Inhibition of interferon γ induced interleukin 12 production: A potential mechanism for the anti-inflammatory activities of tumor necrosis factor (original) (raw)
Abstract
Inflammation is associated with production of cytokines and chemokines that recruit and activate inflammatory cells. Interleukin (IL) 12 produced by macrophages in response to various stimuli is a potent inducer of interferon (IFN) γ production. IFN-γ, in turn, markedly enhances IL-12 production. Although the immune response is typically self-limiting, the mechanisms involved are unclear. We demonstrate that IFN-γ inhibits production of chemokines (macrophage inflammatory proteins MIP-1α and MIP-1β). Furthermore, pre-exposure to tumor necrosis factor (TNF) inhibited IFN-γ priming for production of high levels of IL-12 by macrophages in vitro. Inhibition of IL-12 by TNF can be mediated by both IL-10-dependent and IL-10-independent mechanisms. To determine whether TNF inhibition of IFN-γ-induced IL-12 production contributed to the resolution of an inflammatory response in vivo, the response of TNF+/+ and TNF−/− mice injected with Corynebacterium parvum were compared. TNF−/− mice developed a delayed, but vigorous, inflammatory response leading to death, whereas TNF+/+ mice exhibited a prompt response that resolved. Serum IL-12 levels were elevated 3-fold in _C. parvum-_treated TNF−/− mice compared with TNF+/+ mice. Treatment with a neutralizing anti-IL-12 antibody led to resolution of the response to C. parvum in TNF−/− mice. We conclude that the role of TNF in limiting the extent and duration of inflammatory responses in vivo involves its capacity to regulate macrophage IL-12 production. IFN-γ inhibition of chemokine production and inhibition of IFN-γ-induced IL-12 production by TNF provide potential mechanisms by which these cytokines can exert anti-inflammatory/repair function(s).
Inflammation is normally a localized, protective response to tissue injury. The accumulation and activation of leukocytes at sites of inflammation occurs through a tightly regulated program involving cell adhesion receptors, chemoattractants, and proinflammatory cytokines. Cytokines such as tumor necrosis factor (TNF) and interleukin (IL) 1β are released early and alter blood flow, increase vascular permeability, augment leukocyte adhesion, promote migration into tissue space, and stimulate leukocytes to destroy inciting agents.
Components of the extracellular matrix (ECM), in combination with adhesion receptors, provide cells with the necessary traffic signals to migrate to an inflammatory site (1). The ECM can be modified by an evolving inflammatory process by binding and anchoring proinflammatory cytokines and chemokines and by being processed into biologically active products or fragments. Such modifications can confer proinflammatory activities on matrix components.
Infiltrating leukocytes produce cytokines that amplify the ongoing response. One such cytokine is IL-12, a potent proinflammatory cytokine produced mainly by phagocytic cells in response to bacteria and parasites or, as has been recently demonstrated, by low molecular weight forms of the ECM component hyaluronan (LMW-HA) (2, 3). IL-12 plays a critical role in bridging the innate and adaptive immune responses by inducing interferon (IFN) γ production by T and NK cells and thereby a TH1 type immune response (2). In turn, IFN-γ markedly augments IL-12 production, thus providing an important amplifying mechanism in inflammation (2).
The inflammatory response typically is self-limiting, but the regulatory mechanisms remain unclear. States of chronic inflammation, such as those seen in rheumatoid arthritis, involve the unremitting recruitment and activation of monocytes/macrophages, neutrophils, and T lymphocytes, resulting in excessive cytokine production and ECM turnover and tissue damage. Ultimately, this chronic inflammation can lead to scar tissue formation and end organ dysfunction. However, ECM components and proinflammatory cytokines, although required for an inflammatory response, under appropriate conditions also may play a negative regulatory role.
In this study we investigated the regulation of LMW-HA- and lipopolysaccharide (LPS)-induced chemokine/cytokine production by IFN-γ and TNF. We demonstrate that although IFN-γ enhanced LMW-HA-induced macrophage IL-12 production, it inhibited the production of macrophage inflammatory proteins MIP-1α and MIP-1β in response to LMW-HA, thereby having the potential to promote leukocyte activation at an inflammatory site while limiting further recruitment. Additionally, while concomitant treatment with TNF, IFN-γ, and LMW-HA, or LPS led to increased IL-12 production, pre-exposure to TNF markedly inhibited IFN-γ-enhanced IL-12 production. This activity was specific to TNF, was mediated through the p55 subunit of the TNF receptor (TNFR), and can occur by IL-10-dependent and IL-10-independent mechanisms. Further, TNF inhibits IL-12 production in part by inhibiting the accumulation of IL-12 p40 mRNA. To determine whether TNF inhibition of IL-12 plays a role in the recently reported homeostatic function of TNF in limiting the inflammatory process in vivo, we compared the response of TNF+/+ and TNF−/− mice to heat-killed Corynebacterium parvum. Our results provide further evidence that TNF and IFN-γ are not only critical for the initiation and progression of an inflammatory response, but also for the down-regulation and resolution of the response both in vitro and in vivo.
