Independent Origin of Sex Chromosomes in Two Species of the Genus Silene (original) (raw)
Abstract
Here we introduce a new model species, Silene colpophylla, that could facilitate research of sex chromosome evolution and sex-determining systems. This species is related to the well-established dioecious plant model Silene latifolia. Our results show that S. colpophylla is, similarly to S. latifolia, a male heterogametic species, but its sex chromosomes have evolved from a different pair of autosomes than in S. latifolia. The results of our phylogenetic study and mapping of homologs of S. latifolia X-linked genes indicate that the sex determination system in S. colpophylla evolved independently from that in S. latifolia. We assert that this model species pair will make it possible to study two independent patterns of sex chromosome evolution in related species.
MEMBERS of the genus Silene compose an important model system for the study of the early phases of sex chromosome evolution: several dioecious species in this genus have evolutionarily recent sex chromosomes (∼10–20 MYA, according to Bergero et al. 2007). Most Silene species are either gynodioecious or hermaphroditic and can therefore be used in comparative studies that search for autosomal ancestors of the sex chromosomes. To date, the attention of researchers has been concentrated mainly on the study of dioecious species possessing large heteromorphic sex chromosomes with an XX/XY sex-determining system as in Silene latifolia, Silene dioica, and Silene diclinis (for a review see Vyskot and Hobza 2004). Phylogenetic studies conducted on these species showed that they are closely related and share a common origin of the sex chromosomes (Desfeux et al. 1996; Nicolas et al. 2005). Studies focusing on S. latifolia have been particularly successful in providing new insights into sex chromosome evolution. For example, sex chromosomes in S. latifolia have evolved from a single pair of autosomes (Filatov 2005) that have undergone a stepwise reduction of recombination, as shown by a stepwise-gradient of silent site divergence between X- and Y-linked copies of genes from the pseudoautosomal region toward the distal end of the X chromosome (Nicolas et al. 2005; Bergero et al. 2007). In addition, the mechanism of sex determination in S. latifolia has started to be elucidated (Westergaard 1958; Zluvova et al. 2006). Sex determination in S. latifolia is based on the active role of the Y chromosome that possesses genes for male-promoting functions necessary for anther development, genes necessary for male fertility, and genes for the gynoecium-suppressing function, which prevents the development of female sex organs in males.
In addition to S. latifolia, S. diclinis, and S. dioica, there is also another group of dioecious Silene species consisting of Silene otites and its closely related species. So far, the studied species of this group are characterized by the absence of heteromorphic sex chromosomes (Sansome 1938; Westergaard 1958). This fact, together with the supposed nondioecious origin of the genus Silene (Desfeux et al. 1996), suggests that the sex chromosomes in S. otites and its closely related species are evolutionarily younger than those in S. latifolia. It was also suggested (on the basis of the sequencing of rDNA internal transcribed spacer loci) that sex determination evolved independently in the group of species around S. latifolia and in the group of species around S. otites (Desfeux et al. 1996). Even though the bootstraps supporting this hypothesis are rather low, the hypothesis of the recent and independent origin of sex determination in the species related to S. otites certainly deserves great attention. If it is true, these two groups of species could be suitable and complementary models for the study of early sex chromosome evolution. This type of system could also address important questions relating to mechanisms of sex chromosome evolution such as the suppression of recombination and evolutionary changes of sex-linked genes.
A problem complicating research in the group of species closely related to S. otites is difficulties in taxonomy. This group involves many species that have been difficult to distinguish on the basis of morphology alone, and a proper molecular characterization of these species has not yet been done. We have decided to use S. colpophylla (whose name is from the Greek kolpos, meaning a fold, which refers to its typical leaf shape), a species that is distinguishable from S. otites and other closely related species by its typical leaf shape (see supplemental Figure 1). The flowers of S. colpophylla are relatively large compared to the small flowers of other species related to S. otites (Wrigley 1986; see also supplemental Figure 2 for comparison with S. otites). The morphology of the rudiment of the gynoecium suggests that developmental arrest in S. colpophylla males occurs later than in S. latifolia males. In S. colpophylla, the pistil rudiment closely resembles the gynoecium (see supplemental Figure 3a), while in S. latifolia it is a nondifferentiated rod-like structure (Zluvova et al. 2007). In contrast to gynoecium development in males, the anther development in S. colpophylla females is reduced at an early stage of development similarly to S. latifolia (see supplemental Figure 3b).
