Mitosis-Specific Mechanosensing and Contractile Protein Redistribution Control Cell Shape (original) (raw)
. Author manuscript; available in PMC: 2008 Jul 16.
Published in final edited form as: Curr Biol. 2006 Oct 10;16(19):1962–1967. doi: 10.1016/j.cub.2006.08.027
Summary
Because cell division failure is deleterious, promoting tumorigenesis in mammals [1], cells utilize numerous mechanisms to control their cell-cycle progression [2–4]. Though cell division is considered a well-ordered sequence of biochemical events [5], cytokinesis, an inherently mechanical process, must also be mechanically controlled to ensure that two equivalent daughter cells are produced with high fidelity. Since cells respond to their mechanical environment [6, 7], we hypothesized that cells utilize mechanosensing and mechanical feedback to sense and correct shape asymmetries during cytokinesis. Because the mitotic spindle and myosin-II are vital to cell division [8, 9], we explored their roles in responding to shape perturbations during cell division. We demonstrate that the contractile proteins, myosin-II and cortexillin-I, redistribute in response to intrinsic and externally induced shape asymmetries. In early cytokinesis, mechanical load overrides spindle cues and slows cytokinesis progression while contractile proteins accumulate and correct shape asymmetries. In late cytokinesis, mechanical perturbation also directs contractile proteins but without apparently disrupting cytokinesis. Significantly, this response only occurs during anaphase through cytokinesis, does not require microtubules, is independent of spindle orientation, but is dependent on myosin-II. Our data provide evidence for a mechanosensory system that directs contractile proteins to regulate cell shape during mitosis.
Results and Discussion
To test whether cells monitor their shape during cell division, we examined whether cytokinetic Dictyostelium discoideum cells show asymmetries in myosin-II distribution, which would be suggestive of an active system for correcting shape asymmetries. We collected and analyzed 40 time-lapse movies of dividing myoII::GFP-myosin-II; RFP-tubulin cells. Of these, 57% showed symmetric myosin-II distribution and centralized spindle placement during cytokinesis and an enrichment of myosin-II in the cleavage furrow as cytokinesis progressed (Fig. 1A; Sup. Table 1; Sup. Movie 1). However, the remaining 43% exhibited an asymmetrically positioned mitotic spindle early (anaphase to onset of furrowing) in cytokinesis with GFP-myosin-II distribution concentrated in the cortex furthest from the spindle. These asymmetrical cells had an axial ratio of 1.5±0.08 (mean±SEM), which is significantly greater than the axial ratio of 1.2±0.03 for the symmetrically shaped cells (Student’s _t_-test: P=0.001; Sup. Methods). As the cells progressed through anaphase, the spindle was repositioned to the center, and myosin-II accumulated at the equator, allowing the cells to proceed symmetrically through cytokinesis (Fig. 1B; Sup. Table 1; Sup. Movie 2). These observations suggest that cells redistribute myosin-II to correct shape and/or spindle asymmetries, preventing the cell from producing asymmetric daughter cells.
Fig. 1. Progression of a mitotic cell through the stereotypical shape changes of cytokinesis without mechanical load.