MATERIALS AND METHODS
Reagents.
Purified LMW-HA, fragments of HA from human umbilical cords, was purchased from ICN. The molecular mass of the HA preparation was approximately 2.5 × 105 Da (3). Polymyxin B sulfate (Sigma) was used at a concentration of 10 μg/ml in all stimulants except in conditions where LPS served as a positive control. LPS (Salmonella typhimurium) was purchased from List Biological Laboratories (Campbell, CA). Recombinant murine IFN-γ, TNF, IL-1β, anti-TNF neutralizing antibody (clone XT22), and antagonist antibodies to TNF p55 receptor (clone 55R-170) and p75 receptor (clone TR75–54) were purchased from Genzyme. Anti-IL-10 neutralizing antibody (JES5–2A5) was provided by John Abrams (DNAX Research Institute, Palo Alto, CA) (4). Brewer’s yeast thioglycollate broth (Difco) was prepared according to standard methods (5).
Mice and Cell Culture.
Seven- to 20-week-old female C3H/HeJ (National Cancer Institute, Bethesda MD), C3H/HeN (Taconic Farms), or C57/BL6 (Harlan–Sprague–Dawley) mice were housed under specific pathogen-free conditions. C57/BL6 × 129 TNF−/− mice were generated as described and housed under specific pathogen-free conditions (6). C57/BL6 IL-10−/− mice were originally from Donna Rennick (DNAX Research Institute) and were bred and maintained in the animal facility of the Veterinary School of the University of Pennsylvania (7). Thioglycollate-elicited peritoneal macrophages were harvested and purified as described (3) and cultured in RPMI medium 1640 supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, 50 μM β-ME, antibiotics, and antimycotics.
Cytokine Measurements.
IL-12 p40 and p70 production was measured by RIA using antibody pairs C17.15/C15.6 (p40) and C8.6/C17.15 (p70) as described (3). MIP-1α and MIP-1β production were quantitated by using a modification of a double-ligand method (3). Nitrite production was measured by using Griess reagents (8). IL-1β and IL-6 production were measured by using Quantikine Immunoassay (R & D Systems).
Assessment of Cell Viability and Recovery.
Cell viability was assessed by trypan blue exclusion and cell recovery by the tetrazolium bromide (MTT) reduction assay (9). Briefly, macrophage monolayers were washed with PBS and then incubated with MTT at a final concentration of 0.5 mg/ml in media for 45 min at 37°C. Medium was removed, and 100 μl of dimethyl sulfoxide was added to wells to solubilize formazan crystals. Optical densities were measured at 570 nm in a microplate reader.
Northern Analysis of Steady-State Levels of mRNA.
RNA was extracted from cells by using standard methods as described (10). Fifteen micrograms of total RNA was electrophoresed under denaturing conditions through a 1% formaldehyde-containing agarose gel and transferred and UV cross-linked to Nytran (Schleicher and Schuell) hybridization filters. Blots were hybridized overnight with 106 cpm/ml of 32P-labeled cDNA labeled by the random primed method (Amersham). Blots were washed once in 2× standard saline citrate (SSC)/0.1% SDS at room temperature for 30 min with shaking, then washed twice in 0.1× SSC/0.1% SDS at 50°C with shaking 20 min each wash. Blots were exposed at −70°C against Kodak XAR diagnostic film. RNA loading was normalized based on hybridization with 32P-labeled cDNA for aldolase. Densitometric scanning was performed by using a Molecular Dynamics Personal Densitometer SI.
In Vivo Response to C. parvum.
Mice were injected i.p. with 1 mg of heat-killed C. parvum (Burroughs Wellcome) and sacrificed at days 12, 26, and 35. Mice were retro-orbitally bled, and serum IL-12 p40 levels were measured by RIA. Spleen and liver sections were harvested, embedded in paraffin, and stained with hematoxylin/eosin. Indicated groups of animals were administered 1 mg of anti-IL-12 antibody (clone C17.8; a generous gift from G. Trinchieri, Wistar Institute, Philadelphia) or rat control immunoglobulin (Sigma) 6 days postinjection of C. parvum and weekly thereafter.
Statistical Analysis.