This article concentrates on three basic questions: (1) Is the origin of sex determination in S. colpophylla independent from that of S. latifolia?, (2) Have the sex chromosomes in S. colpophylla evolved from the same pair of autosomes as the sex chromosomes in S. latifolia?, and (3) What is the type of sex determination in S. colpophylla?
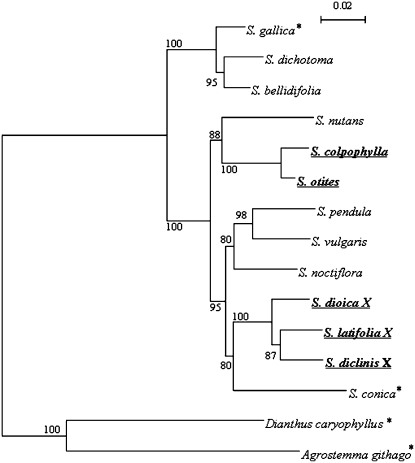
The phylogenetic analysis (Figure 1) using the sequences of homologs of the gene SlX3 (for a characterization of this gene, see Nicolas et al. 2005) clearly shows (the phylogenetic tree is characterized by much higher bootstrap values than the tree published by Desfeux et al. 1996) that the ancestral status in the genus Silene was nondioecious (most probably gynodioecious) and that the sex determination evolved independently in the group including S. colpophylla and S. otites and in the group including the other dioecious species S. latifolia, S. diclinis, and S. dioica. In the hermaphroditic species, any recessive mutation in the nuclear genome arresting the development of the sex organs (male sex organs are supposed to be arrested first) can promote the development of dioecy and sex chromosomes. In the next steps, nuclear gynodioecy is transformed to dioecy via evolution of the female-suppressing function (Charlesworth and Charlesworth 1978). Gynodioecy in the genus Silene is connected with nucleo-cytoplasmic male sterility. In the past, there was a widespread belief that dioecy cannot develop from the nucleo-cytoplasmic sterility because linkage cannot develop between nuclear female sterility and cytoplasmic male sterility (e.g., Ross 1978; Richards 1986). However, other authors (Charlesworth and Ganders 1979; Maurice et al. 1994; Schultz et al. 1994) suggest that, in the case of loss of the male fertile cytotype, male fertility becomes completely controlled by a nuclear gene (fertility restorer), and so it is possible to expect that the development of dioecy and sex chromosomes is feasible in this case. The current mechanism of the suppression of anther development in S. latifolia is not connected with any kind of cytoplasmic sterility but originated via a loss-of-function mutation (on the proto-X chromosome) in the gene that was directly involved in the anther formation, as deduced from the data obtained from the interspecific hybrid S. latifolia × S. viscosa (Zluvova et al. 2005). However, simultaneously some observations have suggested that some nucleo-cytoplasmic system of male sterility at the beginning could be involved (Zluvova et al. 2005). Population studies indicate that nucleo-cytoplasmic male sterility systems are highly dynamic and polymorphic even in the same species (Charlesworth and Laporte 1998). The probability that two different species of the same genus will have the same type of cytotype and the same restorer therefore seems unlikely. The hypothesis that related plant species of the plant genus Silene possess completely different sex-determining pathways is therefore very likely.
Figure 1.—
Tree for orthologs of the gene SlX3. The tree is based on the sequence amplified using primers 11S18 and 11AS13 (Nicolas et al. 2005). This sequence includes both the coding and noncoding part. The tree based on the neighbor-joining method (divergence observed, pairwise gap removal, 10,000 bootstrap replicates) was constructed using Phylo_win software (Galtier et al. 1996). Branch lengths correspond to total sequence divergence under the model assumed (see bar). Values indicated at the nodes are bootstrap values based on 10,000 replicates. Dioecious species are in boldface underlined characters, the hermaphrodite species are marked with an asterisk, and all the other included species are gynodioecious. The tree shows that the distance of S. colpophylla (and its close relative S. otites) from the dioecious Silene species studied so far (S. diclinis, S. dioica, S. latifolia) is relatively large and that there are nondioecious species that are closely related to either S. colpophylla or S. latifolia.
