Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods (original) (raw)
. Author manuscript; available in PMC: 2008 Jul 6.
Published in final edited form as: Eur Heart J. 2006 May 18;27(12):1431–1439. doi: 10.1093/eurheartj/ehi890
Abstract
Aims
Non-potassium-sparing diuretics are commonly used in heart failure (HF). They activate the neurohormonal system, and are potentially harmful. Yet, the long-term effects of chronic diuretic use in HF are largely unknown. We retrospectively analysed the Digitalis Investigation Group (DIG) data to determine the effects of diuretics on HF outcomes.
Methods and results
Propensity scores for diuretic use were calculated for each of the 7788 DIG participants using a non-parsimonious multivariable logistic regression model, and were used to match 1391 (81%) no-diuretic patients with 1391 diuretic patients. Effects of diuretics on mortality and hospitalization at 40 months of median follow-up were assessed using matched Cox regression models. All-cause mortality was 21% for no-diuretic patients and 29% for diuretic patients [hazard ratio (HR) 1.31; 95% confidence interval (CI) 1.11−1.55; P = 0.002]. HF hospitalizations occurred in 18% of no-diuretic patients and 23% of diuretic patients (HR 1.37; 95% CI 1.13−1.65; P = 0.001).
Conclusion
Chronic diuretic use was associated with increased long-term mortality and hospitalizations in a wide spectrum of ambulatory chronic systolic and diastolic HF patients. The findings of the current study challenge the wisdom of routine chronic use of diuretics in HF patients who are asymptomatic or minimally symptomatic without fluid retention, and are on complete neurohormonal blockade. These findings, based on a non-randomized design, need to be further studied in randomized trials.
Keywords: Heart failure, Diuretics, Mortality, Hospitalization, Propensity scores
Most ambulatory chronic heart failure (HF) patients receive therapy with non-potassium-sparing diuretics (referred to as ‘diuretics’ in what follows).1,2 Diuretics are the only drugs that can effectively control fluid retention in HF and are essential for symptomatic treatment of fluid overload.1,2 HF is associated with activation of the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system,1,3,4 which is associated with disease progression and poor prognosis.5,6 Use of diuretics in HF is associated with further activation of these neurohormones.4,7 Diuretics can cause disease progression by increasing myocardial fibrosis in both animal models8 and in human HF.9 Yet, the effect of chronic diuretic therapy on HF outcomes has not been studied in large randomized clinical trials.1,2,10
Current evidence regarding the effects of diuretics on HF outcomes is scant and conflicting. A meta-analysis of three small randomized clinical trials11–13 (n = 236, events = 15, follow up = 4−52 weeks) showed that diuretic use was associated with reduced mortality in HF.14 An observational study, in contrast, demonstrated that diuretics increased mortality and hospitalization in systolic HF.15 Interpretation of findings from observational studies are often limited by potential residual biases from measured confounders and possible biases due to unmeasured confounders.16 The propensity score (PS) technique has recently emerged as an effective tool to address selection and residual biases.17–24 In the current study, we analysed data from the Digoxin Investigation Group (DIG) trial,25 using PS methods, to test the hypothesis that chronic use of diuretics was associated with increased long-term mortality and hospitalization in ambulatory patients with chronic systolic and diastolic HF.
Patients and methods
Study design
We conducted a secondary analysis of the DIG trial. DIG was a multi-center randomized clinical trial (302 centers: 186 in USA and 116 in Canada) conducted over 32 months during 1991−93 and was designed to determine the effect of digoxin in patients with chronic HF.25 Detailed description of the rationale, design, implementation, patient characteristics, and results of the DIG trial have been reported elsewhere.24,25 We obtained a public use copy of the DIG dataset from the National Heart, Lung, and Blood Institute (NHLBI).
Study patients
The DIG trial enrolled 7788 ambulatory chronic systolic [left ventricular ejection fraction (LVEF) ≤45%; n = 6800] and diastolic (LVEF >45%; n = 988) HF patients in normal sinus rhythm, of whom 6076 (78%) were receiving diuretics (excluding spironolactone and other ‘potassium-sparing diuretics’).26 DIG investigators assessed the receipt of diuretic therapy at randomization and data on diuretic use were available for all 7788 participants. Angiotensin-converting enzyme (ACE)-inhibitor therapy was encouraged and 93% were on these drugs. Beta-blockers were not approved for HF during the DIG trial and data on beta-blocker use were not collected. We restricted our main analysis to a subset of 1391 + 1391 2782 DIG patients: 1391 patients receiving diuretics, and 1391 patients who were not receiving diuretics, but had similar probability or propensity to receive diuretics at baseline.
