Inhibitory Role of Plk1 in the Regulation of p73-dependent Apoptosis through Physical Interaction and Phosphorylation (original) (raw)
Abstract
In response to DNA damage, p73 plays a critical role in cell fate determination. In this study, we have found that Plk1 (polo-like kinase 1) associates with p73, phosphorylates p73 at Thr-27, and thereby inhibits its pro-apoptotic activity. During cisplatin-mediated apoptosis in COS7 cells in which the endogenous p53 is inactivated by SV40 large T antigen, p73 was induced to accumulate in association with a significant down-regulation of Plk1. Consistent with these observations, Plk1 reduced the stability of the endogenous p73. Immunoprecipitation and in vitro pulldown assay demonstrated that p73 binds to the kinase domain of Plk1 through its NH2-terminal region. Luciferase reporter assay and reverse transcription-PCR analysis revealed that Plk1 is able to block the p73-mediated transcriptional activation. Of note, kinase-deficient Plk1 mutant (Plk1(K82M)) retained an ability to interact with p73; however, it failed to inactivate the p73-mediated transcriptional activation, suggesting that kinase activity of Plk1 is required for the inhibition of p73. Indeed, in vitro kinase assay indicated that p73 is phosphorylated at Thr-27 by Plk1. Furthermore, small interference RNA-mediated knockdown of the endogenous Plk1 in p53-deficient H1299 cells resulted in a significant increase in the number of cells with sub-G1 DNA content accompanied by the up-regulation of p73 and pro-apoptotic p53AIP1 as well as the proteolytic cleavage of poly(ADP-ribose) polymerase. Thus, our present results suggest that Plk1-mediated dysfunction of p73 is one of the novel molecular mechanisms to inhibit the p53-independent apoptosis, and the inhibition of Plk1 might provide an attractive therapeutic strategy for cancer treatment.
p73 is one of newly identified p53 tumor suppressor gene family members (p53, p73, and p63) that encodes a nuclear transcription factor (1–3). Like the other p53 family members, p73 encodes multiple isoforms, including TA (transactivating), with distinct COOH-terminal extensions arising from alternative splicing events and ΔN (nontransactivating) variants generated by alternative promoter usage (2–4). ΔNp73 has an oncogenic potential (5) and displays a dominant-negative behavior toward TAp73 as well as wild-type p53 (6). TAp73 transactivates overlapping set of p53-target genes implicated in the induction of cell cycle arrest and/or apoptosis, and plays an important role in the regulation of DNA damage response, which is closely linked to its DNA binding activity. The initial studies demonstrated that TAp73 does not induce enough to accumulate in response to DNA damage arising from UV exposure or actinomycin D treatment (1); however, it has been shown that, in response to certain subset of DNA-damaging agents, TAp73 accumulates in the cell nucleus and exerts its pro-apoptotic function (7).
Accumulating evidence suggests that TAp73 is regulated by post-translational modifications such as phosphorylation and acetylation. For example, TAp73 is stabilized in response to DNA damage such as cisplatin (CDDP)2 treatment or exposure to γ-irradiation through the phosphorylation at Tyr-99 mediated by c-Abl (8–10). Ren et al. (11) demonstrated that protein kinase Cδ catalytic fragment phosphorylates TAp73 at Ser-289 in response to CDDP and thereby enhances its stability and activity. Further studies revealed that CDDP-mediated apoptosis is associated with phosphorylation of TAp73 at Ser-47 catalyzed by Chk1 (12). According to their results, Chk1-mediated phosphorylation of TAp73 resulted in an increase in its transcriptional activity. On the other hand, Gaiddon et al. (13) described that cyclin-dependent kinase phosphorylates TAp73 at Thr-86 and thereby reduces its transcriptional activity, suggesting that phosphorylation of TAp73 might not always convert a latent form of TAp73 to an active one. Additionally, several lines of evidence suggest that acetylation of TAp73 mediated by p300/CBP results in its activation (14). Constanzo et al. (15) reported that p300 has an ability to acetylate TAp73 at Lys-321, Lys-327, and Lys-331 in response to DNA damage in a c-Abl-dependent manner, and acetylated forms of TAp73 exert its pro-apoptotic function.
Plk1 (Polo-like kinase 1) is a positive cell cycle regulator (16–18). Plk1 has an NH2-terminal Ser/Thr protein kinase domain and tandem repeats of so-called “Polo-box motif” in its COOH-terminal region that might act as a phosphopeptide-binding domain (19). It has been shown that_Plk1_ is overexpressed in a variety of human tumors as compared with their corresponding normal tissues (20,21). Indeed, enforced expression of Plk1 in mouse fibroblasts causes oncogenic focus formation and promotes tumor growth in nude mice (22), suggesting that Plk1 has an oncogenic potential. In support with this notion, knockdown of the endogenous Plk1 induces G2/M cell cycle arrest and/or apoptosis in various cell lines (23–25). Furthermore, it has been shown that Plk1 is inhibited in response to DNA damage in a mutated in ataxia telangiectasia (ATM) and ATM related kinase-dependent manner (26,27), indicating that Plk1 is one of targets of DNA damage response. In this regard, we have found that Plk1 inhibits pro-apoptotic function of p53 through the physical interaction with it (20). Recently, Liu et al. (28) reported that Plk1 depletion promotes apoptosis of cancerous cells in a p53-independent manner. In this study, we have found that Plk1 has an ability to bind to and phosphorylate TAp73 at Thr-27, thereby inhibiting its transcriptional as well as pro-apoptotic activity.
EXPERIMENTAL PROCEDURES
_Cell Lines and Transfection_—African green monkey kidney COS7, human osteosarcoma SAOS-2, U2OS, and human cervical carcinoma HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 50 μg/ml penicillin, and 50 μg/ml streptomycin (Invitrogen). Human lung carcinoma H1299 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum plus antibiotics mixture. These cells were cultured in a 5% CO2 environment at 37 °C. Where indicated, cells were exposed to CDDP (Sigma). For transient transfection, COS7 and H1299 cells were transfected with the indicated combinations of the expression plasmids using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. pcDNA3 (Invitrogen) was used as a blank plasmid to balance the amount of DNA introduced in transient transfection.
_RNA Extraction and RT-PCR_—Total RNA was prepared from the indicated cells by using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol and reverse-transcribed. The specific primers used were as follows: _p73_α, 5′-TCTGGAACCAGACAGCACCT-3′ and 5′-GTGCTGGACTGCTGGAAAGT-3′; p21WAF1, 5′-ATGAAATTCACCCCCTTTCC-3′ and 5′-CCCTAGGCTGTGCTCACTTC-3′; BAX, 5′-TTTGCTTCAGGGTTTCATCC-3′ and 5′-CAGTTGAAGTTGCCGTCAGA-3′; MDM2, 5′-ACTTGAGCCGAGGAGTTCAA-3′ and 5′-TTGCTCTGTCACCTGGACTG-3′; Plk1, 5′-ATCACCTGCCTGACCATTCCACCAAGG-3′ and 5′-AATTGCGGAAATATTTAAGGAGGGTGATCT-3′; p53AIP1, 5′-GATCTTCCTCTGAGGCGAGCT-3′ and 5′-TTACCCAGCCAGGTGTGTGT-3′; and GAPDH, 5′-ACCTGACCTGCCGTCTAGAA-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The expression of GAPDH was measured as an internal control.
