Mitosis in vertebrate somatic cells with two spindles: Implications for the metaphase/anaphase transition checkpoint and cleavage (original) (raw)
Abstract
During mitosis an inhibitory activity associated with unattached kinetochores prevents PtK1 cells from entering anaphase until all kinetochores become attached to the spindle. To gain a better understanding of how unattached kinetochores block the metaphase/anaphase transition we followed mitosis in PtK1 cells containing two independent spindles in a common cytoplasm. We found that unattached kinetochores on one spindle did not block anaphase onset in a neighboring mature metaphase spindle 20 μm away that lacked unattached kinetochores. As in cells containing a single spindle, anaphase onset occurred in the mature spindles x̄ = 24 min after the last kinetochore attached regardless of whether the adjacent immature spindle contained one or more unattached kinetochores. These findings reveal that the inhibitory activity associated with an unattached kinetochore is functionally limited to the vicinity of the spindle containing the unattached kinetochore. We also found that once a mature spindle entered anaphase the neighboring spindle also entered anaphase x̄ = 9 min later regardless of whether it contained monooriented chromosomes. Thus, anaphase onset in the mature spindle catalyzes a “start anaphase” reaction that spreads globally throughout the cytoplasm and overrides the inhibitory signal produced by unattached kinetochores in an adjacent spindle. Finally, we found that cleavage furrows often formed between the two independent spindles. This reveals that the presence of chromosomes and/or a spindle between two centrosomes is not a prerequisite for cleavage in vertebrate somatic cells.
Keywords: cell cycle checkpoint, kinetochore, chromosomes
During mitosis in animal cells the sister kinetochores on a replicated chromosome rarely attach simultaneously to the opposing poles of the forming spindle. Instead, that kinetochore closest to and facing a pole at the time of nuclear envelope breakdown is usually the first to attach. This results in the initial monoorientation of the chromosome to one pole, and the chromosome remains monooriented until the other kinetochore attaches to the distal pole. To ensure that anaphase and exit from mitosis do not begin until the last monooriented chromosome becomes bioriented, cells have evolved a checkpoint control mechanism that blocks the metaphase/anaphase transition until all chromosomes are attached to the spindle in a bipolar fashion (reviewed in ref. 1). This checkpoint consists of a detector that monitors some aspect of chromosome attachment, and a signal transduction pathway that targets some element in the sequence of molecular events leading to chromatid disjunction and exit from mitosis (see ref. 2).
Recently, it has been shown that the checkpoint controlling entry into anaphase in vertebrate somatic (PtK1) cells is mediated by an inhibitor of the metaphase/anaphase transition produced by unattached kinetochores (ref. 3; see also ref. 4). However, our understanding of the mechanisms by which this checkpoint works remains vague (5, 6). The fact that even a single unattached kinetochore delays anaphase in the whole spindle indicates that the inhibitory agent (or the downstream products of the pathway it activates) must propagate or diffuse away from the signaling kinetochore. However, whether the targets for this signal are located on the spindle, throughout the cytoplasm, or both is unknown (7).
To better understand the characteristics of the signal transduction pathway behind the kinetochore attachment checkpoint we have followed the process of mitosis in living PtK1 cells that formed two adjacent but independent spindles in the same cytoplasm. Previously we have shown that the time required for all kinetochores to attach to the forming PtK1 spindle is quite variable and ranges from 23 min after nuclear envelope breakdown to over 3 hr (8). However, regardless of how long the cell contains monooriented chromosomes it remains stably in mitosis until all kinetochores become attached to a spindle pole. Once the last kinetochore attaches, anaphase occurs 23 ± 1 min later (range 9–48 min; ref. 8). The same is also true for PtK1 cells containing tri- and tetrapolar spindles; they enter anaphase 27 ± 3 and 24 ± 1 min, respectively, after attachment of the last kinetochore (9).