Outcomes
The primary outcome of the DIG trial was all-cause mortality, which was also the primary outcome for this report. We also studied several other pre-specified secondary outcomes: mortality from worsening HF, and hospitalizations due to all causes and worsening HF. Study investigators, who were blinded to patients' treatment assignment, ascertained causes of death or primary diagnoses leading to hospitalizations, by reviewing charts or interviewing relatives. DIG participants were followed for a median of 38 months (the median follow-up in this analysis was 40 months). Vital status was collected up to 31 December 1995 and was ascertained for 99% of the patients.27
Assembly of study cohort: propensity score matching
Because patients in the DIG trial were not randomly assigned to diuretics, we matched patients based on their probability or propensity to receive diuretics at randomization (baseline for this analysis). The PS is the conditional probability of receiving an exposure (e.g. a diuretic) given a vector of measured covariates, and can be used to adjust for selection bias when assessing causal effects in observational studies.22–24,28,29 We estimated the PS for diuretic therapy for each patient using a non-parsimonious multivariable logistic regression model, in which the receipt of diuretics was modelled using all baseline patient characteristics in Table 1,as well as clinically plausible interactions.17,18,20,21,24
Table 1.
Baseline patient characteristics by diuretic use before and after propensity score (PS) matching
| Before PS match | After PS match | |||||
|---|---|---|---|---|---|---|
| No diuretic n = 1712 | Diuretic n = 6076 | _P_-value | No diuretic n = 1391 | Diuretic n = 1391 | _P_-value | |
| Age (years), mean (±SD) | 62.1 (±10.8) | 64.4 (±10.9) | <0.0001 | 62.6 (±10.7) | 62.9 (±10.5) | 0.438 |
| Women (%) | 307 (17.9) | 1619 (26.6) | <0.0001 | 262 (18.8) | 273 (19.6) | 0.597 |
| Non-whites (%) | 181 (10.6) | 947 (15.6) | <0.0001 | 165 (11.9) | 134 (9.6) | 0.058 |
| Body mass index (kg/m2), median | 26.4 | 26.6 | 0.007 | 26.5 | 26.5 | 0.448 |
| Heart failure duration (months), median | 18 | 16 | 0.026 | 17 | 18 | 0.741 |
| Primary cause of heart failure | ||||||
| Ischaemic (%) | 1325 (77.4) | 4035 (66.4) | 1047 (75.3) | 1054 (75.8) | ||
| Hypertensive (%) | 124 (7.2) | 681 (11.2) | <0.0001 | 111 (8.0) | 101 (7.3) | 0.676 |
| Idiopathic (%) | 183 (10.7) | 928 (15.3) | 166 (11.9) | 158 (11.4) | ||
| Others (%) | 80 (4.7) | 432 (7.1) | 67 (4.8) | 78 (5.6) | ||
| Comorbid conditions | ||||||
| Prior myocardial infarction (%) | 1259 (73.5) | 3649 (60.1) | <0.0001 | 989 (71.1) | 997 (71.7) | 0.737 |
| Current angina (%) | 507 (29.6) | 1608 (26.5) | 0.010 | 413 (29.7) | 378 (27.2) | 0.141 |
| Hypertension (%) | 665 (38.8) | 3009 (49.5) | <0.0001 | 563 (40.5) | 550 (39.5) | 0.615 |
| Diabetes (%) | 348 (20.3) | 1870 (30.8) | <0.0001 | 315 (22.6) | 301 (21.6) | 0.523 |
| Chronic kidney disease (%) | 578 (33.8) | 2949 (48.5) | <0.0001 | 500 (35.9) | 520 (37.4) | 0.431 |
| Medications | ||||||
| Digoxin (pre-trial use) (%) | 537 (31.4) | 2828 (46.5) | <0.0001 | 473 (34.0) | 481 (34.6) | 0.749 |
| Digoxin (by randomization) (%) | 865 (50.5) | 3024 (49.8) | 0.580 | 703 (50.5) | 724 (52.0) | 0.426 |
| ACE-inhibitors (%) | 1529 (89.3) | 5745 (94.6) | <0.0001 | 1280 (92.0) | 1280 (92.0) | >0.999 |
| Nitrates and hydralazine (%) | 14 (0.8) | 97 (1.6) | 0.016 | 11 (0.8) | 10 (0.7) | 0.827 |
| Potassium-sparing diuretics (%) | 274 (16.0) | 322 (5.3) | <0.0001 | 183 (13.2) | 191 (13.7) | 0.657 |
| Potassium supplement (%) | 106 (6.2) | 2093 (34.4) | <0.0001 | 105 (7.5) | 108 (7.8) | 0.831 |
| Symptoms and signs of heart failure | ||||||
| Dyspnoea at rest (%) | 225 (13.1) | 1480 (24.4) | <0.0001 | 207 (14.9) | 200 (14.4) | 0.707 |
| Dyspnoea on exertion (%) | 1196 (69.9) | 4666 (76.8) | <0.0001 | 994 (71.5) | 986 (70.9) | 0.738 |
| Activity limitation (%) | 1185 (69.2) | 4718 (77.6) | <0.0001 | 1005 (72.3) | 994 (71.5) | 0.643 |
| Jugular venous distension (%) | 99 (5.8) | 921 (15.2) | <0.0001 | 95 (6.8) | 98 (7.0) | 0.823 |
| Third heart sound (%) | 276 (16.1) | 1570 (25.8) | <0.0001 | 241 (17.3) | 227 (16.3) | 0.478 |
| Pulmonary rales (%) | 146 (8.5) | 1155 (19.0) | <0.0001 | 135 (9.7) | 138 (9.9) | 0.848 |
| Lower extremity oedema (%) | 200 (11.7) | 1433 (23.6) | <0.0001 | 185 (13.3) | 182 (13.1) | 0.867 |
| NYHA functional class | ||||||
| I (%) | 361 (21.1) | 742 (12.2) | 265 (19.1) | 250 (18.0) | ||