Statistical analyses of chemokine/cytokine production was performed by using a nonparametric, matched-pair analysis. Differences with a value of P < 0.05 were considered statistically significant.
RESULTS
IFN-γ Primes for Augmented IL-12 Production But Inhibits Chemokine Production by Thioglycollate-Elicited Macrophages.
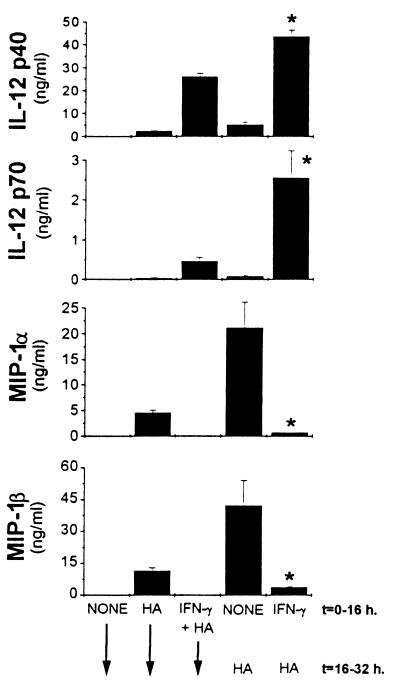
We recently demonstrated that LMW-HA induced the production of the chemokines RANTES, MIP-1α, and MIP-1β (3). In addition, we showed that LMW-HA induced production of IL-12 p40 and biologically active IL-12 p70 heterodimer (3). The levels were not sufficient, however, to detect the IL-12 p70 heterodimer by using a RIA (Fig. 1) (3). However, similar to previous studies of LPS-induced IL-12 production (11–14), pretreatment with IFN-γ for 16 hr primed for markedly enhanced LMW-HA induced IL-12 p40 and p70 production (Fig. 1). Kinetics experiments demonstrated that simultaneous addition of IFN-γ had no effect, but priming was evident within 4 hr and maximal with 16 hr pretreatment with IFN-γ (data not shown). In marked contrast, IFN-γ added simultaneously with, or up to 16 hr before, stimulation with LMW-HA nearly completely inhibited MIP-1α and MIP-1β production (Fig. 1).
Figure 1.
IFN-γ primes for enhanced IL-12 production, but inhibits MIP-1α and MIP-1β production. Thioglycollate-elicited peritoneal macrophages were incubated with 500 units/ml of IFN-γ simultaneously with or 16 hr before the addition of 100 μg/ml of LMW-HA, which was added for an additional 16 hr. Supernatants were harvested and analyzed for IL-12 p40 and IL-12 p70 production by RIA and for MIP-1α and MIP-1β production by ELISA. Data are expressed as the mean ± SD. Similar results were obtained in three independent experiments. ∗, P < 0.05 compared with cells stimulated with HA alone (t = 16–32 h).
TNF Inhibits IFN-γ-Primed LMW-HA-Induced IL-12 Production in Vitro.
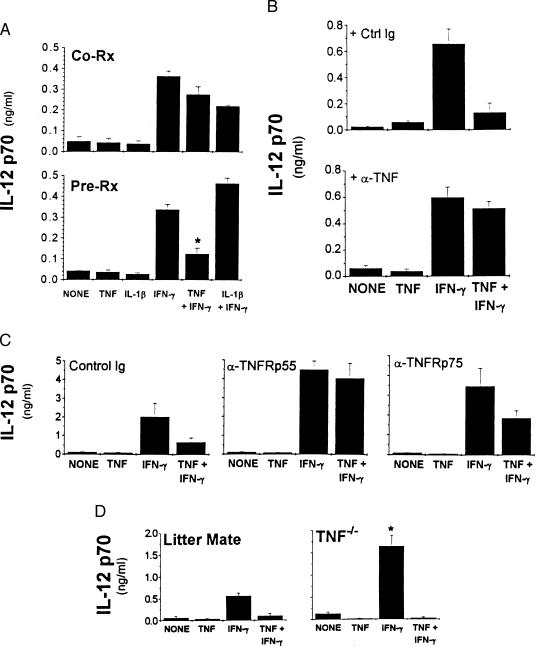
TNF has been demonstrated to synergize with IFN-γ to enhance macrophage antimicrobial, antiviral, and antiparasitic responses (15–19). Although TNF alone or given concomitantly with IFN-γ did not consistently affect LMW-HA-induced IL-12 production, we found that pretreatment with TNF markedly inhibited IFN-γ priming for enhanced IL-12 production (mean inhibition 75 ± 18%, n = 15; Fig. 2A, Lower). TNF, however, did not inhibit the lower levels of IL-12 produced in response to LMW-HA stimulation alone. Inhibition was not caused by cytotoxicity because there were no significant differences in cell viability or cell recovery after the various treatments. Pre-exposure to IL-1β did not consistently affect either the LMW-HA-induced response or priming by IFN-γ (Fig. 2A).