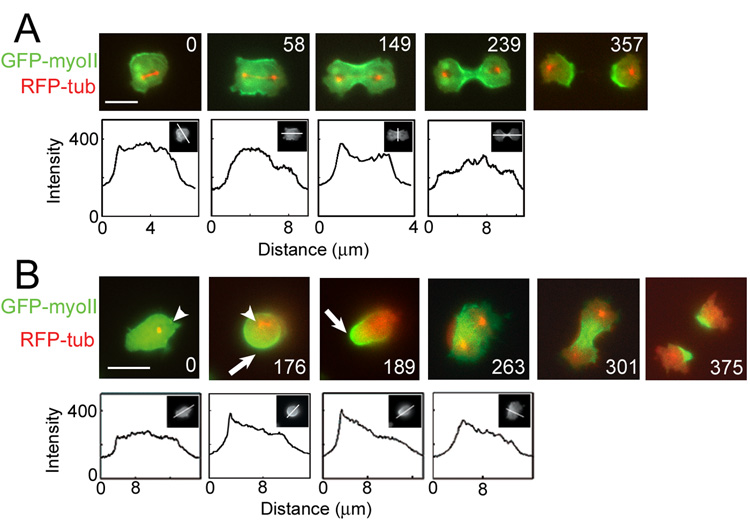
A. Symmetrical GFP-myosin-II is observed in 57% of unloaded dividing cells. The cell rounded up and the mitotic spindle was centrally positioned (0 s). GFP-myosin-II enriched in the cleavage furrow and the mitotic spindle elongated, positioning each daughter nucleus at opposite poles (58 s). The cleavage furrow constricted (149 s) until a bridge was formed (239 s), which finally severed (357 s). Line scans show the magnitude of GFP-myosin-II; insets show the line position. This sequence corresponds to Sup. Movie 1.
B. Asymmetric GFP-myosin-II distribution in early cytokinesis is found in 43% of dividers. Initially, the cell is elongated with the mitotic spindle asymmetrically positioned within the cell (0 s, arrowheads). GFP-myosin-II localized to the polar cortex (arrows; 176 s) furthest from the spindle. As the spindle elongated (189 s and 263 s), myosin-II reoriented to the cleavage furrow, and the cell progressed through symmetric shape changes of cytokinesis (375 s). Line scans revealed the magnitude of the GFP-myosin-II response; insets show the line position. The line scan of the 263 s frame shows the asymmetry of myosin-II in the cleavage furrow cortex (compares to Fig. 1A 149 s). Scale bars, 10 µm. This sequence corresponds to Sup. Movie 2.
Because cell-shape deformation and force are inextricably linked, we tested the possibility that shape asymmetry reflects asymmetric forces acting on the cell cortex. To determine rigorously whether myosin-II asymmetry is a response to shape disturbances, we developed a micropipette aspiration system to apply mechanical loads to dividing cells (Sup. Methods). To ensure that physiologically relevant loads were applied, the minimum pressure required to form a small hemispherical bulge in the pipette was used. Commensurate with the cortical tension of the cell, this load should generate significant cellular deformation (strain) without overwhelming the ability of the cell to respond mechanically. Aspiration pressures applied to myoII::GFP-myosin-II; RFP-tubulin ranged from 0.16–0.60 nN/µm2. Based upon the pipette radius and pressure, the applied force ranged from 8–15 nN, similar to the 4–7 nN of force estimated previously to drive the shape changes of cytokinesis [10, 11].
Using this system, we demonstrate that cells recruit contractile proteins in response to shape deformation to regulate the progression of shape changes during cytokinesis. When aspiration pressure was applied to cells in early (anaphase to the onset of furrowing) or late (onset of furrowing to completion) cytokinesis, myoII::GFP-myosin-II; RFP-tubulin cells responded by sending GFP-myosin-II to the cortex in the pipette (Fig. 2A; Sup. Table 1; Sup. Movie 3). To analyze the responses quantitatively, GFP-myosin-II cortical intensities inside and outside the pipette were measured (Sup. Methods; Fig. 2D–F). Cells aspirated early in cytokinesis generated large scale myosin-II responses, resulting in substantial recruitment of GFP-myosin-II to the pipette, enabling the cell to reject the shape disturbance even though the applied mechanical load was kept constant (Fig. 2A, D). After escaping the pipette, myosin-II redistributed to the furrow and the cell divided symmetrically. A small scale myosin-II response was observed in cells that were loaded late in cytokinesis (Fig. 2B, E). In these late stage cytokinetic cells, myosin-II accumulated in the polar cortex without apparently disrupting the myosin-II accumulation at the contractile ring. Under continuous load, these cells divided symmetrically. Significantly, cells perturbed by the pipette took longer (630±63 s, n=27) to complete cytokinesis than unloaded cells (460±24 s, n=23; Student’s _t_-test: P=0.017). To determine the completion times, we used the initiation of spindle elongation as the reference time (0 s), which meant only movies of cells starting prior to anaphase onset could be included in the completion time analysis. Overall, the majority of aspirated mitotic cells (73%) responded by localizing myosin-II to the site of aspiration, demonstrating their ability to respond to mechanical disturbances during cytokinesis (Sup. Table 1).
