds; P < 0.01). These results demonstrate that the amplification of neurons from both human and mouse NSPCs on laminin is not due to a greater cell density on this substrate.
Generation of astrocytes on the different substrates was assessed by expression of GFAP, and although GFAP has traditionally been used as an astrocyte marker, recent reports have documented its expression in certain precursor cells (Doetsch et al., 1999; Seri et al., 2001; Imura et al., 2003; Sanai et al., 2004). The antibody we used to detect GFAP in our human and mouse cultures marked few, if any, nestin-positive NSPCs and instead labeled cells with characteristic astrocytic morphologies (flat-spread or stellate cells with filamentous GFAP staining in the cytoplasm). We therefore refer to the GFAP-positive cells in our analysis as astrocytes. Equivalent numbers of viable human NSPCs were plated onto substrate-coated coverslips, grown under differentiation conditions for 17–25 days, and stained with antibodies to detect GFAP. As with neurons, astrocyte numbers were also elevated on laminin-containing matrices (Fig. 4B). Quantitation revealed that at day 17, the percentage of astrocytes was higher on matrigel than on laminin; by day 25, the percentages were similar on these two matrices (Fig. 4D). The percentage of astrocytes on fibronectin increased between day 20 and day 25 but remained significantly lower than that on the laminin-containing matrices. Unlike neurons, astrocytes are not terminally postmitotic, so some of the increase in astrocyte number over time could be due to increased proliferation.
Laminin Enhances the Total Neurite Length of Neurons Derived From Cortical NSPCs
NSPCs were plated on substrate-coated coverslips, differentiated, and stained with antibodies (MAP2 or TuJ1) that label both axons and dendrites (collectively termed neurites) at early stages of neuronal differentiation. Neurite outgrowth was analyzed by measuring the number of primary neurites extending from the cell body, the total neurite length per cell, and the number of branch points per neurite length (see Materials and Methods). Human neurons on laminin had more elaborate networks of neurites and greater total neurite length per cell than neurons on fibronectin or matrigel (Fig. 5A,B). Neurons on laminin also had slightly more primary neurites, but this effect was not statistically significant (number of primary neurites per cell body ± SEM: laminin 3.0 ± 0.45, matrigel 2.1 ± 0.26, fibronectin 2.3 ± 0.21; P ≥ 0.1 for all comparisons). There was also no effect of any of the substrates on neurite branching [number of branches per neurite length (mm) ± SEM: laminin 8.0 ± 2.1, matrigel 4.8 ± 2.2, fibronectin 10.5 ± 4.2; P > 0.1 for all comparisons].
Fig. 5.
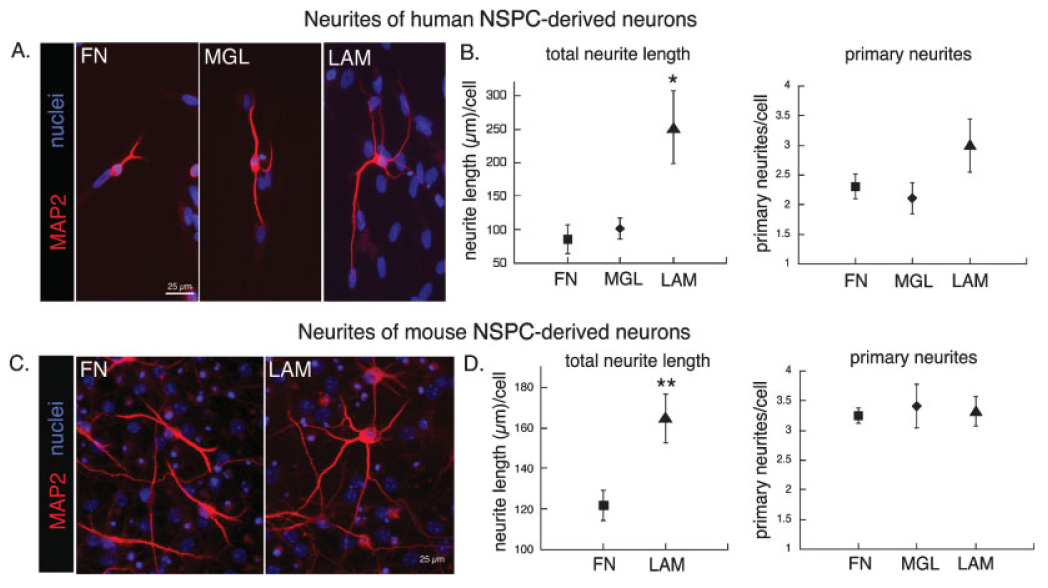