_Immunoblotting_—Whole cells lysates were prepared by incubating cells in lysis buffer containing 25 mm Tris-HCl, pH 8.0, 137 mm NaCl, 2.7 mm KCl, 1% Triton X-100 and a commercial protease inhibitor mixture (Sigma) for 30 min on ice and subjected to a brief sonication for 10 s at 4 °C followed by centrifugation at 15,000 rpm at 4 °C for 10 min to remove insoluble materials. The protein concentrations were measured using the Bradford protein assay according to the manufacturer's instructions (Bio-Rad). The equal amounts of protein (50 μg) were separated by 10% SDS-PAGE and electrophoretically transferred onto polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, MA). The transferred membranes were blocked with Tris-buffered saline containing 5% nonfat dry milk and 0.1% Tween 20 at 4 °C overnight. After blocking, the membranes were incubated with monoclonal anti-p73 (Ab-4; NeoMarkers, Fremont, CA), monoclonal anti-FLAG (M2; Sigma), monoclonal anti-Plk1 (PL2 and PL6; Zymed Laboratories Inc.), monoclonal anti-PARP (F-2; Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-p300 (H-272; Santa Cruz Biotechnology), or with polyclonal anti-actin (20–33; Sigma) antibody for 1 h at room temperature. After incubation with primary antibodies, the membranes were incubated with horseradish peroxidase-coupled goat anti-mouse or anti-rabbit IgG secondary antibody (Cell Signaling, Beverly, MA) for 1 h at room temperature. Immunoblots were visualized by ECL detection reagents according to the manufacturer's instructions (Amersham Biosciences).
_Immunoprecipitation_—HeLa cells were treated with CDDP at a final concentration of 20 μm. Twenty-four hours after CDDP treatment, whole cell lysates (1 mg of protein) were pre-cleared with 30 μl of protein G-Sepharose beads and used for immunoprecipitation with the appropriate antibodies. After the addition of 30 μl of protein G-Sepharose beads, incubations were continued for additional 2 h at 4 °C. The beads were then collected by centrifugation and washed three times with the lysis buffer. The precipitated proteins were analyzed by 10% SDS-PAGE and immunoblotting with the appropriate antibodies as described.
_GST Pulldown Assay_—cDNA fragments encoding the indicated deletion mutants of p73α were generated by PCR-based strategy, and subcloned into GST fusion protein expression plasmid pGEX-4T-3 (Amersham Biosciences). GST and GST-p73α fusion proteins were expressed and purified by glutathione-Sepharose beads (Amersham Biosciences). FLAG-Plk1 was radiolabeled in vitro by using TnT QuickCoupled transcription/translation system (Promega, Madison, WI) in the presence of [35S]methionine and incubated with GST or GST-p73α deletion mutants for 2 h at 4 °C. After the addition of 30 μl of glutathione-Sepharose beads into the reaction mixture, incubations were continued for 1 h at 4 °C. The beads were collected by centrifugation and washed three times with binding buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Nonidet P-40, and 1 mm EDTA. The 35S-labeled bound proteins were eluted by 2× SDS sample buffer and separated by 10% SDS-PAGE. After electrophoresis, the gel was dried and exposed to an x-ray film with an intensifying screen.
_Indirect Immunofluorescence Staining_—HeLa cells were fixed in 3.7% formaldehyde for 30 min at room temperature, permeabilized in 0.2% Triton X-100 for 5 min at room temperature, and then blocked with 3% bovine serum albumin in PBS for 1 h at room temperature. After blocking, cells were washed in PBS and incubated with polyclonal anti-p73 antibody (H-79; Santa Cruz Biotechnology) and monoclonal anti-Plk1 antibody for 1 h at room temperature, followed by the incubation with fluorescein isothiocyanate-conjugated anti-rabbit IgG and rhodamine-conjugated anti-mouse IgG (Invitrogen) for 1 h at room temperature. Cell nuclei were stained with DAPI.
_Flow Cytometry_—After transfection, both floating and attached cells were collected by low speed centrifugation, washed in PBS, and fixed in 70% ethanol at -20 °C overnight. The cells were then stained with propidium iodide (50 μg/ml) in the presence of 50 μg/ml RNase A for 30 min at room temperature. The DNA content indicated by propidium iodide staining was analyzed by FACSCalibur flow cytometer (BD Biosciences).
_Luciferase Reporter Assay_—p53-deficient H1299 cells were plated in 12-well plates at a density of 50,000 cells/well and transiently co-transfected with a constant amount of a luciferase reporter construct driven by p53/p73-responsive element derived from p21WAF1, Mdm2, or Bax promoter, Renilla luciferase expression plasmid (pRL-TK), and the HA-p73α expression plasmid together with or without the increasing amounts of the expression plasmid for Plk1. Forty-eight hours after transfection, cells were lysed, and their luciferase activities were measured by dual luciferase reporter assay system (Promega). The firefly luminescence signal was normalized based on the Renilla luminescence signal. Each experiment was performed at least three times in triplicate.
_Apoptotic Assay_—H1299 cells were transfected with the indicated combinations of the expression plasmids. Forty-eight hours after transfection, cells were washed in PBS, fixed in 3.7% formaldehyde for 1 h at room temperature, and then permeabilized with 0.1% Triton X-100 for 5 min on ice. The cell nuclei were stained by DAPI. The number of GFP-positive cells with apoptotic nuclei was counted.
_Construction of Mutant Forms of GST-p73_α_-(1–130)_—To identify possible phosphorylation site(s) of p73α mediated by Plk1, the T27A mutation was introduced into the GST-p73α-(1–130) using PfuUltra™ high fidelity DNA polymerase (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Details are available upon request. Nucleotide sequences of the PCR products were determined to verify the presence of the desired mutation and the absence of random mutations.
_In Vitro Kinase Reaction_—To identify the possible Thr residue(s) of p73α that could be phosphorylated by Plk1, we used CycLex Polo-like kinase 1 assay kit (CycLex, Nagano, Japan) (29). In brief, the purified Plk1 was added to the reaction mixture containing 50 μm ATP and polyclonal anti-phospho-Thr antibody and incubated for 30 min at room temperature. After the incubation, horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody was mixed with the reaction mixture and incubated for 30 min at room temperature. Finally, GST or the indicated GST-p73α deletion mutants dissolved in substrate solution were added into the reaction mixture and incubated for 5 min at room temperature, followed by the measurement of absorbance in each well using a spectrophotometric plate reader at dual wavelength of 450/540 nm. As a positive control, we used protein X that was supplied by the manufacturer.
