Chromosomal Toxin-Antitoxin Systems May Act as Antiaddiction Modules (original) (raw)
Abstract
Toxin-antitoxin (TA) systems are widespread among bacterial chromosomes and mobile genetic elements. Although in plasmids TA systems have a clear role in their vertical inheritance by selectively killing plasmid-free daughter cells (postsegregational killing or addiction phenomenon), the physiological role of chromosomally encoded ones remains under debate. The assumption that chromosomally encoded TA systems are part of stress response networks and/or programmed cell death machinery has been called into question recently by the observation that none of the five canonical chromosomally encoded TA systems in the Escherichia coli chromosome seem to confer any selective advantage under stressful conditions (V. Tsilibaris, G. Maenhaut-Michel, N. Mine, and L. Van Melderen, J. Bacteriol. 189:6101-6108, 2007). Their prevalence in bacterial chromosomes indicates that they might have been acquired through horizontal gene transfer. Once integrated in chromosomes, they might in turn interfere with their homologues encoded by mobile genetic elements. In this work, we show that the chromosomally encoded Erwinia chrysanthemi ccd (_c_ontrol of _c_ell _d_eath) (ccdEch) system indeed protects the cell against postsegregational killing mediated by its F-plasmid ccd (_ccd_F) homologue. Moreover, competition experiments have shown that this system confers a fitness advantage under postsegregational conditions mediated by the _ccd_F system. We propose that ccdEch acts as an antiaddiction module and, more generally, that the integration of TA systems in bacterial chromosomes could drive the evolution of plasmid-encoded ones and select toxins that are no longer recognized by the antiaddiction module.
Genes are often expected to be an integral part of genetic circuits in an organism and to possess a dedicated role in cellular functioning or adaptation. However, it is also possible that the presence of some genes may rather be the result of past evolutionary processes, implying that they might be devoid of any current physiological role on their own. We propose this view of the chromosomally encoded toxin-antitoxin (TA) systems, which are surprisingly abundant in bacterial chromosomes (24, 32). TA systems were originally discovered on low-copy-number plasmids. On such plasmids, they contribute to plasmid maintenance in growing bacterial populations by selectively eliminating daughter bacteria that do not receive a plasmid copy (postsegregational killing [PSK]) (15). The molecular mechanism underlying PSK is based on a differential stability of the toxin and antitoxin proteins encoded by the TA operon (39, 40). The toxin is a stable protein whose toxic activity is counteracted by the unstable antitoxin protein. In plasmid-free daughter bacteria, since the antitoxin is degraded and not replenished, the toxin is released from the TA complex and is able to exert its lethal activity. Yarmolinsky proposed renaming the plasmid-encoded TA systems as addiction modules, since the progeny of a bacterial cell containing a plasmid-encoded system become dependent on the presence of the otherwise dispensable plasmid (42).
Numerous homologues of plasmid-encoded TA systems are found in bacterial chromosomes. Many of these systems are associated with mobile genetic elements that constitute genomic islands, suggesting that horizontal gene transfer contributes to their dissemination (24). The biological function(s) of the chromosomally encoded TA systems has been an area of intense debate for several years (1, 20, 25, 38). Based on the work on the mazEF and relBE systems of Escherichia coli, the general idea that has emerged is that TA systems are involved in general stress management. mazEF has been shown to be responsible for programmed cell death under a wide variety of seemingly unrelated stressful conditions (e.g., short-term antibiotic treatments, high temperature, and oxidative shock) by the group of Engelberg-Kulka (14, 18, 31). However, chromosomally encoded TA system-dependent programmed cell death was not observed in work by other groups (5, 7, 22, 25, 26, 33, 34) and was recently ruled out by our group (38). The other mainstream hypothesis is that amino acid starvation induces chromosomally encoded TA system-dependent bacteriostasis. This was proposed by the group of Gerdes based mainly on the study of the relBE and mazEF systems (6, 7, 25). In this model, the assumption is that bacteriostasis is an advantage for the bacteria during starvation. However, in competition experiments, no selective advantage conferred by chromosomally encoded TA systems during either nutrient starvation (or other stress conditions) or the poststress recovery phase could be detected (38). Recently published work demonstrated that mRNA cleavage induced by amino acid starvation occurs independently of the five chromosomally encoded TA systems (the toxins of which are endoribonucleases) in E. coli, reinforcing the idea that the TA systems are not involved in stasis induced by starvation (19).
In this paper, we propose to reconsider the function of chromosomally encoded TA systems, taking into account the interactions between plasmid-encoded and chromosomally encoded homologous TA systems. We tested the possibility that chromosomally encoded TA systems may act as antiaddiction modules such that they might protect the host bacteria against PSK mediated by their plasmid-encoded counterparts. The prediction is that their presence might confer a selective advantage under PSK conditions.
MATERIALS AND METHODS
Strains, plasmids, and media.