Fig. 2. GFP-myosin-II localized in response to mechanical load in cells undergoing cytokinesis.
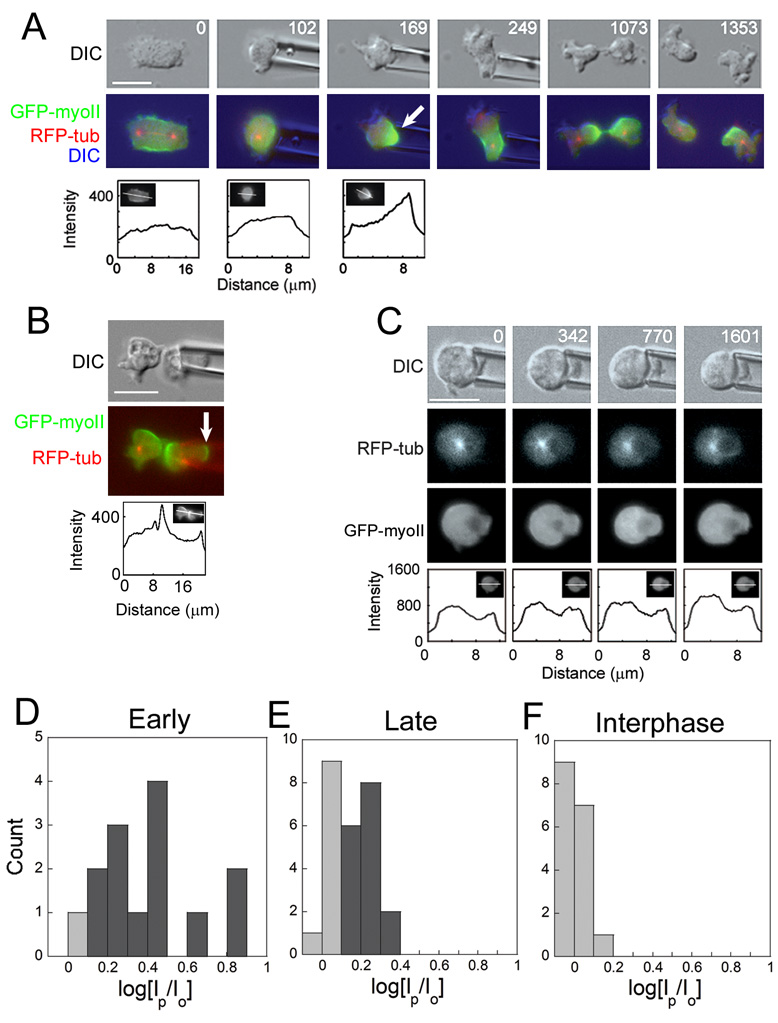
A. A mitotic cell in early cytokinesis (0 s) was captured with the micropipette aspirator (102 s) using a pressure of 0.34 nN/µm2. Although a contractile ring had been initially formed (0s), the cell recruited GFP-myosin-II to the pipette (169 s). Under constant pressure, the cell escaped the pipette (249 s). The cell reoriented myosin-II to the equator, reestablished the correct spindle position, and underwent symmetric cytokinesis (1073 s, 1353 s). Line scans show the magnitude of the GFP-myosin-II response; insets show the line position. The 169 s panel can be compared to the 189 s panel in Fig. 1B, which also shows asymmetric myosin-II. This image sequence corresponds to Sup. Movie 3.