_RNA Interference_—To knock down the endogenous Plk1, H1299 cells were transiently transfected with the chemically synthesized siRNA targeting Plk1 or with the control siRNA (Dharmacon, Chicago) using Lipofectamine™ RNAiMAX (Invitrogen) according to the manufacturer's recommendations. Total RNA and whole cell lysates were prepared 48 h after transfection.
RESULTS
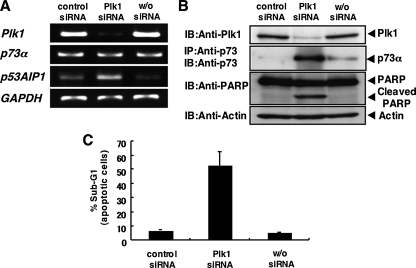
_siRNA-mediated Knockdown of Plk1 Results in a Massive Apoptosis_—To ask whether Plk1 could protect cells from p53-independent apoptosis, p53-deficient human lung carcinoma H1299 cells were transfected with control siRNA or with siRNA against Plk1. As shown inFig. 1_A_, Plk1 was successfully knocked down under our experimental conditions. The expression levels of _p73_α remained unchanged. Of note, the expression levels of pro-apoptotic p53AIP1, which is one o the p53/p73 target genes, were significantly induced in Plk1-knocked down cells. Immunoblot analysis clearly demonstrated that the proteolytic cleavage of PARP, which is one of the substrates of the activated caspase-3 (30), is observed in Plk1-knocked down cells in association with a significant induction of pro-apoptotic p73α (Fig. 1_B_). FACS analysis revealed that siRNA-mediated knockdown of the endogenous Plk1 causes a remarkable increase in number of cells with sub-G1 DNA content relative to control cells (Fig. 1_C_). Similar results were also obtained in H1299 cells transfected with other siRNAs against Plk1 (data not shown), suggesting that Plk1 might have an inhibitory effect on p73-mediated apoptosis. Similar results were also obtained in p53-proficient U2OS cells as well as p53-deficient SAOS-2 cells (supplemental Fig. S1). Thus, it is likely that siRNA-mediated knockdown of the endogenous Plk1 induces apoptosis regardless of p53 status.
FIGURE 1.
siRNA-mediated knockdown of Plk1 induces apoptosis in p53-deficient H1299 cells. A, siRNA-mediated knockdown of the endogenous_Plk1_. H1299 cells were transfected with control siRNA or with Plk1 siRNA (Plk1 siRNA). Forty-eight hours after transfection, total RNA was prepared and subjected to RT-PCR to examine the expression levels of _Plk1, p73_α, and p53AIP1. GAPDH was used as an internal control. B, immunoblotting analysis. H1299 cells were transfected as in A. Forty-eight hours after transfection, whole cell lysates were prepared and processed for immunoblotting (IB) with the indicated antibodies. For the detection of the endogenous p73α, whole cell lysates were subjected to immunoprecipitation (IP) with anti-p73 antibody followed by IB with anti-p73 antibody. Actin expression served as a control for equal loading of proteins in each lane. C, FACS analysis. H1299 cells were transfected as in A. Forty-eight hours after transfection, attached and floating cells were harvested, stained with PI, and their cell cycle distributions examined by flow cytometry.
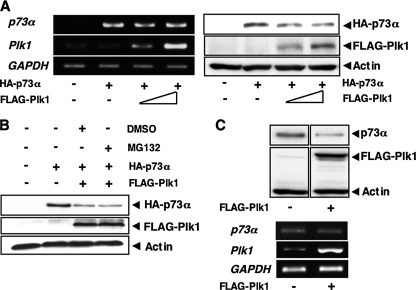
These findings showing that siRNA-mediated knockdown of Plk1 leads to a significant induction of the endogenous p73α at protein level prompted us to examine whether Plk1 could affect the protein stability of p73. For this purpose, H1299 cells were co-transfected with the constant amount of the expression plasmid for HA-p73α together with or without the increasing amounts of FLAG-Plk1 expression plasmid. As shown inFig. 2_A_, left panel, Plk1 had undetectable effect on the expression levels of_p73_α mRNA. On the other hand, immunoblotting analysis revealed that Plk1 reduces the amounts of HA-p73α (Fig. 2_A_, right panel). Intriguingly, Plk1-mediated reduction of HA-p73α was not recovered in the presence of proteasome inhibitor MG-132 (Fig. 2_B_). Similar results were also obtained in cells exposed to lactacystin (data not shown). In addition, enforced expression of FLAG-Plk1 significantly reduced the amounts of the endogenous p73α at protein level but not at the mRNA level (Fig. 2_C_).
FIGURE 2.
Plk1 promotes proteolytic degradation of p73 in a proteasome-independent manner. A, enforced expression of Plk1 reduces the expression levels of p73. H1299 cells were co-transfected with the constant amount of HA-p73α (0.5 μg) expression plasmid together with or without the expression plasmid for FLAG-Plk1 (0.5 and 1.0 μg). Forty-eight hours after transfection, total RNA and whole cell lysates were prepared and subjected to RT-PCR (left panels) and IB with the indicated antibodies (right panels), respectively. B, H1299 cells were transfected with the expression plasmid for HA-p73α alone or with HA-p73α (0.5 μg) plus FLAG-Plk1 (0.5 μg) expression plasmids. Forty-eight hours after transfection, cells were treated with DMSO or with 20 μm of MG-132 for 6 h. Whole cell lysates were then extracted and subjected to IB with the indicated antibodies. C, H1299 cells were transfected with pcDNA3 or with FLAG-Plk1 expression plasmid. Forty-eight hours after transfection, whole cell lysates and total RNA were prepared and subjected to IP with anti-p73 antibody followed by IB with anti-p73 antibody (upper panels) and RT-PCR (lower panels), respectively. Actin was used as a loading control, and GAPDH was used as an internal control.
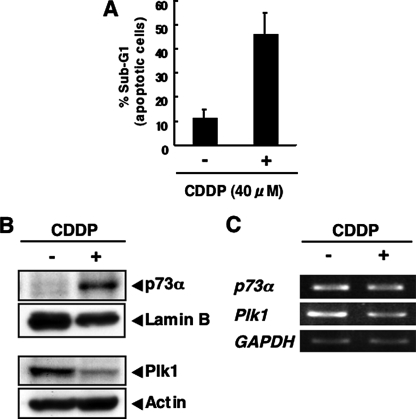
As described previously (31), CDDP treatment led to a stabilization of the endogenous p73α in COS7 cells, whereas the endogenous Plk1 was down-regulated in response to CDDP accompanied by the induction of apoptosis (Fig. 3,A–C), indicating that there exists an inverse relationship between the expression levels of Plk1 and p73α in response to CDDP and that Plk1 might promote proteolytic degradation of p73α in a proteasome-independent manner. Furthermore, enforced expression of Plk1 decreased the sensitivity to CDDP in association with the reduction of CDDP-mediated proteolytic cleavage of PARP (supplemental Fig. S2).
