Glycerol Metabolism and PrfA Activity in Listeria monocytogenes (original) (raw)
Abstract
Listeria monocytogenes is able to efficiently utilize glycerol as a carbon source. In a defined minimal medium, the growth rate (during balanced growth) in the presence of glycerol is similar to that in the presence of glucose or cellobiose. Comparative transcriptome analyses of L. monocytogenes showed high-level transcriptional upregulation of the genes known to be involved in glycerol uptake and metabolism (glpFK and glpD) in the presence of glycerol (compared to that in the presence of glucose and/or cellobiose). Levels of expression of the genes encoding a second putative glycerol uptake facilitator (GlpF2) and a second putative glycerol kinase (GlpK2) were less enhanced under these conditions. GlpK1 but not GlpK2 was essential for glycerol catabolism in L. monocytogenes under extracellular conditions, while the loss of GlpK1 affected replication in Caco-2 cells less than did the loss of GlpK2 and GlpD. Additional genes whose transcription levels were higher in the presence of glycerol than in the presence of glucose and cellobiose included those for two dihydroxyacetone (Dha) kinases and many genes that are under carbon catabolite repression control. Transcriptional downregulation in the presence of glycerol (compared to those in the presence glucose and cellobiose) was observed for several genes and operons that are positively regulated by glucose, including genes involved in glycolysis, N metabolism, and the biosynthesis of branched-chain amino acids. The highest level of transcriptional upregulation was observed for all PrfA-dependent genes during early and late logarithmic growth in glycerol. Under these conditions, a low level of HPr-Ser-P and a high level of HPr-His-P were present in the cells, suggesting that all enzyme IIA (EIIA) (or EIIB) components of the phosphotransferase system (PTS) permeases expressed will be phosphorylated. These and other data suggest that the phosphorylation state of PTS permeases correlates with PrfA activity.
Listeria monocytogenes is known as a facultative intracellular pathogen that can cause severe systemic infections in humans (for recent reviews, see references 15 and 47). This bacterial pathogen has therefore been extensively studied in the last decades preferentially with respect to its virulence genes and the encoded virulence factors. The virulence factors identified were shown to be involved mainly in the intracellular (cytosolic) growth cycle, and their genes were highly expressed under intracellular growth conditions (26). Most of the virulence genes are under the control of the transcription activator PrfA, whose expression is regulated at the transcriptional and the posttranscriptional levels (for recent reviews, see references 21 and 27). In addition, the activity of the PrfA protein is modulated by an as-yet-unknown factor(s) whose production appears to be linked to the metabolism of L. monocytogenes. A low level of PrfA activity was observed upon the growth of L. monocytogenes in a defined minimal medium (MM) in the presence of carbohydrates that are taken up by phosphoenolpyruvate (PEP):phosphotransferase systems (PTS), such as glucose, mannose, and, particularly, the β-glucosides cellobiose and arbutin (19, 34). The inhibitory effect on PrfA activity observed under these growth conditions is relieved by the addition of activated charcoal (40) or Amberlite Xad-4 (38) to the growing L. monocytogenes cultures, suggesting that a component(s) acting directly or indirectly as a negative effector of PrfA activity and produced during active growth may be absorbed by activated charcoal or Xad (16).
The PTS sugars used in these studies lead to carbon catabolite repression (CCR) in L. monocytogenes. It was therefore suggested that components of global CCR control might be involved in the modulation of the PrfA activity (34). In gram-positive bacteria (to which L. monocytogenes belongs), CCR control is mediated by the CcpA protein in complex with HPr-Ser-P (for recent reviews, see references 10, 13, and 25). In short, the phosphorylation of HPr (encoded by the ptsH gene) occurs in two different ways. The phosphate group is either transferred from PEP, catalyzed by enzyme I (EI) (encoded by the ptsI gene), to a histidine residue (His-15) of HPr or transferred from ATP catalyzed by the HPr kinase/phosphorylase (encoded by the hprK gene) to a serine residue (Ser-46). The latter enzyme is activated by metabolites of the glycolysis pathway, especially fructose-1,6-bisphosphate and PEP. HPr-His-P transfers the phosphate group further to EIIA components of all PTS and to dihydroxyacetone (Dha) catalyzed by Dha kinase(s). HPr-His-P is thus involved in the transport of all PTS carbohydrates and of C3 molecules, namely, glycerol and Dha. HPr-His-P also activates glycerol kinase (GlpK) by phosphorylation (12). HPr-Ser-P, on the other hand, becomes part of the active catabolite repressor complex (CcpA-HPr-Ser-P), which binds to the specific cre sites located in most cases downstream of the promoter sequence of CCR-controlled genes.
Insertion mutations in the ptsH and hprK genes of L. monocytogenes were shown to lead to a substantial activation of PrfA (33), while an insertion mutation in ccpA did not activate PrfA (4). Thus, CcpA does not seem to affect PrfA activity, but also, the second key player in CCR control, HPr-Ser-P, does not seem to be directly involved in the modulation of PrfA activity (4, 33).
In addition to various PTS sugars, L. monocytogenes can also utilize glycerol as a carbon source when cultured in a defined MM (37). The transcription of genes involved in glycerol catabolism was shown to be SigB dependent in L. monocytogenes (1). Our recent studies showed that PrfA activity is high throughout growth in the presence of this non-PTS carbon source (33). These data suggested that components of the specific PTS permeases or those controlling their function may participate in the modulation of PrfA activity.
We therefore decided to study the metabolism of glycerol in L. monocytogenes and its effect on PrfA activity in more detail. For this purpose, we compared the levels of gene expression of L. monocytogenes cells grown in a glycerol-containing MM to those in glucose- or cellobiose-containing media.
The results show that L. monocytogenes possesses a rather complex set of genes for the metabolism of glycerol and other C3 metabolites. The glycerol metabolism leads to a high level of activation of PrfA. The data also show that PrfA activity correlates with the phosphorylation state of the PTS permeases.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains used in this study are listed in Table 1. Escherichia coli strains were cultivated in Luria-Bertani (LB) medium at 37°C. L. monocytogenes EGD-e and mutant strains were grown under aerobic conditions in brain heart infusion (BHI) broth (Difco) or in chemically defined MM (37) supplemented with different sugars at 37°C. When necessary, media were supplemented with erythromycin (Sigma, St. Louis, MO) to final concentrations of 300 μg/ml for E. coli or 5 μg/ml for L. monocytogenes. Fresh stock solutions of carbohydrates (glucose, cellobiose, and glycerol) were filter sterilized and added to the culture medium at a final concentration of 50 mM. To determine growth curves, aliquots were removed at regular intervals, and the optical density at 600 nm (OD600) was determined using a spectrophotometer. All growth experiments were performed at least four times independently, and one representative growth curve is shown. For shift experiments, cultures of the strains grown overnight were diluted in fresh BHI broth, allowed to grow to an OD600 of 0.5, and washed once in sterile phosphate-buffered saline (PBS); the pellet was resuspended in MM containing the appropriate carbon source; and growth was subsequently monitored at 37°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| E. coli DH5α | deoR endA1 gyrA96 hsdR17(rK− mK+) recA1 relA1 supE44 λ thi-1 Δ(lacZYA-argF)U169 | 22 |
| L. monocytogenes | ||
| EGD-e | Wild type, derivative of EGD | G. B. Mackaness |
| EGD-e::hprK (lmo2483) | Inactivation of HPrK by insertion of pLSV101 in lmo2483 | 33 |
| EGD-e::ptsH (lmo1002) | Inactivation of HPr by insertion of pLSV101 in lmo1002 | 33 |
| EGD-eΔlmo1167 (Δ_glpF_2) | In-frame deletion of lmo1167 (glycerol uptake facilitator) | This study |
| EGD-eΔlmo1539 (Δ_glpF_1) | In-frame deletion of lmo1539 (glycerol uptake facilitator) | This study |
| EGD-eΔlmo1034 (Δ_glpK_2) | In-frame deletion of lmo1034 (glycerol kinase) | This study |
| EGD-eΔlmo1538 (Δ_glpK_1) | In-frame deletion of lmo1538 (glycerol kinase) | This study |
| EGD-eΔlmo1538-39 (Δ_glpFK_1) | In-frame deletion of lmo1538 (glycerol kinase) and lmo1539 (glycerol uptake facilitator) | This study |
| EGD-eΔlmo1293 (Δ_glpD_) | In-frame deletion of lmo1293 (glycerol-3-P dehydrogenase) | This study |
| EGD-eΔlmo1293 (Δ_glpD_)-C | EGD-eΔlmo1293 (Δ_glpD_) complemented with glpD | This study |
| EGD-eΔlmo1538 (Δ_glpK_1)-C | EGD-eΔlmo1538 (Δ_glpK_1) complemented with _glpK_1 | This study |
General techniques.
PCR amplifications, cloning procedures, isolation of chromosomal DNA, and DNA manipulations were carried out according to standard procedures (41). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed according to standard protocols (28). L. monocytogenes HPr was detected by Western blotting using _Listeria_-specific rabbit polyclonal anti-HPr antibody (1:3,000) (33). The phosphorylation status of HPr (HPr-Ser46/His15-P) in L. monocytogenes was determined as described previously by Mertins et al. (33). Cycle sequencing was conducted using the CEQ Dye Terminator Cycle Sequencing Quick Start kit (Beckman Coulter, Fullerton, CA), and sequencing reactions were run using a XL2000 Beckman Coulter sequencer. In vitro transcription assays were performed as described previously by Luo et al. (30). Data reported on the Listeria homepage of the Institut Pasteur (http://www.genolist.pasteur.fr/ListiList/) were used for sequence comparisons. All oligonucleotides used in this study were synthesized by Sigma Genosys (Steinheim, Germany) and are listed in Table S1 in the supplemental material.
Construction of deletion mutants.
In-frame deletions of _glpF_1 (lmo1539), _glpF_2 (lmo1167), _glpK_1 (lmo1538), and _glpK_2 (lmo1034) were constructed in this study by using L. monocytogenes EGD-e as the parental strain as described previously (26, 51). glpD (lmo1293) was deleted in L. monocytogenes EGD-e using a deletion vector described previously (26).
Construction of complementation mutants.
Complementation mutants of Δ_glpD_ and Δ1538 (Δ_glpK_1) were constructed by homologous recombination using mutagenesis vector pLSV101 (51). To construct the respective plasmids, the coding region along with up- and downstream regions of the gene (around 300 bp) were amplified using the appropriate oligonucleotide pairs, called c-glpD-1/c-glpD-2 and c-glpK1-1/c-glpK1-2 (sequences of the oligonucleotides are listed in Table S1 in the supplemental material). The purified PCR products were digested with the corresponding restriction endonucleases and cloned via the restriction sites into pLSV101 to yield the complementation plasmids. These plasmids were transformed into L. monocytogenes EGD-e by electroporation, and erythromycin-resistant bacteria growing at 42°C due to the presence of a chromosomally integrated plasmid were selected. The integration mutants were subcultured at 30°C over several days, and erythromycin-sensitive clones were screened by PCR to identify a mutant in which the second recombination step has occurred, resulting in the complementation of the gene. Correct in-frame complementation mutants were confirmed by sequencing.
Cell culture and infection experiments.
Human colon epithelial cells (Caco-2; ACC 169) and mouse monocytes-macrophages (J774A.1; ACC 170) from the DSMZ were cultured at 37°C and 5% CO2 in RPMI 1640 medium supplemented with 2 mM l-glutamine (Gibco, Eggenstein, Germany) and 10% heat-inactivated fetal calf serum (Biochrom KG, Berlin, Germany). Cells were seeded into 24-well plates 1 day prior to infection. After a washing step, the cells were infected at a multiplicity of infection (MOI) of 10 bacteria per cell for 1 h (Caco-2 cells) or an MOI of 1 for 45 min (J774 cells). The cells were washed three times (time zero [_t_0]) and incubated with medium containing 100 μg/ml gentamicin, which was replaced with medium containing 10 μg/ml gentamicin after 1 h (_t_1). Cells were lysed at various time points (_t_1, _t_3, _t_5, and _t_7) using cold distilled water, and viable bacterial counts of intracellular bacteria were determined by plating serial dilutions onto BHI agar.
RNA isolation.