| II (%) | 1052 (61.4) | 3192 (52.5) | <0.0001 | 848 (61.0) | 860 (61.8) | 0.079 |
| III (%) | 283 (16.5) | 2004 (33.0) | 263 (18.9) | 264 (19.0) | ||
| IV (%) | 16 (0.9) | 138 (2.3) | 15 (1.1) | 17 (1.2) | ||
| Heart rate (rate/min), median | 75 | 80 | <0.0001 | 76 | 76 | 0.453 |
| Blood pressure | ||||||
| Systolic (mmHg), median | 125 | 125 | 0.494 | 126 | 126 | 0.790 |
| Diastolic (mmHg), median | 76 | 75 | 0.031 | 76 | 76 | 0.491 |
| Chest radiograph findings | ||||||
| Pulmonary congestion (%) | 100 (5.8) | 1009 (16.6) | <0.0001 | 96 (6.9) | 92 (6.6) | 0.763 |
| CT ratio >0.5 (%) | 785 (45.9) | 3905 (64.3) | <0.0001 | 682 (49.0) | 696 (50.0) | 0.596 |
| Serum concentrations, (median) | ||||||
| Creatinine (μmol/L), (mg/dL) | 0.103 (1.17) | 0.108 (1.22) | <0.0001 | 0.105 (1.19) | 0.105 (1.19) | 0.724 |
| Potassium (mmol/L) | 4.40 | 4.30 | <0.0001 | 4.40 | 4.40 | 0.735 |
| Estimated glomerular filtration rate (mL/min/1.73 m2), mean (±SD) | 68.5 (±19.7) | 62.1 (±22.9) | <0.0001 | 67.3 (±19.0) | 66.6 (±18.8) | 0.328 |
| LVEF (%), mean (±SD) | 34.1 (±11.7) | 31.4 (±12.7) | <0.0001 | 33.7 (±11.7) | 33.5 (±12.5) | 0.544 |
We then used PS, the single composite variable, to match each no-diuretic patient with a diuretic patient with a very similar PS, thus matching 1391 (81% of the 1712 not receiving diuretics) no-diuretic patients to 1391 diuretic patients with similar estimated PS.30 In our matching algorithm, we first attempted to match each no-diuretic patient with a diuretic patient who had a similar PS to five decimal places. Then we removed those matched pairs of patients and repeated the process matching to four, three, two, and one decimal places.
Assessment of baseline covariate balance: standardized differences
We compared the balance of all baseline covariates in Table 1 between treatment groups before and after PS matching using the standardized difference, which directly quantifies the bias in the means (or proportions) of covariates across the groups, expressed as a percentage of the pooled standard deviation (SD).18,21,23,24 Our PS model was well calibrated and discriminated effectively between diuretic and no-diuretic patients at baseline (_c_-statistic = 0.833).
Before matching, the mean PS for diuretic use in patients not receiving diuretics (n = 1712) was 0.57535 and in those receiving diuretics (n = 6076) was 0.83789, with an associated standardized difference of 151% (_t_-test _P_-value <0.0001). After matching, the mean PS for diuretic use in the matched patients not receiving diuretics (n = 1391) was 0.63765 and in those receiving diuretics (n = 1391) was 0.63804, which yields a standardized difference of 0.2% (_t_-test _P_-value =0.958).
Statistical analysis
We used Kaplan–Meier survival analyses, and bivariate and multivariable Cox proportional hazards models, with consideration for clinically plausible interactions, to estimate the association of diuretic use with various outcomes. In the multivariable Cox regression model, we also adjusted for PS (raw score entered as a linear term) and the set of covariates. Matching was accounted for in the Cox proportional hazard models by incorporating a variable that identified patients with similar PS. This is essentially a stratified analysis that uses each pair of matched patients as a separate stratum to compare survival within each pair, which is then used to estimate the overall hazard ratio (HR). We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. Covariates used in the multivariable model included all those used in the logistic regression model for PS. All analyses were based on intent-to-treat.
To examine for potential heterogeneity of treatment effect on all-cause mortality, we estimated the effects of diuretics in several subgroups, using the pre-match cohort of 7788 patients. These subgroups included age (cut-off of 65 years), sex, race (whites vs. others), HF aetiology (ischaemic vs. others), New York Heart Association (NYHA) class (III and IV vs. others), LVEF (cut-off of 45%), diabetes, chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2), ACE-inhibitor, and digoxin use. After first calculating absolute risks,31 we then estimated the effect of diuretics in each of the subgroups using Cox regression models, in each case adjusting for propensity to use diuretics. Finally, we formally tested for interactions using multivariable Cox proportional hazards models entering interaction terms between diuretic therapy and the subgroup variables, and adjusting for propensity to use diuretics.