FIGURE 3.
Inverse relationship between the endogenous expression levels of p73 and Plk1 in response to CDDP. A–C, COS7 cells were treated with 40 μm of CDDP or left untreated. Forty-eight hours after the treatment, floating and attached cells were collected, stained with PI, and their cell cycle distributions analyzed by flow cytometry (A). COS7 cells were treated with 40 μm of CDDP or left untreated. Forty-eight hours after the treatment, nuclear (upper panels) and whole cell lysates (lower panels) were prepared and subjected to IB with the indicated antibodies (B). Total RNA was also prepared and analyzed by RT-PCR (C).
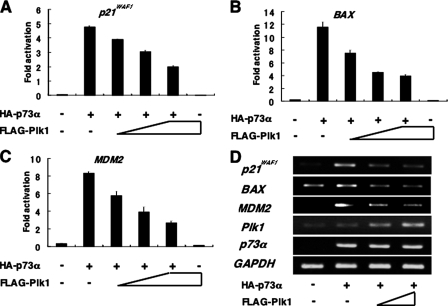
Plk1 Inhibits p73-mediated Transcriptional Activation and Pro-apoptotic Function_—To address whether Plk1 could suppress the transcriptional activity of p73, we performed the luciferase reporter assays. H1299 cells were co-transfected with the constant amount of the expression plasmid for HA-p73α, the luciferase reporter construct carrying p53/p73-responsive_p21WAF1, BAX, or MDM2 promoter, and_Renilla_ luciferase cDNA (pRL-TK) together with or without the increasing amounts of FLAG-Plk1 expression plasmid. As shown inFig. 4, A–C, enforced expression of FLAG-Plk1 significantly reduced the luciferase activities driven by the indicated promoters in a dose-dependent manner. Consistent with these results, HA-p73α-mediated up-regulation of the endogenous p21WAF1, BAX, and MDM2 mRNAs was abrogated by FLAG-Plk1 (Fig. 4_D_), suggesting that Plk1 has an ability to repress the transcriptional activity of p73α.
FIGURE 4.
Plk1 represses the p73-mediated transcriptional activation. A–C, H1299 cells (5 × 104 cells) were co-transfected with the constant amount of HA-p73α expression plasmid (25 ng), 100 ng of p53/p73-responsive luciferase reporter construct bearing_p21WAF1_ (A), BAX (B), or_MDM2_ (C) promoter and 10 ng of Renilla luciferase reporter plasmid (pRL-TK) in the presence or absence of the increasing amounts of FLAG-Plk1 expression plasmid (50, 100, and 200 ng). To standardize the amounts of plasmid DNA per transfection, pcDNA3 was added to yield a total of 510 ng of plasmid. Forty-eight hours after transfection, cells were lysed, and their luciferase activities were measured. Data were normalized and presented as mean values ± S.D. of three independent experiments. D, RT-PCR analysis. H1299 cells were co-transfected with the constant amount of HA-p73α together with or without the increasing amounts of FLAG-Plk1 expression plasmid. Forty-eight hours after transfection, total RNA was prepared and analyzed for the expression levels of p21WAF1, BAX, and MDM2 by RT-PCR. Amplification of GAPDH serves as an internal control.
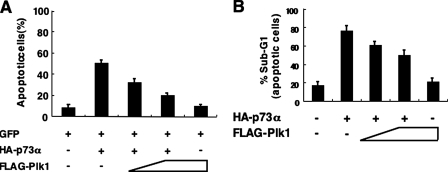
To examine a possible effect of Plk1 on pro-apoptotic activity of p73, we carried out apoptotic assay. H1299 cells were co-transfected with the indicated combinations of the expression plasmids. Consistent with the previous observations (32), HA-p73α alone increased number of cells with apoptotic nuclei (Fig. 5_A_). As expected, FLAG-Plk1 had an ability to decrease GFP-positive cells with apoptotic nuclei caused by exogenous expression of HA-p73α. In accordance with these results, FACS analysis demonstrated that HA-p73α-mediated increase in number of cells with sub-G1 DNA content is inhibited by co-expression with FLAG-Plk1 in a dose-dependent manner (Fig. 5_B_), indicating that Plk1 suppresses the p73α-mediated apoptotic cell death.
FIGURE 5.
Plk1 inhibits the pro-apoptotic activity of p73. A, apoptotic assay. H1299 cells were seeded at a density of 2 × 105 cells/6-well tissue culture plate and allowed to attach overnight. Next day, cells were co-transfected with the constant amount of GFP (100 ng) and HA-p73α (900 ng) expression plasmids together with or without the increasing amounts of FLAG-Plk1 expression plasmid (500 and 1000 ng). Total amount of plasmid DNA was kept constant (2 μg) with pcDNA3. Forty-eight hours after transfection, cells were fixe, and cell nuclei were stained with DAPI. The percentages of GFP-positive cells with apoptotic nuclei were plotted. B, FACS analysis. H1299 cells were co-transfected with the constant amount of the expression plasmid encoding HA-p73α (250 ng) together with or without the increasing amounts of FLAG-Plk1 expression plasmid (100 or 200 ng). Forty-eight hours after transfection, attached and floating cells were collected, stained with PI, and their cell cycle distributions analyzed by flow cytometry.
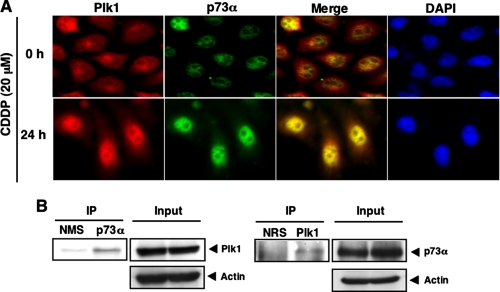
_Interaction between Plk1 and p73 in Cells_—To address whether Plk1 could associate with p73 in cells, we examined their subcellular distributions in response to CDDP. Human cervical carcinoma HeLa cells were treated with CDDP or left untreated for 24 h. Immunofluorescence microscopy demonstrated that the endogenous p73α is detectable in cell nucleus regardless of CDDP treatment (Fig. 6_A_). It was worth noting that the endogenous Plk1 localizes both in the cytoplasm and cell nucleus in the absence of CDDP, whereas CDDP treatment induces the nuclear accumulation of Plk1. Merged images revealed that Plk1 is largely co-localized with p73α in the cell nucleus in response to CDDP. Under our experimental conditions, immunofluorescence staining without primary antibodies did not show any positive signals (data not shown). These observations suggest that Plk1 might interact with p73 in cells exposed to CDDP.
FIGURE 6.