B. A cell aspirated late in cytokinesis accumulated GFP-myosin-II both to the pipette and furrow. Even under continuous load, the cell divided. Line scan shows the magnitude of the GFP-myosin-II response; insets show the line position. This cell can be contrasted to the 239 s panel in Fig. 1A where there is no polar enrichment of myosin-II.
C. An interphase cell was aspirated with a pressure of 0.3 nN/µm2 for 26 minutes. GFP-myosin-II was not recruited to the site of the pipette, indicating that GFP-myosin-II recruitment is mitosis-specific. Line scans show the magnitude of the GFP-myosin-II response; insets show the line position. Scale bars in A–C, 10 µm.
D–F. Frequency histograms of the distribution of magnitudes of Early (D) and Late (E) cytokinesis and Interphase (F) responses. Responders (Sup. Methods) are shaded dark gray and non-responders are shaded light gray. Both Early and Late distributions were significantly greater than the Interphase responses (Student’s _t_-test: P < 0.0001). As a note, we have yet to detect a clear correlation between the magnitudes of the applied loads and responses.
To test whether the redistribution of myosin-II in response to mechanical load is mitosis-specific or a general mechanosensory response, we examined interphase cells. Interphase cells expressing GFP-myosin-II were aspirated with pressures ranging from 0.2–0.6 nN/µm2 for over 25 minutes, ~25-fold longer than needed for a response during mitosis (Fig. 2C). Of 17 interphase cells, none recruited myosin-II to the pipette (Fig. 2C, F; Sup. Table 1). Since the half-life for myosin-II at the cortex is similar in interphase and mitotic cells [12], the response likely depends on a mitosis-specific mechanosensor that is independent of myosin-II thick filament assembly and localization dynamics.
Because aspiration applies force to the cell and myosin-II is a force-generating mechanoenzyme, we tested whether myosin-II is required for resisting the applied force to ensure symmetrical cytokinesis. Without mechanical load, approximately half (compared to only 8% of wild type unloaded divisions and 5% of wild type loaded divisions) of the successful myosin-II mutant cell divisions produced unequally sized daughter cells, while the overall failure rate of cell division was not appreciably higher for myosin-II mutant cells than for wild type (Sup. Table 2). When aspirated, the myosin-II mutant cells showed a three-fold increase in failure rate and lost their ability to control their shape changes, leading to grossly asymmetric divisions (Fig. 3A,B; Sup. Table 2; Sup. Movie 4). Furthermore, the myosin-II mutant cells could only withstand about half of the aspiration pressure as wild type cells without being fully aspirated into the pipette. Thus, myosin-II is essential for resisting mechanical disturbances and for ensuring symmetrical cell division.
Fig. 3. Contractile proteins control cell shape during cytokinesis and redistribute in response to load independent of microtubules.
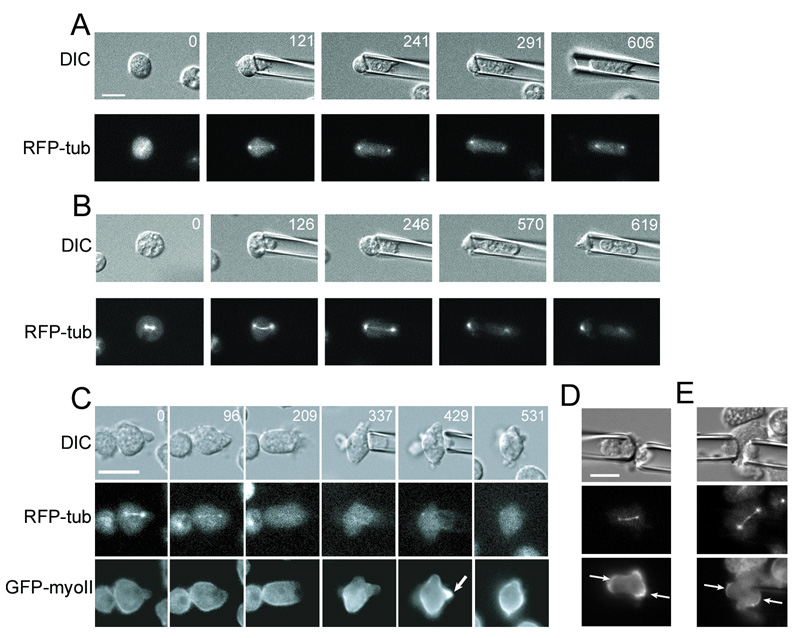
A. The myoII null cell fails to divide under pressure (0.16 nN/µm2). Sup. Movie 4 shows a different myosin-II mutant cell failing cytokinesis under load.