L. monocytogenes EGD-e was grown in MM with the respective carbon sources (glucose, cellobiose, or glycerol), and RNA was isolated from the cells at two different growth phases, namely, early log phase (corresponding to an OD600 of 0.5) and late log phase (corresponding to an OD600 of 1.0), as described previously by Marr et al. (31).
Microarray hybridization and data analysis.
Transcriptome analyses were performed using whole-genome DNA microarrays as described previously by Marr et al. (31). A total of four independently isolated RNA samples from each condition at each growth phase were used for the analysis. RNA from two isolations were pooled and hybridized onto two microarray slides with dye swapping. Another two microarray slides were hybridized using the same principle. In total, we used four RNAs and four microarray slides to generate 16 replicate expression values for each combination except for the comparison between glucose and cellobiose, phase B, where data generated from three microarray slides were used for further analysis. cDNA labeling and hybridization were performed as previously described (33). The slides were scanned using ScanArray HT and analyzed using Scan-Array express software (Perkin-Elmer, Boston, MA). Spots were flagged and eliminated from the analysis when the signal-to-noise ratio was less than 3 or in obvious instances of high background or stray fluorescent signals. The Lowess method of normalization (52) was performed on the background-corrected median intensity of the spots. The normalized ratios were analyzed further with Microsoft Excel (Microsoft, Redmond, WA) and SAM (significance analysis of microarrays) software for statistical significance (46). As described previously (33), genes whose expression values were >1.8 or <0.55 were considered to be differentially regulated. The data discussed in this work are listed in Tables 2 to 4, and the complete list of the differentially regulated genes is available in Table S2 in the supplemental material.
TABLE 2.
Genes upregulated in glycerol compared to glucose and cellobiose at early log phase (phase A) and late log phase (phase B) in MMc
| Gene | Function | Fold induction | |||
|---|---|---|---|---|---|
| YG-A | YG-B | YC-A | YC-B | ||
| qoxA | AA3-600 quinol oxidase subunit II | 2.1 | 3.9 | ||
| qoxBb | AA3-600 quinol oxidase subunit I | 3.1 | |||
| qoxC | AA3-600 quinol oxidase subunit III | 2.6 | |||
| qoxD | Highly similar to quinol oxidase AA3-600 chain IV | 2.2 | |||
| lmo0021 | Similar to PTS; fructose-specific IIA component | 2.7 | 3.5 | 3.7 | |
| lmo0022 | Similar to PTS; fructose-specific IIB component | 4.8 | |||
| lmo0023 | Similar to PTS; fructose-specific IIC component | 4.6 | |||
| lmo0024 | Similar to PTS; mannose-specific IID component | 5.4 | |||
| lmo0039 | Similar to carbamate kinase | 2.5 | 1.9 | ||
| lmo0043 | Similar to arginine deiminase | 2.9 | 3.6 | 3.2 | |
| lmo0084 | Similar to oxidoreductases | 1.8 | |||
| lmo0098b | Similar to PTS; mannose specific, factor IID | 1.9 | |||
| lmo0105b | Highly similar to chitinase B | 5.3 | 6.9 | ||
| lmo0130b | Similar to 5-nucleotidase; putative peptidoglycan-bound protein (LPXTG motif) | 5.2 | 9.2 | 14.7 | |
| lmo0135 | Similar to oligopeptide ABC transport system substrate-binding proteins | 1.8 | |||
| lmo0153 | Similar to a probable high-affinity zinc ABC transporter [Zn(II)-binding lipoprotein] | 2 | 2.8 | ||
| lmo0154 | Similar to high-affinity zinc ABC transporter (ATP-binding protein) | 2.2 | |||
| lmo0155 | Similar to high-affinity zinc ABC transporter (membrane protein) | 2.3 | |||
| lmo0169 | Similar to a glucose uptake protein | 2.2 | 2.1 | 3.1 | 2.8 |
| lmo0180 | Similar to sugar ABC transporter; permease protein | 2.5 | |||
| lmo0181 | Similar to sugar ABC transporter; sugar-binding protein | 2.5 | 3.3 | ||
| lmo0182a | Similar to alpha-xylosidase and alpha-glucosidase | 2.2 | |||
| lmo0183 | Similar to alpha-glucosidase | 2.3 | |||
| lmo0184 | Similar to oligo-1,6-glucosidase | 1.9 | |||
| prfAb,d | Listeriolysin-positive regulatory protein | 3.9 | 31.1 | 78.1 | |
| plcAb,d | Phosphatidylinositol-specific phospholipase C | 5.1 | 2 | 30.5 | 80.6 |
| hlyb,d | Listeriolysin O precursor | 3.3 | 37.4 | 45.5 | |
| mplb,d | Zinc metalloproteinase precursor | 2.5 | 13.9 | 177.5 | |
| actAb,d | Actin assembly-inducing protein precursor | 4.3 | 40 | 106.7 | |
| plcBb,d | Phospholipase C | 4.3 | 54 | 165.5 | |
| lmo0206 | Unknown | 4.7 | 36.9 | 168.8 | |
| lmo0207 | Hypothetical lipoprotein | 4.1 | 26.3 | 85.8 | |
| lmo0231 | Similar to arginine kinase | 1.8 | |||
| lmo0261 | Similar to phospho-beta-glucosidase | 2.2 | 2.2 | 2.4 | |
| lmo0265 | Similar to succinyldiaminopimelate desuccinylase | 4.4 | 3.8 | 7.1 | 4.8 |
| lmo0278 | Similar to sugar ABC transporter; ATP-binding protein | 3 | |||
| lmo0298 | Similar to PTS beta-glucoside-specific enzyme IIC component | 2.5 | 3.3 | ||
| lmo0299a | Similar to PTS beta-glucoside-specific enzyme IIB component | 2.5 | 2.5 | 5.8 | |
| lmo0300 | Similar to phospho-beta-glucosidase and phospho-beta-galactosidase | 2.5 | 2.9 | ||
| lmo0342 | Similar to transketolase | 29.5 | 2.5 | 29.8 | |
| lmo0343 | Similar to transaldolase | 2.7 | 118.1 | 5.1 | 178.1 |
| lmo0344 | Similar to dehydrogenase/reductase | 69.6 | 4.7 | 113.6 | |
| lmo0345 | Similar to sugar-phosphate isomerase | 2.8 | 81.7 | 3 | 105.7 |
| lmo0346 | Similar to triosephosphate isomerase | 180.8 | 6.8 | ||
| lmo0347 | Similar to dihydroxyacetone kinase | 2.8 | 40 | 4.7 | 85.9 |
| lmo0348 | Similar to dihydroxyacetone kinase | 3.1 | 39 | 4.1 | 120.8 |
| lmo0358 | Similar to PTS; fructose-specific enzyme IIBC component | 2.1 | |||
| lmo0384a,b | Similar to B. subtilis IolB protein | 4.6 | 5.4 | ||
| lmo0385 | Similar to B. subtilis IolC protein and to fructokinase | 2.7 | 3 | ||
| lmo0386b | Similar to B. subtilis IolD protein and to acetolactate synthase | 3.4 | 4.4 | ||
| lmo0400 | Similar to fructose-specific phosphotransferase enzyme IIC | 2.4 | 2.5 | ||
| lmo0405 | Similar to phosphate transport protein | 1.9 | 2.1 | 1.9 | |
| lmo0415 | Similar to endo-1,4-beta-xylanase | 2.0 | |||
| lmo0426a,b | Similar to PTS fructose-specific enzyme IIA component | 2.4 | |||
| lmo0427a,b | Similar to PTS fructose-specific enzyme IIB component | 1.8 | 3 | ||
| lmo0428a,b | Similar to PTS fructose-specific enzyme IIC component | 2.8 | 3.4 | 3.3 | 6.4 |
| lmo0429a,b | Similar to sugar hydrolase | 2.7 | 4.5 | 6.9 | |
| lmo0431 | Similar to acetyltransferase | 3.6 | |||
| inlAb,d | Internalin A | 4.3 | 2.2 | 22.2 | 16.4 |
| inlBb,d | Internalin B | 4 | 18.6 | 17.2 | |
| lmo0456 | Similar to permeases | 4.6 | |||
| lmo0458 | Similar to hydantoinase | 2.0 | |||
| lmo0498 | Similar to ribose 5-phosphate isomerase | 7.6 | 8.8 | ||
| lmo0498 | Similar to ribose 5-phosphate isomerase | 7.6 | |||
| lmo0499 | Similar to ribulose-5-phosphate 3 epimerase | 9 | |||
| lmo0500 | Similar to transaldolase | 2.2 | 2.2 | 2.7 | |
| lmo0502a,b | Similar to putative sugar-phosphate isomerase | 9.8 | 19.3 | ||
| lmo0503 | Similar to PTS fructose-specific enzyme IIA component | 9.6 | 22.4 | 9.3 | |
| lmo0505 | Similar to ribulose-5-phosphate 3-epimerase | 9 | 9.5 | ||
| lmo0506 | Similar to polyol (sorbitol) dehydrogenase | 6.5 | 10.3 | 38.4 | |
| lmo0507a,b | Similar to PTS; galactitol-specific IIB component | 11.4 | 25.8 | ||
| lmo0508 | Similar to PTS; galactitol-specific IIC component | 3.5 | 5.5 | 2.8 | |
| lmo0521a | Similar to 6-phospho-beta-glucosidase | 2.2 | 2.4 | 3.3 | |
| lmo0524 | Similar to putative sulfate transporter | 2.3 | 2.3 | ||
| lmo0536b | Similar to 6-phospho-beta-glucosidase | 2.6 | 2.1 | ||
| lmo0539 | Similar to tagatose-1,6-diphosphate aldolase | 4.2 | 3.5 | 6.9 | 5.5 |
| lmo0546 | Similar to putative NAD(P)-dependent oxidoreductase | 1.9 | |||
| lmo0554 | Similar to NADH-dependent butanol dehydrogenase | 7 | 5.3 | 10.2 | 8.5 |
| lmo0555 | Similar to ditripeptide transporter | 2 | 2 | 2.7 | 2.9 |
| lmo0560 | Similar to NADP-specific glutamate dehydrogenase | 3.9 | |||
| lmo0610b | Similar to internalin proteins; putative peptidoglycan-bound protein (LPXTG motif) | 5 | 5.5 | 8 | 5.9 |
| lmo0632b | Similar to PTS; fructose-specific IIC component | 2.2 | |||
| lmo0640a,b | Similar to oxidoreductase | 2.6 | 2.7 | 2.4 | 3.9 |
| lmo0643a,b | Similar to putative transaldolase | 3.4 | 5.1 | ||
| lmo0650 | Conserved membrane protein | 2.5 | 2.2 | 2.2 | 2.7 |
| lmo0669 | Similar to oxidoreductase | 3.5 | 2.8 | 7.3 | 5.0 |
| lmo0722b | Similar to pyruvate oxidase | 3.5 | 3.8 | 5 | 5.1 |
| lmo0727 | Similar to l-glutamine-d-fructose-6-phosphate amidotransferase | 2.5 | 2.6 | ||
| lmo0769 | Similar to alpha-1,6-mannanase | 3 | |||
| lmo0781 | Similar to mannose-specific PTS component IID | 1.9 | 2.4 | 3.3 | 2.7 |
| lmo0782b | Similar to mannose-specific PTS component IIC | 2.8 | 3.3 | 5.6 | 5.0 |
| lmo0783 | Similar to mannose-specific PTS component IIB | 3.7 | 3.5 | 6.1 | 4.8 |
| lmo0784b | Similar to mannose-specific PTS component IIA | 4.3 | 3 | 5.1 | 4.6 |
| lmo0810 | Similar to spermidine/putrescine-binding protein | 2.2 | |||
| lmo0813 | Similar to fructokinases | 2.4 | |||
| uhpTb,d | Highly similar to hexose phosphate transport protein | 5.7 | 4.9 | 10.5 | 93.7 |
| lmo0859 | Similar to putative sugar ABC transporter; periplasmic sugar-binding protein | 1.9 | 1.9 | ||
| lmo0860 | Similar to sugar ABC transporter; permease protein | 2.7 | |||