Interaction between Plk1 and p73 in cells. A, nuclear co-localization of Plk1 with p73 in response to CDDP. HeLa cells were treated with 20 μm CDDP (lower panels) or left untreated (upper panels). Twenty-four hours after the treatment cells were simultaneously probed with polyclonal anti-p73 antibody and monoclonal anti-Plk1 antibody for 1 h at room temperature. After extensive washing in PBS, cells were incubated with rhodamine-conjugated anti-mouse IgG (red) and fluorescein isothiocyanate-conjugated anti-rabbit IgG (green). Cell nuclei were stained with DAPI (blue). Merged images indicate the nuclear co-localization of Plk1 with p73α (yellow). B, immunoprecipitation. HeLa cells were exposed to 20 μm CDDP. Twenty-four hours after CDDP treatment, whole cell lysates were IP with NMS or with anti-p73 antibody followed by IB with anti-Plk1 antibody (left panel). Input lysates were analyzed by IB with the indicated antibodies. Reciprocal experiments are shown in the_right panels_.
To further confirm this notion, we performed immunoprecipitation experiments. HeLa cells were exposed to CDDP for 24 h, and whole cell lysates were immunoprecipitated with normal mouse serum (NMS) or with anti-p73 antibody followed by immunoblotting with anti-Plk1 antibody. As shown inFig. 6_B_, left panels, the anti-p73 immunoprecipitates contained the endogenous Plk1. Reciprocal experiments demonstrated that p73 is co-immunoprecipitated with the endogenous Plk1 (Fig. 6_B_, right panels). Similar results were also obtained in the co-immunoprecipitation experiments using exogenous materials (supplemental Fig. S3). These observations strongly suggest that Plk1 interacts with p73α in cells.
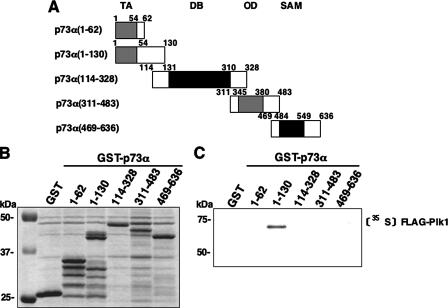
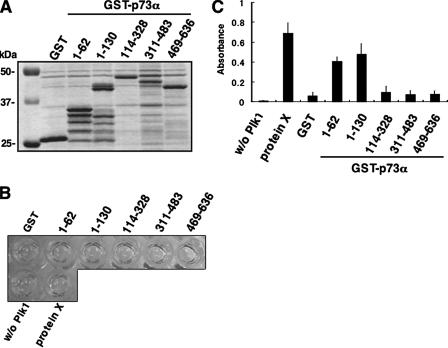
To identify the essential region(s) of p73α required for the interaction with Plk1, we carried out in vitro pulldown assays. The indicated GST-p73α deletion mutants (Fig. 7_A_) were purified by glutathione-Sepharose beads (Fig. 7_B_). Each of these GST fusion proteins were incubated with the radiolabeled FLAG-Plk1, which was generated by in vitro transcription/translation system in the presence of [35S]methionine. As clearly shown inFig. 7_C_, the radiolabeled FLAG-Plk1 was efficiently pulled down by GST-p73α-(1–130) but not by the remaining GST fusion proteins, implying that the region between amino acid residues 63 and 113 of p73α is important for the interaction with Plk1.
FIGURE 7.
NH2-terminal small domain of p73 is required for the interaction with Plk1. A, domain structure of wild-type p73α and schematic representation of GST-tagged p73α deletion mutants. TA, transactivation domain; DB, DNA-binding domain;OD, oligomerization domain; SAM, sterileα-motif domain. Numbers indicate amino acid positions. B, GST and GST-p73α fusion proteins were purified by glutathione-Sepharose beads and separated by 10% SDS-PAGE followed by Coomassie Brilliant Blue staining.C, in vitro pulldown assay. Equal amount of radiolabeled FLAG-Plk1 was incubated with GST or with the indicated GST-p73α fusion proteins. After incubation, GST or GST-p73α fusion proteins were recovered by glutathione-Sepharose beads, and bound materials were resolved by 10% SDS-PAGE followed by autoradiography.
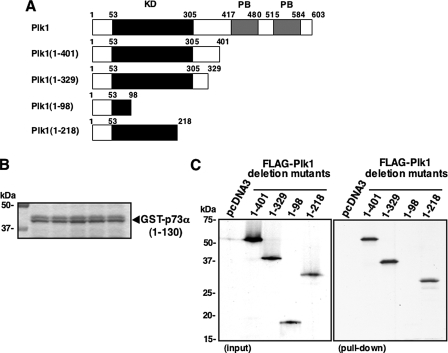
In addition, we sought to determine the region of Plk1 required for the complex formation with p73α. To this end, we generated the indicated Plk1 deletion mutants labeled with [35S]methionine (Fig. 8_A_). These deletion mutants were incubated with GST-p73α-(1–130) (Fig. 8_B_). As shown inFig. 8_C_, FLAG-Plk1-(1–401), FLAG-Plk1-(1–329), and FLAG-Plk1-(1–218) retained an ability to associate with GST-p73α-(1–130), whereas FLAG-Plk1-(1–98) failed to interact with GST-p73α-(1–130). These results indicated that the region between amino acid residues 99 and 218, including a part of the kinase domain of Plk1, is essential for the interaction with p73α.
FIGURE 8.
Kinase domain of Plk1 is essential for the interaction with p73. A, schematic drawing of wild-type Plk1 and its deletion mutants.KD, kinase domain; PB, polo-box domain. B, Coomassie Brilliant Blue staining of GST-p73α-(1–130) used for this study. C, in vitro pulldown assay. Equal amount of GST-p73α-(1–130) was incubated with radiolabeled FLAG-Plk1 deletion mutants (left panel). After incubation, GST-p73α-(1–130) was precipitated by glutathione-Sepharose beads, and bound materials were separated by SDS-PAGE followed by autoradiography.
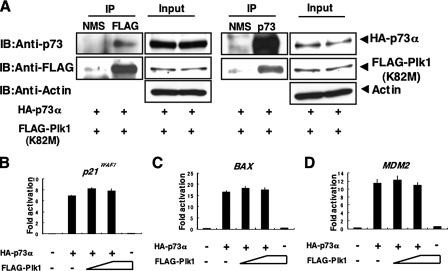
_Kinase Activity of Plk1 Is Required for the Inhibition of p73 Function_—To examine whether the kinase activity of Plk1 could be necessary for the inhibition of p73 function, we tested a possible effect of the kinase-deficient mutant form of Plk1 (Plk1(K82M)) (20) on p73α. For this purpose, we first examined whether Plk1(K82M) could interact with p73α in cells. COS7 cells were co-transfected with the expression plasmids encoding HA-p73α and FLAG-Plk1(K82M). Forty-eight hours after transfection, whole cell lysates were immunoprecipitated with NMS or with anti-FLAG antibody followed by immunoblotting with anti-p73 or with anti-FLAG antibody. As seen in Fig. 9_A_, left panels, HA-p73α was co-immunoprecipitated with FLAG-Plk1(K82M). Similarly, the anti-p73 immunoprecipitates contained FLAG-Plk1(K82M) (Fig. 9_A_, right panels). These results suggest that the kinase-deficient mutant form of Plk1 retains an ability to interact with p73 in cells.