Sensitivity analyses
We conducted sensitivity analyses using several alternative approaches to assess the robustness of our findings regarding the effect of diuretics on all-cause mortality to changes in the analytic approach. First, to address concerns about incomplete matching, we analysed data from all 7788 patients, using both direct regression adjustment for PS, and sub-classification based on quin-tiles of PS. Second, to account for initiation and discontinuation of diuretic therapy during follow-up, we re-estimated the effect of diuretics in a subset of patients who were always receiving diuretics during the first 24 months of follow up compared with patients who were never receiving diuretics during the same time period. Finally, even though our PS match achieved excellent balance in our measured covariates, we could not rule out bias due to imbalances in unmeasured covariates. Therefore, we conducted formal sensitivity analyses to describe the weight of our evidence by quantifying the degree of hidden bias that would need to be present to invalidate our main conclusions. All statistical tests were evaluated using two-tailed 95% confidence intervals (CI), and data analyses were performed using SPSS for Windows version 13.0.2.
Results
Patient characteristics
The mean (±SD) age of the 2782 PS-matched patients was 63 (±11) years, (median: 64; range: 22−92), 535 (19%) were women and 299 (11%) were non-whites. Table 1 compares baseline patient characteristics by diuretic use before and after PS matching. Before matching, diuretic patients were older and sicker. They were more likely to have diabetes and chronic kidney disease, dyspnoea, higher NYHA classes, elevated jugular venous pressure, a third heart sound, pulmonary rales, cardiomegaly, pulmonary congestion, and lower LVEF. However, diuretic users were also more likely to be women, have shorter HF duration, higher body mass index, and non-ischaemic etiology for HF.
After matching, diuretic and no-diuretic patients were similar with regards to all of the 34 baseline covariates (Table 1 and Figure 1). Our PS matching reduced standardized differences for all observed covariates below 10% in absolute value, demonstrating substantial improvement in covariate balance across the treatment groups (Figure 1).18,21,23
Figure 1.
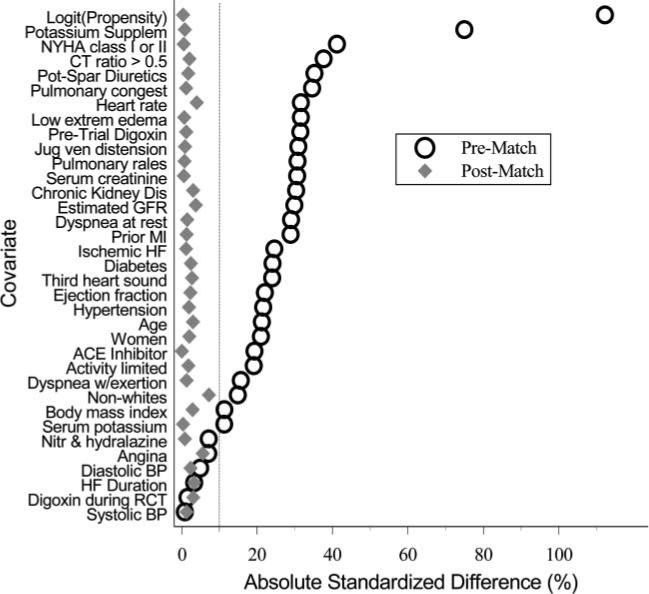
Absolute standardized differences before and after propensity score matching comparing covariate values for patients receiving and not receiving diuretics.
Diuretics and mortality
During a median 40 months of follow-up, 695 (25%) patients died from all causes and 202 (7%) died due to worsening HF. Compared with 21% deaths in patients of the no-diuretic group, 29% of those in the diuretic group died from all causes (HR 1.31, 95% CI 1.11−1.55; P = 0.002; Table 2). Mortality due to HF occurred in 6% of patients in the no-diuretic group and 9% of those in the diuretic group (HR 1.36, 95% CI 0.99−1.87; P = 0.056; Table 2). These associations remained essentially unchanged after adjustment for baseline covariates and PS (Table 2). Kaplan–Meier survival curves for all-cause and HF mortality are displayed in Figures 2A and B. Mean (95% CI) survival times for diuretic vs. no-diuretic patients were, respectively, 47 (46−48) and 50 (49−51) months.
Table 2.
Hazard ratios and 95% CI (adjusted for matching) for death and hospitalizations by diuretic use in the matched cohort
| Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI)a | |
|---|---|---|
| Mortality due to all causes | 1.31 (1.11−1.55); P = 0.002 | 1.32 (1.09−1.60); P = 0.004 |
| Mortality due to heart failure | 1.36 (0.99−1.87); P = 0.056 | 1.41 (0.99−2.01); P = 0.055 |
| Hospitalization due to all causes | 1.15 (1.02−1.29); P = 0.023 | 1.16 (1.03−1.30); P = 0.017 |
| Hospitalization due to heart failure | 1.37 (1.13−1.65); P = 0.001 | 1.57 (1.25−1.96); P < 0.0001 |
Figure 2.