B. The myoII null cell divides under pressure (ranging from 0.1–0.2 nN/µm2), producing two grossly asymmetric daughter cells. Scale bar, 10 µm; applies to all panels in A, B.
C. Microtubules were inhibited with nocodazole (added at 21 s). Aspiration pressure (0.35 nN/µm2) was applied after the microtubules disappeared (337 s). GFP-myosin-II localized to the pipette without microtubules (arrow, 429 s). This image sequence corresponds to Sup. Movie 6.
D. With the pipettes aligned parallel to the spindle axis, crescents (arrows) of myosin-II assembled at each polar cortex. Applied pressure, 0.45 nN/µm2.
E. Crescents (arrows) of myosin-II assembled on opposing sides of the spindle with two pipettes oriented perpendicularly to the mitotic spindle but placed near one centrosome. Applied pressure, 0.35 nN/µm2.
We also examined whether other actin-associated cytoskeletal proteins respond to mechanical load. Cells expressing GFP-cortexillin-I, an actin cross-linker that localizes to the cleavage furrow cortex during cytokinesis, were aspirated with pressures ranging from 0.15–0.30 nN/µm2. Cortexillin-I was recruited to the site of the applied load in 67% of dividing cells (Sup. Fig. 1; Sup. Table 1; Sup. Movie 5). However, similar to myosin-II, cortexillin-I failed to redistribute in response to mechanical disturbance during interphase (Sup. Table 1). In contrast, dynacortin, which enriches in the polar cortex during cytokinesis, did not relocalize to the pipette during mitosis (Sup. Fig. 2). Thus, the mechanosensory system is a contractile protein response, rather than a myosin-II-specific or a general cytoskeleton response.
The mitotic spindle delivers positive and negative signals to the overlying cortex, which triggers contractile ring assembly [8]. In our experiments, all of the unloaded cells and 64% of the loaded cells that had asymmetric GFP-myosin-II distribution during early cytokinesis also had an asymmetrically positioned mitotic spindle. Despite aspirating during all stages of mitosis, we observed that the cell could only respond to mechanical load during anaphase through the end of cytokinesis. These observations raise the question of whether the GFP-myosin-II recruitment is a response to an asymmetrically positioned anaphase spindle, applied load, or both. To separate the roles of force from spindle position, we used nocodazole to depolymerize the microtubules during anaphase (Sup. Methods). Myosin-II redistributed in response to aspiration in the absence of microtubules, and further, the myosin-II response dissipated when the pressure was released, indicating that force was sufficient to direct myosin-II recruitment (Fig. 3C; Sup. Table 1; Sup. Movie 6). Using two pipettes to apply load to different regions of the cortex simultaneously, we also verified that spindle orientation did not influence the ability to respond to mechanical load. Crescents of myosin-II could be recruited to the cortex under each pipette with the spindle in any orientation with respect to the pipettes (Fig. 3D,E).
These observations, coupled with the response in late cytokinesis (Fig. 2B), demonstrate that mechanical force deforms the cortex, leading to the redistribution of contractile proteins and that applied force can override normal spindle signals (Fig. 1A vs. Fig. 3D). Thus, while the mitotic spindle position correlates with the asymmetric distribution of contractile proteins, its role is not essential for mechanosensing. Therefore, the asymmetry in spindle position may be reflective of asymmetric cell shape and/or mechanical strain in the cortical network.