FIGURE 9.
Kinase activity of Plk1 is required for the inhibition of p73. A, Plk1(K82M) retains an ability to interact with p73 in cells. COS7 cells were transiently co-transfected with the expression plasmids for HA-p73α and FLAG-Plk1(K82M). Forty-eight hours after transfection, whole cell lysates were prepared and subjected to IP with NMS or with anti-FLAG antibody. The immunoprecipitates were analyzed by IB with anti-p73 (1st panel) or with anti-FLAG (2nd panel) antibody. Input lysates were processed for IB with the indicated antibodies. Right panels show the results of the reciprocal experiments. B–D, luciferase reporter assay. H1299 cells were transiently co-transfected with the constant amount of HA-p73α expression plasmid (25 ng), 100 ng of luciferase reporter construct carrying p53/p73-responsive element derived from_p21WAF1_ (B), Bax (C), or_MDM2_ (D) promoter and 10 ng of pRL-TK together with or without the increasing amounts of the expression plasmid for FLAG-Plk1(K82M) (50 and 100 ng). Forty-eight hours after transfection, cells were lysed and their luciferase activities determined. Firefly luminescence signal was normalized based on the Renilla luminescence signal. Results were shown as fold induction of the firefly luciferase activity compared with control cells transfected with the empty plasmid alone.
To further examine the effect of the kinase activity of Plk1 on p73 function, we performed luciferase reporter assays. H1299 cells were co-transfected with the constant amount of the expression plasmid for HA-p73α, luciferase reporter construct bearing p53/p73-responsive_p21WAF1, BAX,_ or MDM2 promoter, and_Renilla_ luciferase cDNA along with or without the increasing amounts of FLAG-Plk1(K82M). As shown in Fig. 9,B–D, FLAG-Plk1(K82M) had negligible effect on the transcriptional activity of HA-p73α. In contrast to wild-type Plk1, enforced expression of FLAG-Plk1(K82M) in H1299 cells had marginal effect on the expression levels of the endogenous p73α (supplemental Fig. S4). Collectively, these results indicate that kinase activity of Plk1 is required for the inhibition of p73 function.
_Plk1 Phosphorylates p73 in Vitro_—To address whether Plk1 could phosphorylate p73, we performed in vitro kinase reaction (29). To this end, we employed a CycLex Plk1 assay kit. As a positive control, we used protein X, which was provided by the manufacturer. According to the manufacturer's instructions, the active form of Plk1 was incubated with anti-phospho-Thr antibody, and then substrate proteins, including protein X, GST, or the indicated GST-p73α fusion proteins (Fig. 10_A_) were added to the reaction mixture. After the incubation, horseradish peroxidase-conjugated secondary antibody was mixed with the reaction mixtures. As shown inFig. 10_B_,yellow color was observed in the reaction mixtures containing protein X, GST-p73α-(1–62), and GST-p73α-(1–130).Fig. 10_C_ shows the results of quantification of the reactions, suggesting that Plk1 might phosphorylate Thr residue(s) within the region between amino acid residues 1 and 130 of p73α.
FIGURE 10.
Plk1 has an ability to phosphorylate p73 at its NH2-terminal region in vitro. A, Coomassie Brilliant Blue staining of GST or GST-p73α fusion proteins used for in vitro kinase reaction. B, in vitro kinase assay. GST or the indicated GST-p73α deletion mutants bound to glutathione-Sepharose beads were washed with washing buffer and resuspended in 90 μl of kinase reaction buffer. Protein X, which was supplied by manufacturer, was used as a positive control. 10 μl of the active form of Plk1 were added to the reaction mixtures and incubated at 30 °C for 30 min. The reaction mixtures were washed with washing buffer and then incubated with 100 μl of polyclonal anti-phospho-Thr antibody at room temperature for 30 min followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG at room temperature for 30 min. After incubation, 100 μl of substrate reagent were added to the reaction mixtures and incubated at room temperature for 5 min.Yellow coloration indicates the Plk1-mediated phosphorylation at Thr residue. After the addition of 100 μl of stop solution, supernatant was transferred into 96-well tissue culture plate, and the absorbance reading was carried out at 450/540 nm using the microplate reader (C).
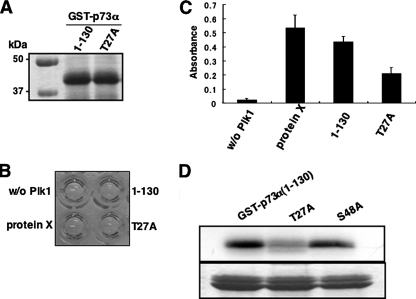
As described previously (33), a sequence (D/E)X(S/T)Ψ_X_(D/E) (where X is any amino acid; Ψ is hydrophobic amino acid) was identified as a consensus motif for Plk1-dependent phosphorylation. During the search for a putative phosphorylation site(s) targeted by Plk1 within the amino acid sequence of p73α (residues 1–130), we found out a related motif (25DSTYFD30) was in the NH2-terminal portion of p73α. To further confirm whether Thr-27 of p73α could be phosphorylated by Plk1, we generated a mutant form of GST-p73α-(1–130), termed T27A, where Thr-27 was substituted to Ala. Purified GST fusion proteins (Fig. 11_A_) were subjected to the in vitro kinase reaction. As shown in Fig. 11, B and C, the amino acid substitution resulted in a significant reduction of Plk1-mediated phosphorylation level of GST-p73α-(1–130). Consistent with these results, GST-p73α-(1–130) and S48A mutant were strongly radiolabeled in the presence of Plk1, whereas T27A mutant was labeled to a lesser degree (Fig. 11_D_), indicating that Thr-27 of p73α is at least one of the phosphorylation sites targeted by Plk1. Taken together, our current results have exposed a novel molecular mechanism behind Plk1-mediated protection of cells from p73-dependent apoptosis.
FIGURE 11.
Thr-27 of p73 is phosphorylated by Plk1 in vitro.A, Coomassie Brilliant Blue staining of GST-p73α-(1–130) and the mutant form of GST-p73α-(1–130) termed T27A where Thr-27 was substituted to Ala. B, in vitro kinase assay. In vitro kinase reactions were performed using GST-p73α-(1–130) and T27A as described in Fig. 10_B_. C, absorbance reading of the in vitro kinase reactions. D, standard in vitro kinase assay. GST-p73α-(1–130), T27A, and S48A were incubated with the active form of Plk1 in the presence of [γ-32P]ATP. The reaction mixtures were separated by SDS-PAGE and subjected to autoradiography (top panel). Bottom panel showed the Coomassie Brilliant Blue staining of the GST-p73α fusion proteins.