We reasoned that the variability in time for the completion of kinetochore attachment for any one spindle should produce instances, in cells containing two independent spindles, where one spindle had completed chromosome congression while the other still had one or more unattached kinetochores. The subsequent behavior of these spindles should then provide important information on two facets of how the metaphase/anaphase transition is regulated. First, it would reveal whether the inhibitory activity produced by an unattached kinetochore acts locally just on its spindle, or globally on both spindles. If the inhibitory signal propagates or diffuses through the cytoplasm (see ref. 7), then neither spindle should initiate anaphase until the last kinetochore in the cell becomes attached. On the other hand, if one or more unattached kinetochores on one spindle do not block anaphase onset in a neighboring spindle with fully congressed chromosomes, then the inhibitor produced by unattached kinetochores must act locally within the spindle containing the unattached kinetochore(s). Second, information on the synchrony of anaphase onset between the two independent spindles would also reveal whether the molecular changes associated with the metaphase/anaphase transition are initiated locally within the spindle or globally.
Finally, our system also allows us to evaluate whether chromosomes are involved in determining where cleavage furrows form in somatic cells. Do cleavage furrows form only between spindle poles containing intervening chromosomes (as predicted by the chromosomal passenger protein model of cleavage, refs. 10 and 11), or can they also form between two neighboring poles that lack an intervening spindle or chromosomes (as predicted by the astral stimulation model, ref. 12)?
MATERIALS AND METHODS
Cell Culture.
PtK1 cells were cultured as previously described (8). In brief, stock cultures were maintained in 5% CO2 in antibiotic-free Ham’s F-12 medium supplemented with 10% fetal calf serum. The PtK1 line was initially purchased from the American Type Culture Collection at passage number 66, and only cells at passage numbers 70–100 were used for this study.
For experiments the stock cells were subcultured onto 25 × 25 mm coverslips which were then mounted in Rose chambers containing L-15 medium supplemented with 10% fetal calf serum and 10 mM Hepes. These chambers were then placed on the stage of a Nikon Diaphot inverted microscope and maintained at 35–37°C with a custom-built incubator (described in ref. 8).
Cell Fusion.
We electrofused PtK1 cells with a ProGenetor II electroporator (Hoefer). During this process subconfluent mitotically active coverslip cultures were placed in fusing media (280 mM sucrose/2 mM Hepes, pH 6.9/1 mM MgCl2) over two platinum electrodes, separated by 10 mm, that were mounted on a glass microscope slide. The cells were then exposed to a single electric pulse of 350 V for 2 ms and then quickly transferred back into conditioned culture medium. After 1 hr the medium was changed and the coverslips were incubated overnight in fresh F-12 medium supplemented with 10% fetal calf serum and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin).
Light Microscopy.
Single cells containing two spindles, separated by a distance of 20–100 μm, were followed from prometaphase through anaphase by time-lapse video light microscopy using a framing rate of 15 frames per min. Cells were illuminated with shuttered, monochromatic (546-nm) heat-filtered light obtained from a 100-W tungsten filament. They were viewed and followed with either 40× phase-contrast (numerical aperture = 0.7) or 60× phase-contrast (numerical aperture = 1.40) objectives and a 0.85 numerical aperture condenser. Video images, obtained with a VE1000 Newvicon camera (Dage–MTI, Michigan City, IN), were enhanced by a Hamamatsu DVS-3000 image processor (Hamamatsu Photonics, Hamamatsu City, Japan). Optical and electronic noise was reduced by background subtraction and averaging of 16 consecutive frames. Processed images were then stored using a Sony laser videodisk LVS 500 recorder or a Panasonic AG 6760 time-lapse SVHS VCR.
Immunofluorescent Microscopy.
Coverslip cultures of fused PtK1 cells were fixed with 1% glutaraldehyde in phosphate buffer, permeabilized for 10 min with 1% Triton X-100 in buffer, reduced in NaBH4, and then stained with an α-tubulin primary antibody (Sigma, catalog no. T5168) followed by a fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody (Sigma, catalog no. F0257). They were then imaged using a Photometrics KAF-1400 cooled CCD camera (Photometrics, Tuscon, AZ) and then processed using SGI-based (Silicon Graphics, Mountain View, CA) isee software (Inovision, Durham, NC).
Data Analysis.