In spite of the fact that sex determination evolved independently, it is still relevant to ask whether there is some region (or whole chromosome) in the Silene genome with a higher tendency to be recruited for the sex chromosome formation. To answer this question, we have mapped the homologs of the genes that were mapped in previously studied dioecious Silene species and/or in the gynodioecious S. vulgaris (Filatov 2005; Nicolas et al. 2005; Bergero et al. 2007). To continue the nomenclature used in previous articles concerning the genes of S. latifolia and their homologs that were isolated from hermaphrodite species (Filatov 2005; Nicolas et al. 2005; Bergero et al. 2007), we use the terms ScolXY3, ScolXY4, ScolDD44XY, and ScolCypXY for the homologs of the genes SlX3/SlY3, SlX4/SlY4, SlDD44X/SlDD44Y, and SlCypX/SlCypY, respectively. The sequences of primers used for the amplification of homologs and the corresponding PCR profiles are summarized in the supplemental Table 1. The polymorphisms used for the segregation analysis and recombination mapping were based either on length polymorphisms of the PCR products or on SNPs, which were identified by sequencing PCR products. For the detection of SNPs, we used the dCAPS method (Michaels and Amasino 1998; Neff et al. 1998).
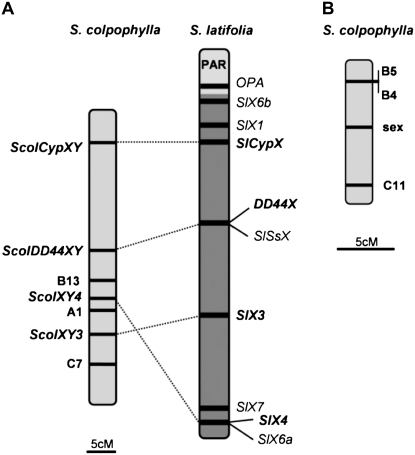
Genetic analysis using the genes ScolXY3, ScolXY4, ScolDD44, and ScolCyp showed that these genes are located in one linkage group in S. colpophylla, similarly to S. latifolia (the difference between the observed gene orders in S. latifolia and S. colpophylla is represented only by the inversion of the chromosomal part including gene ScolXY3 and ScolXY4 (eventually SlX3 and SlX4; Figure 2A). However, in contrast to S. latifolia for which sex linkage of these genes has been shown (Moore et al. 2003; Nicolas et al. 2005; Bergero et al. 2007), none of these genes show sex linkage in S. colpophylla. The results obtained by Nicolas et al. (2005) and Bergero et al. (2007) suggest that a number of markers (including SlDD44X, SlX3, SlX4, and SlCypX whose homologs are used in this study) are well spread over the X chromosome of S. latifolia. Therefore, we can conclude that the pair of sex chromosomes in S. colpophylla probably evolved from a different pair of autosomes than the sex chromosomes in S. latifolia. There is yet a rather hypothetical possibility that sex-determining regions evolved in S. colpophylla on a different pair of autosomes but were subsequently translocated to the homolog of the S. latifolia X chromosome. In this case, the sex linkage of the studied genes could be undetectable in spite of the fact that the sex-determining region would be located on the same chromosome. However, even in this case our data support the view that the evolution of the sex determination in S. colpophylla proceeded independently on S. latifolia and started in a region that was not genetically linked to the region homologous to the current X linked region of S. latifolia.
Figure 2.—
Results of the genetic mapping in S. colpophylla. (A) Linkage group containing three AFLP loci and homologs of the genes that are sex linked in S. latifolia compared with the S. latifolia X chromosome map based on the data of Bergero et al. (2007) (pseudoautosomal region is marked as PAR; homologs are connected by dotted lines). (B) Linkage group containing the sex-determining region (sex) and three AFLP loci.