Figure 2.
(A) Pre-exposure to TNF inhibits IFN-γ priming for enhanced IL-12 production. Elicited macrophages were incubated with cytokines simultaneously with (Co-Rx, Upper), or 16 hr before (Pre-Rx, Lower), the addition of LMW-HA. Conditioned media was collected after 32 hr. ∗, P < 0.05 compared with IFN-γ-primed, HA-stimulated cultures. (B) Inhibition of IFN-γ priming is blocked by neutralizing anti-TNF antibody. Elicited macrophages were treated with cytokines in the presence of anti-TNF antibody (10 μg/ml) or control antibody (53.672; 10 μg/ml) for 16 hr before the addition of LMW-HA for an additional 16 hr. (C) Role of TNFR p55 and TNFR p75 in TNF-mediated inhibition of IFN-γ priming. Elicited macrophages were pretreated with antibodies specific for the p55 or the p75 subunit of the TNFR (10 μg/ml) or isotype-matched control antibody (2C11, 10 μg/ml) for 45 min at 4°C. Cells were washed and stimulants added. (D) Elicited macrophages were harvested from TNF−/− mice. Cells were treated with the indicated cytokines 16 hr before the addition of LMW-HA. ∗, P < 0.05 compared with IFN-γ-primed cultures. IL-12 p70 levels were measured by RIA.
Neutralizing anti-TNF antibody abrogated TNF inhibition of IFN-γ-augmented IL-12 production in response to LMW-HA in comparison to control rat Ig-treated cultures (Fig. 2B). Furthermore, blocking the p55 subunit of the TNFR completely abrogated TNF-mediated inhibition of IFN-γ-enhanced IL-12 production, whereas blocking the p75 subunit partially reversed the inhibition by TNF (46 ± 17%, n = 3) (Fig. 2C). IL-12 levels in cultures treated with anti-TNFRp55 antibodies were higher compared with control Ig-treated samples, suggesting that endogenous TNF down-regulates IL-12 production similar to the effect of exogenous TNF. This finding is consistent with our observation of increased IL-12 production by macrophages derived from TNFR- and TNF-deficient mice (J.H.-D. and E.P., unpublished observations and Fig. 2D).
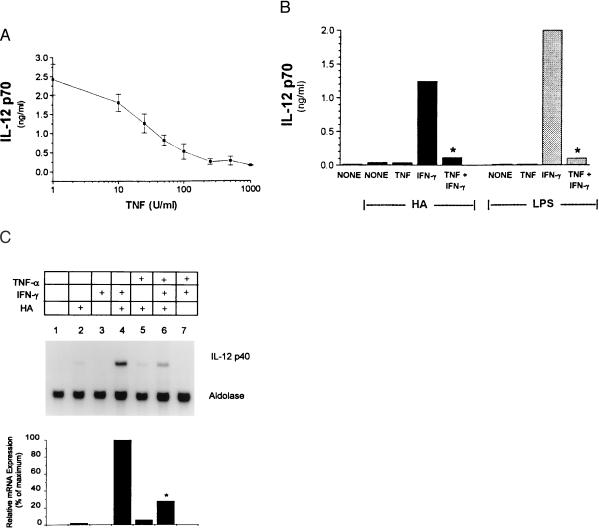
Inhibition of IFN-γ-primed IL-12 production by TNF was dose dependent with optimal inhibition observed between 250–500 units/ml of TNF (Fig. 3A). Kinetic experiments demonstrated that inhibition occurred when TNF was added simultaneously with, or up to 16 hr before, the addition of IFN-γ provided both cytokines were added before stimulation with LMW-HA. IFN-γ priming of enhanced IL-12 production in response to LPS was similarly abrogated by TNF (Fig. 3B).
Figure 3.
(A) TNF-mediated inhibition of IFN-γ-primed IL-12 production is dose dependent. Cells were treated with indicated doses of TNF and 500 units/ml IFN-γ 16 hr before addition of LMW-HA. IL-12 p70 production was measured by RIA. (B) Pre-exposure to TNF inhibits IFN-γ priming for enhanced IL-12 production to LPS and HA. Elicited macrophages were incubated with the indicated cytokines 16 hr before stimulation with LMW-HA (100 μg/ml) or LPS (1 μg/ml) for an additional 16 hr. IL-12 p70 levels were measured by RIA. ∗, P < 0.05 compared with IFN-γ-primed cultures. (C) TNF-mediated inhibition of IFN-γ-primed, HA-induced IL-12 p40 mRNA accumulation. Elicited macrophages were incubated with the indicated cytokines for 16 hr before the addition of LMW-HA for 8 hr. Total RNA was extracted and the levels of IL-12 p40 mRNA were detected by Northern blot. Aldolase controls are shown. ∗, P < 0.05 compared with lane 4.