For this study we filmed more than 30 PtK1 cells containing two independent spindles. Congression of the last monooriented chromosome and anaphase onset were determined for each spindle as described by Rieder et al. (8), and all timings were rounded to the nearest minute. Statistical analyses were performed using Quattro Pro 6.1 (Novell, Orem, UT).
RESULTS
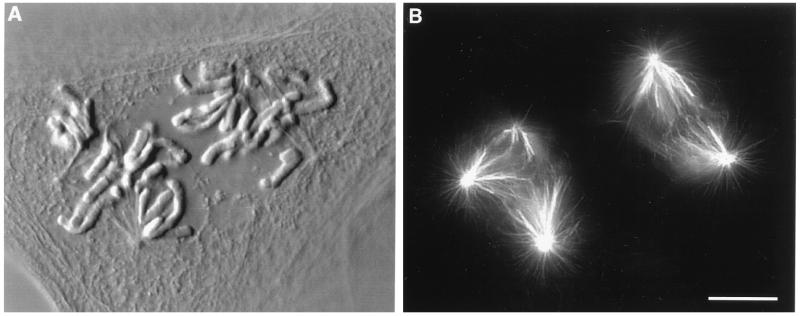
In our study we analyzed the progress of mitosis in over 30 cells containing two independent spindles that were separated by 20–100 μm. That these spindles were truly independent was clear from two observations. First, immunofluorescent analyses of spindle microtubule distribution in fixed cells containing two spindles, similar to the ones we followed in vivo for our study, revealed that the two spindles were not connected by overlapping astral or spindle microtubules (Fig. 1). More importantly, however, we could always determine functionally when separate spindles lost their independence in living cells because the attachment of any chromosome to two spindles led to a rapid fusion of the spindles into a single multipolar spindle (data not shown). Indeed, we found that such a fusion invariably occurred whenever the two spindles wandered within less than 20 μm of each other. When this occurred the cell was eliminated from further consideration.
Figure 1.
Differential interference contrast (A) and epifluorescent (B) photomicrographs of a fused PtK1 cell that contains independent bipolar and tripolar spindles. Note that the two spindles do not share a common chromosome and that their microtubule arrays do not overlap. (Bar = 10 μm.)
For descriptive purposes we have defined the leading spindle as the first one in the cell to initiate anaphase, or in the case of simultaneous anaphase in both spindles, the first spindle to congress all of its chromosomes.
Mitosis in Untreated PtK1 Cells That Form Two Independent Spindles in a Common Cytoplasm.
Approximately 3% of the cells in an untreated PtK1 culture are binucleated (13) and normally these nuclei lie in close proximity. As these cells enter mitosis chromosome condensation and nuclear envelope breakdown occur synchronously in both nuclei but, because of their proximity, the two groups of chromosomes become incorporated into a single large multipolar spindle (see ref. 9). In some binucleates, however, two independent spindles are formed in a common cytoplasm. Although such cells are rare we were able to find and follow three during this study.