| lmo0861 | Similar to sugar ABC transporter; permease protein | 2.5 | |||
| lmo0862 | Similar to oligo-1,6-glucosidase | 3 | |||
| lmo0865 | Similar to phosphomannomutase | 3 | 3.7 | ||
| lmo0875 | Similar to PTS; beta-glucoside enzyme IIB component | 2.2 | |||
| lmo0876 | Similar to PTS; lichenan-specific enzyme IIC component | 1.9 | |||
| lmo0877 | Similar to B. subtilis NagB protein (glucosamine-6-phosphate isomerase) | 1.9 | |||
| lmo0878 | Similar to oxidoreductases | 1.9 | |||
| lmo0880 | Similar to succinate semialdehyde dehydrogenase | 3.2 | 2.8 | 2.7 | |
| lmo0913b | Similar to succinate semialdehyde dehydrogenase | 3.7 | 3 | 5.3 | 4.7 |
| lmo0914 | Similar to PTS; IIB component | 2.2 | 3.2 | ||
| lmo0915b | Similar to PTS EIIC | 2 | 3 | 2.7 | |
| lmo0916 | Similar to PTS EIIA | 6.2 | |||
| lmo0917 | Similar to beta-glucosidase | 4.8 | |||
| lmo0956 | Similar to _N_-acetylglucosamine-6P-phosphate deacetylase (EC 3.5.1.25) | 2.3 | 2.7 | 3.2 | 4.2 |
| lmo0957 | Similar to glucosamine-6-P isomerase (EC 5.3.1.10) | 2.4 | 2 | 3.9 | |
| lmo0979 | Similar to daunorubicin resistance ATP-binding proteins | 2.4 | |||
| lmo1034 | Similar to glycerol kinase | 2 | 2 | ||
| lmo1057 | Similar to l-lactate dehydrogenase | 2 | 2.9 | ||
| lmo1097 | Similar to integrases | 3.3 | 5.1 | ||
| lmo1099 | Similar to a protein encoded by Tn_916_ | 2.5 | |||
| cadA | Cadmium resistance protein | 2.4 | 4.9 | ||
| lmo1103 | Highly similar to Tn_916_ ORF13 | 2 | 2.1 | ||
| lmo1142 | Similar to Salmonella enterica PduS protein | 1.9 | 2.2 | 8.8 | |
| lmo1143 | Similar to Salmonella enterica PduT protein | 1.9 | 2.8 | ||
| lmo1151 | Similar to Salmonella enterica serovar Typhimurium PduA protein | 9.2 | |||
| lmo1152 | Similar to S. enterica serovar Typhimurium PduB protein | 14.3 | 1.9 | 10.7 | |
| lmo1154 | Similar to diol dehydrase (diol dehydratase) gamma subunit | 17.4 | |||
| lmo1155 | Similar to diol dehydrase (diol dehydratase) gamma subunit (pddC) | 14.1 | |||
| lmo1157 | Similar to diol dehydratase-reactivating factor small chain | 2 | 15.5 | ||
| lmo1159 | Similar to carboxysome structural protein | 27.2 | |||
| lmo1160 | Similar to Salmonella enterica PduL protein | 22.2 | 20.8 | ||
| lmo1161 | Similar to ethanolamine utilization protein EutJ | 1.8 | 17.9 | ||
| lmo1164 | Highly similar to Salmonella enterica PduO protein | 13.5 | 12.1 | ||
| lmo1165 | Similar to ethanolamine utilization protein EutE | 11.3 | |||
| lmo1166b | Similar to NADPH-dependent butanol dehydrogenase | 6.4 | |||
| glpF | Similar to glycerol uptake facilitator protein | 13.3 | 15.3 | ||
| ackA2 | Similar to acetate kinase | 3.6 | |||
| lmo1180 | Similar to putative carboxysome structural protein | 2.5 | |||
| lmo1205 | Similar to putative cobalt transport protein CbiN | 6.1 | |||
| lmo1207 | Similar to cobalt transport ATP-binding protein CbiO | 8.7 | |||
| glpDa,b | Similar to glycerol-3-phosphate dehydrogenase | 29.6 | 24.5 | 46.5 | 77.7 |
| glnA | Highly similar to glutamine synthetases | 2.3 | 1.9 | ||
| lmo1349a,b | Similar to glycine dehydrogenase (decarboxylating) subunit 1 | 2.4 | 2 | 3.6 | |
| lmo1350a,b | Similar to glycine dehydrogenase (decarboxylating) subunit 2 | 2 | 2 | 2.6 | |
| lmo1375 | Similar to aminotripeptidase | 1.8 | 2.1 | ||
| lmo1389 | Similar to sugar ABC transporter, ATP-binding protein | 1.8 | |||
| lmo1390 | Similar to ABC transporter (permease proteins) | 2 | |||
| lmo1391 | Similar to sugar ABC transporter, permease protein | 1.9 | |||
| pflBb | Pyruvate formate-lyase | 2 | 2.7 | 3.0 | |
| pflC | Pyruvate formate-lyase-activating enzyme | 2 | 2.2 | ||
| lmo1421 | Similar to glycine betaine/carnitine/choline ABC transporter (ATP-binding protein) | 1.9 | |||
| opuCD | Similar to betaine/carnitine/choline ABC transporter (membrane protein) | 1.8 | 2.6 | 2.1 | |
| opuCC | Similar to glycine betaine/carnitine/choline ABC transporter (osmoprotectant-binding protein) | 2.3 | 1.9 | ||
| opuCB | Similar to glycine betaine/carnitine/choline ABC transporter (membrane protein) | 2.8 | |||
| opuCA | Similar to glycine betaine/carnitine/choline ABC transporter (ATP-binding protein) | 2 | 2.3 | ||
| zurA | Metal (zinc) transport protein(ABC transporter, ATP-binding protein) | 2.3 | |||
| glyQ | Similar to glycyl-tRNA synthetase alpha chain | 2 | |||
| lmo1538a,b | Similar to glycerol kinase | 13.5 | 17.8 | 26.3 | 51.1 |
| lmo1539 | Similar to glycerol uptake facilitator | 17.1 | 21.6 | 43.8 | 57.1 |
| thrS | Threonyl-tRNA synthetase | 1.9 | 3.0 | ||
| lmo1579 | Similar to alanine dehydrogenase | 1.9 | |||
| argJ | Highly similar to ornithine acetyltransferase and amino-acid acetyltransferases | 2.1 | |||
| argC | Similar to _N_-acetylglutamate gamma-semialdehyde dehydrogenases | 2.7 | |||
| trpA | Highly similar to tryptophan synthase (alpha subunit) | 2.2 | |||
| trpB | Highly similar to tryptophan synthase (beta subunit) | 2 | |||
| trpF | Phosphoribosyl anthranilate isomerase | 2.6 | |||
| trpC | Highly similar to indol-3-glycerol phosphate synthases | 2.7 | |||
| trpD | Highly similar to anthranilate phosphoribosyltransferase | 2.8 | |||
| trpG | Highly similar to anthranilate synthase beta subunit | 2.3 | |||
| trpE | Highly similar to anthranilate synthase alpha subunit | 1.9 | |||
| lmo1671 | Similar to ABC transporter and adhesion proteins | 1.8 | |||
| inlCb | Internalin C | 5.5 | 3.9 | 16.3 | |
| pyrE | Highly similar to orotate phosphoribosyltransferases | 3.5 | 2.1 | ||
| pyrF | Highly similar to orotidine 5 -phosphate decarboxylases | 4.4 | 2.2 | ||
| pyrD | Highly similar to dihydroorotase dehydrogenase | 4 | 2.1 | ||
| pyrDII | Highly similar to dihydroorotate dehydrogenase (electron transfer subunit) | 2.4 | |||
| pyrAB | Highly similar to carbamoyl-phosphate synthetase (catalytic subunit) | 2.8 | 2.2 | ||
| pyrAa | Highly similar to carbamoyl-phosphate synthetase (glutaminase subunit) | 2 | 2.8 | ||
| pyrC | Highly similar to dihydroorotase | 4.5 | |||
| lmo1867 | Similar to pyruvate phosphate dikinase | 4.2 | 8.6 | 7.1 | 10.0 |
| lmo1883a,b | Similar to chitinases | 2.3 | 4.1 | 2.8 | 6.6 |
| pflAa,b | Similar to pyruvate formate-lyase | 1.8 | |||
| pnpb | Similar to purine-nucleoside phosphorylase | 2.2 | 2.1 | ||
| drm | Similar to phosphopentomutase | 2.5 | 2.7 | 3.5 | 4.1 |
| fhuG | Similar to ferrichrome ABC transporter (permease) | 2.1 | |||
| lmo1972 | Similar to pentitol PTS; EIIB component | 2 | 1.9 | 2.3 | |
| lmo1992 | Similar to alpha-acetolactate decarboxylase | 2.5 | |||
| lmo1997 | Similar to PTS mannose-specific enzyme IIA component | 2.2 | |||
| lmo1998 | Similar to opine catabolism protein | 3.1 | 5.8 | ||
| lmo1999b | weakly similar to glucosamine-fructose-6-phosphate aminotransferase | 2.6 | 3.4 | 3.2 | |
| lmo2000 | Similar to PTS mannose-specific EIID component | 4.8 | 7.6 | ||
| lmo2001a,b | Similar to PTS mannose-specific EIIC component | 3.4 | 6.2 | 5.3 | |
| lmo2002 | Similar to PTS mannose-specific EIIB component | 3.2 | 4 | 2.3 | |
| alsS | Similar to alpha-acetolactate synthase protein (AlsS) | 2.3 | 2.0 | ||
| lmo2007 | Weakly similar to putative sugar-binding lipoproteins | 1.8 | |||
| lmo2008 | Similar to putative ABC transporter; permease protein | 2.1 | 2.6 | ||
| lmo2015 | Similar to alpha-mannosidase | 1.9 | |||
| ileS | Isoleucyl-tRNA synthetase | 2.4 | |||
| lmo2067 | Similar to conjugated bile acid hydrolase | 6.0 | 4.8 | 4.8 | 9.0 |
| lmo2085b | Putative peptidoglycan-bound protein (LPXTG motif) | 3.8 | 4.7 | 6.3 | 9.0 |
| lmo2098 | Similar to PTS; galactitol-specific EIA component | 3.3 | 3.2 | ||
| lmo2108 | Similar to _N_-acetylglucosamine-6-phosphate deacetylase | 2.1 | |||
| lmo2109 | Similar to hydrolase | 1.8 | 2.5 | 2.5 | |
| lmo2115 | Similar to ABC transporter (permease) | 3.2 | |||
| lmo2121a,b | Similar to maltosephosphorylase | 3.6 | |||
| lmo2122 | Similar to maltodextrose utilization protein MalA | 2.2 | 4 | ||
| lmo2123 | Similar to maltodextrin ABC transport system (permease) | 2.7 | 4.7 | ||
| lmo2124 | Similar to maltodextrin ABC transport system (permease) | 2 | 3.2 | ||
| lmo2125b | Similar to maltose/maltodextrin ABC transporter (binding protein) | 2.7 | 4.5 | ||
| lmo2134 | Similar to fructose-1,6-biphosphate aldolase type II | 2.5 | |||
| lmo2135 | Similar to PTS; fructose-specific EIIC component | 2.9 | |||
| lmo2136 | Similar to PTS; fructose-specific EIIB component | 3.2 | |||
| lmo2143 | Weakly similar to mannose-6-phosphate isomerase | 2.3 | |||
| lmo2159b | Similar to oxidoreductase | 2.2 | 2.6 | 3.1 | |
| lmo2175 | Similar to dehydrogenase | 3.9 | 6.3 | ||
| fruA | Highly similar to PTS fructose-specific EIIABC component | 2.2 | 2.0 | ||
| lmo2341 | Similar to carbohydrate kinases | 2.2 | 3.2 | 2.6 | |
| lmo2389 | Similar to NADH dehydrogenase | 1.8 | |||
| lmo2434 | Highly similar to glutamate decarboxylases | 3 | 2.5 | 4.2 | 2.7 |
| lmo2463 | Similar to transport protein | 2.1 | 2.5 | ||
| lmo2469 | Similar to amino acid transporter | 2.0 | |||
| lmo2569 | Similar to dipeptide ABC transporter (dipeptide-binding protein) | 2 | 4.2 | ||
| lmo2573b | Similar to zinc-binding dehydrogenase | 4.9 | 6.4 | 9.2 | 9.0 |
| lmo2580 | Similar to ABC transporter; ATP-binding protein | 1.8 | 5.1 | ||
| lmo2584a,b | Similar to formate dehydrogenase-associated protein | 7.6 | 8.4 | ||
| lmo2586a,b | Similar to formate dehydrogenase alpha chain | 13.9 | 28 | 39.8 | 40.5 |
| lmo2592 | Similar to oxidoreductase; aldo/keto reductase family | 2.0 | |||