To investigate the mechanism by which TNF inhibited IFN-γ-enhanced IL-12 production, we measured mRNA accumulation of the IL-12 p40 subunit. The transcript for the p35 subunit of IL-12 is constitutively expressed, whereas the p40 transcript is induced only in cells stimulated to produce functional p70 heterodimer (20, 21). LMW-HA induced modest p40 mRNA accumulation (Fig. 3C, lane 2), which was significantly augmented by IFN-γ priming (Fig. 3C, lane 4). Although TNF alone had no demonstrable effect on LMW-HA-induced IL-12 p40 mRNA (Fig. 3C, lane 5), pretreatment with TNF and IFN-γ together markedly reduced IFN-γ-induced IL-12 p40 mRNA accumulation (62 ± 12%, n = 3; Fig. 3C, lane 6).
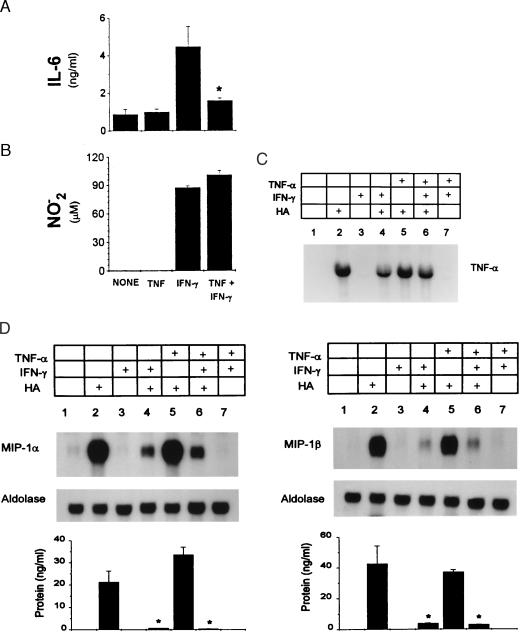
We investigated whether pre-exposure to TNF resulted in a generalized suppressive effect. As with IL-12, pre-exposure to TNF inhibited IFN-γ-primed IL-6 (52 ± 11.5%, n = 3) production in response to LMW-HA (Fig. 4A). In contrast, neither nitric oxide release as measured by the detection of its stable end-product nitrite nor the accumulation of TNF mRNA was inhibited under these same conditions (Fig. 4 B and C). TNF also did not reverse the IFN-γ inhibition of LMW-HA-induced MIP-1α or MIP-1β production or mRNA accumulation (Fig. 4D).
Figure 4.
Effect of TNF on other inflammatory mediators. Elicited macrophages were incubated with the indicated cytokines for 16 hr before addition of LMW-HA. IL-6 production was measured by Quantikine ELISA (A), and nitrite production was measured by reaction with Griess reagents (B); ∗, P < 0.05 compared with IFN-γ primed cultures. Elicited macrophages were incubated with the indicated cytokines for 16 hr before the addition of HA for 8 hr. Total cellular RNA was extracted, and TNF (C) and MIP-1α and MIP-1β (D) mRNA were detected by Northern blot. Protein levels for MIP-1α and MIP-1β were determined and are shown below. ∗, P < 0.05 compared with IFN-γ-primed cultures.
Role of IL-10 in TNF Inhibition of IFN-γ-Primed IL-12 Production.