Plasmids and strains used in this work are listed in Table 1. The sequences of the primers used in this work are listed in Table 2.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| MG1655 | Wild-type E. coli K-12 | 12 |
| NM529 | MG1655 Δ_ara leu_::Tet Δ_lac_ | From N. Majdalani |
| NM532 | MG1655 Δ_ara_ Δ_lac lacI_q | From N. Majdalani |
| MS7 | MG1655 ccd_O157+_ | This work |
| MS8 | MG1655 ccdEch + | This work |
| MS10 | MG1655 ccdEch+ Δ_ara leu_::Tet | This work |
| MS20 | MG1655 Δ_ara leu_::Tet | This work |
| NC397 | W3110 gal490* pgl_Δ_8 attL int-N pL _cI857_Δ (cro-bioA) _bio_Δ lacI <_kan_ - T1 - _cat- sacB_> plac | From N. Majdalani |
| MS2 | MG1655 cat-sacB | This work |
| SG22622 | MC4100 cpsB::lacZ Δ_ara malP_::_lacI_q | From S. Gottesman |
| SG22622_gyrA_462 | SG22622 gyrA462 zei::Tn_10_ | Our laboratory |
| Plasmids | ||
| pBAD33 | p15A, Cmr, pBAD promoter | 13 |
| pBAD-ccdBF | _ccdB_F under the control of the pBAD promoter | 41 |
| pBAD-ccdBEch | ccdBEch under the control of the pBAD promoter | This work |
| pKK223-3 | ColE1, Ampr, pTac promoter | 4 |
| pKK-ccdAF (pULB2709) | _ccdA_F under the control of the pTac promoter | 29 |
| pKK-ccdAEch | ccdAEch under the control of the pTac promoter | This work |
| pMLO59 | pGB2 ts derivative, Specr | From M. Labocka |
| pMLO-ccdF (pULB2710) | pMLO59 containing the _ccd_F operon | 40 |
| pMLO-ccdEch | pMLO59 containing the ccd Ech operon | This work |
TABLE 2.
Sequences of the primers used in this work
| Primer | Sequence (5′ to 3′) |
|---|---|
| _ccdAEch_-for | GAATTCATGAAACACCGCGTC |
| _ccdAEch_-rev | CTGCAGTCACCAGTTCCTG |
| _ccdBEch_-for | TCTAGAAGGAGGTGATCATGCAATTCATT |
| _ccdBEch_-rev | CTGCAGTCAAATCCCCCAA |
| _ccdEch_-for | CTATATCGTGCCCAACCCGC |
| _ccdEch_-rev | GCGAACACTCTGACTCCCCC |
| _catsac_-for | TCGCGTCTTATCCGGCCTTCCTATATCAGGCTGTGTTTAAAAAATGAGACGTTGATCGGC |
| _catsac_-rev | CGTCGAACCGGCATAAGGATTTGGGCGAAGCGGCGGCGTCATCAAAGGGAAAACTGTCCAT |
| _IccdEch_-for | CCGGTCGCGTCTTATCCGGCCTTCCTATATCAGGCTGTGTTCAATAGCGTGAAATACGCCAT |
| _IccdEch_-rev | CGTCGAACCGGCATAAGGATTTGGGCGAAGCGGCGGCGTCTTAAATCCCCCAAAACATAAGAT |
| IccdO157-for | CCGGTCGCGTCTTATCCGGCCTTCCTATATCAGGCTGTGTGGTATTCAGCGAATTCCACG |
| IccdO157-rev | CGTCGAACCGGCATAAGGATTTGGGCGAAGCGGCGGCGTCTTAAATCCCGTCGAGCATAA |
Construction of plasmids. (i) Expression plasmids. (a) pBAD-ccdB Ech plasmid.
The Erwinia chrysanthemi ccd (_c_ontrol of _c_ell _d_eath) (ccdBEch) gene was amplified by PCR using E. chrysanthemi 3937 chromosomal DNA as the template and the ccdBEch-for and ccdBEch-rev primers. The PCR product was cloned into the TOPO-XL vector (Invitrogen). The resulting plasmid was then digested using XbaI and PstI. The fragment containing ccdBEch was inserted into the pBAD33 vector cut with the same enzymes.
(b) pKK-ccdA Ech plasmid.
The ccdAEch gene was amplified by PCR using E. chrysanthemi 3937 chromosomal DNA as the template and the ccdAEch-for and ccdAEch-rev primers. The PCR product was cloned into the TOPO-XL vector (Invitrogen). The resulting plasmid was then digested using EcoRI and PstI. The fragment containing ccdAEch was inserted into the pKK223-3 vector cut with the same enzymes.
(ii) PSK plasmid (pMLO-ccd Ech plasmid).
The ccdEch operon was amplified by PCR using E. chrysanthemi 3937 chromosomal DNA as the template and the ccdEch-for and ccdEch-rev primers. The PCR product was cloned into the TOPO-XL vector (Invitrogen). The resulting plasmid was then digested by EcoRI and inserted into the PMLO59 vector cut using the same enzyme.