| lmo2650b | Similar to hypothetical PTS enzyme IIB component | 2 | |||
| lmo2651a,b | Similar to mannitol-specific PTS EIIA component | 2.1 | |||
| lmo2659a | Similar to ribulose-phosphate 3-epimerase | 2.6 | 2.9 | ||
| lmo2660a | Similar to transketolase | 2.2 | |||
| lmo2663a | Similar to polyol dehydrogenase | 2.3 | 3 | 7.2 | |
| lmo2664a | Similar to sorbitol dehydrogenase | 3.5 | 7.2 | 4.9 | 10.9 |
| lmo2665a | Similar to PTS; galactitol-specific EIIC component | 4.4 | 6.4 | 7.4 | 8.2 |
| lmo2666a,b | Similar to PTS; galactitol-specific EIIB component | 5.2 | 5 | 7.6 | 8.2 |
| lmo2667a | Similar to PTS; galactitol-specific EIIA component | 5.9 | 4.6 | 8.8 | 6.5 |
| lmo2674 | Similar to ribose 5-phosphate epimerase | 2.9 | 2.6 | 3.3 | 4.5 |
| kdpB | Potassium-transporting ATPase B chain | 4.4 | |||
| lmo2683 | Similar to cellobiose phosphotransferase EIIB component | 2.3 | 2.2 | ||
| lmo2684 | Similar to cellobiose phosphotransferase EIIC component | 6.3 | 3.1 | ||
| lmo2685b | Similar to cellobiose phosphotransferase EIIA component | 7.4 | 5.6 | ||
| lmo2689 | Highly similar to Mg2+ transport ATPase | 2.4 | 3.1 | ||
| lmo2695b | Similar to dihydroxyacetone kinase | 4.7 | 4.9 | 9.3 | 9.8 |
| lmo2696b | Similar to hypothetical dihydroxyacetone kinase | 3.7 | 4.3 | 6.7 | 8.6 |
| lmo2708 | Similar to PTS; cellobiose-specific EIIC | 18.5 | 11.1 | 11.0 | |
| lmo2733 | Similar to PTS; fructose-specific IIABC component | 3.3 | |||
| lmo2735b | Similar to sucrose phosphorylase | 2.1 | |||
| lmo2743 | Similar to transaldolase | 3.3 | 3 | 4.7 | 4.0 |
| lmo2760 | Similar to ABC transporter (ATP-binding protein) | 3.4 | 3.1 | ||
| lmo2764a,b | Similar to xylose operon regulatory protein and to glucose kinase | 2.2 | 1.9 | ||
| lmo2772a,b | Similar to beta-glucoside-specific EIIABC | 1.9 | |||
| bvrBa,b | Beta-glucoside-specific phosphotransferase EIIABC component | 1.8 | |||
| lmo2797b | Similar to PTS mannitol-specific EIIA | 3.5 | 5.6 | ||
| lmo2798b | Similar to phosphatase | 3.1 | 6.4 | ||
| lmo2799a,b | Similar to PTS mannitol-specific EIIBC | 3.9 | 2.7 | 11.5 | |
| lmo2800 | Similar to dehydrogenase | 2.9 | 4 | ||
| lmo2848 | Highly similar to l-rhamnose isomerase | 1.9 | |||
| lmo2849 | Similar to rhamnulokinase | 2.2 | 2.3 | ||
| lmo2850 | Similar to sugar transport proteins | 2.1 |
TABLE 4.
Genes differentially regulated in glucose compared to cellobiose at early log phase (phase A) and late log phase (phase B) in MMa
| Gene | Function | Fold induction | |
|---|---|---|---|
| GC-A | GC-B | ||
| lmo0018 | Beta-glucosidase | 0.2 | |
| lmo0096b | Similar to PTS; mannose-specific, factor IIAB | 15.8 | 10.3 |
| lmo0097 | Similar to PTS; mannose-specific, factor IIC | 13.4 | 9.5 |
| lmo0098b | Similar to PTS; mannose-specific, factor IID | 12.6 | 8.6 |
| prfAc | Listeriolysin positive regulatory protein | 5.8 | 46.2 |
| plcAb,c | Phosphatidylinositol-specific phospholipase c | 9.2 | 48.6 |
| hlyb,c | Listeriolysin O precursor | 10.4 | 46.1 |
| mplc | Zinc metalloproteinase precursor | 4.5 | 47.3 |
| actAc | Actin assembly-inducing protein precursor | 15.2 | 64.8 |
| plcBb,c | Phospholipase C | 12.6 | 117 |
| lmo0271 | Highly similar to phospho-beta-glucosidase | 0.3 | |
| inlAb,c | Internalin A | 4.5 | 7.7 |
| inlBb,c | Internalin B | 3.2 | 5.9 |
| lmo0560 | Similar to NADP-specific glutamate dehydrogenase | 2.1 | |
| uhpTb,c | Highly similar to hexose phosphate transport protein | 61.8 | |
| lmo0914 | Similar to PTS, IIB component | 4.1 | |
| pheS | Phenylalanyl-tRNA synthetase alpha subunit | 0.3 | |
| pheT | Phenylalanyl-tRNA synthetase beta subunit | 0.4 | |
| tcsA | CD4+ T-cell-stimulating antigen; lipoprotein | 0.5 | |
| zurA | Metal (zinc) transport protein(ABC transporter, ATP-binding protein) | 1.9 | |
| valS | Valyl-tRNA synthetase | 3.4 | |
| aroA | 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase | 0.5 | |
| lmo1625 | Similar to putative transporters | 0.4 | |
| trpA | Highly similar to tryptophan synthase (alpha subunit) | 2.9 | 0.3 |
| trpB | Highly similar to tryptophan synthase (beta subunit) | 4.7 | 0.3 |
| trpF | Phosphoribosyl anthranilate isomerase | 7 | 0.4 |
| trpC | Highly similar to indol-3-glycerol phosphate synthases | 7.8 | 0.4 |
| trpD | Highly similar to anthranilate phosphoribosyltransferase | 8.1 | 0.4 |
| lmo1719 | Similar to PTS lichenan-specific enzyme IIA component | 0.4 | |
| lmo1734 | Similar to glutamate synthase (large subunit) | 2 | |
| inlCc | Internalin C | 55.5 | |
| ilvB | Similar to acetolactate synthase (acetohydroxy acid synthase) (large subunit) | 2 | |
| ilvN | Similar to acetolactate synthase (acetohydroxy acid synthase) (small subunit) | 2 | |
| ilvCb | Similar to ketol acid reductoisomerase (acetohydroxy acid isomeroreductase) | 1.9 | |
| ilvA | Similar to threonine dehydratase | 1.9 | |
| lmo2114 | Similar to ABC transporter (ATP-binding protein) | 7.2 | |
| lmo2115 | Similar to ABC transporter (permease) | 7 | |
| arpJ | Similar to amino acid ABC transporter; permease protein | 0.5 | |
| lmo2390 | Similar to hypothetical thioredoxin reductase | 1.9 | |
| lmo2469 | Similar to amino acid transporter | 2.3 | |
| glyA | Highly similar to glycine hydroxymethyltransferase | 0.4 | |
| lmo2580 | Similar to ABC transporter; ATP-binding protein | 2.3 | |
| lmo2650b | Similar to hypothetical PTS EIIB component | 3.4 | |
| lmo2651 | Similar to mannitol-specific PTS EIIA component | 2.7 | |
| lmo2684 | Similar to cellobiose phosphotransferase EIIC component | 0.1 | 0.3 |
| lmo2685 | Similar to cellobiose phosphotransferase EIIA component | 0.1 | 0.2 |
| cydD | Highly similar to ABC transporter (ATP-binding protein) required for expression of cytochrome bd | 0.5 | |
| cydC | Highly similar to ABC transporter required for expression of cytochrome bd | 0.5 | |
| serS | Seryl-tRNA synthetase | 0.4 |
Real-time RT-PCR.
Real-time reverse transcriptase PCR (RT-PCR) was conducted as described previously (26), with total RNA isolated independently from that used for transcriptome analysis experiments.
Determination of hemolytic activity.
Culture supernatants of wild-type L. monocytogenes and the glycerol metabolism mutants were assayed for hemolytic activity as described previously (40). The strains, the wild type and the Δ_glpK_1 and Δ_glpD_ mutants, were grown in BHI broth to an OD600 of 0.5 and washed once in PBS, and the pellets were resuspended in MM with glucose or glycerol. After 2 h of incubation in this medium at 37°C, 50 μl of the culture supernatant was incubated in 1 ml of a 4% sheep erythrocyte suspension for 30 min at 37°C. After incubation, the tubes were centrifuged at 2,500 rpm for 5 min at room temperature. The hemolytic activity was determined by the released hemoglobin measured using the OD543.
Microarray data accession number.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE11459.
RESULTS
Growth of L. monocytogenes in the presence of glycerol compared to that in the presence of glucose and cellobiose.
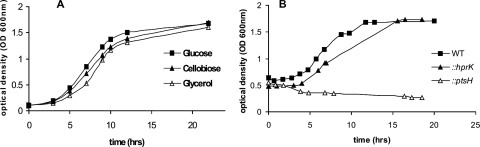
L. monocytogenes EGD-e can grow in a defined MM with glycerol as a carbon source (33, 37). The growth rate in this medium was only slightly lower than that observed in the same medium with either of the two PTS sugars glucose and cellobiose (Fig. 1A). As shown in Fig. 1B, growth in glycerol-containing medium still occurred with an hprK mutant (deficient in HPr kinase/phosphorylase) but not with a ptsH mutant (deficient in HPr production) (33), suggesting that the activity of glycerol kinase initiating glycerol catabolism depends on HPr-His-P-mediated phosphorylation, similar to what has been described for the glycerol kinase (GlpK) of Bacillus subtilis. Indeed, the listerial GlpK1 (encoded by lmo1538) contains a histidyl residue (His-231) equivalent to His-230 of GlpK of B. subtilis and other low-G+C gram-positive bacteria, which acts as a phosphorylation site (11).
FIG. 1.
(A) Growth of wild-type L. monocytogenes EGD-e in MM supplemented with 50 mM glucose (filled squares), cellobiose (filled triangles), and glycerol (open triangles). The time points during exponential growth, where L. monocytogenes EGD-e cells were harvested for RNA isolation, are indicated (OD600 of 0.5 [phase A] and OD600 of 1.0 [phase B]). (B) Shift from BHI at an OD600 of 0.5 to glycerol-containing MM. Shown are data for the growth of wild-type L. monocytogenes EGD-e (WT) (filled squares) and insertion mutants (hprK [filled triangles] and ptsH [open triangles]).
Comparison of the L. monocytogenes transcript profiles upon growth in the presence of glycerol, glucose, and cellobiose as carbon sources.
For a better understanding of the entire metabolism of L. monocytogenes during growth in the presence of glycerol, we carried out comparative transcriptome analyses using transcripts from L. monocytogenes cultured in MM with glucose, cellobiose, or glycerol. L. monocytogenes cells were harvested at an early time point (OD600 of 0.5 [∼5 × 108 bacteria/ml]) (phase A) and a later time point (OD600 of 1.0 [∼109 bacteria/ml]) (phase B) during exponential growth. Equal amounts of RNA from the different combinations, namely, glycerol (phase A)/glucose (phase A), glucose (phase A)/cellobiose (phase A), glycerol (phase A)/cellobiose (phase A), glycerol (phase B)/glucose (phase B), glucose (phase B)/cellobiose (phase B), and glycerol (phase B)/cellobiose (phase B), were hybridized to whole-genome microarrays as described previously (26).
