The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization (original) (raw)
Abstract
PTEN is a tumor suppressor frequently mutated in cancer. Recent reports implicated Nedd4-1 as the E3 ubiquitin ligase for PTEN that regulates its stability and nuclear localization. We tested the physiological role of Nedd4-1 as a PTEN regulator by using cells and tissues derived from two independently generated strains of mice with their Nedd4-1 gene disrupted. PTEN stability and ubiquitination were indistinguishable between the wild-type and Nedd4-1-deficient cells, and an interaction between the two proteins could not be detected. Moreover, PTEN subcellular distribution, showing prominent cytoplasmic and nuclear staining, was independent of Nedd4-1 presence. Finally, activation of PKB/Akt, a major downstream target of cytoplasmic PTEN activity, and the ability of PTEN to transactivate the Rad51 promoter, a measure of its nuclear function, were unaffected by the loss of Nedd4-1. Taken together, our results fail to support a role for Nedd4-1 as the E3 ligase regulating PTEN stability and subcellular localization.
Keywords: E3 ligase, tumor suppressor, P13K signal, WW domain
P_TEN_ is one of the most frequently mutated genes in human cancer (1–3). In the cytoplasm, PTEN functions as a lipid phosphatase by dephosphorylating the D3 position of phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], and directly antagonizing PI3K (4–6). Consistent with a negative regulatory role for PTEN in regulation of PI3 signaling, PTEN-deficient cells and tissues exhibit defects in cell proliferation, growth, survival, death, protein translation, metabolism, migration, and structural organization (2, 3, 7, 8).
One of the most intriguing features of PTEN is its subcellular localization. Although PTEN was originally found to be a cytoplasmic protein (9), its nuclear localization in many cell types has been reported by using various independently developed monoclonal and polyclonal anti-PTEN antibodies (10–14). A number of attempts have been made to uncouple the cytoplasmic and nuclear roles of PTEN, yielding incongruous results. For example, differing accounts of the respective levels of cytoplasmic and nuclear PTEN throughout the stages of the cell cycle have been documented (15, 16). Functionally, nuclear PTEN has been shown to induce cell cycle arrest in certain cell types (17), whereas in others, nuclear accumulation of PTEN increased upon stimulation with proapoptotic factors and correlated with induction of apoptosis (18). Finally, nuclear-targeted PTEN was shown to impede the growth of U251MG glioblastoma cells and down-regulate p70S6K in a PKB/Akt-independent manner but was unable to inhibit cell invasion (19).
Recently, published work revealed a phosphatase activity-independent nuclear role for PTEN in the regulation of chromosomal stability and repair of DNA damage (20). Wang et al. (21) and Trotman et al. (22) reported that PTEN stability and nuclear translocation were regulated by ubiquitination mediated by the ubiquitin ligase Nedd4-1. Although the Nedd4-1-mediated polyubiquitination of PTEN in the cytosol caused PTEN degradation (21), its Nedd4-1-dependent monoubiquitination led to the translocation of this tumor suppressor to the nucleus, where it was protected from degradation (22).
Nedd4-1 is an E3 ubiquitin ligase that contains a C2 domain, three or four WW domains, and a ubiquitin ligase Hect domain, and it belongs to the Nedd4 family of Hect E3 ligases that also includes its relative Nedd4-2 (23–25). The WW domain of Nedd4 proteins recognizes and binds a short amino acid sequence in substrate proteins, called the PY motif (L/PPxY) (26–30). By using two independent approaches, we recently generated distinct strains of mice with disrupted Nedd4-1 gene, leading to a complete loss of Nedd4-1 protein and embryonic lethality at mid to late gestation (F.F. and D.R., unpublished observations; H.K., A.N., and N.B., unpublished observations). Surprisingly, by using a comprehensive analysis of PTEN levels, subcellular distribution, and cytoplasmic and nuclear function in tissues and cells from Nedd4-1 knockout mice, we demonstrate here that Nedd4-1 is dispensable for regulation of PTEN stability, subcellular localization, and activity.
Results
Analysis of PTEN Levels and Ubiquitination in Nedd4-1-Deficient Cells.