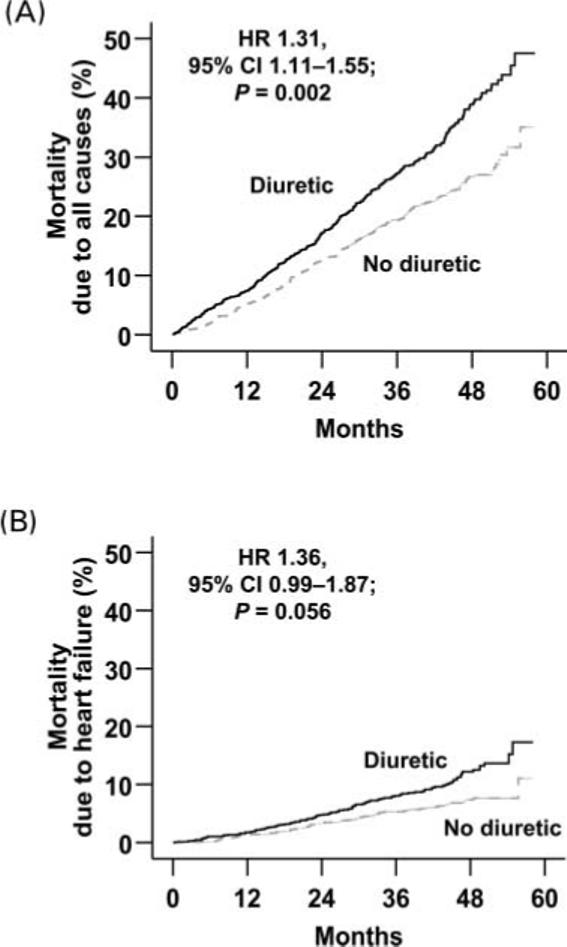
Kaplan–Meier plots for cumulative risk of mortality due to (A)all causes and (B) worsening heart failure.
Sensitivity analyses
In the full (pre-match) cohort (n = 7788), 2606 (34%) patients died. Compared with 21% deaths in no-diuretic patients, 37% of diuretic patients died (unadjusted HR 1.97, 95% CI 1.77−2.21; P < 0.0001). When we adjusted for PS, the association became weaker but remained significant (HR 1.29; 95% CI 1.14−1.46; P < 0.0001). Additional multivariable adjustment did not alter this association (HR 1.28; 95% CI 1.13−1.45; P < 0.0001). Among patients in PS quintiles two and three (n = 3116), we observed similar diuretic effect on all-cause death: unadjusted (HR 1.59; 95% CI 1.33−1.89; P < 0.0001), PS-adjusted (HR 1.49; 95% CI 1.25−1.78; P < 0.0001), and full model adjusted (HR 1.51; 95% CI 1.27−1.81; P < 0.0001).
Receipt of diuretics was ascertained during visits at 1, 4, 8, 12, 16, 20, and 24 months. During the first 24 months of follow-up, 781 patients never received diuretics and 2984 patients were always receiving diuretics. Compared with 8% deaths among patients never receiving diuretics during the first 24 months of follow-up, 19% of those who always received diuretics during the same time died from all causes (multivariable adjusted HR 1.81, 95% CI 1.38−2.38; P < 0.0001).
Like any non-randomized study, the conclusions of our study may be sensitive to potential hidden biases, and so we completed a formal check of the sensitivity of these results to potential hidden biases. In the absence of hidden bias, a sign-score test for matched data with censoring provides strong evidence (P = 0.0006) that treatment with diuretics decreases survival time, even after adjustment by PS matching. To attribute the lower survival time to an unobserved binary covariate unrelated to our PS model rather than to the effect of the exposure to diuretics, that unobserved covariate would need to increase the odds of a patient receiving diuretics by about 12% and would also need to be a near perfect predictor of mortality.
Diuretics and hospitalization
During the follow-up, 1705 (61%) patients were hospitalized from all causes and 563 (20%) were hospitalized due to worsening HF. Compared with 59% hospitalizations due to all causes among no-diuretic patients, 64% of patients in the diuretic group were hospitalized (HR 1.15; 95% CI 1.02−1.29; P = 0.023; Table 2). Hospitalizations due to worsening HF occurred in 18% patients of the no-diuretic group and 23% patients in the diuretic group (HR 1.37; 95% CI 1.13−1.65; P = 0.001; Table 2). These associations remained essentially unchanged after multivariable risk adjustment (Table 2). The Kaplan–Meier survival curves for all-cause and HF-hospitalizations are, respectively, displayed in Figures 3A and B.
Figure 3.

Kaplan–Meier plots for cumulative risk of hospitalizations due to (A) all causes and (B) worsening heart failure.
Subgroup analyses
The association of diuretic therapy with mortality was noted across a wide spectrum of HF patients (Figure 4). Diuretic use was associated with increased mortality in HF patients who were asymptomatic or minimally symptomatic (NYHA class I and II) and those who were receiving inhibitors of RAAS (ACE-inhibitors). There were no significant interactions between diuretic use and any of the covariates, except for diabetes (P for interaction = 0.022). However, after multivariable adjustment, that interaction was no longer significant.
Figure 4.

Hazard ratios (95% CI) for all-cause mortality in subgroups of patients with heart failure (ACE-inhibitor, angiotensin-converting enzyme-inhibitor).