In the following section, we concentrate on the major results of these analyses. The complete list of differentially regulated genes under the various conditions can be found in Table S2 in the supplemental material. All PrfA-regulated genes, including prfA itself, showed high levels of upregulation when phase A transcripts from L. monocytogenes grown in glycerol-containing medium were compared to those from L. monocytogenes grown in glucose-containing medium (Table 2). The upregulation of these genes was much lower (at most, twofold) in the comparative profiles with phase B transcripts.
In contrast, phase A as well as phase B transcripts of PrfA-dependent genes of glycerol-grown L. monocytogenes cultures showed very high levels of upregulation (almost 200-fold) compared to those of cellobiose-grown L. monocytogenes cultures (Table 2), indicating that PrfA activity is high throughout the growth phase when L. monocytogenes grows in the presence of glycerol and low in the presence of cellobiose. In the presence of glucose, PrfA activity is low during early (balanced) growth phases (phase A) but is considerably enhanced in phase B, when bacterial growth may no longer be balanced, probably due to reduced glucose uptake (33).
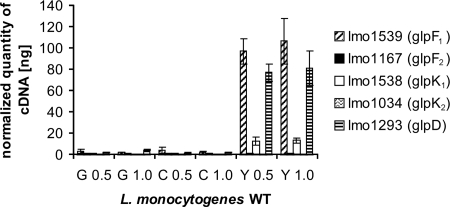
In addition to the upregulated expression of the PrfA- dependent genes, the expression of many other genes was found to be upregulated in glycerol-containing medium compared to that in glucose- and cellobiose-containing media. In particular, these genes included genes involved in glycerol uptake and metabolism: two genes encoding putative glycerol uptake facilitators, GlpF1 and GlpF2 (encoded by lmo1539 and lmo1167, respectively); two genes for putative glycerol kinases, GlpK1 and GlpK2 (encoded by lmo1538 and lmo1034, respectively); and one gene for putative glycerol-3-P dehydrogenase (glpD [lmo1293]). While lmo1293, lmo1538, and lmo1539 (which show high levels of homology to glpD and glpFK of B. subtilis, respectively) are highly upregulated in phases A and B in the presence of glycerol, lmo1034 (specific for L. monocytogenes) shows a twofold upregulation in phase A, and lmo1167 is upregulated in phase B only. The upregulation of these genes was confirmed by RT-PCR (Fig. 2). These data indicate that lmo1167 and lmo1034 are poorly expressed compared to lmo1538 and lmo1539, suggesting that the two latter genes are involved mainly in the metabolism of glycerol under extracellular growth conditions.
FIG. 2.
Transcriptional analysis with real-time RT-PCR to study the expression of genes involved in glycerol metabolism (glycerol uptake facilitators _glpF_1 and _glpF_2, glycerol kinases _glpK_1 and _glpK_2, and glycerol-3-P dehydrogenase glpD). L. monocytogenes EGD-e was grown in MM supplemented with 50 mM glucose (G), cellobiose (C), and glycerol (Y) to an OD600 of 0.5 or 1.0. The relative expression levels of the genes studied were normalized to the housekeeping gene rpoB as described elsewhere previously (35, 43). RT-PCR was performed with three independently isolated RNAs from L. monocytogenes EGD-e grown in the different media and at different time points in duplicate. Error bars indicate the standard deviations from the means. WT, wild type.
In addition to the genes involved directly in glycerol metabolism, the induced expression of the genes encoding two Dha kinases (DhaK1, encoded by lmo0347 and lmo0348, and DhaK2, encoded by lmo2695 and lmo2696) was observed. Both Dha kinases belong to category C of the DhaK family (3). The expression of these enzymes is differentially controlled during growth. While the genes (lmo2695 and lmo2696) encoding DhaK2 are upregulated in phase A and B, the upregulation of the genes (lmo0347 lmo0348) encoding DhaK1 is seen only in phase B. The _dhaK_1 genes are part of an extended operon, and the entire operon is highly upregulated in phase B but not in phase A. This operon (lmo0341 to lmo0351) encodes, among other proteins, a putative transketolase, a transaldolase, a dehydrogenase, a sugar-phosphate isomerase, and a triosephosphate isomerase, enzymes that may also be involved in C3 metabolism (Table 2 and see Table S2 in the supplemental material).
Of interest in this context is also the upregulation of genes encoding enzymes involved in pyruvate metabolism, like pyruvate oxidase (lmo0722), pyruvate formate lyase (pflB [lmo1406]), pyruvate phosphate dikinase (lmo1867), acetolactate synthetase (alsS [lmo2006]), and acetolactate decarboxylase (lmo1992).
Other major upregulated genes include genes for several PTS specific for mannose (lmo0781 to lmo0784 and lmo2000 to lmo2002), cellobiose (lmo2683 to lmo2685 and lmo2708), fructose (lmo0426 to lmo0428), and galactitol (lmo2665 to lmo2667); the gene for a conjugated bile acid hydrolase (lmo2067); genes for proteins with an LPXTG motif (lmo0130, lmo0610, and lmo2085); and several genes with unknown functions. There are several genes that are specifically upregulated in glycerol compared to glucose but not to cellobiose and vice versa (Table 2 and see Table S2 in the supplemental material).
Among the genes downregulated in the presence of glycerol compared to glucose and cellobiose (Table 3) are the genes for a mannose-specific (lmo0096 to lmo0098) PTS (in the presence of glucose); several operons encoding ABC transporters for metal cations, amino acids, and oligopeptides; and, in particular, genes known to be controlled by the cellular glucose level (5), like lmo1298 and lmo1299 (glnR and glnA, respectively [glutamine synthetase]), lmo1424, lmo1516 and lmo1517 (NrgA and PII, respectively), lmo1827, and the _ilv_-leu operon. There is also a downregulation of all genes (especially in phase B) involved in glycolysis (eno, pgm, tpi, pgk, and gap). The complete list of the downregulated genes is given in Table S2 in the supplemental material.
TABLE 3.
Genes downregulated in glycerol compared to glucose and cellobiose at early log phase (phase A) and late log phase (phase B) in MMc
| Gene | Function | Fold induction | |||
|---|---|---|---|---|---|
| YG-A | YG-B | YC-A | YC-B | ||
| lmo0018 | Beta-glucosidase | 0.3 | 0.3 | ||
| lmo0050 | Similar to sensor histidine kinase (AgrC from Staphylococcus) | 0.4 | |||
| purA | Similar to adenylosuccinate synthetase | 0.3 | 0.3 | ||
| lmo0096 | Similar to PTS; mannose-specific, factor IIAB | 0.1 | 0.1 | ||
| lmo0097 | Similar to PTS; mannose-specific, factor IIC | 0.1 | 0.1 | ||
| lmo0098 | Similar to PTS; mannose-specific, factor IID | 0.1 | 0.1 | ||
| lmo0135 | Similar to oligopeptide ABC transport system substrate-binding proteins | 0.5 | |||
| lmo0152 | Similar to oligopeptide ABC transporter-binding protein | 0.4 | 0.6 | ||
| lmo0176 | Similar to glucose uptake protein | 0.4 | 0.4 | 0.4 | |
| lmo0218 | Polyribonucleotide nucleotidyltransferase domain present | 0.5 | 0.5 | 0.4 | |
| lmo0219 | Fusion protein; N-terminal part similar to B. subtilis YacA protein; C-terminal part similar to hypoxanthine-guanine phosphoribosyltransferase | 0.5 | 0.4 | ||
| cysE | Similar to serine _O_-acetyltransferase | 0.4 | 0.5 | ||
| lmo0269 | Similar to transporter | 0.4 | |||
| lmo0271 | Similar to phospho-beta-glucosidase | 0.4 | 0.3 | ||
| lmo0279 | Similar to anaerobic ribonucleoside-triphosphate reductase | 0.5 | 0.4 | ||
| lmo0280 | Similar to anaerobic ribonucleotide reductase activator protein | 0.3 | 0.5 | ||
| lmo0286 | Similar to aminotransferase | 0.5 | 0.4 | ||
| lmo0519b | Similar to multidrug resistance protein | 0.5 | |||
| lmo0537 | Similar to _N_-carbamyl-l-amino acid amidohydrolase | 0.5 | |||
| lmo0560b | Similar to NADP-specific glutamate dehydrogenase | 0.5 | 0.5 | ||
| hisD | Similar to histidinol dehydrogenases | 0.5 | |||
| hisZ | Histidyl-tRNA synthetase | 0.5 | 0.5 | ||
| lmo0611 | Similar to acyl-carrier protein phosphodiesterase and NAD(P)H dehydrogenase | 0.5 | 0.5 | ||
| lmo0645 | Similar to amino acid transporter | 0.5 | 0.5 | ||
| lmo0787 | Similar to amino acid transporter | 0.5 | |||
| lmo0798 | Similar to lysine-specific permease | 0.3 | |||
| lmo0802 | Weakly similar to GTP-pyrophosphokinase | 0.5 | 0.4 | ||
| lmo0837 | Similar to ABC transporter (ATP-binding protein) | 0.5 | |||
| lmo0841 | Similar to cation (calcium) transporting ATPase | 0.5 | 0.4 | ||
| lmo0847 | Similar to glutamine ABC transporter (binding and transport protein) | 0.5 | |||
| lmo0897 | Similar to transport proteins | 0.4 | |||
| lmo0912a | Similar to transporters (formate) | 0.5 | |||
| lmo0945 | Similar to C-terminal part of B. subtilis ComEC protein and to ComEA | 0.5 | |||
| lmo0947 | Hypothetical transport protein | 0.5 | |||
| lmo0960 | Similar to proteases | 0.4 | 0.4 | 0.5 | |
| lmo0981 | Similar to efflux transporter | 0.4 | |||
| gbuA | Similar to glycine betaine ABC transporter (ATP-binding protein) | 0.4 | 0.5 | ||
| gbuB | Similar to glycine betaine ABC transporters (permease) | 0.5 | 0.4 | 0.4 | |
| gbuCa,b | Similar to glycine betaine ABC transporters (glycine betaine-binding protein) | 0.5 | 0.4 | ||
| lmo1017 | Similar to phosphotransferase system glucose-specific enzyme IIA | 0.5 | |||
| lmo1073 | Similar to metal binding protein (ABC transporter) | 0.5 | |||
| pheS | Phenylalany-tRNA synthetase alpha subunit | 0.2 | |||
| pheT | Phenylalanyl-tRNA synthetase beta subunit | 0.3 | |||
| proA | Gamma-glutamyl phosphate reductase | 0.5 | |||
| proB | Gamma-glutamyl kinase | 0.6 | |||
| glnR | Similar to glutamine synthetase repressor | 0.1 | 0.1 | ||
| glnA | Similar to glutamine synthetases | 0.1 | |||
| lmo1300 | Similar to arsenic efflux pump protein | 0.4 | |||
| smbA | Similar to uridylate kinases | 0.4 | 0.5 | ||
| lmo1424 | Similar to manganese transport proteins NRAMP | 0.3 | 0.3 | 0.3 | 0.4 |
| lmo1431 | Similar to ABC transporter (ATP-binding protein) | 0.5 | |||
| zurM | Metal (zinc) transport protein (ABC transporter, permease protein) | 0.5 | |||