DISCUSSION
In this study, we have found for the first time that p73 plays a crucial role in the induction of massive apoptosis in p53-deficient cells caused by siRNA-mediated depletion of the endogenous Plk1. Thus, it is likely that Plk1 protects p53-deficient cells from p73-mediated apoptosis. In this connection, inhibition of Plk1 function might provide a novel therapeutic strategy to treat tumors with p53 mutation.
We have previously demonstrated that Plk1 interacts with p53 and suppresses its transcriptional as well as pro-apoptotic activity (20). According to our previous results, Plk1 bound to a DNA-binding domain of p53 and inhibited its transcriptional activity. For p73, Plk1 interacted with the region between NH2-terminal transactivation domain and a DNA-binding domain of p73α, which includes the proline-rich domain (amino acid residues 81–113). Because NH2-terminal proline-rich domain is involved in the transcriptional activity of p73 (34), it is possible that Plk1 masks the proline-rich domain to render p73α latent form. Thus, it is likely that Plk1-mediated inhibitory mechanisms of p73 is distinct from those of p53.
In response to CDDP, p73α as well as p53 is induced to accumulate in association with a down-regulation of Plk1 (20). Under our experimental conditions, Plk1 has an ability to promote a proteolytic degradation of p73 in a proteasome-independent manner. As described (35), calpain promoted the proteolytic cleavage of p73. However, our preliminary experiments demonstrated that the calpain inhibitor had an undetectable effect on Plk1-mediated proteolytic degradation of p73 (data not shown). Although the precise molecular mechanisms behind the Plk1-mediated proteolytic degradation of p73 remain unclear, our present results indicate that, under normal conditions, Plk1 might contribute to maintain pro-apoptotic p73 at extremely low levels and also suggest that Plk1-mediated proteolytic degradation of p73α might be an alternative molecular mechanism of p73 dysfunction. Similarly, Liu and Erikson (25) described that Plk1 depletion stabilizes p53 in HeLa cells. In support with this notion, Chen et al. (36) found that Plk1 inhibits UV-mediated phosphorylation of p53 at Ser-15 and thereby facilitates its nuclear export and proteolytic degradation.
As described (2,3), p73 function is modulated by post-translational modifications such as phosphorylation and acetylation. Several lines of evidence suggest that phosphorylation of p73 does not always enhance its function (8–13). Based on our present results, Plk1 inhibited the p73α-mediated transcriptional activation, whereas the kinase-deficient mutant form of Plk1 did not, indicating that Plk1-dependent phosphorylation of p73 plays an inhibitory role in the regulation of p73 activity. In accordance with this notion, our in vitro kinase reactions demonstrated that GST-p73α-(1–130) is phosphorylated by Plk1. During the extensive search for putative phosphorylation site(s) within the NH2-terminal portion of p73α, we found out two canonical phosphorylation sites targeted by Plk1, including (25DSTYFD30) and (46DSSMDV51). Thr-27 substituted to Ala (T27A) resulted in a significant reduction of Plk1-mediated phosphorylation of GST-p73α-(1–130), whereas S48A mutant was efficiently phosphorylated by Plk1, suggesting that Thr-27 is at least one of the phosphorylation site(s) of p73α. Considering that Thr-27 exists within the transactivation domain of p73, it is likely that Plk1-mediated phosphorylation of p73α at Thr-27 might induce the conformational change of its transactivation domain and/or the dissociation of the co-activator such as p300/CBP from p73α, and thereby inhibiting transcriptional as well as pro-apoptotic activity of p73α. Indeed, Plk1 inhibited the complex formation between p73 and p300 (supplemental Fig. S5). In addition, it has been shown that p300-mediated acetylation of p73 increases the stability of p73 (15). Thus, it is possible that Plk1-mediated phosphorylation of p73 inhibits p73 function through the dissociation of p300 from p73 and also induces the degradation of latent forms of p73. Further studies should be required to address this issue.
Another finding of this study was that the endogenous Plk1 begins to accumulate in the cell nucleus in response to CDDP treatment. Based on our present results, Plk1 interacts with p73 and inhibits the apoptosis mediated by p73. Because the expression levels of Plk1 decreased in cells exposed to CDDP, p73 might be liberated from the inhibitory complex containing Plk1 to exert its pro-apoptotic activity. The precise molecular mechanisms behind the CDDP-induced nuclear accumulation of Plk1 remain unclear.
It has been well documented that the elevated levels of Plk1 expression show various human tumors (21,37), and patients with tumors showed a clear correlation between lower survival rates and higher Plk1 expression levels (37–39). Taken together with our present and previous results (20), Plk1 has an ability to block the p53- and p73-dependent pro-apoptotic pathway, raising the possibility that Plk1 is one of the potential targets in cancer treatment.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Y. Nakamura for technical assistance.
*
This work was supported in part by a grant-in-aid from the Ministry of Health, Labor, and Welfare for Third Term Comprehensive Control Research for Cancer, a grant-in-aid for scientific research on priority areas from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, a grant-in-aid for scientific research from Japan Society for the Promotion of Science, and a grant from Uehara Memorial Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
S⃞
The on-line version of this article (available athttp://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
2
The abbreviations used are: CDDP, cisplatin; DAPI, 4, 6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorter;GAPDH, glyderaldehyde-3-phosphate dehydrogenase; GST, glutathione_S_-transferase; IB, immunoblotting; IP, immunoprecipitation; NMS, normal mouse serum; PARP, poly(ADP-ribose) polymerase; PBS, phosphate-buffered saline; RT, reverse transcription; siRNA, small interference RNA; GFP, green fluorescent protein.