Construction of bacterial strains. (i) ccdEch and _ccd_O157 strains.
The cat-sacB cassette was amplified by PCR using genomic DNA of the NC397 strain as the template and the catsac-for and catsac-rev primers. The resulting amplification product contained homologous sequences to the insertion point, i.e., the _folA_-apaH intergenic region at its 5′ end and homologous sequence of the apaH 5′ end at its 3′ end. The product was inserted in the E. coli MG1655 chromosome by use of the method described in reference 8. Chloramphenicol-resistant recombinants were selected and screened for sucrose sensitivity. The recombinant MG1655 cat-sacB strain was called MS2. The ccdEch and E. coli O157:H7 ccd (_ccd_O157) TA systems were then introduced at that same location. The systems were amplified by PCR using the IccdEch-for and IccdEch-rev or the IccdO157-for and IccdO157-rev primers, and E. chrysanthemi 3937 or E. coli O157:H7 genomic DNA, respectively, as the template. The resulting amplification products containing homologous sequences to the flanking regions of the cat-sacB cassette were inserted in the chromosome of MS2. Sucrose-resistant strains were selected. The ccdEch (MS8) and _ccd_O157 (MS7) strains were obtained and the inserted sequences and flanking regions were sequenced.
(ii) MG1655 Δ_ara_ (MS20) and ccdEch Δ_ara_ (MS10) strains.
The MG1655 Δ_ara_ and ccdEch Δ_ara_ strains were constructed by transducing Δ_ara leu_::Tet (Tet indicates tetracycline resistance) from the MG1655 derivate NM529 by use of P1_vir_ as described in reference 21.
Media.
Luria-Bertani liquid and agar medium (LB) (Invitrogen) and MacConkey agar medium (Difco) were used.
DNA manipulations.
Transformations with appropriate plasmids were performed according to reference 21, and most routine DNA manipulations were done as described in reference 30.
Toxicity and antitoxicity assay.
Strains carrying the pKK223-3 vector or its derivatives expressing either _ccdA_F (pKK-ccdAF) or ccdAEch (pKK-ccdAEch) were transformed with the pBAD33 vector or its pBAD-ccdBF and pBAD-ccdBEch derivatives. Transformation mixtures were plated on LB with appropriate antibiotics (ampicillin at 500 μg/ml and chloramphenicol at 20 μg/ml) with or without arabinose (1%). Plates were incubated overnight (ON) at 37°C. The efficiency of transformation was calculated as the ratio of the number of transformants obtained on 1% arabinose plates to the number of transformants obtained on plates without arabinose.
PSK assay.
The MG1655 strain or its ccdEch (MS8) and _ccd_O157 (MS7) derivatives containing the pMLO59 vector or its derivates encoding the F-plasmid ccd (_ccd_F) system (pMLO-ccdF) were grown ON at 30°C in LB liquid medium containing spectinomycin (100 μg/ml). ON cultures were diluted 800-fold in LB prewarmed to 42°C. Tenfold dilutions were performed after 2 and 3 h to maintain the cultures in log phase. Every 60 min, samples were plated on LB agar plates with or without spectinomycin (50 μg/ml).
Competition experiments.
ON cultures of the MG1655 strain or its Δ_ara_ derivative (MS20) containing either the pMLO59 vector or the pMLO-ccdF plasmid were mixed at a 1:1 ratio with the ccdEch strain (MS8) or its Δ_ara_ derivative (MS10) containing the pMLO-ccdF plasmid. Cocultures were diluted 400-fold in prewarmed LB medium. Competition experiments were carried out as the PSK experiments described above, except that dilutions of the cultures were plated on MacConkey agar plates containing 1% arabinose with or without spectinomycin (50 μg/ml) to distinguish between ara+ and Δ_ara_ strains.
Phylogenetic analysis.
Chromosomally encoded and plasmid-encoded homologues of the toxin and antitoxin proteins belonging to the _ccd_F and _ccd_O157 systems were selected using TBlastN on complete sequenced genomes of gammaproteobacteria. Twenty-eight CcdA and 24 CcdB homologue open reading frames (ORFs) with a maximum E value of 10−6 were considered. Phylogenetic analyses were carried using the neighbor-joining method (28). The optimal trees for CcdA and CcdB homologues with the sums of branch length of 4.1 and 4.0, respectively, are shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (10). The tree is drawn to scale, with branch lengths in units the same as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the matrix-based method of Dayhoff (9) and are in the units of the number of amino acid substitutions per site. All positions containing gaps and missing data were eliminated from the data set (complete deletion option). There were a total of 72 positions in the final data set of CcdA homologues and a total of 70 positions in the final data set of CcdB homologues. Phylogenetic analyses were conducted by MEGA4 (36).
RESULTS
The chromosomally encoded ccdEch system encodes a TA gene pair.