| udk | Similar to uridine kinase | 0.5 | 0.5 | ||
| lmo1516b | Similar to ammonium transporter NrgA | 0.02 | 0.03 | ||
| lmo1517b | Similar to nitrogen regulatory PII protein | 0.02 | 0.03 | ||
| relA | Similar to (p)ppGpp synthetase | 0.5 | |||
| valS | Valyl-tRNA synthetase | 0.5 | |||
| tyrS | Tyrosyl-tRNA synthetase | 0.5 | 0.4 | ||
| aroA | 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase | 0.4 | 0.2 | 0.1 | |
| lmo1603 | Similar to aminopeptidase | 0.5 | |||
| lmo1617 | Similar to multidrug-efflux transporter | 0.5 | 0.4 | ||
| daaA | d-Amino acid aminotransferase | 0.5 | |||
| lmo1624 | Similar to putative transporters | 0.2 | |||
| lmo1625 | Similar to putative transporters | 0.3 | 0.1 | ||
| trpA | Similar to tryptophan synthase (alpha subunit) | 0.2 | 0.1 | ||
| trpB | Similar to tryptophan synthase (beta subunit) | 0.2 | 0.1 | ||
| trpF | Phosphoribosyl anthranilate isomerase | 0.3 | 0.2 | ||
| trpC | Similar to indol-3-glycerol phosphate synthases | 0.3 | 0.1 | ||
| trpD | Similar to anthranilate phosphoribosyltransferase | 0.4 | 0.2 | ||
| trpG | Similar to anthranilate synthase beta subunit | 0.4 | 0.3 | ||
| trpE | Similar to anthranilate synthase alpha subunit | 0.5 | 0.4 | ||
| lmo1634 | Similar to alcohol-acetaldehyde dehydrogenase | 0.2 | 0.3 | 0.5 | 0.1 |
| ansB | Similar to asparaginyl-tRNA synthetases | 0.5 | 0.4 | ||
| metK | Similar to _S_-methionine adenosyltransferase | 0.5 | |||
| lmo1682 | Similar to transmembrane transport proteins | 0.4 | |||
| lmo1705 | Similar to deoxyguanosine kinase/deoxyadenosine kinase(I) subunit | 0.5 | |||
| lmo1719 | Similar to PTS lichenan-specific EIIA component | 0.5 | |||
| lmo1720 | Similar to PTS lichenan-specific EIIB component | 0.4 | |||
| lmo1730 | Similar to sugar ABC transporter-binding protein | 0.3 | |||
| lmo1739 | Similar to amino acid (glutamine) ABC transporter (ATP-binding protein) | 0.6 | |||
| lmo1749 | Similar to shikimate kinase | 0.3 | 0.3 | ||
| purD | Phosphoribosylglycinamide synthetase | 0.5 | 0.5 | ||
| purH | Bifunctional phosphoribosylaminoimidazole carboxy formyl formyltransferase and inosine-monophosphate cyclohydrolase | 0.5 | 0.5 | ||
| purN | Similar to phosphoribosylglycinamide formyltransferases | 0.5 | 0.4 | ||
| purM | Phosphoribosylaminoimidazole synthetase | 0.5 | |||
| purF | Glutamine phosphoribosylpyrophosphate amidotransferase | 0.4 | |||
| purQ | Phosphoribosylformylglycinamidine synthetase I | 0.4 | 0.4 | ||
| purQ | Phosphoribosylformylglycinamidine synthetase I | 0.4 | 0.5 | 0.4 | |
| purL | Similar to phosphoribosylformylglycinamidine synthetase II | 0.4 | 0.4 | 0.2 | 0.4 |
| purC | Phosphoribosylaminoimidazole succinocarboxamide synthetase | 0.5 | 0.5 | 0.2 | 0.4 |
| purB | Adenylosuccinate lyase | 0.3 | 0.5 | 0.2 | |
| purK | Phosphoribosylaminoimidazole carboxylase II | 0.4 | 0.3 | ||
| purE | Phosphoribosylaminoimidazole carboxylase I | 0.2 | 0.4 | 0.1 | |
| lmo1778 | Similar to ABC transporter (ATP-binding protein) | 0.4 | |||
| rncS | Similar to RNase III | 0.4 | |||
| lmo1827 | Similar to guanylate kinases | 0.5 | 0.5 | ||
| pyrP | Similar to uracil permease | 0.4 | |||
| lmo1847 | Similar to adhesion binding proteins and lipoproteins with multiple specificity for metal cations (ABC transporter) | 0.4 | 0.5 | ||
| lmo1848 | Similar metal cations ABC transporter (permease protein) | 0.4 | 0.4 | 0.4 | 0.5 |
| lmo1849 | Similar to metal cation ABC transporter, ATP-binding proteins | 0.3 | 0.4 | 0.2 | 0.5 |
| lmo1884 | Similar to xanthine permeases | 0.3 | 0.4 | 0.3 | 0.4 |
| lmo1885 | Similar to xanthine phosphoribosyltransferase | 0.3 | 0.4 | 0.4 | 0.4 |
| aroE | Similar to 5-enolpyruvylshikimate-3-phosphate synthase | 0.3 | |||
| tyrA | Similar to prephenate dehydrogenase | 0.4 | 0.2 | ||
| hisC | Similar to histidinol-phosphate aminotransferase and tyrosine/phenylalanine aminotransferase | 0.5 | 0.3 | ||
| lmo1926 | Similar to chorismate mutase | 0.4 | 0.3 | ||
| aroB | Similar to 3-dehydroquinate synthase | 0.4 | 0.2 | ||
| aroF | Similar to chorismate synthase | 0.4 | 0.2 | ||
| gpsA | Similar to NAD(P)H-dependent glycerol-3-phosphate dehydrogenase | 0.6 | |||
| lysA | Similar to diaminopimelate decarboxylase | 0.5 | |||
| fhuC | Similar to ferrichrome ABC transporter (ATP-binding protein) | 0.5 | |||
| lmo1976 | Similar to oxidoreductase | 0.5 | |||
| lmo1978 | Similar to glucose-6-phosphate 1-dehydrogenase | 0.4 | 0.5 | 0.4 | 0.3 |
| ilvD | Similar to dihydroxy acid dehydratase | 0.4 | 0.4 | 0.5 | 0.3 |
| ilvB | Similar to acetolactate synthase (acetohydroxy acid synthase) (large subunit) | 0.5 | |||
| leuA | Similar to 2-isopropylmalate synthase | 0.5 | |||
| leuBb | Similar to 3-isopropylmalate dehydrogenase | 0.5 | |||
| lmo2075 | Similar to glycoprotein endopeptidase | 0.5 | |||
| lmo2110 | Similar to mannnose-6 phosphate isomerase | 0.3 | 0.5 | ||
| lmo2114 | Similar to ABC transporter (ATP-binding protein) | 0.3 | |||
| lmo2152 | Similar to thioredoxin | 0.5 | |||
| lmo2153 | Similar to flavodoxin | 0.5 | |||
| lmo2192b | Similar to oligopeptide ABC transporter (ATP-binding protein) | 0.5 | |||
| lmo2193b | Similar to oligopeptide ABC transporter (ATP-binding protein) | 0.5 | |||
| lmo2194b | Similar to oligopeptide ABC transporter (permease) | 0.4 | 0.5 | ||
| lmo2195b | Similar to oligopeptide ABC transporter (permease) | 0.5 | 0.5 | ||
| lmo2196 | Similar to pheromone ABC transporter (binding protein) | 0.4 | 0.3 | ||
| lmo2238 | Similar to transport system permease protein | 0.5 | 0.3 | ||
| arpJ | Similar to amino acid ABC transporter, permease protein | 0.5 | 0.3 | ||
| lmo2346 | Similar to amino acid ABC transporter, ATP-binding protein | 0.5 | |||
| lmo2348 | Similar to amino acid ABC transporter (permease) | 0.4 | 0.5 | ||
| lmo2349 | Similar to amino acid ABC transporter (binding protein) | 0.5 | |||
| lmo2355 | Similar to multidrug resistance protein | 0.5 | 0.5 | ||
| lmo2371 | Similar to putative ABC transporter transmembrane subunit | 0.5 | |||
| lmo2372 | Similar to ABC transporter ATP binding proteins | 0.5 | |||
| lmo2374 | Similar to aspartate kinase | 0.5 | 0.5 | ||
| lmo2377 | Similar to multidrug resistance efflux pump | 0.5 | 0.6 | ||
| lmo2421 | Similar to two-component sensor histidine kinase | 0.5 | |||
| lmo2430 | Similar to B. subtilis ferrichrome ABC transporter (permease) FhuG | 0.5 | 0.5 | ||
| lmo2431 | Similar to B. subtilis ferrichrome ABC transporter fhuD precursor (ferrichrome-binding protein) | 0.5 | |||
| eno | Similar to enolase | 0.4 | |||
| pgm | Similar to phosphoglycerate mutase | 0.4 | 0.3 | ||
| tpi | Similar to triose phosphate isomerase | 0.5 | 0.3 | ||
| pgk | Similar to phosphoglycerate kinase | 0.3 | 0.3 | ||
| gap | Similar to glyceraldehyde-3-phosphate dehydrogenase | 0.5 | 0.4 | 0.3 | |
| atpH | Similar to H+-transporting ATP synthase chain delta | 0.4 | |||
| atpF | Similar to H+-transporting ATP synthase chain b | 0.5 | |||
| atpE | Similar to H+-transporting ATP synthase chain c | 0.5 | |||
| atpB | Similar to H+-transporting ATP synthase chain a | 0.5 | |||
| atpI | Similar to ATP synthase subunit i | 0.5 | |||
| upp | Similar to uracil phosphoribosyltransferase | 0.5 | |||
| glyA | Similar to glycine hydroxymethyltransferase | 0.5 | |||
| hom | Similar to homoserine dehydrogenase | 0.5 | 0.5 | 0.4 | 0.4 |
| fbaA | Similar to fructose-1,6-bisphosphate aldolase | 0.5 | 0.4 | ||
| lmo2601 | Similar to ABC transporter (ATP-binding protein) | 0.5 | |||
| lmo2684 | Similar to cellobiose phosphotransferase EIIC component | 0.6 | |||
| lmo2720 | Similar to acetate-coenzyme A ligase | 0.5 | 0.4 | 0.4 | |
| serS | Seryl-tRNA synthetase | 0.5 | 0.2 | ||
| guaB | Similar to inosine-monophosphate dehydrogenase | 0.5 | |||
| lmo2769 | Similar to ABC transporter, ATP-binding protein | 0.5 | 0.5 | ||
| lmo2824 | Similar to d-3-phosphoglycerate dehydrogenase | 0.6 | |||
| serC | Similar to phosphoserine aminotransferase | 0.4 |
Transcript profiles of L. monocytogenes cultures grown in glucose compared to those of cultures grown in cellobiose.
In the course of the above-described studies, we noticed significant qualitative and quantitative differences in the expression profiles when we compared transcripts from cultures grown in glycerol/glucose and glycerol/cellobiose. This led us to directly compare the transcript profiles of L. monocytogenes cultures grown in glucose to those of L. monocytogenes cultures grown in cellobiose. This analysis was carried out with RNAs from L. monocytogenes harvested again at an OD600 of 0.5 to 0.6 (phase A) and an OD600 of 1.0 (phase B). Genes that were upregulated in glucose compared to cellobiose in phases A and B (Table 4) included the mannose-specific PTS (lmo0096 to lmo0098), the _ilv_-leu operon, and, most significantly, all genes of the PrfA-controlled LIPI-1 virulence gene cluster as well as inlAB. Interestingly, the PrfA-regulated genes hpt (uhpT) and inlC show high upregulation only in phase B but not in phase A. Among the few genes that are more downregulated in the presence of glucose than in the presence cellobiose in phases A and B (Table 4) are most noticeably the lmo2684 and lmo2685 genes, determining a cellobiose-specific PTS.
Genes that are specifically upregulated in the presence of glucose in phase A only include those determining the enzymes involved in the synthesis of aromatic amino acids (particularly tryptophan). The trp genes are, however, downregulated in phase B (Table 4).
Growth of mutants defective in glycerol uptake and metabolism under extra- and intracellular conditions.
To study the functions of the genes that are most likely involved in glycerol uptake and metabolism, we constructed mutants carrying in-frame deletions of various genes involved in glycerol metabolism in L. monocytogenes (Table 1) and tested their growth in MM supplemented with glycerol as a carbon source in comparison to that of the wild-type strain.