Discussion
Our findings demonstrate that the use of non-potassium-sparing diuretics was associated with increased risk of mortality and hospitalization in a wide spectrum of ambulatory chronic systolic and diastolic HF patients. Over 90% of these patients were receiving ACE-inhibitors and about 80% belonged to NYHA class I and II. These findings are important as diuretics and ACE-inhibitors remain the most commonly used regimen for HF therapy.1
Possible mechanism of action
A mechanistically plausible explanation of our findings is that the unfavourable effects of diuretics are mediated through the activation of the neuroendocrine system, in particular, the RAAS.4,7,32 Activation of angiotensin-II and aldosterone is associated with the stimulation of proinflammatory cytokines, myocardial fibrosis, disease progression, and poor outcomes in HF.8–10,33 It is also possible that the deleterious effects of diuretics are extensions of their common clinical adverse effects such as electrolyte imbalance, hypotension, and worsening kidney function.1 Serum creatinine and potassium levels, and BP were well balanced at baseline in our study, and are unlikely to explain our findings. However, diuretics might have altered some of these parameters during the course of therapy.
Clinical implications: asymptomatic heart failure
HF guidelines recommend that most HF patients ‘should be routinely managed with’ a diuretic, an ACE-inhibitor, or an angiotensin receptor blocker (ARB), and a beta-blocker and that diuretics are essential for symptomatic management of fluid overload in HF.1,2 Diuretics are also recommended for HF patients with prior symptoms.1 However, these recommendations are based on Level C evidence (only consensus opinion of experts, case studies, or standard-of-care) as there are no randomized clinical trial data on the effect of diuretics on survival in HF.1,2
We noted that in a wide spectrum of PS-matched HF patients, use of diuretics was associated with a significant 31% increased risk of death (Table 2). Among NYHA class I and II patients, diuretic use was associated with a significant 25% increased risk of death (Figure 4). These findings question the wisdom of routine chronic diuretic use in euvolemic HF patients who are asymptomatic (NYHA I) or minimally symptomatic (NYHA II) and are already receiving an ACE-inhibitor or an ARB and a beta-blocker approved for HF.
It is currently unknown if blocking of the aldosterone-receptor would favourably influence the clinical course of HF patients with NYHA I or II symptoms receiving diuretic therapy. This will be addressed in the upcoming trial ‘a comparison of the endpoints of death from a cardiovascular cause and hospitalization for worsening heart failure in patients who have NYHA Class II heart failure when they are treated with eplerenone or placebo in addition to standard heart failure medicines’.34
Clinical implications: symptomatic heart failure
Diuretic use was also associated with increased mortality in HF patients with NYHA class III and IV symptoms (Figure 4). However, it may not be practically feasible to withhold or discontinue diuretics in HF patients with fluid overload and NYHA class III and IV symptoms.1 The deleterious effects of diuretics may be attenuated by stricter restriction of salt intake. Diuretics increase salt appetite,35 and aldosterone-induced myocardial damage is worse at a high-salt environment.36,37 Clinicians might also consider adding low-dose digoxin, instead of increasing the dose of diuretics, to relieve HF symptoms. Digoxin reduces HF hospitalization regardless of LVEF and serum digoxin concentration, and if care is taken to achieve a low serum digoxin concentration (0.5−0.9 ng/mL), it also reduces all-cause mortality and all-cause hospitalizations.24,25 Furosemide is the most commonly used diuretic in HF. There is a growing body of evidence that suggests that use of furosemide may be associated with activation of neurohormones,3,8,10,38 and that torsemide may reverse neurohormone-induced myocardial fibrosis.9,39 Some preliminary studies have also demonstrated improved HF outcomes associated with torsemide use.40–42 However, torsemide is expensive and the long-term effects of torsemide in HF are largely unknown.
Comparison with other published studies
Despite widespread clinical acceptance, there have been no large-scale randomized clinical trials of diuretic therapy with long-term follow-up in HF.1,2 Recently, Domanski et al.15 demonstrated that in patients with chronic HF, use of non-potassium-sparing diuretics (vs. no-diuretics) was associated with increased risk of total mortality (adjusted HR 1.28, 95% CI 1.19−1.49) and HF hospitalization (adjusted HR 1.38, 95% CI 1.11−1.71). However, in that study, diuretic patients were generally sicker at baseline and the multivariable risk adjustment model did not include all potential confounders.43 Balancing all measured baseline covariates by PS matching, inclusion of patients with LVEF >45%, adjustment for all prognostically important covariates, and use of sensitivity analyses distinguish our study from theirs.
Limitations
The findings of our study might be potentially limited by biases related to unmeasured or hidden covariates, and incomplete and/or inexact matching. The results of our study were fairly sensitive to a potential hidden covariate.44,45 However, sensitivity analysis cannot determine if such a bias existed. It is unlikely that a hidden variable could be completely unrelated to any of the 34 covariates used in our PS analysis. In HF, diuretics are generally prescribed in response to fluid overload and for symptom management, and, less often, for BP control, all of which are overt and measurable covariates, and most of those were measured and accounted for in our analysis. This is important, as our conclusions' apparent sensitivity depends on a hidden variable being strongly related to both use of diuretics and to survival.21 From a clinical standpoint, it is difficult to contemplate that such a hidden covariate could exist that would be associated with both use of diuretics and death or hospitalizations, and yet remain unmeasured or essentially unassociated with any of the large number of clinically significant covariates used in our analysis.