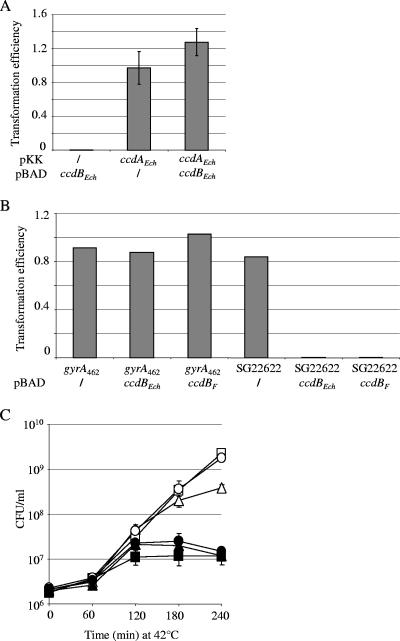
To test the antiaddiction hypothesis, chromosomally encoded ORFs closely related to that of the _ccd_F system of the E. coli F plasmid were selected using the TBlastN software. Two adjacent ORFs encoded in the chromosome of E. chrysanthemi 3937 appeared as candidates, since they presented 65% and 61% identity with the CcdAF antitoxin and the CcdBF toxin, respectively (data not shown). They were named ccdAEch and ccdBEch, respectively. The effect of their ectopic expression on bacterial viability was assessed in the E. coli strain MG1655. Figure 1A shows that the ccdBEch gene encodes a functional toxin whose toxic activity is counteracted by coexpression of the ccdAEch gene. Like its CcdBF and CcdBO157 counterparts (3, 41), CcdBEch targets the DNA gyrase, since a CcdBF-resistant mutant (GyrA462 mutant) is also resistant to CcdBEch (Fig. 1B). As observed for the chromosomally encoded _ccd_O157 system from the E. coli O157:H7 strain (41), ccdEch is unable to mediate PSK when cloned in the replication-thermosensitive pMLO59 vector; this contrasts with _ccd_F, which leads to PSK. Figure 1C shows that the viability of MG1655 is not affected by the loss of the pMLO-ccdEch, while the loss of pMLO-_ccd_F leads to a 1-log loss of viability, as previously described in references 16, 40, and 41. This experiment was conducted for longer time without gaining any effect in PSK (data not shown).
FIG. 1.
Characterization of the chromosomally encoded ccdEch system. (A) ccdEch encodes functional antitoxin and toxin proteins. MG1655 strains carrying either the pKK223-3 vector (/) or its pKK-ccdAEch derivative (ccdAEch) were transformed with the compatible pBAD33 vector (/) or its pBAD-ccdBEch derivative (ccdBEch). The efficiency of transformation was calculated as the ratio of the number of transformants obtained on arabinose plates to the number of transformants obtained on plates without arabinose. Values correspond to the mean of three independent experiments. (B) The CcdBEch toxin targets the GyrA subunit of DNA gyrase. The SG22622 strain and its _gyrA_462 derivative were transformed with the pBAD33 vector (/) or its pBAD33-ccdBEch (ccdBEch) and pBAD33-ccdBF (_ccdB_F) derivatives. The efficiency of transformation was calculated as the ratio of the number of transformants obtained on 1% arabinose plates to the number of transformants obtained on plates without arabinose. (C) The ccdEch system is unable to mediate PSK. MG1655 strains carrying either pMLO59 (squares) or its derivatives encoding ccdEch (pMLO-ccdEch; circles) and _ccd_F (pMLO-ccdF; triangles) were grown at 42°C. Viability (CFU/ml) was monitored for 240 min by plating serial dilutions on LB agar plates with (filled symbols) or without (open symbols) spectinomycin. Values correspond to the mean of three independent experiments.
The chromosomally encoded ccdEch system acts as an antiaddiction module.
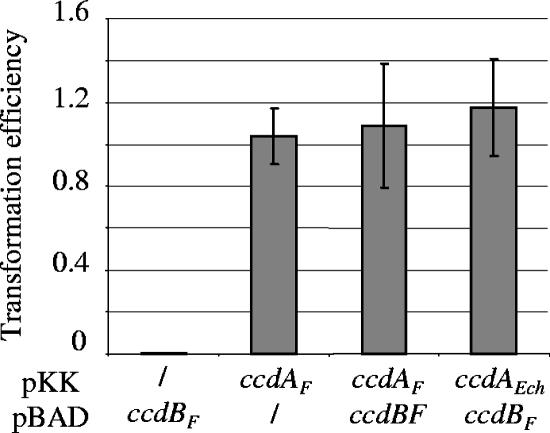
Our definition of an antiaddiction module would be genes that help to protect the cell from the loss of a plasmid carrying an addiction system. To evaluate whether ccdEch might constitute an antiaddiction module for a plasmid carrying _ccd_F, we tested first the ability of CcdAEch to inhibit the toxic activity of the F-plasmid-encoded CcdBF toxin. A pBAD plasmid carrying the _ccdB_F toxin gene cannot be successfully transformed into cells unless they also express the antitoxin to this toxin (Fig. 2, compare column 1 and column 3). Ectopic overexpression of CcdAEch is able to inhibit the toxic activity of CcdBF as efficiently as the CcdAF antitoxin (Fig. 2, compare column 4 to column 3).