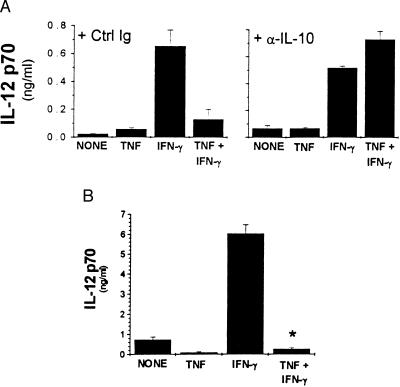
To test whether the inhibitory effect of TNF was caused by production of IL-10, macrophages were pre-exposed to TNF in the presence of neutralizing anti-IL-10 antibodies or control Igs. Neutralizing IL-10 reversed the TNF-mediated inhibition of IFN-γ-primed IL-12 production by wild-type macrophages when given simultaneously with TNF and IFN-γ 16 hr before the addition of LMW-HA (Fig. 5A), indicating that endogenous IL-10 can mediate the inhibitory effect of TNF on wild-type macrophages. Consistent with this finding, macrophages from IL-10-deficient mice produced higher levels of IL-12 in response to LMW-HA and IFN-γ, suggesting that production of endogenous IL-10 may dampen the production of IL-12 by wild-type macrophages (Fig. 5B and data not shown). However, pre-exposure to exogenous TNF resulted in a comparable percent inhibition of IFN-γ-induced IL-12 production in wild-type and IL-10-deficient macrophages, indicating that endogenous IL-10 was not required to mediate the inhibitory effect of TNF (Fig. 5B). Together, these data indicate that although the production of IL-10 is a major regulatory mechanism for controlling production of IL-12, there are also other mechanisms of TNF-mediated inhibition of IFN-γ priming that are evident in the absence of endogenous IL-10.
Figure 5.
Inhibition of IFN-γ priming by TNF is mediated by IL-10-dependent and IL-10-independent mechanisms. (A) Elicited macrophages were treated with cytokines in the presence of anti-IL-10 antibody (10 μg/ml) or control antibody (53.672, 10 μg/ml) for 16 hr before addition of LMW-HA for an additional 16 hr. (B) Elicited macrophages were harvested from IL-10−/− mice. Cells were treated with the indicated cytokines 16 hr before the addition of LMW-HA. ∗, P < 0.05 compared with IFN-γ-primed cultures. IL-12 p70 levels were measured by RIA.
TNF Limits the Production of IL-12 and Thereby Limits an Inflammatory Response in Vivo.
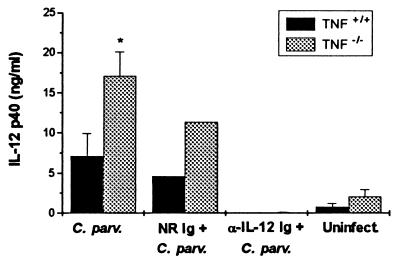
Recent evidence suggested that TNF has an essential role in limiting the inflammatory response to heat-killed C. parvum in mice (6). We found that C. parvum injection resulted in approximately 3-fold higher levels of serum IL-12 in TNF−/− mice compared with TNF+/+ mice at days 12, 26, and 35 postinjection (Fig. 6). Furthermore, although untreated and normal rat Ig-treated _C. parvum-_injected TNF−/− mice exhibited a delayed but vigorous inflammatory response leading to death, administration of neutralizing anti-IL-12 antibody weekly, starting 6 days postinjection of C. parvum, led to resolution of the granulomatous response to C. parvum in the livers and spleens of TNF−/− mice, similar to that observed in TNF+/+ mice injected with C. parvum alone (data not shown).
Figure 6.
_C. parvum-_treated TNF-deficient mice have exaggerated serum levels of IL-12. Littermate control (TNF+/+) and TNF−/− mice were injected i.p. with C. parvum and treated with control or neutralizing anti-IL-12 antibody weekly beginning 6 days postinjection with C. parvum. Serum levels of IL-12 p40 were measured by RIA.
DISCUSSION
The inflammatory response typically is self-limiting. Although resolution of an inflammatory response may be partly caused by elimination or dilution of the eliciting agent, evidence indicates that active regulatory mechanisms are also important. This study establishes that IFN-γ and TNF, both of which possess well-defined pro-inflammatory activities, also can play potentially important roles in the negative regulation of an inflammatory response. Thus, we demonstrate that IFN-γ inhibited the production of the chemokines MIP-1α and MIP-1β, which are important to the persistent recruitment of inflammatory cells required to sustain a chronic inflammatory response. Expression of two other chemokines (KC and MCP-1) also was inhibited by IFN-γ whereas RANTES was unaffected (22, 23). Furthermore, we demonstrate that TNF can inhibit IFN-γ priming for enhanced IL-12 production and thereby interrupt the amplification loop involving IFN-γ and IL-12. TNF did not reverse the inhibition of MIP-1α and MIP-1β production by IFN-γ. Therefore, TNF can inhibit the proinflammatory activities of IFN-γ without overriding the negative regulatory mechanism of IFN-γ herein described.