Inexact matching and/or incomplete matching might also affect the results of our study. We were able to find near-exact matching diuretic patients for 81% of our no-diuretic patients, with the worst match having a PS difference <0.02 in absolute value. This is in contrast to <60% adequate matching in other studies.23,28 The estimated mean PS for our 321 unmatched no-diuretic patients were generally small (mean PS, 0.3054), but were (with one exception) within the range of PS associated with our 1391 matched no-diuretic patients (mean PS, 0.6376). The estimated mean PS for our 4685 unmatched diuretic patients were generally large (0.8972), and were within the range of PS associated with 1391 matched diuretic patients (mean PS, 0.6380).
Incomplete matching has the potential to introduce biases and overestimate treatment effects towards worse effects for the treatment. However, compared with matched diuretic patients, unmatched diuretic patients generally were older and sicker. Conversely, compared with matched no-diuretic patients, unmatched no-diuretic patients generally were younger and less sick. Inclusion of unmatched older and sicker diuretic patients, and younger and less sick no-diuretic patients would have inflated the diuretic effect towards a greater harm than that observed in our matched cohort. Therefore, while exclusion of unmatched patients might have compromised to some degree the external validity of our findings, our matching procedure improves internal validity.
The results of our study are based on predominantly white, male, and relatively younger HF patients with normal sinus rhythm, thus limiting their generalizability. We had no data on specific diuretics and their dosages, and more importantly, on patients' salt status and blood urea nitrogen levels. We also had no data on the use of beta-blockers and aldosterone antagonists, thus limiting generalizability to today's HF patients. As beta-blockers blunt the activation of the sympathetic nervous system and attenuate renin release from the kidneys,3 further studies of patients in the current era of HF therapy are needed. The effect of diuretics on HF outcomes should be examined in well-designed prospective studies or randomized clinical trials. More resources need to be dedicated to the development of newer generations of diuretics with no or minimal neurohormonal activation. Effect of salt intake on the deleterious effects of diuretics, and the effect of diuretics on salt-craving need to be further studied.
Conclusions
We observed associations between chronic diuretic use and poor long-term outcomes in a wide spectrum of ambulatory patients with chronic, mild to moderate systolic and diastolic HF. These findings are mechanistically plausible and consistent with previous laboratory and observational data, and suggest that symptomatic relief achieved by diuretic therapy might be at the cost of increased mortality and hospitalization. It is tempting to suggest that these results challenge the wisdom of routine chronic use of non-potassium-sparing diuretics for HF patients who are asymptomatic (NYHA I) or minimally symptomatic (NYHA II) without any fluid retention, and are on appropriate neurohormonal blockade. However, the findings of our study, based on a non-randomized design, are largely hypothesis generating, and call for similar analyses of larger and more recent databases, prospective follow-up studies, and for confirmation in randomized clinical trials.
Acknowledgements
The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This manuscript has been reviewed by NHLBI for scientific content and consistency of data interpretation with previous The DIG publications and significant comments have been incorporated prior to submission for publication.
The authors wish to thank Raynald Levesque, Aon Consulting, Montreal, Canada for his generous assistance with the SPSS macro and other programs.
Grant supports
A.A. is supported by a grant 1-K23-AG19211-01 from the National Institutes of Health/National Institute on Aging. A.H. and L.J.D. are supported by a Specialized Center for Clinically Oriented Research (SCCOR) in Cardiac Dysfunction grant P50HL077100 from the National Institutes of Health/National Heart, Lung and Blood Institute. L.J.D. is also supported by a grant from the Office of Research and Development, Medical Service, Department of Veteran Affairs.
Footnotes
Authors' contributions
A.A. conceived the study hypothesis and design, and wrote the first draft in collaboration with G.G. and R.C.B. A.A. conducted the statistical analyses with assistance from T.E.L. and S.M. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. A.A. and S.M. had full access to the data.
Abstract presentation
An abstract based on this analysis was presented in an oral session at the American Heart Association Annual 2005 Scientific Sessions on November 16, 2005 in Dallas, TX, USA.