FIG. 2.
CcdAEch antagonizes CcdBF toxic activity. MG1655 strains containing either the pKK223-3 vector (/) or pKK-ccdAEch (ccdAEch) and pKK-cidA F (ccdA F) were transformed with the pBAD33 vector (/) or its pBAD-ccdBF derivative (_ccdB_F). The efficiency of transformation was calculated as the ratio of the number of transformants obtained on 1% arabinose plates to the number of transformants obtained on plates without arabinose. Values correspond to the mean of three independent experiments.
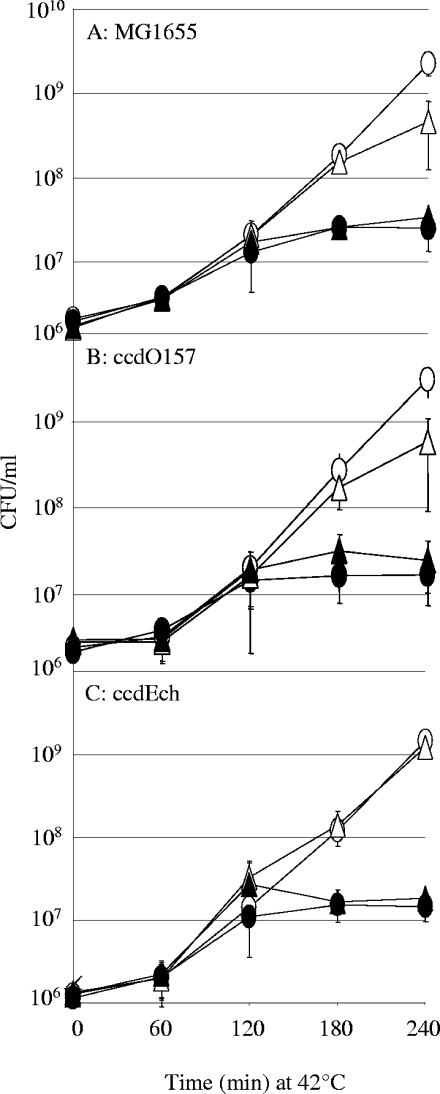
We then tested whether the ccdEch system could interfere with _ccd_F-mediated PSK. For this purpose, the ccdEch system was inserted in the MG1655 chromosome. As a control, we chose to insert the _ccd_O157 system, which naturally occurs in E. coli isolates such as O157:H7 and O55:H7 and was previously shown to be unable to interfere with _ccd_F-mediated PSK (41). Both systems were inserted in MG1655 between the folA and apaH genes (at the natural location of the _ccd_O157 system in O157:H7 and O55:H7 strains), giving rise to the ccdEch (MS8) and _ccd_O157 (MS7) strains, respectively (see Materials and Methods). The loss of pMLO-ccdF affects the viability of the MG1655 and _ccd_O157 strains similarly, with a decrease of viability of ∼1 log (Fig. 3A and B). However, the presence of ccdEch in the ccdEch strain prevents this loss in viability and therefore protects the cell against _ccd_F-mediated PSK (Fig. 3C). These data show that ccdEch acts as an antiaddiction module with respect to the plasmid-encoded _ccd_F system.
FIG. 3.
ccdEch is an antiaddiction module. The MG1655 (A), _ccd_O157 (B), and ccdEch (C) strains containing either the pMLO59 replication-thermosensitive plasmid (spectinomycin resistance) (circles) or its derivative encoding the _ccd_F system (pMLO-ccdF; triangles) were grown at 42°C. Viability (CFU/ml) was monitored for 240 min by plating serial dilutions on LB agar plates with (filled symbols) or without (open symbols) spectinomycin. Values correspond to the mean of three independent experiments.
_ccdEch_-dependent gain of fitness under _ccd_F-mediated PSK conditions.
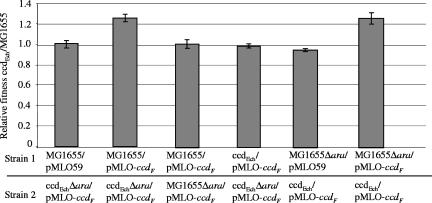
To test whether the ccdEch conferred a selective advantage under our experimental conditions, competition experiments between the MG1655 and ccdEch strains were carried out. A deletion of the arabinose operon (Δ_ara_) was introduced in both strains to allow their discrimination during competition experiments. Figure 4 shows that the strain carrying the ccdEch module had an advantage over a strain that did not carry it (relative fitness, 1.25 [columns 2 and 6]) only when both carried the pMLO-ccdF plasmid as well (compare column 2 to column 1 and column 6 to column 5). The fitness of the ccdEch/pMLO-ccdF and MG1655/pMLO-ccdF strains was similar when they were cocultivated with their Δ_ara_ derivatives, showing that the ara deletion does not interfere with the growth rate (columns 3 and 4). These results show that the ccdEch system confers a selective advantage of 25% in _ccd_F-mediated PSK conditions. This gain in fitness may not be more dramatic because the _ccd_F system is not a very efficient stabilization system (10-fold stabilization) (Fig. 1B) (16, 40, 41).