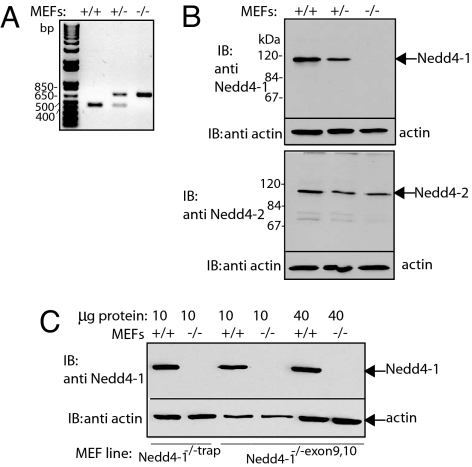
To examine the physiological function of Nedd4-1, its gene in the mouse was disrupted by using two independent approaches. First, by using gene-trap methodology, a β-gal cDNA was inserted into the mouse Nedd4-1 genomic locus between exons 6 and 7 (ES trap clone XB786; BayGenomics) (F.F. and D.R., unpublished observations). In a separate effort, exons 9 and 10 of mouse Nedd4-1 (Ensemble NC_000075.4) were disrupted by homologous recombination in ES cells (H.K., A.N., and N.B., manuscript in preparation). Nedd4-1−/− embryos resulting from both knockout strategies die at mid to late gestation displaying complete loss of Nedd4-1 protein (F.F. and D.R., unpublished observations; and H.K., A.N., and N.B., unpublished observations). To explore the physiological function of Nedd4-1 in greater detail, day 13.5–14.5 mouse embryonic fibroblasts (MEFs) from Nedd4-1−/− embryos, as well as from their sibling wild-type (WT) and heterozygote controls, were generated by using two different strategies. Serial passage was used to derive the Nedd4-1−/−trap MEFs (31), whereas MEFs from Nedd4-1−/−exon9,10 were immortalized by retrovirally mediated expression of the SV40 Large-T antigen. Immunoblotting of protein lysates from these cells demonstrated complete loss of Nedd4-1 expression in Nedd4-1−/−trap and Nedd4-1−/−exons9,10 cells, whereas the expression of its close relative Nedd4–2 remained unaffected (Fig. 1).
Fig. 1.
Loss of Nedd4-1 expression in MEFs derived from Nedd4-1 knockout mice. (A) Genotyping of MEFs generated from Nedd4-1+/+, Nedd4-1+/−trap, and Nedd4-1−/−trap embryos. PCR analysis of genomic DNA from the indicated cells is shown. A 447-bp fragment corresponds to the WT allele and a 680-bp fragment to the trapped allele. (B) Immunoblotting of lysates from Nedd4-1+/+, Nedd4-1+/−trap, and Nedd4-1−/−trap MEFs. Cell lysates were probed for Nedd4-1 and Nedd4–2 by using anti-Nedd4-1 antibodies (Upper) and anti-Nedd4–2 antibodies (Lower), respectively. Actin levels were determined with anti-actin antibodies. (C) Immunoblotting of lysates from Nedd4-1+/+ and Nedd4-1−/−exons9,10 MEFs. Ten or 40 μg of proteins obtained from cell lysates of the indicated MEFs were probed for Nedd4-1 by immunoblotting with anti-Nedd4-1 antibodies, as in B. Actin levels were determined with anti-actin antibodies.
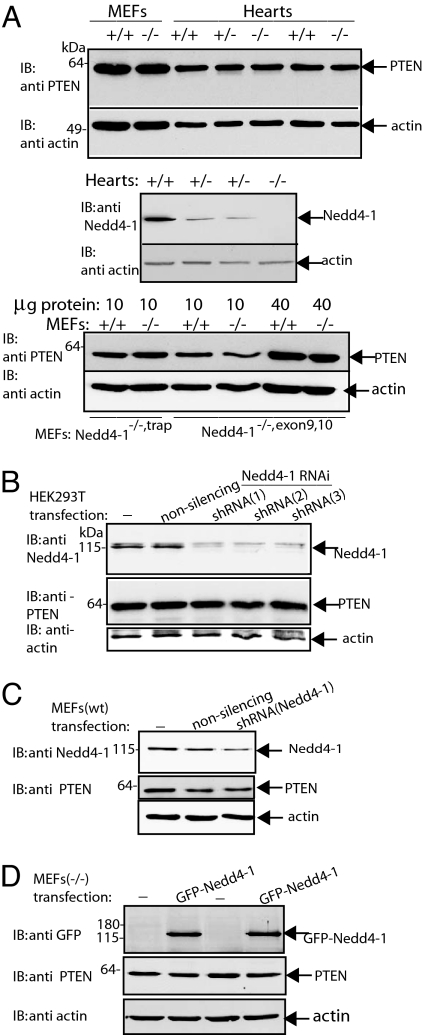
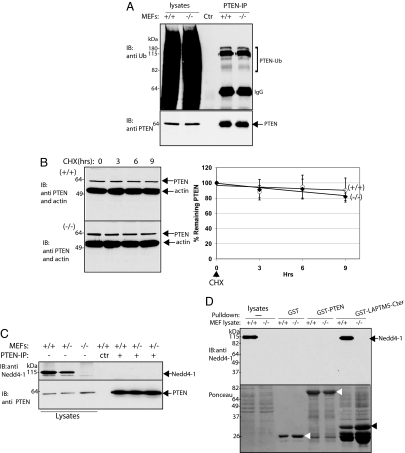
By using in vitro ubiquitination assays and monitoring PTEN ubiquitination upon overexpression or knockdown of Nedd4-1 in cultured cells, a direct role of Nedd4-1, but not Nedd4-2, in regulation of PTEN ubiquitination and stability was recently proposed (21). To explore this relationship in our system, we examined PTEN levels in Nedd4-1−/− MEFs. Judged by immunoblotting, PTEN protein levels were independent of the presence of Nedd4-1 (Fig. 2A Top and Bottom). Moreover, PTEN protein levels in the hearts of the Nedd4-1−/− embryos (a tissue that expresses high levels of Nedd4-1; Fig. 2A Middle) were indistinguishable from those in Nedd4-1 WT or heterozygote hearts (Fig. 2A Top). To determine overall levels of