Our data are consistent with recent evidence for a role of TNF in vivo in limiting the magnitude and duration of the inflammatory response (6, 24–29). For example, in contrast to the prompt granulomatous response followed by resolution of the granuloma in _C. parvum_-injected TNF +/+ mice, similarly treated TNF−/− mice showed very little or no initial response, but then developed a vigorous, disorganized inflammatory response leading to death (6). In this study we establish that this response is associated with enhanced production of IL-12 and inhibited by administration of a neutralizing anti-IL-12 mAb. Others also reported that TNFRp55−/− mice infected with Leishmania developed larger lesions than control mice, and although the parasites were eliminated, failed to resolve the lesions (24). Our data indicate that one mechanism for the lack of resolution of the inflammatory response in these mutant mice is the failure to down-regulate IL-12 and thereby IFN-γ production, leading to excessive leukocyte recruitment and activation.
IL-12 is produced mainly by monocytes/macrophages as one of the first host responses to infection and induces IFN-γ production by T and NK cells (2, 20, 30). This early IFN-γ activates macrophages and initiates differentiation of TH1 type T lymphocytes. IL-12 also can down-regulate TH2 cell expansion, which may lead to exacerbation of diseases in which infections are characterized by a protective TH2 cytokine response (2). IFN-γ priming of enhanced IL-12 production is particularly significant because IL-12 itself is a potent inducer of IFN-γ production. Further, the priming effect of IFN-γ plays an important role in response to infectious agents with limited ability to induce IL-12 production on their own such as mycobacteria, where IFN-γ is required for optimal production of IL-12 in vitro and in vivo (31).
The importance of down-regulating IL-12 is reflected in the number of diseases characterized by excessive IL-12 production (2, 32). There are several mechanism(s) by which TNF may inhibit IFN-γ-enhanced IL-12 production. It is not likely that down-regulation of the IFN-γ receptor is responsible because TNF does not affect IFN-γ inhibition of MIP-1α and MIP-1 β production. Further, TNF was reported to increase IFN-γ receptor levels on monocytic cells (33). Similarly, expression of the primary receptor for LMW-HA, CD44, was not affected (J.H.-D. and E.P., unpublished observations). Northern blot analysis indicated, however, that TNF inhibits the accumulation of IL-12 p40 mRNA in response to IFN-γ and LMW-HA. Further studies are in progress to determine whether TNF inhibits IL-12 at the level of transcription.
The fact that exogenous IL-10 can inhibit LMW-HA-induced IL-12 production together with the data that neutralizing antibody to IL-10 abrogates TNF-mediated inhibition suggests that TNF may promote macrophage production of IL-10, which inhibits IFN-γ-primed IL-12 production. Although the finding that the percent inhibition of IL-12 production by TNF was comparable in macrophages derived from IL-10-deficient mice to control mice may appear to be a paradox, in fact, the absolute levels of IFN-γ-induced IL-12 produced by IL-10-deficient macrophages in the presence of TNF and the levels of IL-12 produced by wild-type macrophages in the presence of IL-10 neutralizing antibodies were the same (approximately 0.6 ng/ml). In other words, TNF inhibition does not reduce IL-12 production by IL-10-deficient macrophages down to the level of TNF-treated wild-type macrophages. Thus, IL-10 can play a role in TNF-mediated inhibition, but in the absence of IL-10 there is exaggerated IL-12 production and additional mechanisms by which TNF can inhibit IFN-γ-primed IL-12 production.
TNF did not reverse the IFN-γ inhibition of MIP-1α and MIP-1β production, suggesting that there may be two pathways (or a bifurcation of the pathway) of IFN-γ signaling, one path leading to increased IL-12 production that is sensitive to TNF and a second that inhibits MIP-1α and MIP-1β production that is resistant to negative regulation by TNF. IFN-γ binding to its receptor results in the rapid induction of tyrosine phosphorylation of the transcription factor STAT-1α mediated by JAK1 and JAK2 tyrosine kinases. Phosphorylated STAT-1α binds directly to the γ-activating sequence of a number of IFN-γ primary response genes (34). However, a number of IFN-γ responses, such as induction of major histocompatibility complex (MHC) class II (35), are considered “secondary” because expression after IFN-γ treatment is delayed and protein synthesis is required. Multiple signaling pathways are involved in induction of MHC class II, including activation of tyrosine kinases, protein kinase C, the Na+/H+ antiporter, and Ca2+/calmodulin. Further studies are needed to determine whether pre-exposure to TNF interrupts these IFN-γ signaling pathways and at what stage.
In conclusion, this study provides potential mechanisms for the putative anti-inflammatory activities of IFN-γ and TNF. Furthermore, these results indicate that the kinetics and balance of the multiple cytokines produced during the course of an inflammatory response are likely to determine its magnitude and duration.