Neurons generated from human and mouse NSPCs extend longer neurites on laminin. Human or mouse NSPCs were differentiated on the various substrates to allow formation of neurons with concomitant extension of neurites. A: Human NSPCs (SC23) were differentiated for 25 days and neurites visualized by immunostaining for MAP2. Nuclei were counterstained with Hoechst. B: Total neurite length and number of primary neurites were determined for each human NSPC-derived neuron (see Materials and Methods for details of quantification; *P < 0.05 for total neurite length on LAM vs. MGL or FN; error bars represent SEM). No difference was found in the number of neurite branches (see text). C: Mouse NSPCs were differentiated for 10 days on the various substrates and immunostained for MAP2 to visualize neurites (nuclei counterstained with Hoechst). D: Total neurite length and numbers of primary neurites were quantified for mouse NSPC-derived neurons as for human neurons (**P < 0.01 for total neurite length on LAM vs. FN; error bars represent SEM). As for human cells, there was no difference in neurite branching (see text).
Similar to the human cells, mouse NSPC-derived neurons on laminin had more extensive neurites and greater neurite length per cell than neurons on fibronectin (Fig. 5C,D). The numbers of primary neurites per cell were not different among mouse NSPC-derived neurons grown on fibronectin, matrigel, and laminin (Fig. 5D) but were significantly lower for mouse NSPC-derived neurons on PLO (number of primary neurites per cell body ± SEM: laminin 3.3 ± 0.24, matrigel 3.4 ± 0.36, fibronectin 3.2 ± 0.13, PLO 2.6 ± 0.22; P < 0.05 for PLO compared with fibronectin or laminin). As seen with the human cells, the number of neurite branches was not significantly different for mouse NSPC-derived neurons grown on the various substrates [number of branches per neurite length (mm) ± SEM: laminin 9.3 ± 1.3, matrigel 6.5 ± 1.5, fibronectin 12.8 ± 2.0, PLO 7.0 ± 1.4; _P_ > 0.1 for all comparisons]. For both human and mouse NSPC-derived neurons, the lack of a difference among fibronectin, matrigel, and laminin on the number of primary neurites or number of branches per unit length indicates that the effect of laminin on total neurite length is due primarily to neurite elongation.
Human Cortical NSPCs Express Cell Surface Integrins That Mediate Their Responses to ECM Molecules
Because the human NSPCs responded strongly to laminin in all of the cellular assays, we used flow cytometry to determine the cell surface expression of all the identified laminin-binding integrins: α1β1, α2β1, α3β1, α6β1, α6β4, and α7β1 (Milner and Campbell, 2002; Fig. 6A). Almost all the human NSPCs expressed the β1 integrin subunit, but only a small percentage expressed β4. Antibodies to the α1 and α2 subunits did not label human NSPCs. A significant percentage of human NSPCs expressed α3, α6, or α7 integrin subunits. The α3β1 heterodimer binds both laminin and fibronectin, whereas the α6 heterodimers and α7β1 are specific for laminin. Because α6 forms heterodimers with both β1 and β4, we further examined their coexpression and found that all the α6-positive cells also expressed β1, although only a small percentage coexpressed β4. Human NSPCs also expressed the α5 integrin subunit (~56% of cells were positive), which couples with β1 to generate a fibronectin specific heterodimer, but we did not detect expression of the fibronectin-binding α4 integrin subunit.
Fig. 6.