PTEN ubiquitination, Nedd4-1−/−trap and control WT MEFs were pretreated with a proteasome inhibitor (MG132) for 3 h and lysed. Lysates were then boiled in SDS (to dissociate any putative PTEN-interacting proteins), and endogenous PTEN was immunoprecipitated from these cell lysates (after diluting the SDS) and probed with anti-ubiquitin antibodies (Fig. 3A). Our results show that the pattern and intensity of bands recognized by the anti-ubiquitin antibodies (most likely representing ubiquitin-modified endogenous PTEN) were comparable in cells with or without Nedd4-1 (Fig. 3A). Moreover, pulse–chase experiments performed either by the addition of cycloheximide to block protein synthesis (Fig. 3B) or by using metabolic labeling with [35S]Met/Cys (data not shown) revealed no difference in PTEN stability or rate of degradation between MEFs containing or lacking Nedd4-1 (Fig. 3B), by using either the Nedd4-1−/−trap or Nedd4-1−/−exons9,10 MEFs. Thus, our results suggest that deletion of Nedd4-1 had no effect on the stability or ubiquitination of PTEN in our system.
Fig. 2.
PTEN stability is not affected by Nedd4-1. (A) PTEN levels are unchanged in the absence (knockout) of Nedd4-1. Levels of PTEN in MEFs and heart tissue from Nedd4-1+/+, Nedd4-1+/−trap, and Nedd4-1−/−trap embryos (Top), and MEFs from Nedd4-1+/+ and Nedd4-1−/−exons9,10 embryos (Bottom) were analyzed by immunoblotting. Expression of Nedd4-1 in embryonic hearts of Nedd4-1+/+ and Nedd4-1+/−trap (but not Nedd4-1−/−trap) mice is depicted in the Middle. (B and C) PTEN levels are unchanged by knockdown of Nedd4-1. (B) HEK293T cells were either not transfected or were transfected individually with three different shRNAmir (in pGIPZ) directed toward the human Nedd4-1 sequence or pGIPZ plasmid containing a nonsilencing control. After 72 h, cells were lysed, and levels of Nedd4-1, PTEN, and actin were determined by immunoblotting with their respective antibodies. Endogenous levels of Nedd4-1 were reduced 62%, 70%, and 71% for shRNA (1), shRNA (2) and shRNA (3), respectively (average of n = 5, relative to cells transfected with nonsilencing control). Average PTEN levels in these experiments remained close to 100% for each of the knockdown constructs [93%, 90%, and 97% for shRNA (1), shRNA (2) and shRNA (3), respectively (n = 5, relative to nonsilencing control)]. Actin levels remained constant as well (99%, 105%, and 102% compared with nonsilencing control, respectively). The experiment shown is a representative of three independent experiments, each performed in duplicate. (C) WT MEFs (Nedd4-1+/+,exons9,10) were transfected with shRNA directed against mouse Nedd4-1. Cells were then analyzed for the extent of Nedd4-1 knockdown and levels of PTEN and actin, as described above. Average Nedd4-1 knockdown was 56% (n = 4), whereas actin levels remained unchanged. (D) Reconstitution of Nedd4-1 in Nedd4-1−/−exons9,10 MEFs does not alter PTEN levels. Nedd4-1−/−exons9,10 MEFs were transfected with GFP-Nedd4-1, and 24 h later cells were analyzed for expression of Nedd4-1 by using anti-GFP antibodies, and for the levels of PTEN and actin, as described above.
Fig. 3.