FIG. 4.
ccdEch increases fitness under the PSK condition. Competition experiments between the MG1655 strain containing either the pMLO59 vector (column 1) or the pMLO-ccdF plasmid (column 2) and the ccdEch Δ_ara_ strain containing the pMLO-ccdF plasmid were carried out. As controls, (i) each strain was cocultivated with its Δ_ara_ derivative, i.e., MG1655/pMLO-ccdF in competition with MG1655 Δ_ara_/pMLO-ccdF (column 3) and ccdEch/pMLO-ccdF in competition with ccdEchΔ_ara_/pMLO-ccdF (column 4); and (ii) the “mirror” competition experiment was carried out with the MG1655 Δ_ara_ strain containing either the pMLO59 vector (column 5) or the pMLO-ccdF plasmid (column 6) and the ccdEch strain containing the pMLO-ccdF plasmid. Cultures were mixed at a 1:1 ratio and cocultures grown under conditions similar to those used for the PSK experiments (see Materials and Methods). Viability (CFU/ml) was monitored for 240 min by plating serial dilutions on MacConkey arabinose (1%) agar plates. Relative fitness was calculated as described in reference 11 and represents the advantage of the ccdEch strain relative to MG1655. Values correspond to the mean of three independent experiments.
DISCUSSION
The wide occurrence of TA systems in bacterial chromosomes is quite striking (24, 32). In bacterial species such as Vibrio cholerae and Nitrosomonas europaea, they tend to cluster in large superintegron structures (24, 27), and recent data have shown that they stabilize large genomic regions by reducing large-scale deletion (33). Thus, hypotheses regarding the biological functions of chromosomally encoded TA systems other than the stress management ones are emerging. Moreover, recent data questioned this last biological role, at least in the E. coli model and under the experimental conditions tested (38). However, as TA systems are extremely diversified, one cannot exclude the possibility that some are integrated in genetic circuits, notably in specific bacterial species which undergo development, as was recently described for Myxococcus xanthus (23).
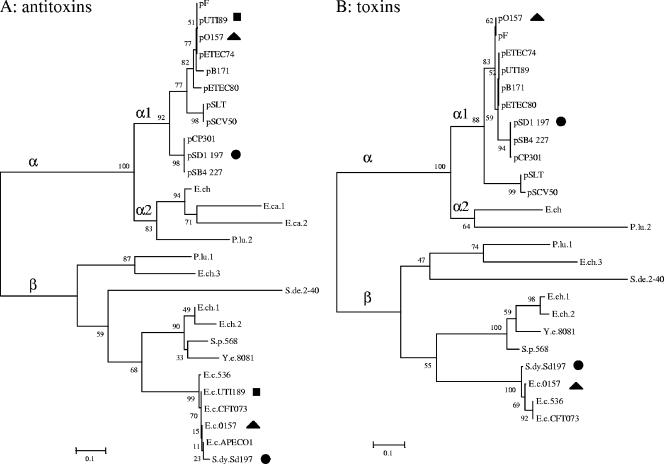
The abundance of chromosomally encoded TA systems has been proposed to be correlated to the bacterial lifestyle, since Gerdes and colleagues have observed that TA systems are more abundant in free-living bacterial species chromosomes than in obligate intracellular bacteria (24). This observation has been interpreted in light of the stress management hypothesis, i.e., free-living bacteria are subjected to ever-changing environments and have to cope with a multitude of different stresses (24). An alternative view is that the occurrence of TA systems in bacterial chromosomes is correlated to the rate of horizontal gene transfer. Once integrated in chromosomes, these systems might in turn interfere with their mobile genetic element-encoded homologues. This raises the possibility that chromosomally encoded TA systems might serve as a defense mechanism against invading DNA (plasmids or phages) in a way that was proposed by Mazel and his collaborators (27) and reminiscent of restriction-modification systems and CRISPR regions (2, 17, 37). It was notably shown by the group of Kobayashi that solitary chromosomally encoded methylase can protect the bacterial host against PSK mediated by a plasmid-encoded restriction-modification system (35). The data presented in the present work demonstrate that the chromosomally encoded ccdEch system is indeed able to interfere with its close _ccd_F homologue and thereby to protect the cell against _ccd_F-mediated PSK. Competition experiments under PSK conditions showed that a gain in fitness is conferred by ccdEch. These results lead us to propose that newly integrated chromosomally encoded TA systems could act de facto as antiaddiction modules due to their new location and their high similarity with plasmid-encoded homologues. The positive effect of