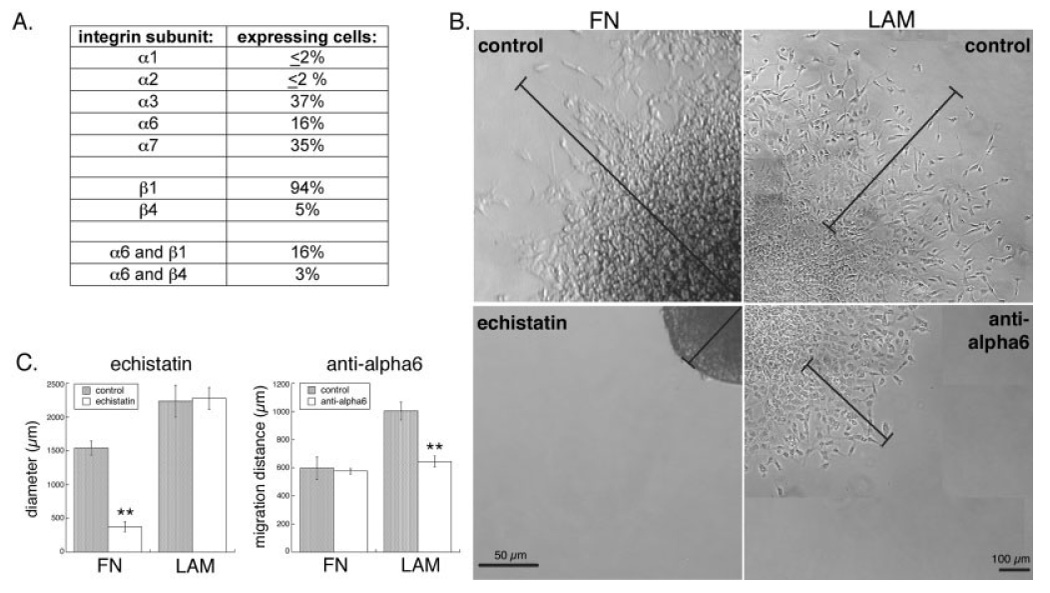
Cell surface integrins mediate responses of human NSPCs to extracellular matrices. A: Human NSPCs (SC27) were analyzed by flow cytometry to determine the mean percentages of cells expressing the laminin-binding α and β integrin subunits and the percentage of cells that coexpress the α6 subunit with its binding partners β1 and β4. Similar levels of integrin subunit expression were detected on SC30 human NSPCs. B: Human NSPC spheres (SC27) were adhered to FN- or LAM-coated coverslips, then treated with the disintegrin echistatin (FN panels) or an α6 integrin blocking antibody (LAM panels). Bars in the FN panels denote the distance from the middle of the sphere to the edge of migrating cells at 24 hr (control) or to the edge of the nonadherent sphere (echistatin). Bars in the LAM panels indicate the extent of NSPC migration by marking the position of the front of migrating cells prior to antibody addition and after 24 hr. C: Quantitation of spheres and migrating cells at 24 hr shows that human NSPCs migrate out of control spheres on FN, but spheres treated with echistatin were no longer adhered and thus cells were unable to migrate (**P < 0.01). Migration on LAM was partially blocked by an α6 antibody (see Materials and Methods for description of quantitation). Controls in B and C are no treatment (for echistatin) or incubation with an isotype-matched control antibody (for anti-α6).
We used blockers of integrin function to assess whether the identified integrins mediate the responses of human NSPCs to ECM molecules. Because of the complicated interrelationships among proliferation, cell density, cell–cell contact, and differentiation, we utilized the assay with the shortest time scale (migration) for the function-blocking studies to assay the cellular response most unlikely to be secondarily confounded. Human NSPC spheres were adhered to fibronectin- or laminin-coated coverslips prior to incubation with blockers, so that effects on the cells migrating from the spheres could be analyzed. Fibronectin-binding integrins, in particular α5β1, are efficiently blocked by echistatin, a disintegrin that contains multiple RGD sequences (Wierzbicka-Patynowski et al., 1999). Human NSPC spheres on fibronectin that were treated with echistatin rapidly lost adhesion to the surface and began to float in the medium (Fig. 6B). This effect was consistent for all spheres and occurred at the lowest echistatin concentration tested (0.5 µM). Therefore, our measure of sphere diameters after 24 hr reflects the sphere and surrounding rim of migrating cells for control spheres on fibronectin but just the diameter of the floating spheres for those treated with echistatin (Fig. 6C). In contrast to the striking effects of echistatin on cells on fibronectin, human NSPCs on laminin treated with echistatin showed no defects in adhesion or cell migration (Fig. 6C).