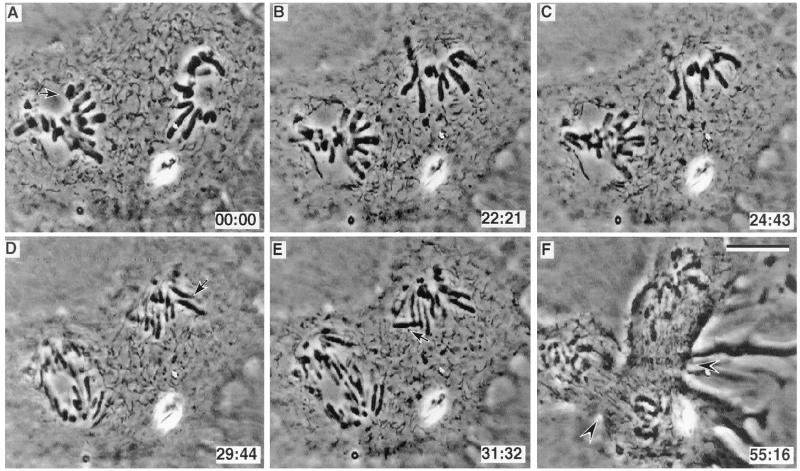
In two of these cells the trailing spindle did not possess a monooriented chromosome long enough (i.e., 49 min; see refs. 8 and 9) after the leading spindle congressed its last chromosome to reveal any potential inhibitory effect. However, in one cell a monopolar spindle was formed 20 μm from a normal bipolar spindle (Fig. 2). Twenty-four minutes after the last unattached kinetochore on the bipolar spindle became attached to its spindle (Fig. 2A, arrow) the spindle initiated anaphase (see Fig. 2 A_–_C) even though the neighboring monopolar spindle contained numerous unattached kinetochores (seven in the plane of focus shown in Fig. 2 C–E). Then, 3–4 min after anaphase onset in the bipolar spindle, anaphase was initiated in the monopolar spindle as evidenced by chromatid disjunction (Fig. 2D). During the ensuing anaphase the attached chromatids on the monopolar spindle moved closer to the poles, while some of the unattached chromatids were seen to be ejected away from the pole into the cytoplasm (Fig. 2, arrows in D and E). Later this cell initiated two cleavage furrows (Fig. 2F, arrowheads): one between the separated groups of chromosomes on the bipolar spindle and another between the monopolar spindle and the bipolar spindle.
Figure 2.
(A–F) Selected video images from an untreated PtK1 cell with independent bipolar and monopolar spindles. In this cell the bipolar spindle entered anaphase (C) 24 min after its last monooriented chromosome initiated congression (arrow in A), and in the presence of numerous monooriented chromosomes on the monopolar spindle. Arrows in D and E indicate unattached anaphasic chromatids on the monopolar spindle. Note that a cleavage furrow is initiated between the poles of the bipolar spindle, and also between the poles of the bipolar and monopolar spindles where no chromosomes were present (arrowheads in F). (Bar = 10 μm.)
Mitosis in Fused PtK1 Cells That Form Two Independent Spindles in a Common Cytoplasm.
To obtain a larger sample size we electrofused subconfluent cultures of PtK1 cells. After this procedure mitotic cells could occasionally be found, 18–24 hr after fusion, that contained two independent multipolar or bipolar spindles separated by a variable distance (e.g. Fig. 3). We followed 30 cells that contained independent bipolar spindles and/or multipolar spindles. It should be emphasized that the use of multipolar spindles is relevant to our study because the interval between when the last kinetochore attaches and anaphase onset on a multipolar PtK1 spindle is the same as in a normal bipolar spindle (see ref. 9).
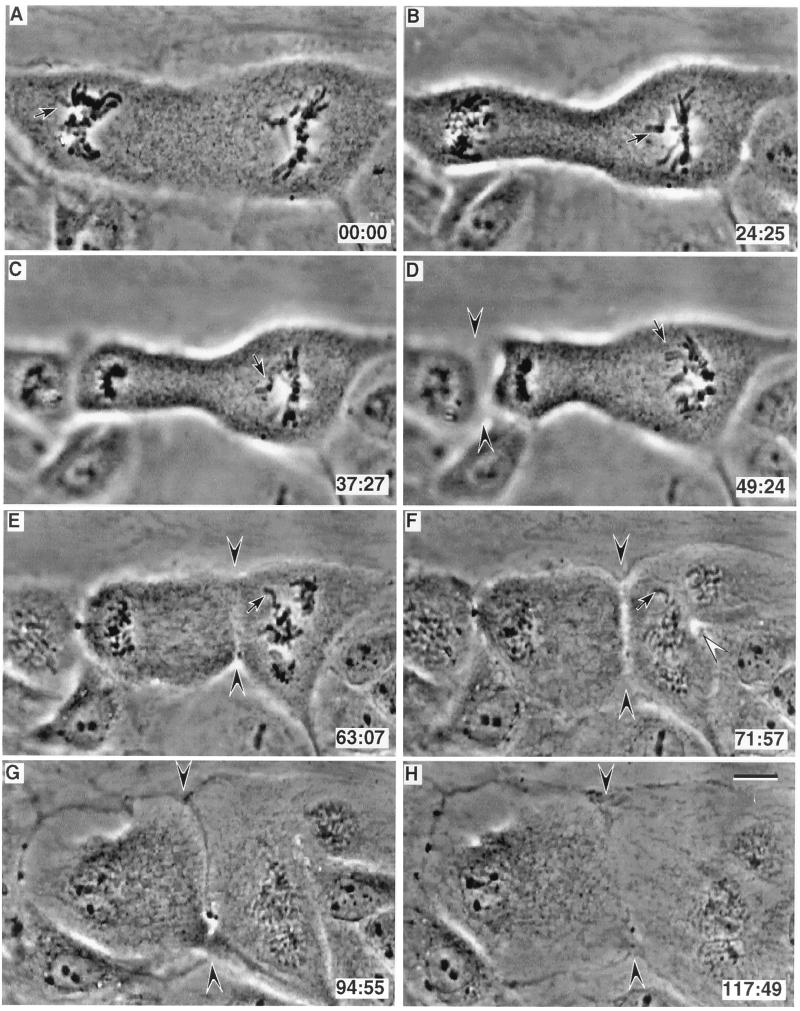
Figure 3.