PTEN ubiquitination is comparable in Nedd4-1+/+ and Nedd4-1−/−trap MEFs and lack of association between PTEN and Nedd4-1. (A) PTEN ubiquitination: Nedd4-1+/+ and Nedd4-1−/−trap MEFs were pretreated with a proteasome inhibitor (20 μM MG132). They were then lysed, and the lysate was boiled in SDS to remove PTEN-associated proteins. After dilution of the SDS, PTEN was immunoprecipitated from the lysates, and immunoprecipitates were immunoblotted with anti-ubiquitin antibody to detect ubiquitinated PTEN (PTEN-Ub; Upper) or anti-PTEN antibody (Lower). Control (Ctr): Immunoprecipitate with beads alone (without anti-PTEN antibodies). The left two lanes represent total ubiquitination of the MEF lysates used for the experiment. (B) PTEN stability is unchanged in the absence of Nedd4-1. Nedd4-1+/+ MEFs (Top Left) or Nedd4-1−/−trap MEFs (Bottom Left) were exposed to cycloheximide (CHX) and the amount of PTEN remaining was analyzed over time (0, 3, 6, 9 h) by immunoblotting. Identical results were obtained in the Nedd4-1−/−exons9,10 MEFs (data not shown). Quantification of the pulse–chase data from both the Nedd4-1−/−trap and Nedd4-1−/−exons9,10 MEFs (and their wild-type controls) is shown in the Right (mean± SD, n = 4). (C and D) PTEN does not bind Nedd4-1. (C) PTEN was immunoprecipitated from Nedd4-1+/+ and Nedd4-1−/−trap MEFs, and the precipitates were immunoblotted for Nedd4-1 (Upper) or PTEN (Lower) with their respective antibodies. Control (Ctr): beads alone (without anti-PTEN antibodies). (D) (Upper) Immobilized GST-PTEN, GST alone (negative control), or GST-LAPTM5(C terminus) (GST-LAPTM5-Cter; positive control) were incubated with lysates from Nedd4-1+/+ and Nedd4-1−/−trap MEFs, and the precipitate was immunoblotted for Nedd4-1. (Lower) GST fusion proteins (arrowheads) used for the pulldowns, analyzed by Ponceau S staining. Binding experiments were repeated two to four times with identical results.
Because the above experiments used cells or tissues that chronically lack Nedd4-1 because of a germ-line deletion (and thus could have adapted to the absence of this E3 ligase during development), we tested PTEN stability in HEK293T cells in which the endogenous Nedd4-1 level was acutely reduced with RNA interference. As seen in Fig. 2B, microRNA-adapted short hairpin RNA (shRNAmirs) directed toward three different regions of human Nedd4-1 led to a ≈70% reduction in levels of endogenous Nedd4-1; such reduction, however, did not alter PTEN abundance (Fig. 2B). To investigate this observation further, we determined PTEN levels in Nedd4-1+/+ MEFs with their Nedd4-1 knocked down and in Nedd4-1−/−exons9,10 MEFs reconstituted to express Nedd4-1 by transfection. As seen in Fig. 2C, knockdown of endogenous Nedd4-1 by shRNA (≈50%) in WT MEFs did not lead to increased levels of PTEN. Moreover, GFP-Nedd4-1 transfection into Nedd4-1−/−exons9,10 MEFs did not alter PTEN levels (Fig. 2D). Thus, chronic loss of Nedd4-1 by gene knockout or acute loss by gene knockdown did not affect PTEN stability.
Lack of Physical Interaction Between PTEN and Nedd4-1.
By using pulldowns of purified GST-PTEN and Nedd4-1 proteins, as well as coimmunoprecipitation from HEK293 cells transfected with PTEN and Nedd4-1, Wang et al. (21) reported a direct interaction between PTEN and Nedd4-1. We thus tested whether endogenous PTEN and Nedd4-1 can coimmunoprecipitate from WT MEFs. As shown in Fig. 3C, no association between endogenous Nedd4-1 and PTEN in WT MEFs could be detected. To test further for Nedd4-1–PTEN interactions, we performed a pulldown experiment whereby lysates from Nedd4-1+/+ or Nedd4-1−/−trap MEFs were incubated with immobilized GST-PTEN, GST alone (negative control), or GST-LAPTM5(C terminus), which readily interacts with Nedd4-1 and was used as a positive control (32). As shown in Fig. 3D, unlike the GST-LAPTM5(C terminus), GST-PTEN failed to precipitate Nedd4-1 from cell lysates. Thus, by using two robust methods of detecting Nedd4-1 protein–protein interactions, we were unable to detect its association with PTEN.
Normal PI3K Signaling Throughput in Nedd4-1-Deficient Cells.