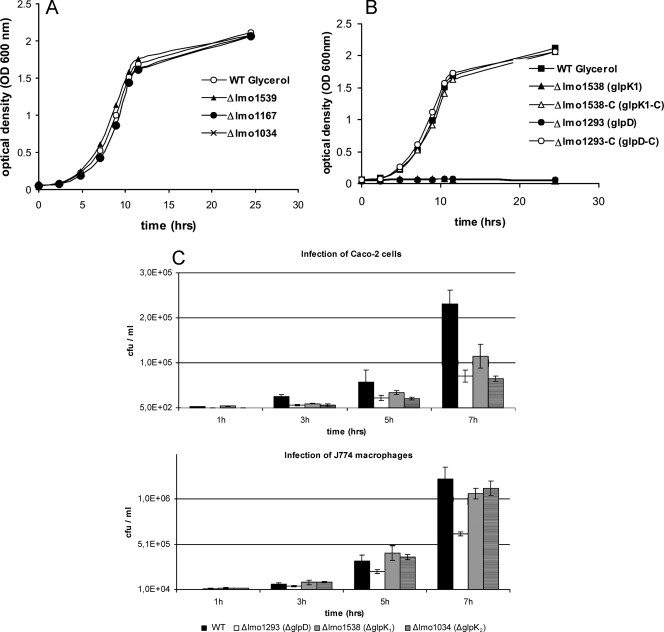
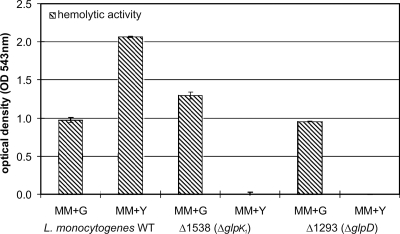
The deletion of genes encoding the two putative glycerol uptake facilitators had little effect on growth (Fig. 3A), suggesting that in the presence of 50 mM glycerol, which was used in these studies, the free diffusion of glycerol provides sufficient substrate for glycerol-driven metabolism. In contrast, the deletion of the glycerol kinase 1 (GlpK1) encoded by lmo1538 (part of the glpFK operon) abolished the ability to grow in glycerol-containing MM entirely, indicating that the second putative glycerol kinase (GlpK2), encoded by lmo1034, cannot replace the loss of GlpK1, at least not under the applied in vitro growth conditions. In accord with this assumption is the observation that the deletion of lmo1034 did not affect growth in glycerol-containing medium (Fig. 3A). The deletion of lmo1293 (glpD), encoding glycerol-3-P dehydrogenase, also led to the complete loss of growth in the presence of glycerol (Fig. 3A). To further characterize these mutants (Δ_glpk_1 and Δ_glpD_), these genes were complemented in the deletion mutants, and as can be seen in Fig. 3B, a wild-type phenomenon could be restored in these complemented strains with respect to growth in MM containing glycerol.
FIG. 3.
(A) Growth of wild-type L. monocytogenes EGD-e (WT), and glycerol metabolism mutants Δlmo1539, Δlmo1167, and Δlmo1034 in glycerol-containing MM at 37°C under aeration. (B) Growth of wild-type L. monocytogenes EGD-e and glycerol metabolism mutants Δlmo1538 (_glpK_1), Δlmo1293 (glpD), and the complementation mutants of _glpK_1 and glpD in glycerol-containing MM at 37°C under aeration. (C) Effect of nonpolar deletions of lmo1293 (glpD), lmo1538 (_glpK_1), and lmo1034 (_glpK_2) on the intracellular replication of L. monocytogenes EGD-e. Caco-2 epithelial cells or J774 macrophages were infected with either the wild-type strain or the mutants to an MOI of 10 (Caco-2) or an MOI of 1 (J774), and the numbers of bacteria recovered after 1, 3, 5, and 7 h of infection were determined. Three independent infections were performed for each strain. Error bars represent the standard deviations from the means.
As recently reported (26), mutants with insertions in _glpK_1 and glpD obtained from a random insertion mutant library showed reduced levels of growth in Caco-2 cells. We therefore tested the intracellular replication of the above-mentioned deletion mutants in Caco-2 cells and J774 macrophages. In these growth studies, the mammalian host cells were precultured in a glucose-containing cell culture medium. The glpD (lmo1293) deletion mutant as well as the mutant with a deletion in _glpK_1 (lmo1538) showed a modest but significant reduction in intracellular replication in Caco-2 cells (Fig. 3B). Interestingly, the mutant with the deletion in lmo1034, which encodes GlpK2 (an _L. monocytogenes_-specific glycerol kinase), also exhibited a modest but significant reduction in levels of intracellular replication in Caco-2 cells, which was more pronounced than that in the _glpK_1 (lmo1538) deletion mutant. In J774 macrophages, only the inactivation of glpD led to a significant growth reduction (Fig. 3B), suggesting a cell type-specific dependency on the glycerol kinase activity.
Growth of L. monocytogenes in the presence of dihydroxyacetone.
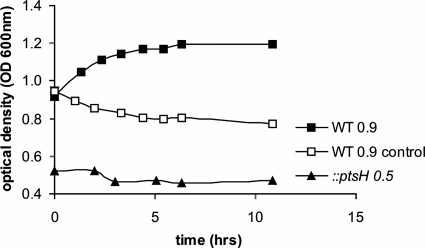
The presence of two Dha kinases in L. monocytogenes and the high level of upregulation of the encoding genes in the presence of glycerol suggest that Dha may also be a carbon source for L. monocytogenes. When MM was supplemented with 50 mM Dha instead of glycerol, no growth of L. monocytogenes was observed (data not shown), but growth in the presence of Dha was observed when the bacteria where preincubated in glycerol-containing medium in order to induce the two DhaKs (Fig. 4).
FIG. 4.
Growth of wild-type L. monocytogenes EGD-e (WT) (filled squares) and the L. monocytogenes ptsH mutant (filled triangle) in MM supplemented with 50 mM Dha. Wild-type L. monocytogenes was grown in MM with 50 mM glycerol to an OD600 of 0.9 to induce genes involved in Dha metabolism and was then shifted to MM with Dha. The L. monocytogenes ptsH mutant was unable to grow in MM with glycerol and was therefore shifted from BHI broth (OD600 of 0.5) to MM with Dha. The control (open squares) is the shift of WT to MM without an additional carbon source to show that the preceding growth in glycerol does not lead to the storage of intermediates of glycerol metabolism.
The ptsH mutant was unable to grow in Dha-containing medium, which suggests that listerial DhaKs (both share the typical structure of category C DhaK) (3) are activated by HPr-His-P and transfer the energy-rich phosphate to Dha, generating Dha-P (17).
PrfA activation is due to glycerol metabolism and not to glycerol itself.
A recent study (33) and the data described above (Table 2) indicated that PrfA is activated in glycerol-containing MM. A previous structural analysis of PrfA showed that glycerol can tightly bind to PrfA (Protein Data Bank record 41 [http://www.rcsb.org/pdb/explore.do?structureId=10MI]). To test whether the binding of glycerol may directly activate PrfA, we studied PrfA activity in the glpD and _glpK_1 mutants, which are still able to take up but are unable to catabolize glycerol. For this goal, the wild-type strain and the two mutants were grown in BHI broth to early log phase (OD600 of 0.5). After a wash in PBS, one half was shifted into glycerol-containing MM, while the other half was shifted into glucose-containing MM. The hemolytic activity, taken as a measure for the PrfA activity, was determined 2 h after the shift. As shown in Fig. 5, the wild-type strain was still able to express the PrfA-dependent hly gene after shift into glycerol- or glucose-containing medium, as expected, while the _glpK_1 or the glpD mutant expressed the hly gene only in the glucose-containing but not in the glycerol-containing medium, suggesting that glycerol by itself does not activate PrfA. (The hemolytic activity of the wild-type strain grown in BHI [not shown in Fig. 5] is very low [<0.1 OD543 units], and the hemolytic activity observed in the _glpK_1 or the glpD mutant after the shift from BHI broth into glycerol-containing MM remains at this low level.)
FIG. 5.
Hemolytic activities of wild-type L. monocytogenes EGD-e (WT) and glycerol metabolism mutants shifted to MM supplemented with 50 mM glucose (MM+G) or glycerol (MM+Y). The bacteria were grown in BHI broth to an OD600 of 0.5 and then incubated for 2 h in glucose- or glycerol-containing MM. The hemolytic activity was determined in three independently performed experiments; the error bars indicate standard deviations of the means for the three experiments.
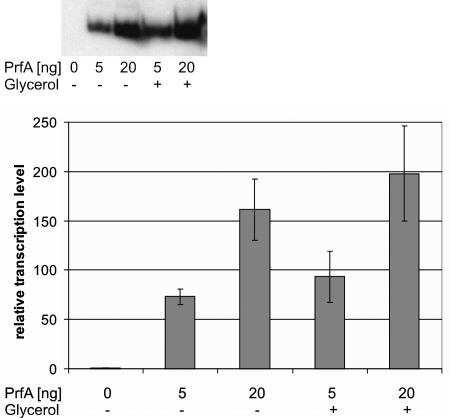
In addition, we purified PrfA using buffers without glycerol and determined the specific activities of both PrfA preparations in the previously established in vitro runoff transcription assay using reaction buffers with and without glycerol (6, 29, 30). In vitro transcription was initiated at the PrfA-dependent hpt (uhpT) promoter (P_hpt_) as previously described (48). As shown in Fig. 6, PrfA activities with glycerol and those without added glycerol were identical. These results along with those of the above-described hemolytic activity assays suggest that glycerol by itself does not activate PrfA directly but rather that components connected with glycerol metabolism may modulate PrfA activity.
FIG. 6.
In vitro transcription starting at the hpt (uhpt) promoter (Phpt). UTP was used as 32P-labeled rNTP present in the lowest concentration, 0.08 mM, in the assay. The amount of PrfA and the addition of glycerol (1 M) are indicated. Quantification of the transcripts was performed by phosphorimaging and is shown in the lower graph. The lowest transcription efficiency (transcription from Phpt in the absence of PrfA) is taken as 1, and all other values are normalized to it. Error bars indicate standard deviations of the means for three independently performed experiments.
CCR control and phosphorylation of HPr in L. monocytogenes cultures grown in the presence of glycerol.
The comparative transcript profiles obtained with RNAs from glycerol-grown and glucose- or cellobiose-grown L. monocytogenes cultures indicated an induced expression of many genes in the glycerol-grown L. monocytogenes cultures that were recently shown to be upregulated in a ccpA mutant, an hprK mutant, or both mutants (33) (Tables 2 to 4) and, hence, are probably under CCR control. These results suggest that CCR control is (at least partially) relieved in the presence of glycerol compared to that in the presence glucose and cellobiose as carbon sources. Increased levels of expression of these genes were more pronounced in growth phase A than in phase B, which is expected due to the higher carbohydrate concentration in phase A. More CcpA/HPr-Ser-P- and HPrK-controlled genes were identified as being upregulated in the glycerol/cellobiose transcript pattern than in the glycerol/glucose transcript pattern, suggesting that cellobiose may exert a stronger catabolite repression than glucose.
The level of HPr-Ser-P, the second component of CCR control in gram-positive bacteria (for recent reviews, see references 7, 45, and 49), was low in L. monocytogenes when cells were grown in glycerol (Fig. 7C), which may explain the (at least partial) derepression of CCR-controlled genes in glycerol-grown L. monocytogenes cultures.
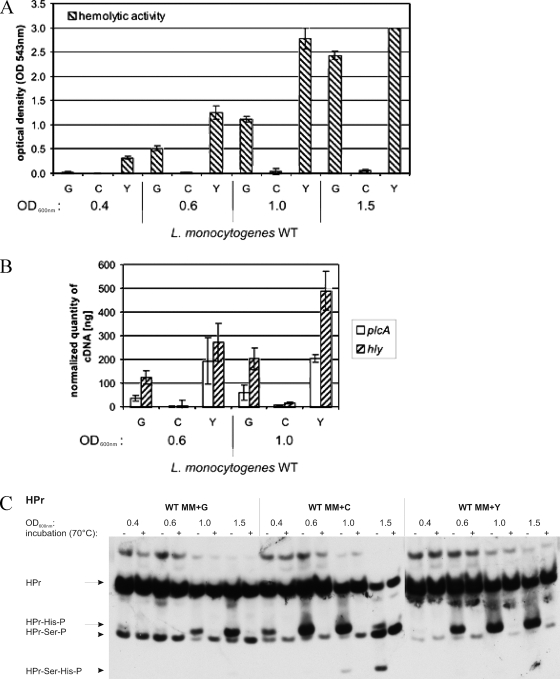
FIG. 7.