(A–H) Selected video images of a PtK1 cell 20 hr after fusing. This cell contains independent bipolar (left side of A) and tripolar (right side of A) spindles. In this example anaphase started in the bipolar spindle (B) 24 min after its last monooriented chromosome initiated congression (arrow in A), and anaphase was initiated in the presence of a monooriented chromosome on the tripolar spindle (arrow in B). The tripolar spindle then entered anaphase 25 min later (D) even though it still contained a monooriented chromosome (arrow in D and E). This cell cleaved between the poles of the bipolar spindle (arrowheads in D). It then also initiated cleavage between two spindle poles in the tripolar spindle (open arrowhead in F) and also between the independent spindles (arrowheads in F–H). Note that this latter furrow did not contain a midbody, and that it completely cleaved the cell between two spindle poles that lacked intervening chromosomes. (Bar = 10 μm.)
In all 30 cells containing two separate spindles the leading spindle congressed all of its chromosomes before initiating anaphase (e.g., Fig. 3 A–C). Moreover, as in controls containing one spindle, anaphase started in these leading spindles 24 ± 4 min after the last monooriented chromosome initiated congression. In eight of 30 cells anaphase started in the leading spindle while one or more monooriented chromosomes were still present on the adjacent trailing spindle (one example is shown in Fig. 3). In seven of these cells the trailing spindle also ultimately entered anaphase while it possessed one or more monooriented chromosomes (in the other cell the mono-oriented chromosome on the trailing spindle congressed before the trailing spindle entered anaphase). In the other 22 cells there were no monooriented chromosomes on either spindle at the moment the leading spindle entered anaphase, and with the exception of one cell, the leading spindle always entered anaphase within the range of times normally seen in a large population of control bipolar or multipolar spindles (9–49 min after attachment of the last kinetochore; see refs. 8 and 9). In the exceptional cell both spindles congressed their last monooriented chromosome at about the same time, but both remained in metaphase for over 1 hr (61 and 68 min). Thus, in no case did a mature spindle with all kinetochores attached wait for the adjacent spindle to complete kinetochore attachment before initiating anaphase.
Once anaphase was initiated in the mature spindle it then started in the trailing spindle 0–39 min later. We found no correlation between the distance separating the two spindles and the degree of asynchrony in anaphase onset. We then asked whether a correlation exists between the asynchrony in anaphase onset and the presence or absence of monooriented chromosomes on the trailing spindle. Again, we found no correlation between these two parameters: anaphase started in the trailing spindle, on average, 9 min after anaphase onset in the leading spindle regardless of whether the trailing spindle lacked (x̄ = 8.6 min; range = 0–39 min; n = 20) or contained (x̄ = 8.9 min; range = 0–26 min; n = 8) monooriented chromosomes.