Conflict of interest: none declared.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. Published online before print September 13, 2005, doi:10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez-Sendon JL, Nieminen MS, Pierard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 3.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 4.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdsson A, Swedberg K. Neurohormonal activation and congestive heart failure: today's experience with ACE inhibitors and rationale for their use. Eur Heart J. 1995;16(Suppl N):65–72. doi: 10.1093/eurheartj/16.suppl_n.65. [DOI] [PubMed] [Google Scholar]
- 6.Packer M. Beta-blockade in the management of chronic heart failure. Another step in the conceptual evolution of a neurohormonal model of the disease. Eur Heart J. 1996;17(Suppl B):21–23. doi: 10.1093/eurheartj/17.suppl_b.21. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44:1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–2035. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 10.Weber KT. Furosemide in the long-term management of heart failure: the good, the bad, and the uncertain. J Am Coll Cardiol. 2004;44:1308–1310. doi: 10.1016/j.jacc.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Burr ML, King S, Davies HE, Pathy MS. The effects of discontinuing long-term diuretic therapy in the elderly. Age Ageing. 1977;6:38–45. doi: 10.1093/ageing/6.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Myers MG, Weingert ME, Fisher RH, Gryfe CI, Shulman HS. Unnecessary diuretic therapy in the elderly. Age Ageing. 1982;11:213–221. doi: 10.1093/ageing/11.4.213. [DOI] [PubMed] [Google Scholar]
- 13.Sherman LG, Liang CS, Baumgardner S, Charuzi Y, Chardo F, Kim CS. Piretanide, a potent diuretic with potassium-sparing properties, for the treatment of congestive heart failure. Clin Pharmacol Ther. 1986;40:587–594. doi: 10.1038/clpt.1986.228. [DOI] [PubMed] [Google Scholar]
- 14.Faris R, Flather M, Purcell H, Henein M, Poole-Wilson P, Coats A. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol. 2002;82:149–158. doi: 10.1016/s0167-5273(01)00600-3. [DOI] [PubMed] [Google Scholar]
- 15.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 2003;42:705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 16.Michels KB, Braunwald E. Estimating treatment effects from observational data: dissonant and resonant notes from the SYMPHONY trials. JAMA. 2002;287:3130–3132. doi: 10.1001/jama.287.23.3130. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 18.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 20.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 21.Love TE. Using propensity scores: stratification, adjustment, sensitivity and strategies. 2005 June 4; http://www.chrp.org/propensity/
- 22.Aronow HD, Topol EJ, Roe MT, Houghtaling PL, Wolski KE, Lincoff AM, Harrington RA, Califf RM, Ohman EM, Kleiman NS, Keltai M, Wilcox RG, Vahanian A, Armstrong PW, Lauer MS. Effect of lipid-lowering therapy on early mortality after acute coronary syndromes: an observational study. Lancet. 2001;357:1063–1068. doi: 10.1016/S0140-6736(00)04257-4. [DOI] [PubMed] [Google Scholar]
- 23.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 26.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 27.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Contr Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. JAMA. 2001;286:1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 29.Stenestrand U, Wallentin L. Early revascularisation and 1-year survival in 14-day survivors of acute myocardial infarction: a prospective cohort study. Lancet. 2002;359:1805–1811. doi: 10.1016/S0140-6736(02)08710-X. [DOI] [PubMed] [Google Scholar]
- 30.Levesque RM. In: SPSS® Programming Data Management. A Guide for SPSS® and SAS® Users. 2nd ed. Levesque R, editor. SPSS Inc.; Chicago, IL, USA: 2005. http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 31.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 32.Knight RK, Miall PA, Hawkins LA, Dacombe J, Edwards CR, Hamer J. Relation of plasma aldosterone concentration to diuretic treatment in patients with severe heart disease. Br Heart J. 1979;42:316–325. doi: 10.1136/hrt.42.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J. 1995;16(Suppl O):107–109. doi: 10.1093/eurheartj/16.suppl_o.107. [DOI] [PubMed] [Google Scholar]
- 34.Clinical Trials. Gov. National Institutes of Health A comparison of the endpoints of death from a cardiovascular cause and hospitalization for worsening heart failure, in patients who have NYHA Class II heart failure when they are treated with eplerenone or placebo in addition to standard heart failure medicines. 2006 January 20; http://www.clinicaltrials.gov/ct/show/NCT00232180?order=3://www.clinicaltrials.gov/ct/show/NCT00232180?order=3.
- 35.Rowland NE, Morian KR. Roles of aldosterone and angiotensin in maturation of sodium appetite in furosemide-treated rats. Am J Physiol. 1999;276:R1453–R1460. doi: 10.1152/ajpregu.1999.276.5.R1453. [DOI] [PubMed] [Google Scholar]
- 36.Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 37.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 38.Cataliotti A, Boerrigter G, Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM, Chen HH, Malatino LS, Burnett JC., Jr Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]
- 39.Muniz P, Fortuno A, Zalba G, Fortuno MA, Diez J. Effects of loop diuretics on angiotensin II-stimulated vascular smooth muscle cell growth. Nephrol Dial Transplant. 2001;16(Suppl 1):14–17. doi: 10.1093/ndt/16.suppl_1.14. [DOI] [PubMed] [Google Scholar]
- 40.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, Smith FE, Lane KA, Adams LD, Tierney WM, Brater DC. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–520. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 41.Cosin J, Diez J. Torsemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–513. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 42.Muller K, Gamba G, Jaquet F, Hess B. Torsemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 43.Ghali JK. Diuretic use, progressive heart failure, and death in patients in SOLVD. J Am Coll Cardiol. 2004;43:1723. doi: 10.1016/j.jacc.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum PR. Sensitivity analysis for matching with multiple controls. Biometrika. 1988;75:577–581. [Google Scholar]
- 45.Rosenbaum PR. Observational Studies. 2nd ed Springer-Verlag; New York: 2002. Sensitivity to hidden bias. pp. p110–124. [Google Scholar]