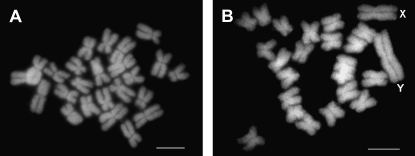
A potential limitation of S. colpophylla as a future model plant is a lack of knowledge concerning sex determination in this species. The type of sex determination system (XX/XY or ZW/ZZ) has not yet been experimentally tested in this species and no exact data are available for the related species. Studies on S. otites indicate even that both ZW/ZZ (Correns 1928; Sansome 1938) and XX/XY (Warmke 1942) sex-determining systems could be present in this species. We began research in this area by studying mitotic chromosomes to test if the sex chromosomes of S. colpophylla are homomorphic, as they are in the related species S. otites. To obtain karyotypes of high quality, we used a technique based on the bulk preparation of mitotic protoplasts from root tips of seedlings (for details see Lengerova et al. 2004). Multiple metaphase plates (50) were evaluated to ensure the presence of both sexes in the mixed sample. The results show either that there is a homomorphic sex chromosome pair in this species or that at least the difference between heterochromosomes is much less prominent than in S. latifolia. The total number of chromosomes is 24 as in most Silene species (for typical metaphase plate, see Figure 3). Because of the supposed homomorphic character of the sex chromosomes in S. colpophylla, we decided to use molecular markers to further investigate the sex-determining system. To obtain a sufficient number of markers, we used the AFLP technique as described by Bratteler et al. (2006). In the initial experiment, we tested 16 primer pair combinations (see supplemental Table 2) on a representative group of males and females and looked for markers that cosegregated with sex. To determine the heterogametic sex, we used an approach that was based on the study of two subsequent generations—the parental and F1 generation. This approach would enable us to identify the heterogametic sex even if none of the markers were completely sex linked. The results expected under two different models (XX/XY and ZW/ZZ) are displayed in supplemental Figure 4.
Figure 3.—
(A) Typical mitotic metaphase plate of S. colpophylla. The observed number of chromosomes (2_n_ = 24) suggests that S. colpophylla is not a polyploid species. No heteromorphic sex chromosomes can be distinguished. (B) Typical mitotic metaphase plate of the S. latifolia male (sex chromosomes are marked by “X” and “Y”). Bars, 5 μm.
We found 45 segregating AFLP markers, but only 3 showed sex linkage. Two markers (B4, C11) from the pollen donor cosegregated predominantly with the female sex in the progeny (supplemental Tables 3 and 4). This pattern of inheritance suggests that these markers are linked to the X chromosome. One marker (B5) showed cosegregation mostly with the male sex, suggesting that the B5 marker is linked to the Y chromosome (supplemental Table 5). In total, these data reveal that males in S. colpophylla, rather than females, are the heterogametic sex. Synthesis of the data obtained by genetic mapping of the three chosen genes and the AFLP markers (done using JoinMap 3.0 software; Van Ooijen and Voorrips 2001) enabled us to define two separate linkage groups for S. colpophylla (Figure 2). One of these linkage groups carries three AFLP markers (A1, B13, and C7) and homologs of genes that are sex linked in S. latifolia: genes ScolXY3, ScolXY4, ScolDD44XY, and ScolCypXY (Figure 2A). The second linkage group involves a sex-determining region (marked “sex”) and three AFLP markers: B4, B5, and C11 (Figure 2B). None of these 3 sex-linked AFLP loci shows linkage to the genes ScolXY3, ScolXY4, ScolDD44XY, and ScolCypXY. These results further support the idea that the sex chromosomes of S. colpophylla and S. latifolia have evolved from different pairs of autosomes. In conclusion, the finding that sex chromosomes in S. colpophylla have evolved from a different pair of autosomes further supports the view that the genus Silene is an excellent model for studying the evolution of sex chromosomes and sex determination because of the diversity of evolutionary patterns represented within it.
Acknowledgments
This work was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (IAA600040801 to B.J.), the Grant Agency of the Czech Republic (521/05/2076 to B.J. and 204/05/H505 to B.V.), the Ministry of Education of the Czech Republic (LC06004 to B.V.), Institutional Research Plan (AVOZ50040507), and the Swiss National Science Foundation grant 3100A0-116455 to A.W.
References
- Bergero, R., A. Forrest, E. Kamau and D. Charlesworth, 2007. Evolutionary strata on the X chromosomes of the dioecious plant Silene latifolia: evidence from new sex-linked genes. Genetics 175 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratteler, M., C. Lexer and A. Widmer, 2006. A genetic linkage map of Silene vulgaris based on AFLP markers. Genome 49 320–327. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 1978. Model for evolution of dioecy and gynodioecy. Am. Nat. 112 975–997. [Google Scholar]
- Charlesworth, D., and F. R. Ganders, 1979. Population genetics of gynodioecy with cytoplasmic-genic male sterility. Heredity 43 213–218. [Google Scholar]
- Charlesworth, D., and V. Laporte, 1998. The male-sterility polymorphism of Silene vulgaris: analysis of genetic data from two populations and comparison with Thymus vulgaris. Genetics 150 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correns, C., 1928. Handbuch der Vererbungswissenschaft, Vol. 2. E. Baur and M. Hartmann, Berlin.