Of the 30 fused cells filmed for our study 15 were followed long enough to determine cleavage patterns. Of these 14 initiated or completed cleavage between those spindle poles that had chromosomes positioned between them. Importantly, eight of these cells also initiated cleavage between spindle poles derived from adjacent spindles at positions that did not have intervening chromosomes (Fig. 3, see also Fig. 1 E and F). In some of these cases, furrows formed between spindles that were separated by ≥60 μm, a distance that precluded an overlap of astral microtubules (see Fig. 3). Of these, four completed the cleavage (e.g., Fig. 3, arrowheads in E–H) and the furrow ultimately regressed in the other four.
DISCUSSION
Although studies on fixed cells have reported that fused cells entering mitosis sometimes form multiple independent spindles (e.g., for animals see refs. 14 and 15; for plants see ref. 16), to our knowledge this is the first study to detail the process of mitosis in vivo in vertebrate somatic cells containing two independent spindles.
Initially, polykaryons formed from fusing cells in an asynchronous population contain nuclei from different points of the cell cycle. However, within the duration of a single cell cycle (about 24 hr in PtK1; see ref. 17) these heterophasic nuclei become synchronized so that by 18–20 hr after fusion most cells entering mitosis contain nuclei at the same stage of the cell cycle (18–20). In addition, after fusion the multiple cytoskeletal networks usually become converted into a single common network in which the nuclei are aggregated near the center of the cell (21). As a result a large common multipolar spindle is formed when the polykaryon enters mitosis. However, we found it possible to find polykaryons entering mitosis with well separated nuclei in cultures 18–20 hr after fusion. Under this condition multiple independent mitotic spindles were formed within the cell, and those cells forming just two spindles were selected for our study. The fact that we obtained similar data from untreated and electrofused cultures reveals that the fusion process does not influence our results.
The Inhibitor of the Metaphase/Anaphase Transition Produced by Unattached Kinetochores Is Not Freely Diffusible.
We know through laser microsurgery (3) and micromanipulation (4) studies that unattached or weakly attached kinetochores produce an inhibitor of anaphase onset. The fact that even a single unattached (8) or weakly attached (22) kinetochore prevents anaphase for many hours suggests that its inhibitory activity must, to some extent, propagate away from the kinetochore to affect at least the rest of the spindle and perhaps the cell as a whole. The nature of the “wait anaphase” signal produced by unattached kinetochores, and its mode of action, is currently unknown (reviewed in ref. 6).
The primary goal of our study was to determine whether the inhibitor of anaphase produced by unattached kinetochores is freely diffusible (e.g., see ref. 7). We followed the process of mitosis in PtK1 cells containing two adjacent but independent spindles. Our rationale was that if the inhibitor is diffusible, or if it targets a diffusible cytoplasmic component that constitutes the “wait anaphase” signal, then anaphase should be delayed in two adjacent spindles until the last unattached kinetochore in the cell attaches to its spindle.
We found that anaphase onset in a mature spindle was not delayed by one or more unattached kinetochores in an adjacent spindle. Indeed, as in controls containing a single spindle, anaphase was initiated in the leading spindle with fully congressed chromosomes x̄ = 24 min after the last kinetochore attached (Figs. 2 and 3). From these observations we conclude that the action of the inhibitor produced by unattached kinetochores is not freely diffusible and that it targets something on the spindle containing the unattached kinetochore. One obvious ramification of this conclusion is that completely unattached chromosomes, well separated from the forming spindle, should not inhibit anaphase onset and this appears to be the case, at least in newt pneumocytes (ref. 23, see also ref. 24).
It could be argued that the inhibitor produced by an unattached kinetochore is freely diffusible, but that the cytoplasmic volume in cells containing two spindles is at least 2-fold larger than in normal cells, and that in these cells the inhibitor is diluted to a level below its threshold. However, if this was true the concentration of the inhibitor would not be sufficient to delay the metaphase/anaphase transition even in the leading spindle which it clearly does (these spindles enter anaphase only after the last kinetochore attaches). Moreover, if one unattached kinetochore can arrest one normal cell volume in mitosis, the many (at least seven) unattached kinetochores on an adjacent monopolar spindle should be more than sufficient to arrest a cell of 2-fold greater volume if the inhibitor was freely diffusible.