References
- 1.Kaghad, M., Bonnet, H., Yang, A., Creancier, L., Biscan, J. C., Valent, A., Minty, A., Chalon, P., Lelias, J. M., Dumont, X., Ferrara, P., McKeon, F., and Caput, D. (1997) Cell 90 809-819 [DOI] [PubMed] [Google Scholar]
- 2.Melino, G., De Laurenzi, V., and Vousden, K. H. (2002) Nat. Rev. Cancer 2 605-615 [DOI] [PubMed] [Google Scholar]
- 3.Ozaki, T., and Nakagawara, A. (2005) Cancer Sci. 96 729-737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourdon, J. C., Fernandes, K., Murray-Zmijewski, F., Liu, G., Diot, A., Xirodiamas, D. P., Saville, M. K., and Lane, D. P. (2005) Genes Dev. 19 2122-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiewe, T., Zimmermann, S., Frilling, A., Esche, H., and Putzer, B. M. (2002) Cancer Res. 62 3598-3602 [PubMed] [Google Scholar]
- 6.Pozniak, C. D., Radinovic, S., Yang, A., McKeon, F., Kaplan, D. R., and Miller, F. D. (2000) Science 289 304-306 [DOI] [PubMed] [Google Scholar]
- 7.Irwin, M., Kondo, K., Marin, M. C., Cheng, L. S., Hahn, W. C., and Kaelin, W. G., Jr. (2003) Cancer Cell 3 403-410 [DOI] [PubMed] [Google Scholar]
- 8.Gong, J., Costanzo, A., Yang, H.-Q., Melino, G., Kaelin, W. G., Jr., Levreno, M., and Wang, J. Y. (1999) Nature 399 806-809 [DOI] [PubMed] [Google Scholar]
- 9.Agami, R., Blandino, G., Oren, M., and Shaul, Y. (1999) Nature 399 809-813 [DOI] [PubMed] [Google Scholar]
- 10.Yuan, Z.-M., Shioya, H., Ishiko, T., Sun, X., Gu, J., Huang, Y. Y., Lu, H., Kharbanda, S., Weichselbaum, R., and Kufe, D. (1999) Nature 399 814-817 [DOI] [PubMed] [Google Scholar]
- 11.Ren, J., Datta, R., Shioya, H., Li, Y., Oki, E., Biedermann, V., Bharti, A., and Kufe, D. (2002) J. Biol. Chem. 277 33758-33765 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez, S., Prives, C., and Cordon-Cordo, C. (2003) Mol. Cell. Biol. 23 8161-8171 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Gaiddon, C., Lokshin, M., Gross, I., Levasseur, D., Taya, Y., Loeffler, J.-P., and Prives, C. (2003) J. Biol. Chem. 278 27421-27431 [DOI] [PubMed] [Google Scholar]
- 14.Zeng, X., Li, X., Miller, A., Yuan, Z., Yuan, W., Kwok, R. P., Goodman, R., and Lu, H. (2000) Mol. Cell. Biol. 20 1299-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo, A., Merlo, P., Pediconi, N., Fulco, M., Sartorelli, V., Cole, P. A., Fontemaggi, G., Fanciulli, M., Schiltz, L., Blandino, G., Balsano, C., and Levrero, M. (2002) Mol. Cell 9 175-186 [DOI] [PubMed] [Google Scholar]
- 16.Barr, F. A., Sillje, H. H., and Nigg, E. A. (2004) Nat. Rev. Mol. Cell Biol. 5 429-440 [DOI] [PubMed] [Google Scholar]
- 17.Xie, S. Xie, B., Lee, M. Y., and Dai, W. (2005) Oncogene 24 277-286 [DOI] [PubMed] [Google Scholar]
- 18.Dai, W. (2005) Oncogene 24 214-216 [DOI] [PubMed] [Google Scholar]
- 19.Elia, A. E., Cantley, L. C., and Yaffe, M. B. (2003) Science 299 1228-1231 [DOI] [PubMed] [Google Scholar]
- 20.Ando, K., Ozaki, T., Yamamoto, H., Furuya, K., Hosoda, M., Hayashi, S., Fukuzawa, M., and Nakagawara, A. (2004) J. Biol. Chem. 279 25549-25561 [DOI] [PubMed] [Google Scholar]
- 21.Eckerdt, F., Yuan, J., and Strebhardt, K. (2005) Oncogene 24 267-276 [DOI] [PubMed] [Google Scholar]
- 22.Smith, M. R., Wilson, M. L., Hamanaka, R., Chase, D., Kung, H., Longo, D. L., and Ferris, D. K. (1997) Biochem. Biophys. Res. Commun. 234 397-405 [DOI] [PubMed] [Google Scholar]
- 23.Spankuch-Schmitt, B., Bereiter-Hahn, J., Kaufmann, M., and Strebhardt, K. (2002) J. Natl. Cancer Inst. 94 1863-1877 [DOI] [PubMed] [Google Scholar]
- 24.Spankuch-Schmitt, B., Wolf, G., Solbach, C., Loibl, S., Knecht, R., Stegmuller, M., von Minckwitz, G., Kaufmann, M., and Strebhardt, K. (2002) Oncogene 21 3162-3171 [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., and Erikson, R. L. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 5789-5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits, V. A., Klompmaker, R., Arnaud, L., Rijksen, G., Nigg, E. A., and Medema, R. H. (2000) Nat. Cell Biol. 2 672-676 [DOI] [PubMed] [Google Scholar]
- 27.van Vugt, M. A., Smits, V. A., Klompmaker, R., Medema, R. H. (2001) J. Biol. Chem. 276 41656-41660 [DOI] [PubMed] [Google Scholar]
- 28.Liu, X., Lei, M., and Erikson, R. L. (2006) Mol. Cell. Biol. 26 2093-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., Shreder, K. R., Gai, W., Corral, S., Ferris, D. K., and Rosenblum, J. S. (2005) Chem. Biol. 12 99-107 [DOI] [PubMed] [Google Scholar]
- 30.Niizuma, T., Nakamura, Y., Ozaki, T., Nakanishi, H., Ohira, M., Isogai, E., Kageyama, H., Imaizumi, M., and Nakagawara, A. (2006) Oncogene 26 5046-5055 [DOI] [PubMed] [Google Scholar]
- 31.Hosoda, M., Ozaki, T., Miyazaki, K., Hayashi, S., Furuya, K., Watanabe, K., Nakagawa, T., Hanamoto, T., Todo, S., and Nakagawara, A. (2005) Oncogene 24 7156-7169 [DOI] [PubMed] [Google Scholar]
- 32.Zeng, X., Chen, L., Jost, C. A., Maya, R., Keller, D., Wang, X., Kaelin, W. G., Jr., Oren, M., Chen, J., and Lu, H. (1999) Mol. Cell. Biol. 19 3257-3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima, H., Toyoshima-Morimoto, F., Taniguchi, E., and Nishida, E. (2003) J. Biol. Chem. 278 25277-25280 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, Y., Kameoka, M., Itaya, A., Ota, K., and Yoshihara, K. (2004) Biochem. Biophys. Res. Commun. 317 865-872 [DOI] [PubMed] [Google Scholar]
- 35.Munarriz, E., Bano, D., Sayan, A. E., Rossi, M., Melino, G., and Nicotera, P. (2005) Biochem. Biophys. Res. Commun. 333 954-960 [DOI] [PubMed] [Google Scholar]
- 36.Chen, J., Dai, G., Wang, Y. Q., Wang, S., Pan, F. Y., Xue, B., Zhao, D. H., and Li, C. J. (2006) FEBS Lett. 580 3624-3630 [DOI] [PubMed] [Google Scholar]
- 37.Takai, N., Hamanaka, R., Yoshimatsu, J., and Miyakawa, Y. (2005) Oncogene 24 287-291 [DOI] [PubMed] [Google Scholar]
- 38.Knecht, R., Elez, R., Oechler, M., Solbach, C., von Ilberg, C., and Strebhardt, K. (1999) Cancer Res. 59 2794-2797 [PubMed] [Google Scholar]
- 39.Knecht, R., Oberhauser, C., and Strebhardt, K. (2000) Int. J. Cancer 89 535-536 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]