To test directly the role of a laminin-specific integrin in mediating the responses of human NSPCs to laminin, we performed α6 function-blocking experiments with a well-characterized antibody (Sonnenberg et al., 1988; Hall et al., 1990; Jacques et al., 1998). We were unable to identify a function-blocking α7 integrin antibody proven to work on human cells. Human NSPCs on laminin treated with an α6 integrin function-blocking antibody exhibited decreased migration compared with cells treated with isotype-matched antibody controls (Fig. 6B). To facilitate quantitation of migration in the antibody blocking experiments, we tracked the movement of cells out of individual spheres over time and were able to measure the distance traveled by the front of human NSPCs over 24 hr (Fig. 6C). Whereas blocking of α6 function decreased migration of human NSPCs on laminin, cells on fibronectin treated with α6 antibodies or isotype-matched controls showed no difference in cell migration (Fig. 6C), confirming the specificity of the α6 antibody, since as α6 heterodimers do not bind fibronectin. Reasons for the greater-than-expected α6 antibody effect (only 16% of NSPCs were α6 positive by flow cytometry) are unknown but could include the preferential localization of β1 integrin-expressing cells to the neurosphere periphery (Campos et al., 2004). Nonetheless, the antibody blocking experiments demonstrate that the laminin-binding α6 integrin subunit mediates at least part of the effect of laminin on human NSPC migration.
DISCUSSION
Our results demonstrate that human NSPC migration, expansion, differentiation, and neurite outgrowth of NSPC-derived neurons are all enhanced on laminin-containing matrices compared with PLO or fibronectin. Differentiation of human NSPCs on laminin-coated surfaces leads to greater numbers of neurons, consistent with data showing that a laminin priming step increases generation of cholinergic neurons from human NSPCs (Wu et al., 2002; Tarasenko et al., 2004). We have identified the laminin-binding integrins α3, α6, α7, β1, and β4 on the surface of human NSPCs and found that the α6 integrin subunit (likely via α6β1) is involved in the migration of these cells on laminin. Additionally, the expression of α7 on ~35% of the human NSPCs suggests the involvement of α7β1 laminin-binding integrins in the laminin-mediated behaviors. Although α7 integrin expression on rodent NSPCs has not been described, it does play a role in neurite outgrowth and axonal regeneration of differentiated mouse neurons (Werner et al., 2000; Mercado et al., 2004; Gardiner et al., 2005). Further studies will be necessary to determine which laminin-binding integrins and downstream signaling events mediate particular NSPC behaviors, in that distinct integrins may influence specific behaviors (Jacques et al., 1998).
The responses of human and mouse NSPCs to laminin were remarkably similar, considering the differences in species and developmental stage at the time of isolation (during early neurogenesis in mouse and after neurogenesis is largely complete in human). This suggests both phylogenetic and ontogenetic conservation of laminin effects on NSPCs, an idea supported by the ability of α6 integrin antibody to inhibit the migration of human NSPCs (Fig. 6) and mouse ganglionic eminence NSPCs on laminin (Tate et al., 2004), and chain migration of rat forebrain NSPCs (Jacques et al., 1998). The conservation of integrin-mediated responses extends to nonlaminin-binding integrins, such as the α5β1 integrin (Fig. 6), which mediates the adhesion of human NSPCs to fibronectin and has been implicated in the adhesion and migration of mouse ganglionic eminence NSPCs (Tate et al., 2004) and the proliferation of postnatal rat forebrain NSPCs (Jacques et al., 1998) on fibronectin. The evolutionary conservation of the responses of NSPCs to ECM molecules implies that these signals are potent regulators of these cells.