Historically, micromechanical studies have contributed significantly to our understanding of the changes in mechanical behavior that correlate with cytokinetic furrowing [9, 13–17]. However, the molecular bases for these changes, how they relate to cleavage furrow ingression, and how they regulate the evolution of cell shape are not understood [9, 18]. Our study raises the possibility that some of the mechanical changes uncovered by traditional micromechanical techniques may have been influenced by mechanosensory responses of dividing cells. Previous studies also demonstrated that contractile proteins can be directed around the cell by moving the spindle [19, 20]; however, our observations indicate that applied force can override normal spindle signals, allowing myosin-II to accumulate wherever cell-shape deformation is induced (Fig. 4).
Fig. 4. Mechanical force triggers the re-distribution of contractile proteins.
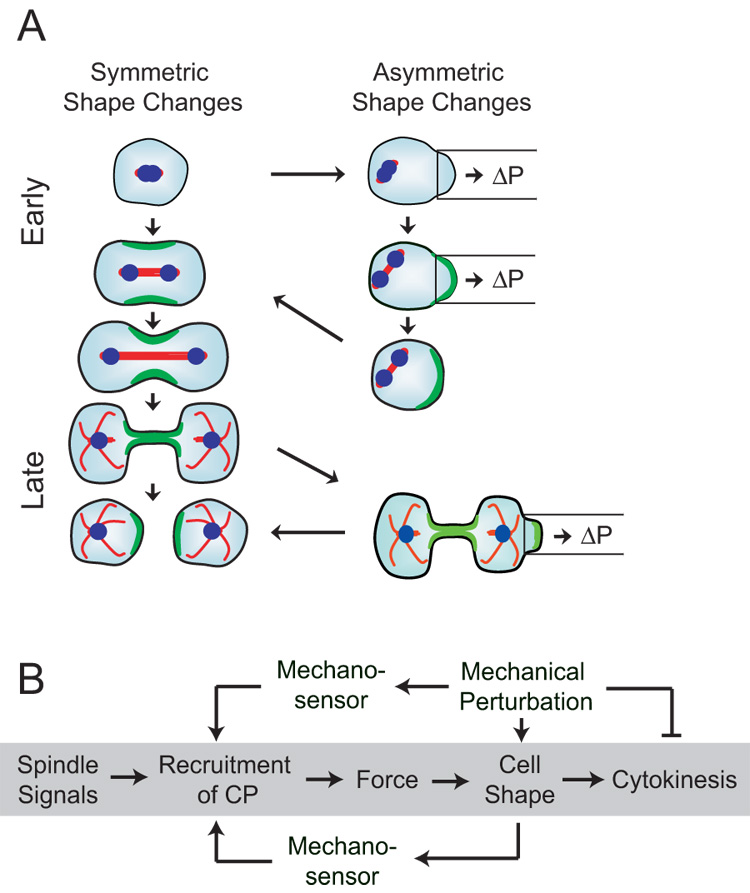
A. Symmetrical vs. asymmetrical shape changes of cytokinesis. The left column shows the symmetrical shape changes of cytokinesis. The right column depicts the asymmetrical shape changes that occur either naturally or that are induced by aspirating the cell. Cells aspirated early during anaphase recruit contractile proteins to the site of aspiration in conjunction with spindle elongation. After escaping the pipette, the cell repositions the spindle centrally and reorients myosin-II and cortexillin-I to the cleavage furrow, progressing through symmetrical cytokinesis. Cells aspirated late in cytokinesis recruited myosin-II both to the furrow and aspirated polar region. Under continuous load, these cells typically complete symmetric division.