Cytoplasmic activity of PTEN has been shown to antagonize the PI3K pathway throughput via direct dephosphorylation of PI(3,4,5)P3, a second messenger product of PI3K activity (4, 6). We reasoned that if Nedd4-1 promoted ubiquitination and degradation of PTEN (21), then the absence of Nedd4-1 might lead to impairment of the PI3K signaling pathway. To test this possibility, we investigated the activation-specific phosphorylation of PKB/Akt, a major downstream target of PI3K activity, in our Nedd4-1-deficient cells. As shown in Fig. 4, there were no differences in PKB/Akt phosphorylation under either serum starvation or serum restimulation conditions between the Nedd4-1−/−, Nedd4-1+/−, or Nedd4-1+/+ MEFs, suggesting no change in PTEN cytoplasmic function upon Nedd4-1 loss.
Fig. 4.
PKB/Akt activation does not depend on Nedd4-1. Subconfluent Nedd4-1+/+, Nedd4-1+/−trap, and Nedd4-1−/−trap MEFs (A) or Nedd4-1+/+ and Nedd4-1−/−exons9,10 MEFs (B) were starved overnight followed by either no treatment, 10-min stimulation with 10% FBS, or 50 μM LY294002 treatment for 1 h followed by FBS stimulation. Equal amounts of protein lysates were immunoblotted with anti-phospho-PKB (Ser-473), anti-PKB, anti-PTEN, or anti-GAPDH antibodies. Results are representative of three independent experiments.
Characterization of Nuclear Localization and Function of PTEN in Nedd4-1-Deficient Cells.
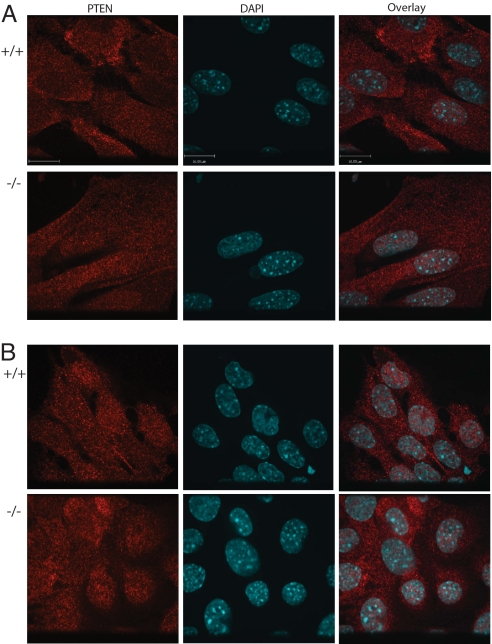
It was recently shown that in both HeLa cells and Trp53−/− MEFs, PTEN nuclear localization depended on its Nedd4-1-mediated monoubiquitination (22). These findings infer that in cells lacking Nedd4-1, nuclear translocation of PTEN should be impaired. Contrary to this prediction, however, we found that endogenous PTEN was localized in the nucleus and in the cytoplasm of both Nedd4-1−/− and Nedd4-1+/+ MEFs (Fig. 5). Quantitation of PTEN subcellular localization revealed that 100% of the Nedd4-1-deficient cells exhibited both cytosolic and nuclear localization. Namely, 213 of 213 Nedd4-1+/+trap MEFs and 290 of 290 Nedd4-1+/+ exons9,10 MEFs used as controls, and 155 of 155 Nedd4-1−/−trap MEFs and 297 of 297 Nedd4-1−/−exons9,10 MEFs exhibited indistinguishable, coordinate PTEN cytoplasmic and nuclear localization (Fig. 5).
Fig. 5.
PTEN subcellular localization is independent of Nedd4-1. Nedd4-1+/+ and Nedd4-1−/−trap MEFs (A) or Nedd4-1+/+ and Nedd4-1−/−exons9,10 MEFs (B) were immunostained for PTEN (red) and DAPI (blue, to mark the nucleus) and analyzed by confocal microscopy to evaluate cellular distribution of PTEN. (Scale bar: 16 μm.)
One of the proposed nuclear functions of PTEN is its influence on the activity of the promoter for Rad51, a protein essential for the repair of double-strand DNA breaks and maintenance of genomic integrity (20, 33–35). Consistent with previous studies in PC-3 cells, we observed transactivation of the Rad51 promoter by PTEN upon coexpression of E2F1 (Fig. 6). We then tested the activity of the Rad51 promoter in response to PTEN in Nedd4-1-deficient cells. Significantly, the presence of Nedd4-1 had no effect on the ability of PTEN to activate the Rad51 promoter (Fig. 6). Accordingly, cellular levels of transfected PTEN were indistinguishable between WT and Nedd4-1-deficient cells (data not shown). Thus, Nedd4-1 disruption failed to prevent PTEN nuclear localization or affect its nuclear function.
Fig. 6.