antiaddiction modules on host fitness could favor their fixation within populations subjected to the lethal activity of the plasmid-encoded homologues. In turn, this could drive the directional selection of natural variants of these plasmid-encoded systems, in which the toxin is no longer recognized by the antiaddiction module. This evolution of plasmid-encoded TA systems toward a group distinct enough to be resistant to chromosomally encoded antitoxin is supported by our phylogenetic analysis of the ccd systems found in gammaproteobacteria. Figure 5 shows that plasmid-encoded ccd systems form a monophyletic group (α1) that is distantly related to the chromosomally encoded one (β). Interestingly, the ccdEch antiaddiction module belongs to the chromosomally encoded α2 group, which is closely related to the α1 plasmid-encoded group. Coevolution of TA systems might have led to the divergence of chromosomally encoded TA systems and their plasmid-encoded homologues into two distinct groups which might allow for the “harmonious” coexistence of both types of systems, as observed for the E. coli O157:H7 strain (41). In this case, the chromosomally encoded _ccd_O157 system does not interfere with PSK mediated by the _ccd_F system harbored by the F-related pO157 virulence plasmid (41). This situation might represent the result of evolutionary events that have led to the selection of plasmid-encoded TA systems having the capacity to avoid any functional interference caused by their chromosomal homologues. Therefore, some chromosomally encoded TA systems might simply be remnants of past evolutionary events and could be devoid of any current physiological role. Frameshift mutations found in the CcdB ORFs might indicate the decay of these systems. A more global understanding of TA system evolution, distribution, and mobility will certainly help to decipher the biological meaning of their presence in bacterial genomes.
FIG. 5.
Phylogenetic analysis of the _ccd_F homologues. By use of the TBlastN software, 27 and 23 homologues of CcdAF and CcdBF, respectively, were selected in the complete sequenced genomes of gammaproteobacteria. Phylogenetic relationships were inferred by analyzing amino acids sequences of antitoxins and toxins as described in Materials and Methods. Names of bacterial species are abbreviated as follows: E. chrysanthemi 3937 (E.ch, E.ch.1, E.ch.2, and E.ch.3), E. carotovora subsp. atroseptica SCRI1043 (E.ca.1 and E.ca.2), Photorabdus luminescens subsp. laumondii TTO1 (P.lu), E. coli O157:H7 (E.c.O157), E. coli UTI189 (E.c.UTI189), E. coli CFT073 (E.c.CFT073), E. coli 536 (E.c.536), E. coli APEC01 (E.c.APEC01), Shigella dysenteriae Sd197 (S.dy.Sd197), Saccharophagus degradans 2-40 (S.de.2-40), Yersinia enterocolitica subsp. enterocolitica 8081 (Y.e.8081), and Serratia proteamaculans 568 (S.p.568). Naturally coexisting plasmid-encoded TA systems and chromosomally encoded TA systems are indicated by symbols as follows: O157:H7 contains the pO157 plasmid (triangles), UTI189 contains the pUTI89 plasmid (squares), and Sd197 contains the pSD1 197 plasmid (circles). Note that for E.ca.1, E.ca.2, E.c.APEC01, and E.c.UTI189, CcdA ORFs were detected, while cognate CcdB-encoding ORFs either were not (E.ca.1) or contained frameshift mutations (E.ca.2, E.c.APEC01, and E.c.UTI189) that are predicted to inactivate CcdB.
Acknowledgments
We are grateful to Abram Aertsen, Susan Gottesman, Nadim Majdalani, and Didier Mazel for critical reading of the manuscript and to the members of the lab for exciting and constructive discussions. We thank Nadim Majdalani for providing numerous bacterial strains.
This work was supported by the Fonds de la Recherche Scientifique (FRSM-3.4530.04), the European Union (LSHM-CT-2005-019023), the Fonds Jean Brachet, and the Fondation Van Buuren. M.S.D.B. is supported by the FRIA.
Footnotes
▿
Published ahead of print on 25 April 2008.