B. The diagram outlines a proposed mechanical feedback system. In the unloaded (traditional) pathway (shaded gray), spindle signals initiate the process of cytokinesis, recruiting contractile proteins (CP) to the cleavage furrow. These contractile proteins generate force, driving cell shape changes that produce cytokinesis. By applying a mechanical perturbation (load), a mechanical feedback is suggested. Mechanosensors may measure mechanical perturbations directly by measuring molecular scale strain (upper pathway) or indirectly by monitoring cell shape (lower pathway). The essential differences between these two types of mechanosensors are the length-scales and magnitudes of the strains that they detect. The feedback system then leads to the recruitment of contractile proteins, which correct for shape asymmetries.
Numerous biological systems achieve robustness by utilizing feedback loops [21, 22]. We propose that this mechanosensory system of redistributing proteins in response to shape perturbations is part of a feedback mechanism that monitors cell shape. In this feedback system, mechanical strain in the network triggers activation of a mechanosensor, leading to recruitment of myosin-II and cortexillin-I to the shape disturbance. These proteins then increase the local viscoelastic resistance of the cortex [9, 23, 24], slowing further shape deformation. During early cytokinesis, furrowing is delayed until myosin-II, which promotes dynamic cortical rearrangements through its mechanochemistry [24], contracts the cortex away from the pipette. During late cytokinesis, myosin-II recruited to the pipette at the polar cortex resists the local deformation while equatorial myosin-II contractility along with other myosin-II-independent processes (Laplace pressures and membrane trafficking [11, 25]) continue furrow thinning. Further, the ability to correct mechanical perturbation is dependent on myosin-II, and cells deficient in either myosin-II or cortexillin-I have increased frequencies of asymmetrical cell divisions, indicating an inability to correct shape asymmetries (Sup. Table 2, [23, 26]). Importantly, loaded wild type cells achieve similar levels of cytokinesis completion as unloaded cells (Sup. Table 1), demonstrating the robustness of this system.
Significantly, this system is mitosis-specific, suggesting a novel pathway for mechanosensation. Cells must sense mechanical strain in the cortex, perhaps by opening ion channels or by stretching cortical cytoskeletal proteins to create new binding sites [6, 7], triggering contractile protein recruitment. Previous studies on interphase Dictyostelium and mammalian cells have shown that extreme cell deformation leads to cortex-membrane rupture and myosin-II recruitment to the rupture site [27, 28]. However, our response is specific to anaphase through cytokinesis and does not appear to involve cortex-membrane rupture. Microtubule depolymerization also promotes oscillatory myosin-II-dependent contractions of interphase cells [29, 30]. In contrast, our response occurs in the presence of microtubules with and without external mechanical perturbation and is not oscillatory. The mitosis-specificity might arise because the contractile system is more mobile as the cell prepares for cytokinesis. During interphase, cells may mask the mitotic system and achieve shape control by other mechanisms such as strain-hardening the cytoskeleton through the action of other actin cross-linkers and cytoskeletal structures [6]. Overall, this study establishes a framework for a systems-level analysis of the biochemical and mechanical regulation of cell shape that provides for robust cytokinesis.