Nedd4-1 does not affect activation of the Rad51 promoter by PTEN. Nedd4-1+/+, Nedd4-1−/−exons9,10, or PC-3 cells were transiently transfected with the indicated plasmids, and lysates were assayed for luciferase activity. The results are represented as the mean ± SD (n = 4 samples, representative of three independent experiments). Luciferase activity is shown as fold induction normalized to basal Rad51 promoter activity. There is no statistically significant difference in Rad51 activation by PTEN between the WT and Nedd4-1(−/−) cells.
Discussion
In contrast to a comprehensive understanding of PTEN genetic alterations in cancer and a generally accepted role as a major negative regulator of PI3K signaling, relatively little is known about modes of PTEN regulation. PTEN C-terminal phosphorylation has been implicated in control of PTEN stability (36–40), whereas phosphorylation-dependent polyubiquitination was proposed as a potential molecular mechanism leading to PTEN degradation (41). Recent work has postulated that PTEN is subject to both poly- and monoubiquitination mediated by the ubiquitin ligase Nedd4-1, leading to PTEN degradation and nuclear localization, respectively (21, 22). Importantly, a potential phosphatase-independent nuclear function of PTEN in tumor suppression and the monoubiquitination-dependent molecular mechanism of PTEN nuclear translocation have been described, revealing a nuclear molecular cascade involving PTEN (20).
By using cells deficient for Nedd4-1, we demonstrate here that Nedd4-1 is dispensable for the regulation of PTEN stability, localization, or activity. There are several possible reasons for the apparent discrepancy between our results and published findings (21, 22). Our work used two independently generated Nedd4-1 knockout mouse models and MEFs derived from them, whereas the previous reports relied entirely on overexpression and knockdown of Nedd4-1 to examine its function. Because it is likely that Nedd4-1 has numerous physiological targets (refs. 32, 42 and 43 and F.F. and D.R., unpublished observations), some of which may have roles in PTEN turnover, their forced ubiquitination upon Nedd4-1 overexpression may lead to false conclusions assuming an immediate relationship. Further, indiscriminate ubiquitin ligase activity of strongly overexpressed Nedd4-1 may have nonphysiological consequences leading to PTEN modulation. In an attempt to assess the effects of transient, acute knockdown of Nedd4-1 on PTEN, we performed such an analysis in HEK293T cells and WT MEFs (Fig. 2). Regardless of the experimental system, we failed to detect the effect of rapid reduction of Nedd4-1 levels on PTEN. The fact that the high levels of expression of particular siRNAs may lead to substantial off-target effects raises the possibility that such events may have contributed to the discrepancy of the data reported by Wang et al. and Trotman et al. (21, 22) with our results.
Our genetic approach to disrupt the Nedd4-1 gene in all mouse tissues and the analysis of cells and tissues derived from these mice offers a more physiological system for the analysis of Nedd4-1 ubiquitin ligase targets. This is exemplified by the fact that even though there was a statistically significant inverse correlation between Nedd4-1 mRNA and PTEN protein levels in human bladder cancer samples (21), complete loss of Nedd4-1 expression in cells and tissues did not affect PTEN levels (Fig. 2). Thus, our results are not consistent with the existence of a linear relationship between Nedd4-1 and regulation of PTEN. We did notice, however, that primary Nedd4-1-deficient MEFs grow more slowly than their wild-type counterparts (data not shown), consistent with a potential role of Nedd4-1 in control of cell proliferation and transformation, likely via a yet unidentified substrate. These observations may explain the ability of overexpressed Nedd4-1 to enhance Ras-induced transformation of p53-deficient fibroblasts (21) and the apparent negative effect of Nedd4-1 knockdown on growth of certain prostate cancer cell lines as xenografts (21).
Our biochemical analysis did not support a role for Nedd4-1 in PTEN ubiquitination or binding. Although endogenous Nedd4-1 from WT MEFs readily interacted with the GST fusion protein containing the C terminus of its known substrate LAPTM5 (32), GST-PTEN could not precipitate Nedd4-1 from the same cells (Fig. 3). Unlike known Nedd4-1 substrates, including LAPTM5, which typically contain PY motifs (27–30, 32), PTEN does not possess this motif, possibly explaining the observed lack of its interaction with Nedd4-1 (Fig. 3 C and D) and unaffected PTEN ubiquitination in Nedd4-1-deficient cells (Fig. 3A).