- Desfeux, C., S. Maurice, J. P. Henry, B. Lejeune and P. H. Gouyon, 1996. Evolution of reproductive systems in the genus Silene. Proc. R. Soc. Lond. Biol. 263 409–414. [DOI] [PubMed] [Google Scholar]
- Filatov, D. A., 2005. Evolutionary history of Silene latifolia sex chromosomes revealed by genetic mapping of four genes. Genetics 170 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier, N., M. Gouy and C. Gautier, 1996. SeaView and Phylo_win, two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12 543–548. [DOI] [PubMed] [Google Scholar]
- Lengerova, M., E. Kejnovsky, R. Hobza, J. Macas, S. R. Grant et al., 2004. Multicolor FISH mapping of the dioecious model plant, Silene latifolia. Theor. Appl. Genet. 108 1193–1199. [DOI] [PubMed] [Google Scholar]
- Maurice, S., E. Belhassen, D. Couvet and P. H. Gouyon, 1994. Evolution of dioecy: Can nuclear-cytoplasmic interactions select for maleness? Heredity 73 346–354. [DOI] [PubMed] [Google Scholar]
- Michaels, S. D., and R. M. Amasino, 1998. A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 14 381–385. [DOI] [PubMed] [Google Scholar]
- Moore, R. C., O. Kozyreva, S. Lebel-Hardenack, J. Siroky, R. Hobza et al., 2003. Genetic and functional analysis of DD44, a sex-linked gene from the dioecious plant Silene latifolia provides clues to early events in sex chromosome evolution. Genetics 163 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M. M., J. D. Neff, J. Chory and A. E. Pepper, 1998. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J. 14 387–392. [DOI] [PubMed] [Google Scholar]
- Nicolas, M., G. Marais, V. Hykelova, B. Janousek, V. Laporte et al., 2005. A gradual process of recombination restriction in the evolutionary history of the sex chromosomes in dioecious plants. PLoS Biol. 3 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, A. J., 1986. Plant Breeding Systems. Allen & Unwin, London.
- Ross, M. D., 1978. The evolution of gynodioecy and subdioecy. Evolution 32 174–188. [DOI] [PubMed] [Google Scholar]
- Sansome, F. W., 1938. Sex determination in Silene otites and related species. J. Genet. 35 387–396. [Google Scholar]
- Schultz, S. T., 1994. Nucleo-cytoplasmic male sterility and alternative routes to dioecy. Evolution 48 1933–1945. [DOI] [PubMed] [Google Scholar]
- Van Ooijen, J. W., and R. E. Voorrips, 2001. JoinMap 3.0, Software for the Calculation of Genetic Linkage Maps. Plant Research International, Wageningen, The Netherlands.
- Vyskot, B., and R. Hobza, 2004. Gender in plants: sex chromosomes are emerging from the fog. Trends Genet. 20 432–438. [DOI] [PubMed] [Google Scholar]
- Warmke, H. E., 1942. A new method for determining the sex heterozygote in species with morphologically undifferentiated sex chromosomes and its application to Silene otites. Genetics 27 174. [Google Scholar]
- Westergaard, M., 1958. The mechanism of sex determination in dioecious flowering plants. Adv. Genet. 9 217–281. [DOI] [PubMed] [Google Scholar]
- Wrigley, F., 1986. Taxonomy and chorology of Silene section Otites (Caryophyllaceae). Ann. Bot. Fenn. 23 69–81. [Google Scholar]
- Zluvova, J., M. Lengerova, M. Markova, R. Hobza, M. Nicolas et al., 2005. The inter-specific hybrid Silene latifolia × S.viscosa reveals early events of sex chromosome evolution. Evol. Dev. 7 327–336. [DOI] [PubMed] [Google Scholar]
- Zluvova, J., M. Nicolas, A. Berger, I. Negrutiu and F. Moneger, 2006. Premature arrest of the male flower meristem precedes sexual dimorphism in the dioecious plant Silene latifolia. Proc. Natl. Acad. Sci. USA 103 18854–18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zluvova, J., S. Georgiev, B. Janousek, D. Charlesworth, B. Vyskot et al., 2007. Early events in the evolution of the Silene latifolia Y chromosome: male specialization and recombination arrest. Genetics 177 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]