Supplementary Material
01
02
03
04
05
06
07
Acknowledgements
We thank the members of the Robinson Lab for many helpful discussions and Sue Craig (Johns Hopkins) and Yixian Zheng (Carnegie Institution) for reading the manuscript. This work was supported by a Burroughs-Wellcome Career Development Award (D.N.R.), a Beckman Young Investigator Award (D.N.R.), the NIH (R01#GM066817 to D.N.R., GM071920 to P.A.I.) and the NSF (DMS0083500 to P.A.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 2.Stukenberg PT. Triggering p53 after cytokinesis failure. J. Cell Biol. 2004;165:607–608. doi: 10.1083/jcb.200405089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connell CB, Wang Y. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell. 2000;11:1765–1774. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The nocut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Wu J-Q, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 6.Janmey PA, Weitz DA. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem. Sci. 2004;29:364–370. doi: 10.1016/j.tibs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev. Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DN, Spudich JA. Mechanics and regulation of cytokinesis. Curr. Opin. Cell Biol. 2004;16:182–188. doi: 10.1016/j.ceb.2004.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichl EM, Effler JC, Robinson DN. The stress and strain of cytokinesis. Trends Cell Biol. 2005;15:200–206. doi: 10.1016/j.tcb.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DN, Cavet G, Warrick HM, Spudich JA. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biology. 2002;3:4. doi: 10.1186/1471-2121-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc. Natl. Acad. Sci. USA. 2005;102:7186–7191. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yumura S. Myosin II dynamics and cortical flow during contractile ring formation in Dictyostelium cells. J. Cell Biol. 2001;154:137–145. doi: 10.1083/jcb.200011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolpert L. The mechanical properties of the membrane of the sea urchin egg during cleavage. Exp. Cell Res. 1966;41:385–396. doi: 10.1016/s0014-4827(66)80146-5. [DOI] [PubMed] [Google Scholar]
- 14.Hiramoto Y. Mechanical properties of sea urchin eggs II. Changes in mechanical properties from fertilization to cleavage. Exp. Cell Res. 1963;32:76–88. doi: 10.1016/0014-4827(63)90070-3. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport R, Ebstein RP. Duration of stimulus and latent periods preceding furrow formation in sand dollar eggs. J. Exp. Zool. 1965;158:373–382. doi: 10.1002/jez.1401580311. [DOI] [PubMed] [Google Scholar]
- 16.Hiramoto Y. Mechanical properties of the cortex before and during cleavage. Ann. N.Y. Acad. Sci. 1990;582:22–30. doi: 10.1111/j.1749-6632.1990.tb21664.x. [DOI] [PubMed] [Google Scholar]
- 17.Matzke R, Jacobson K, Radmacher M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat. Cell Biol. 2001;3:607–610. doi: 10.1038/35078583. [DOI] [PubMed] [Google Scholar]
- 18.Wang YL. The mechanism of cortical ingression during early cytokinesis: thinking beyond the contractile ring hypothesis. Trends Cell Biol. 2005;15:581–588. doi: 10.1016/j.tcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Shuster CB, Burgess DR. Parameters that specify the timing of cytokinesis. J. Cell Biol. 1999;146:981–992. doi: 10.1083/jcb.146.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsop GB, Zhang D. Microtubules continuously dictate distribution of actin filaments and positioning of cell cleavage in grasshopper spermatocytes. J. Cell Sci. 2003;117:1591–1602. doi: 10.1242/jcs.01007. [DOI] [PubMed] [Google Scholar]
- 21.Brandman O, Ferrell JE, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelling J, Sauer U, Szallasi Z, Doyle FJ, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23:1536–1546. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard KD, Kuo SC, Robinson DN. Dictyostelium myosin-II mechanochemistry promotes active behavior of the cortex on long time-scales. Proc. Natl. Acad. Sci. USA. 2006;103:2103–2108. doi: 10.1073/pnas.0508819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Weber I, Neujahr R, Du A, Köhler J, Faix J, Gerisch G. Two-step positioning of a cleavage furrow by cortexillin and myosin II. Curr. Biol. 2000;10:501–506. doi: 10.1016/s0960-9822(00)00452-8. [DOI] [PubMed] [Google Scholar]
- 27.Merkel R, Simson R, Simson DA, Hohenadl M, Boulbitch A, Wallraff E, Sackmann E. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys. J. 2000;79:707–719. doi: 10.1016/S0006-3495(00)76329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pletjushkina OJ, Rajfur Z, Pomorski P, Oliver TN, Vasiliev JM, Jacobson K. Induction of cortical oscillations in spreading cells by depolymerization of microtubules. Cell Motil. Cytoskeleton. 2001;48:235–244. doi: 10.1002/cm.1012. [DOI] [PubMed] [Google Scholar]
- 30.Paluch E, Piel M, Prost J, Bornens M, Sykes C. Cortical actomyosin breakage triggers shape oscillations in cells and cell fragments. Biophys. J. 2005;89:724–733. doi: 10.1529/biophysj.105.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05
06
07