Our data on the stimulatory effects of laminin on NSPCs in vitro suggest that this matrix molecule may regulate NSPCs in vivo. Laminin is expressed in the developing rodent VZ (the NSPC niche of the developing cortex; Georges-Labouesse et al., 1998; Campos et al., 2004) and the human cortex during early developmental stages, when many of the cells are NSPCs (expression is evident at approximately week 17 of gestation; Anlar et al., 2002). Cells in the VZ also express α6 and β1 integrin subunits, and colabelling with the NSPC marker nestin and markers of actively dividing cells strongly suggests that these cells are NSPCs (Campos et al., 2004). The α6 integrin subunit likely plays a critical role in these cortical cells, insofar as mice deficient in α6 expression exhibit abnormal cortical development (Georges-Labouesse et al., 1998). The expression pattern of laminin and the laminin-binding integrin α6β1 in vivo implies that laminin might have effects on endogenous NSPCs analogous to those we describe for cells in vitro. A detailed understanding of the influence of ECMs and their integrin ligands on human NSPCs will help us in studying the basic biology of these cells and might lead to therapies utilizing defined exogenous NSPCs or mobilized endogenous cells to treat injury or disease.
ACKNOWLEDGMENTS
Contract grant sponsor: NIMH; Contract grant sponsor: NIA; Contract grant sponsor: CHOC Foundation for Children; Contract grant sponsor: United Mitochondrial Disease Foundation; Contract grant sponsor: March of Dimes Birth Defects Foundation.
The authors thank Dr. Stephen J. Kaufman (University of Illinois, Urbana-Champaign) for the H36 α7 integrin antibody and Hubert Nethercott for assistance with human NSPC cultures for flow cytometry experiments. Support was provided by NIH grants AG23583 (L.A.F.) and K08 MH02029 (E.S.M.), the CHOC Foundation for Children (P.H.S), the United Mitochondrial Disease Foundation (P.H.S.), and the March of Dimes Birth Defects Foundation (E.S.M.).
REFERENCES
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Anlar B, Atilla P, Cakar AN, Kose MF, Beksac MS, Dagdeviren A, Akcoren Z. Expression of adhesion and extracellular matrix molecules in the developing human brain. J Child Neurol. 2002;17:707–713. doi: 10.1177/088307380201700913. [DOI] [PubMed] [Google Scholar]
- Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta 1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Drago J, Nurcombe V, Bartlett PF. Laminin through its long arm E8 fragment promotes the proliferation and differentiation of murine neuroepithelial cells in vitro. Exp Cell Res. 1991;192:256–265. doi: 10.1016/0014-4827(91)90184-v. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155:511–517. doi: 10.1083/jcb.200105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Fernyhough P, Tomlinson DR, Mayer U, von der Mark H, Streuli CH. Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol Cell Neurosci. 2005;28:229–240. doi: 10.1016/j.mcn.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp Neurol. 2003;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- Mercado ML, Nur-e-Kamal A, Liu HY, Gross SR, Movahed R, Meiners S. Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal alpha7beta1 integrin. J Neurosci. 2004;24:238–247. doi: 10.1523/JNEUROSCI.4519-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Schwartz PH, Bryant PJ, Fuja TJ, Su H, O’Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Palmer TD, Gage FH. The search for neural progenitor cells: prospects for the therapy of neurodegenerative disease. Mol Med Today. 1999;5:474–480. doi: 10.1016/s1357-4310(99)01596-8. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens C, Gage F. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- Tarasenko YI, Yu Y, Jordan PM, Bottenstein J, Wu P. Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J Neurosci Res. 2004;78:625–636. doi: 10.1002/jnr.20316. [DOI] [PubMed] [Google Scholar]
- Tate MC, Garcia AJ, Keselowsky BG, Schumm MA, Archer DR, La-Placa MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Niewiarowski S, Marcinkiewicz C, Calvete JJ, Marcinkiewicz MM, McLane MA. Structural requirements of echistatin for the recognition of alpha(v)beta(3) and alpha(5)beta(1) integrins. J Biol Chem. 1999;274:37809–37814. doi: 10.1074/jbc.274.53.37809. [DOI] [PubMed] [Google Scholar]
- Wu P, Tarasenko YI, Gu Y, Huang LY, Coggeshall RE, Yu Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]