Another formal possibility is that the inhibitory factor is freely diffusible but its range is limited ≤20 μm, which is the minimum separation distance which spindles in PtK1 cells can maintain their independence. However, this is highly unlikely considering the fact that an unattached kinetochore located near one of the poles on a large multipolar PtK1 spindle inhibits anaphase onset in all parts of the spindle, even those parts located ≥20 μm from the monooriented chromosome (e.g., see figures 5–9 in ref. 9). Functionally, the inhibitor can propagate greater distances within a spindle than between adjacent independent spindles. This suggests the inhibitor of the metaphase/anaphase transition produced by monooriented chromosomes becomes structurally associated with the spindle containing the unattached kinetochore, and that it then spreads throughout the spindle. However, regardless of whether the inhibitor structurally binds to spindle microtubules or not, our conclusion that it targets something on or near the spindle raises the possibility that it regulates the activity of anaphase promoting complexes (25) or the CDC2/cyclin B kinase, both of which have been reported to be located primarily in the spindle (for anaphase promoting complexes see refs. 26 and 27; reviewed in ref. 28; for CDC2/cyclin B see refs. 29 and 30).
Dominance of the Molecular Changes Accompanying the Metaphase/Anaphase Transition.
Our data reveal that once the metaphase/anaphase transition is initiated in one part of the cell, it spreads throughout the cell and that it can override the “wait anaphase” signal associated with a spindle containing unattached kinetochores. This observation, coupled with our conclusion that the inhibitor of anaphase produced by unattached kinetochores targets something associated with the spindle, suggests that the events leading to chromatid separation and exit from mitosis are initially catalyzed in the mature spindle and then spread throughout the cell (see also ref. 24).
Does the presence of monooriented chromosomes forestall anaphase onset in the trailing spindle after the leading spindle starts anaphase? In this regard we found no difference in the asynchrony between anaphase in the two spindles regardless of whether the trailing spindle lacked or contained monooriented chromosomes at the moment the mature spindle entered anaphase—the asynchrony in both populations averaged 8–9 min. However, laser irradiation studies reveal that the “wait anaphase” signal produced by unattached kinetochores is only gradually shut off as the kinetochore attaches, and that this process takes ≈6 min (3). When we factored this consideration into our analyses we found that the degree of asynchrony in anaphase onset was significantly greater (x̄ = 12 ± 3 min; n = 15; range = 0–39 min vs. x̄ = 5 ± 1 min; n = 13; range = 0–13 min) for cells in which the trailing spindle contained a monooriented chromosome, or congressed its last monooriented chromosome, ≤6 min before anaphase in the leading spindle. The fact that we obtain two different results from the same data set, by just minimally changing the criteria for defining whether the trailing spindle is still under a checkpoint control at the time of anaphase in the leading spindle, reveals that our sample size is too small to draw any reliable conclusions regarding this issue.
The Role of the Chromosomes and Spindle Midzone in Determining the Plane of Cytokinesis.
Mackay and Earnshaw (10) proposed that the location of the cleavage furrow in vertebrate somatic cells is mediated, in part, by passenger proteins released from the chromosomes at the spindle equator at anaphase onset. In a similar vein, Margolis and Andreassen (31) suggest that cleavage furrow formation is mediated in animal cells by a telophase disk, that is templated by the mitotic spindle during anaphase. That the spindle midzone supplies a requisite signal for cytokinesis is also suggested from the micromanipulation experiments on normal rat kidney epithelial cells (11, 32).
Our data, however, clearly reveal that cleavage furrows can form and cleave the cell between two spindle poles that have no intervening chromosomes, even when these poles belonging to different spindles were separated by up to 60 μm (e.g., Fig. 3). We found that furrow formation and cytokinesis occurred with no detectable astral overlap, as evidenced by the fact that a spindle midbody failed to form. Thus, our data support Rappaport’s (ref. 12; see also ref. 33) contention that in animal cells neither chromosomes nor central spindles are necessary for cleavage furrow formation or cytokinesis. Our results are also consistent with Zhang and Nicklas’ (4) finding that cytokinesis occurs in grasshopper spermatocytes after all of the chromosomes are removed.