Acknowledgments
We thank Drs. Lloyd J. Old, Aili L. Lazaar, and Giorgio Trinchieri for many helpful discussions and critical review of this manuscript. This work was supported by National Institutes of Health Grants HL50057 (to R.M.S), HL60539 (to P.W.N), and AI42334 (to C.A.H) and a grant from the Arthritis Foundation (to E.P.). J.H.D. is supported by National Institutes of Health Training Grant CA 09171.
ABBREVIATIONS
ECM
extracellular matrix
TNF
tumor necrosis factor
IL
interleukin
IFN
interferon
HA
hyaluronan
LMW-HA
low molecular weight hyaluronan
LPS
lipopolysaccharide
MIP
macrophage inflammatory protein
TNFR
tumor necrosis factor receptor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Gilat D, Cahalon L, Hershkoviz R, Lider O. Immunol Today. 1996;17:16–20. doi: 10.1016/0167-5699(96)80563-9. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 3.Hodge-Dufour J, Noble P W, Horton M R, Bao C, Wysocka M, Burdick M D, Strieter R M, Trinchieri G, Puré E. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- 4.Hunter C A, Subauste C S, van Cleave V H, Remington J S. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishell B B, Shiigi S M. Selected Methods in Cellular Immunology. San Francisco: Freeman; 1980. [Google Scholar]
- 6.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, et al. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neyer L E, Grunig G, Fort M, Remington J S, Rennick D M, Hunter C A. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green S J, Nacy C A. Curr Opin Infect Dis. 1993;6:384–396. [Google Scholar]
- 9.Miki I, Ishihara N, Otoshi M, Kase H. J Immunol Methods. 1998;164:255–261. doi: 10.1016/0022-1759(93)90318-2. [DOI] [PubMed] [Google Scholar]
- 10.Noble P W, Lake F R, Henson P M, Riches D W H. J Clin Invest. 1993;91:2368–2377. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Chow J M, Gri G, Carra G, Gerosa F, Wolf S F, Dzialo R, Trinchieri G. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skeen M J, Miller M A, Shinnick T M, Ziegler H K. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 13.Hayes M P, Wang J, Norcross M A. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 14.Chensue S W, Ruth J H, Warmington K, Lincoln P, Kunkel S L. J Immunol. 1995;155:3546–3551. [PubMed] [Google Scholar]
- 15.Wietzerbin J, Gaudelet C, Catinot L, Chebath J, Falcoff R. J Leukocyte Biol. 1990;48:149–155. doi: 10.1002/jlb.48.2.149. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Fernandez M A, Fernandez M A, Fresno M. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 17.Ding A H, Nathan C F, Stueher D J. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 18.Esparza I, Mannel D, Ruppel A, Falk W, Krammer P. J Exp Med. 1987;166:589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes T, Kaspar T, Coppenhaver D H. Antiviral Res. 1988;10:1–9. doi: 10.1016/0166-3542(88)90010-1. [DOI] [PubMed] [Google Scholar]
- 20.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, et al. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf S F, Sieburth D S, Perussia B, Yetz-Adalpe J, D’Andrea A. FASEB J. 1992;6:A1335. [Google Scholar]
- 22.Ohmori Y, Hamilton T. J Immunol. 1994;153:2204–2212. [PubMed] [Google Scholar]
- 23.Horton M R, Burdick M D, Strieter R M, Bao C, Noble P W. J Immunol. 1998;160:3023–3030. [PubMed] [Google Scholar]
- 24.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 25.Cope A P, Liblau R S, Yang X-D, Congia M, Laudanna C, Schreiber R D, Probert L, Kollias G, McDevitt H O. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo S, Wang B, Fujisawa H, Shivji G M, Echtenacher B, Mak T W, Sauder D N. J Immunol. 1995;155:3801–3805. [PubMed] [Google Scholar]
- 27.Bruce A J, Boling W, Kindy M S, Peschon J, Kraemer P J, Carpenter M K, Holtsberg F W, Mattson M P. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T, Edwards C K, Yang P, Wang Z, Bluethmann H, Mountz J D. J Immunol. 1996;156:2661–2665. [PubMed] [Google Scholar]
- 29.Elkon K B, Liu C C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Song X, Zhou J-L, Old L J, Crystal R G, Falck-Pedersen E. Proc Natl Acad Sci USA. 1997;97:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 31.Flesch I E A, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H E. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng S X, Elias J A. J Immunol. 1997;159:2161–2168. [PubMed] [Google Scholar]
- 33.Sanceau J, Merlin G, Wietzerbin J. J Immunol. 1997;149:1671–1675. [PubMed] [Google Scholar]
- 34.Bach E A, Aguet M, Schreiber R D. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 35.Glimcher L H, Kara C J. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]