The proposition that PTEN is subject to monoubiquitination-dependent nuclear translocation, leading to profound effects on its tumor suppressor function, deserves further attention because it may have significant clinical implications (21, 22); namely, PTEN nuclear localization has been associated with various stages of tumorigenesis. For instance, in the thyroid, normal follicular cells display preferential nuclear staining, which gradually weakens as the normal cells progress toward follicular adenoma and eventually carcinoma (12). These progressive changes in PTEN nuclear localization seem to precede the eventual loss of PTEN expression associated with more advanced disease, likely because of genetic and epigenetic means. The observed lack of coordinate increase in cytoplasmic staining accompanying decreased nuclear PTEN localization during genesis of thyroid tumors argues against a single activity regulating PTEN nuclear localization and cytoplasmic turnover. Contrary to this view, however, in both normal pancreatic islet cells and melanocytes, PTEN is preferentially found in the nucleus, whereas in tumors originating from these cells, PTEN nuclear localization diminishes and is associated with increased cytoplasmic accumulation (10, 14). Thus, it is conceivable that PTEN mono- and polyubiquitination may be subject to regulation by multiple ubiquitin ligases, possibly acting in cell- and tissue-specific manners. In that context, the discovery of potential phosphatase-independent nuclear roles of PTEN in tumor suppression (20) and the monoubiquitination-dependent molecular mechanism of PTEN nuclear translocation (22) offers a significant insight into this largely underappreciated aspect of PTEN function. As our knowledge in this area continues to expand, the identification of the physiological ubiquitin ligase(s) for PTEN remains a major challenge on the road to a comprehensive understanding of cellular roles of this tumor suppressor.
Experimental Procedures
Materials.
The sources of all reagents can be found in supporting information (SI) Experimental Procedures.
Methods.
The detailed experimental procedures can be found in SI Experimental Procedures.
Generation of Nedd4-1 Knockout Mice and MEFs.
For generation of Nedd4-1_−/−_trap mice, the Nedd4-1-trapped ES clone (XB786) was obtained from BayGenomics; the Nedd4-1_−/−_exons9,10 conventional knockout mice were generated by homologous recombination in ES cells (H.K., A.N., and N.B., unpublished observations). Nedd4-1_−/−_trap MEFs from E14.5 were generated as described in ref. 31. Nedd4-1_−/−_exons9,10 MEFs were prepared from E13.5 embryos and immortalized after infection with retroviruses expressing the SV40 Large T-antigen (44).
Immunofluorescence Confocal Microscopy.
Immunofluorescence confocal microscopy was performed as described in ref. 32. To visualize PTEN, cells were stained with the anti-PTEN antibody (1:50) (clone 6H2.1) (Cascade Bioscience).
Pulldown and Coimmunoprecipitation Assays.
Pulldown and coimmunoprecipitation assays were performed as described in ref. 32. Bound Nedd4-1 was identified by immunoblotting with anti-Nedd4-1 antibody (1:1,000, clone 15; BD Biosciences).
Knockdown Experiments.
Three different shRNAmirs directed toward the human Nedd4-1 RNA {V2LHS_254872 [shRNA (construct 1)], V2LHS_72553 [shRNA (construct 2)], V2LHS_72555 [shRNA (construct 3)], all in pGIPZ} (OpenBiosystems) were used for Nedd4-1 knockdown in 293 cells. For knockdown of mouse Nedd4-1 shRNAmir directed against mouse Nedd4-1 (V2LMM_17409; OpenBiosystems) was used. Nedd4-1 was reexpressed as a GFP-tagged Nedd4-1 described in ref. 32.
Ubiquitination Assays.
Nedd4-1+/+ and Nedd4-1−/−trap MEFs were pretreated with 20 μM MG132 for 3 h, lysed, and cell lysates (1 mg of protein) were boiled for 5 min in 1% SDS to ensure dissociation of any PTEN-associated proteins. After 11-fold dilution of the lysate with lysis buffer (to dilute the SDS), PTEN was precipitated from the boiled lysate with 10 μg of anti-PTEN antibody and 25 μl of protein G–Sepharose beads (GE Healthcare) followed by immunoblotting with anti-ubiquitin and anti-PTEN antibodies.
Determination of PKB/Akt Activation.
PKB/Akt activation was determined as described in ref. 6, and in the SI.
Analysis of Rad51 Promoter Activity.
The activity of the pGL3-hRad51-luc promoter in PC-3 cells and Nedd4-1+/+ or Nedd4-1−/−exons9,10 MEFs was assessed as described in ref. 20.
Supplementary Material
Supporting Information
Acknowledgments.
We thank C. Jiang and A. Griffin for technical support and Dr. Yuxin Yin (Columbia University, New York) for the gift of pGL3-hRad51-luc and pCMV-E2F1 plasmids. This work was supported by grants from the Canadian Institute of Health Research (177834-31865) and the National Cancer Institute of Canada, with support from the Canadian Cancer Society (to D.R.) and the Canadian Breast Cancer Foundation (to V.S.). D.R. and V.S. hold Canada Research Chairs.