Acknowledgments
We thank Ms. C. Hughes for tissue culture assistance. Much of this work was conducted within the Wadsworth Center’s Video-LM core facility, and was supported by National Institutes of Health Grants GM 40198 (to C.L.R.), GM 30758 (to G.S.), and RR 01219, awarded by the Biomedical Research Technology Program, National Center for Research Resources, Department of Health and Human Resources/U.S. Public Health Service), to support the Wadsworth Centers Biological Microscopy and Image Reconstruction facility as a National Biotechnological Resource.
References
- 1.Wells W A E. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- 2.Hardwick K G, Murray A W. J Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieder C L, Cole R W, Khodjakov A, Sluder G. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D, Nicklas R B. Nature (London) 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen R-H, Waters J C, Salmon E D, Murray A W. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 6.Rudner A D, Murray A W. Curr Opin Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh J R. Cold Spring Harbor Symp Quant Biol. 1991;56:613–619. doi: 10.1101/sqb.1991.056.01.070. [DOI] [PubMed] [Google Scholar]
- 8.Rieder C L, Schultz A, Cole R W, Sluder G. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sluder G, Thompson E A, Miller F J, Hayes J, Rieder C L. J Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Mackay A M, Earnshaw W C. Cold Spring Harbor Symp Quant Biol. 1993;58:697–706. doi: 10.1101/sqb.1993.058.01.077. [DOI] [PubMed] [Google Scholar]
- 11.Cao L-G, Wang Y-L. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rappaport R. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 13.Heneen W K. Chromosoma. 1970;29:88–117. doi: 10.1007/BF01183663. [DOI] [PubMed] [Google Scholar]
- 14.Gosh S, Paweletz N, Gosh I. Chromosoma. 1978;65:293–300. doi: 10.1007/BF00286409. [DOI] [PubMed] [Google Scholar]
- 15.Peterson S P, Berns M W. Exp Cell Res. 1979;120:223–236. doi: 10.1016/0014-4827(79)90382-3. [DOI] [PubMed] [Google Scholar]
- 16.Giménez-Martín G, López-Sáez J F, Moreno P, González-Fernández A. Chromosoma. 1968;25:282–296. [Google Scholar]
- 17.Aubin J E, Osborn M, Franke W W, Weber K. Exp Cell Res. 1980;129:149–165. doi: 10.1016/0014-4827(80)90340-7. [DOI] [PubMed] [Google Scholar]
- 18.Rao P N, Johnson R T. Nature (London) 1970;225:159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R T, Rao P N. Biol Rev. 1971;46:97–155. doi: 10.1111/j.1469-185x.1971.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 20.Gosh S, Paweletz N. Chromosoma. 1984;89:197–200. doi: 10.1007/BF00294999. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Q, Chang C S. Cell Motil Cytoskeleton. 1990;17:45–355. doi: 10.1002/cm.970170409. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Nicklas R B. Nature (London) 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 23.Rieder C L, Alexander S P. In: Mechanisms of Chromosome Distribution and Aneuploidy. Resnick M A, Vig B K, editors. New York: Liss; 1989. pp. 185–194. [Google Scholar]
- 24.Sluder G, Miller F J, Thompson E A, Wolf D E. J Cell Biol. 1994;126:189–198. doi: 10.1083/jcb.126.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King R W, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner M W. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 26.Mirabito P M, Morris N R. J Cell Biol. 1993;120:959–968. doi: 10.1083/jcb.120.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tugendreich S, Tomkiel J, Earnshaw W, Heiter P. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 28.King R W, Deshaies R J, Peters J-M, Kirschner M W. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 29.Zubiak J Z, Weber M, de Pennart H, Winston N J, Maro B. EMBO J. 1993;12:3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreassen P R, Margolis R L. J Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis R L, Andreassen P R. BioEssays. 1993;15:201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley S P, Wang Y-L. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devore J J, Conrad G W, Rappaport R. J Cell Biol. 1989;109:2225–2232. doi: 10.1083/jcb.109.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]