REFERENCES
- 1.Amitai, S., Y. Yassin, and H. Engelberg-Kulka. 2004. MazF-mediated cell death in Escherichia coli: a point of no return. J. Bacteriol. 1868295-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrangou, R., C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, D. A. Romero, and P. Horvath. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 3151709-1712. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, P., and M. Couturier. 1992. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J. Mol. Biol. 226735-745. [DOI] [PubMed] [Google Scholar]
- 4.Brosius, J., and A. Holy. 1984. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc. Natl. Acad. Sci. USA 816929-6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budde, P. P., B. M. Davis, J. Yuan, and M. K. Waldor. 2007. Characterization of a higBA toxin-antitoxin locus in Vibrio cholerae. J. Bacteriol. 189491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, S. K., M. Mikkelsen, K. Pedersen, and K. Gerdes. 2001. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 9814328-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, S. K., K. Pedersen, F. G. Hansen, and K. Gerdes. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J. Mol. Biol. 332809-819. [DOI] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayhoff, M. O. 1974. Computer analysis of protein sequences. Fed. Proc. 332314-2316. [PubMed] [Google Scholar]
- 10.Efron, B., E. Halloran, and S. Holmes. 1996. Bootstrap confidence levels for phylogenetic trees. Proc. Natl. Acad. Sci. USA 9313429-13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elena, S. F., and R. E. Lenski. 2003. Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4457-469. [DOI] [PubMed] [Google Scholar]
- 12.Gentry, D., H. Xiao, R. Burgess, and M. Cashel. 1991. The omega subunit of Escherichia coli K-12 RNA polymerase is not required for stringent RNA control in vivo. J. Bacteriol. 1733901-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazan, R., and H. Engelberg-Kulka. 2004. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol. Genet. Genomics 272227-234. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe, A., T. Ogura, and S. Hiraga. 1985. Effects of the ccd function of the F plasmid on bacterial growth. J. Bacteriol. 163841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, R. B., E. Grohmann, H. Schwab, R. Diaz-Orejas, and K. Gerdes. 1995. Comparison of ccd of F, parDE of RP4, and parD of R1 using a novel conditional replication control system of plasmid R1. Mol. Microbiol. 17211-220. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi, I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 293742-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodkin-Gal, I., and H. Engelberg-Kulka. 2006. Induction of Escherichia coli chromosomal mazEF by stressful conditions causes an irreversible loss of viability. J. Bacteriol. 1883420-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, X., M. Yagi, T. Morita, and H. Aiba. 2008. Cleavage of mRNAs and role of tmRNA system under amino acid starvation in Escherichia coli. Mol. Microbiol. 68286-297 [DOI] [PubMed] [Google Scholar]
- 20.Magnuson, R. D. 2007. Hypothetical functions of toxin-antitoxin systems. J. Bacteriol. 1896089-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Morganroth, P. A., and P. C. Hanawalt. 2006. Role of DNA replication and repair in thymineless death in Escherichia coli. J. Bacteriol. 1885286-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nariya, H., and M. Inouye. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 13255-66. [DOI] [PubMed] [Google Scholar]
- 24.Pandey, D. P., and K. Gerdes. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen, K., S. K. Christensen, and K. Gerdes. 2002. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol. Microbiol. 45501-510. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112131-140. [DOI] [PubMed] [Google Scholar]
- 27.Rowe-Magnus, D. A., A. M. Guerout, L. Biskri, P. Bouige, and D. Mazel. 2003. Comparative analysis of superintegrons: engineering extensive genetic diversity in the Vibrionaceae. Genome Res. 13428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 29.Salmon, M. A., L. Van Melderen, P. Bernard, and M. Couturier. 1994. The antidote and autoregulatory functions of the F plasmid CcdA protein: a genetic and biochemical survey. Mol. Gen. Genet. 244530-538. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Laboratory Press, Cold Spring Harbor, NY.
- 31.Sat, B., R. Hazan, T. Fisher, H. Khaner, G. Glaser, and H. Engelberg-Kulka. 2001. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethality. J. Bacteriol. 1832041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sevin, E. W., and F. Barloy-Hubler. 2007. RASTA-Bacteria: a web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome Biol. 8R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szekeres, S., M. Dauti, C. Wilde, D. Mazel, and D. A. Rowe-Magnus. 2007. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Mol. Microbiol. 631588-1605. [DOI] [PubMed] [Google Scholar]
- 34.Tachdjian, S., and R. M. Kelly. 2006. Dynamic metabolic adjustments and genome plasticity are implicated in the heat shock response of the extremely thermoacidophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 1884553-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi, N., Y. Naito, N. Handa, and I. Kobayashi. 2002. A DNA methyltransferase can protect the genome from postdisturbance attack by a restriction-modification gene complex. J. Bacteriol. 1846100-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 37.Tock, M. R., and D. T. Dryden. 2005. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8466-472. [DOI] [PubMed] [Google Scholar]
- 38.Tsilibaris, V., G. Maenhaut-Michel, N. Mine, and L. Van Melderen. 2007. What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome? J. Bacteriol. 1896101-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchimoto, S., Y. Nishimura, and E. Ohtsubo. 1992. The stable maintenance system pem of plasmid R100: degradation of PemI protein may allow PemK protein to inhibit cell growth. J. Bacteriol. 1744205-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Melderen, L., P. Bernard, and M. Couturier. 1994. Lon-dependent proteolysis of CcdA is the key control for activation of CcdB in plasmid-free segregant bacteria. Mol. Microbiol. 111151-1157. [DOI] [PubMed] [Google Scholar]
- 41.Wilbaux, M., N. Mine, A. M. Guerout, D. Mazel, and L. Van Melderen. 2007. Functional interactions between coexisting toxin-antitoxin systems of the ccd family in Escherichia coli O157:H7. J. Bacteriol. 1892712-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarmolinsky, M. B. 1995. Programmed cell death in bacterial populations. Science 267836-837. [DOI] [PubMed] [Google Scholar]