Footnotes
The authors declare no conflict of interest.
References
- 1.Eng C. PTEN: One gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 2.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 3.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 4.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 5.Myers MP, et al. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 9.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 10.Perren A, et al. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared with normal islet cells. Am J Pathol. 2000;157:1097–1103. doi: 10.1016/S0002-9440(10)64624-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–2338. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]
- 12.Gimm O, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachibana M, et al. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:1955–1960. doi: 10.1002/cncr.0678. [DOI] [PubMed] [Google Scholar]
- 14.Whiteman DC, et al. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer. 2002;99:63–67. doi: 10.1002/ijc.10294. [DOI] [PubMed] [Google Scholar]
- 15.Deleris P, et al. SHIP-2 and PTEN are expressed and active in vascular smooth muscle cell nuclei, but only SHIP-2 is associated with nuclear speckles. J Biol Chem. 2003;278:38884–38891. doi: 10.1074/jbc.M300816200. [DOI] [PubMed] [Google Scholar]
- 16.Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0–G1 in MCF-7 cells. Cancer Res. 2003;63:282–286. [PubMed] [Google Scholar]
- 17.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 18.Gil A, et al. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 2006;17:4002–4013. doi: 10.1091/mbc.E06-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JL, et al. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211–6224. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trotman LC, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: Role of Nedd4/Rsp5p family of ubiquitin–protein ligases. J Membr Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 24.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: Functional diversity within a common modular architecture. Oncogene. 2004;23:1972–1984. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 25.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 26.Staub O, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 27.Kanelis V, Bruce MC, Skrynnikov NR, Rotin D, Forman-Kay JD. Structural determinants for high-affinity binding in a Nedd4 WW3* domain-Comm PY motif complex. Structure. 2006;14:543–553. doi: 10.1016/j.str.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Kanelis V, Rotin D, Forman-Kay JD. Solution structure of a Nedd4 WW domain-ENaC peptide complex. Nat Struct Biol. 2001;8:407–412. doi: 10.1038/87562. [DOI] [PubMed] [Google Scholar]
- 29.Kasanov J, Pirozzi G, Uveges AJ, Kay BK. Characterizing class I WW domains defines key specificity determinants and generates mutant domains with novel specificities. Chem Biol. 2001;8:231–241. doi: 10.1016/s1074-5521(01)00005-9. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, et al. Ubiquitination screen using protein microarrays for comprehensive identification of Rsp5 substrates in yeast. Mol Syst Biol. 2007;3(116):1–12. doi: 10.1038/msb4100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman A. In: Cells, A Laboratory Manual. Spector DL, Goldman RD, Leinwand LA, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1998. [Google Scholar]
- 32.Pak Y, Glowacka WK, Bruce MC, Pham N, Rotin D. Transport of LAPTM5 to lysosomes requires association with the ubiquitin ligase Nedd4, but not LAPTM5 ubiquitination. J Cell Biol. 2006;175:631–645. doi: 10.1083/jcb.200603001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thacker J. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 2005;219:125–135. doi: 10.1016/j.canlet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Hays SL, Firmenich AA, Berg P. Complex formation in yeast double-strand break repair: Participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc Natl Acad Sci USA. 1995;92:6925–6929. doi: 10.1073/pnas.92.15.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolkacheva T, Chan AM. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene. 2000;19:680–689. doi: 10.1038/sj.onc.1203331. [DOI] [PubMed] [Google Scholar]
- 38.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 39.Torres J, et al. Phosphorylation-regulated cleavage of the tumor suppressor PTEN by caspase-3: Implications for the control of protein stability and PTEN–protein interactions. J Biol Chem. 2003;278:30652–30660. doi: 10.1074/jbc.M212610200. [DOI] [PubMed] [Google Scholar]
- 40.Okahara F, Ikawa H, Kanaho Y, Maehama T. Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. J Biol Chem. 2004;279:45300–45303. doi: 10.1074/jbc.C400377200. [DOI] [PubMed] [Google Scholar]
- 41.Tolkacheva T, et al. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res. 2001;61:4985–4989. [PubMed] [Google Scholar]
- 42.Pham N, Rotin D. Nedd4 regulates ubiquitination and stability of the guanine-nucleotide exchange factor CNrasGEF. J Biol Chem. 2001;276:46995–47003. doi: 10.1074/jbc.M108373200. [DOI] [PubMed] [Google Scholar]
- 43.Blot V, et al. Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking toward the multivesicular body pathway prior to virus budding. J Cell Sci. 2004;117:2357–2367. doi: 10.1242/jcs.01095. [DOI] [PubMed] [Google Scholar]
- 44.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information