(A) Hemolytic activity of wild-type L. monocytogenes EGD-e (WT) grown in MM supplemented with 50 mM glucose (G), cellobiose (C), or glycerol (Y). The bacteria were grown to an OD600 of 0.4, 0.6, 1.0, or 1.5, and hemolytic activity was determined in three independently performed experiments; the error bars indicate standard deviations of the means for the three experiments. (B) Transcriptional analysis with real-time RT-PCR to study the expression of the virulence genes plcA and hly. Wild-type L. monocytogenes EGD-e was grown in MM supplemented with 50 mM glucose (G), cellobiose (C), or glycerol (Y) to an OD600 of 0.5 or 1.0, and RT-PCR was performed as described in the legend to Fig. 2. (C) Western blot analysis of HPr and its phosphorylated forms (HPr-His15-P, HPr-Ser46-P, and double-phosphorylated HPr-Ser46-P-His15-P). Equal amounts of cell extracts untreated (−) or incubated at 70°C for 10 min (+) to hydrolyze the heat-labile HPr-His15-P were separated on a 15% nondenaturing polyacrylamide gel and immunoblotted using specific rabbit polyclonal antibodies against HPr. The positions of HPr, HPr-Ser46-P, HPr-His15-P, and HPr-Ser46-P-His15-P are indicated. Equivalent loading of the gels was controlled by Coomassie staining (data not shown). Wild-type L. monocytogenes EGD-e was grown in MM supplemented with 50 mM glucose (G), cellobiose (C), or glycerol (Y) to OD600 values of 0.4, 0.6, 1.0, and 1.5.
PrfA activity and the phosphorylation state of HPr during growth in the presence of glycerol, glucose, and cellobiose.
Previous data showed that neither CcpA nor HPr-Ser-P acts as a modulator of PrfA activity. On the other hand, HPr seems to somehow be involved in the modulation of PrfA activity, since a ptsH mutant (deficient in the production of HPr) shows greatly increased levels of PrfA activity (33). The other phosphorylated HPr derivative, HPr-His-P, is critical for the activation of all PTS permeases but also for the activation of GlpK, DhaK, and different transcription regulators. The data described above seem to rule out GlpK and DhaK or its substrates and products as potential modulators of PrfA activity.
To better understand how PrfA activity is linked to the phosphorylation state of HPr (and hence to that of the PTS permeases), we determined PrfA activity (by measuring the activity of PrfA-dependent listeriolysin [Fig. 7A] and the transcript levels of the PrfA-dependent genes hly and plcA [Fig. 7B]) and the amount of HPr-His-P and HPr-Ser-P (Fig. 7C) throughout the growth of L. monocytogenes cells in the presence of glucose, cellobiose, and glycerol.
As shown in Fig. 7A, PrfA activity in the presence of glycerol was low at the start of growth (lag phase) (Fig. 1); under these conditions, little phosphorylated HPr (mainly HPr-Ser-P) was observed. Levels of PrfA activity then increased quickly and remained high throughout the logarithmic growth and the early stationary growth phases. During the entire active growth period, a rather high level of HPr-His-P and a low level of HPr-Ser-P were observed (Fig. 7C).
In the presence of glucose, PrfA activity remained low throughout the early logarithmic growth phase, where the level of HPr-His-P was low (consumed by the phosphorylation of the transported glucose) and that of HPr-Ser-P was high. In the late log phase and the early stationary phase, the PrfA activity increased and reached levels comparable to those observed in the presence of glycerol. In this growth phase, the level of HPr-His-P also increased significantly (reduced glucose concentration in the medium and hence decreased uptake of glucose by PTS), while that of HPr-Ser-P slightly decreased.
In the presence of cellobiose, PrfA activity was very low throughout the logarithmic growth phase, and this correlated with a rather low level of HPr-Ser-P and a rather high level of HPr-His-P. The latter may be due to the fact that the uptake of the disaccharide cellobiose (equivalent of two glucose moieties) requires the same amount of HPr-His-P as the uptake of the monosaccharide glucose. The unexpected low level of HPr-Ser-P suggests that cellobiose catabolism may not activate the HPr kinase as efficiently as glucose catabolism, possibly by a lesser accumulation of glycolytic intermediates (e.g., fructose-1,6-diphosphate), which are known to activate HPr kinase activity (18, 39). There was a slight increase in levels of PrfA activity in the stationary phase, and this was accompanied by an increased level of HPr-Ser-P and the appearance of double-labeled P-Ser-HPr-His-P.
DISCUSSION
L. monocytogenes is a heterotrophic microorganism capable of utilizing a variety of carbohydrates. For the efficient uptake of these substrates, it carries genes for up to 30 complete PEP:PTS specific for mono- and disaccharides and several genes encoding single EIIA, EIIB, or EIIC components only (3, 20; R. Stoll, personal communication). The genes for these PTS appear to be differently regulated. Some of them were shown to be under global CCR control and, hence, induced in a ccpA mutant and/or an hprK mutant (33). Others are substrate induced, more or less constitutively expressed, or even silent under the applied experimental growth conditions (BHI broth, LB medium, and MM) (R. Stoll, personal communication). Previous studies indicated that during active PTS-mediated sugar transport, the activity of the central regulator of virulence gene transcription PrfA is low in general (31, 40). There seems to be a hierarchy among the PTS sugars with respect to their inhibitory effects on PrfA activity. By far, the strongest inhibition was observed during PTS-mediated uptake and subsequent metabolism of the β-glucoside cellobiose, while the uptake of glucose, mannose, or fructose as a carbon source inhibited PrfA activity to a lesser extent (19, 34; our unpublished results).
In this study, we used glycerol as a non-PTS carbon source, which allows the growth of L. monocytogenes cultures in defined MM (37) with a growth rate similar to that observed with PTS sugars when applied at equimolar concentrations. Under these growth conditions, the levels of expression of all genes involved in the uptake and metabolism of glycerol are highly upregulated, similar to what has been observed for B. subtilis (11). These genes include (i) the operon of lmo1538 and lmo153939, showing a high level of homology to glpK and glpF of B. subtilis and other gram-positive bacteria (these genes encode the glycerol uptake facilitator GlpF and the glycerol kinase GlpK), and (ii) lmo1293, a gene with a high level of homology to glpD of B. subtilis, which encodes glycerol-3-phosphate dehydrogenase. The organization of the genes involved in glycerol catabolism is slightly different in L. monocytogenes in comparison to that in B. subtilis. In the latter microorganism, the above-mentioned genes are physically clustered together as glpP (regulator of glpD) in a glpPFKD operon. L. monocytogenes lacks a homolog of glpP, and glpD is separated from the bicistronic glpFK unit. The levels of expression of the genes encoding a second putative glycerol uptake facilitator (lmo1167) and a second, _L. monocytogenes_-specific glycerol kinase (lmo1034) are not as high as those of glpFK and glpD.
Together with these genes essential for glycerol metabolism, two sets of genes encoding two Dha kinases (DhaK) are upregulated. Dha kinases are the key enzymes for the metabolism of Dha, another C3 component that, according to our results, may also act as a carbon source for L. monocytogenes. Dha kinases have been identified in many organisms. Based on their different structures, DhaKs can be divided into categories A to F. The two DhaKs of L. monocytogenes belong to category C (3).
The common part of the PTS pathway is linked to DhaK and GlpK in two different ways (2, 39). The DhaK-catalyzed phosphorylation of its substrate dihydroxyacetone by HPr-His-P occurs in a way similar to that of the phosphorylation of the EIIA components of PTS permeases and the subsequent transfer of the phosphate group to its transported carbohydrate. Indeed, homologous domains essential for phosphorylation are present in DhaK and EIIA, respectively (17). The glycerol kinase (GlpK) uses ATP for the phosphorylation of its substrate glycerol. However, in order to become active, this enzyme has to be phosphorylated by HPr-His-P, as shown in B. subtilis and other gram-positive bacteria (11).
The inability of the L. monocytogenes ptsH mutant (deficient in the synthesis of functional HPr) to grow in the presence of either glycerol or Dha indicates similar requirements for the listerial GlpK and DhaK homologues. Indeed, listerial GlpK1 (a gene product of lmo1538) contains the same conserved phosphorylation site (histidyl residue at position 231 surrounded by Y and FF) as GlpK of B. subtilis and other low-G+C gram-positive bacteria (9, 50). The second listerial glycerol kinase (GlpK2, encoded by lmo1034) lacks this conserved site but contains a histidyl residue, which may also be phosphorylated by HPr-His-P, at position 232.
Interestingly, GlpK2, encoded by lmo1034, seems to be more important for intracellular growth than for extracellular growth. The opposite is the case for GlpK1; i.e., the deletion of lmo1538 affects intracellular growth little, although this gene is absolutely required for extracellular growth in the presence of glycerol. To the contrary, the mutant lacking GlpK2 grows in glycerol-containing MM at a rate similar to that of the wild-type strain. The intracellular replication of the _glpK_2 mutant in Caco-2 cells is reduced almost to the same extent as that of the glpD deletion mutant, which is unable to oxidize glycerol-3-phosphate to dihydroxyacetone-phosphate. Surprisingly, neither of the two glycerol kinases seems to play a major role in the macrophage cell line J774, which could mean that the supply of glycerol is different in the two cell types.
In addition to these genes involved in C3 metabolism (which seem to also be under CCR control) (33), many other CCR-regulated genes, including genes for several PTS, are likewise upregulated during growth in the presence of glycerol. However, the transcription of these CCR-controlled genes is not as enhanced as that of the genes involved in glycerol catabolism, and the transcriptional upregulation of these genes is more pronounced in the comparative glycerol/cellobiose than in the glycerol/glucose transcript profiles.
This relief of the CCR in the presence of glycerol can be explained by the rather low level of HPr-Ser-P produced in L. monocytogenes cells growing in the presence of glycerol (compared to glucose), while the amount of CcpA seems to be expressed rather independently of carbon sources, as also shown for Bacillus (23, 24) or Lactobacillus (36). However, the amount of HPr-Ser-P in cellobiose-grown L. monocytogenes cultures also seems to be lower than that in the presence of glucose (at least during logarithmic growth), although CCR-regulated genes are clearly more repressed in the presence of cellobiose than in the presence of glycerol. A possible explanation could be that in addition to its involvement as a cofactor in CcpA-mediated CCR, HPr-Ser-P participates in inducer exclusion of non-PTS carbon compounds by binding to the corresponding non-PTS transporters (53). Since more of these transporter genes seem to be expressed in the presence of glycerol than in the presence of cellobiose, it is likely that a substantial amount of HPr-Ser-P is titrated out by binding to such transporters.
A considerable portion of the energy-rich phosphate of PEP derived from glycerol oxidation is apparently utilized for the generation of HPr-His-P by EI-P, as indicated by the high concentration of cellular HPr-His-P in glycerol-grown cells. As mentioned above, this component is necessary for the activation of the glycerol kinase (GlpK) and will also lead to phosphorylation of the EIIA components of the PTS expressed in the presence of glycerol as a carbon source. The EI-mediated phosphorylation of HPr to HPr-His-P by PEP will leave pyruvate behind. The observed upregulation of genes encoding pyruvate-metabolizing enzymes, like pyruvate-formate lyase, pyruvate oxidase, acetolactate synthetase, and pyruvate dehydrogenase, in the presence of glycerol may therefore be necessary to remove excess pyruvate.
The comparative transcript patterns also indicate that in the presence of glycerol, the genes for glycolysis enzymes (especially those involved in the upper part of glycolysis) are turned down, while genes involved in gluconeogenesis and the pentose phosphate pathway are upregulated. The level of fructose-1,6-bisphosphate (and possibly even that of ATP) may therefore be too low for the activation of the HPr kinase, which would explain the small amount of HPr-Ser-P in glycerol-growing L. monocytogenes cells. The downregulation of the genes for ATP synthase and the upregulation of the qox genes furthermore indicate that aerobic respiration and hence the production of ATP by the respiratory pathway are also less efficient in the presence of glycerol.
The most remarkable feature of L. monocytogenes cells growing in the presence of glycerol is, however, the high levels of upregulation of all PrfA-controlled genes of the LIPI-1 cluster as well as of inlAB, inlC, and hpt. The comparative expression profiles (L. monocytogenes in glycerol/glucose and glycerol/cellobiose) indeed identify these genes as being the highest-induced ones during growth in glycerol. The profiles also show that in contrast to cellobiose, which appears to inactivate PrfA throughout the logarithmic growth phase, glucose inhibits PrfA activity less strongly and only during the balanced growth phase but not in the late logarithmic growth phase. High levels of HPr-His-P and relatively low levels of HPr-Ser-P are observed throughout logarithmic growth in the presence of glycerol and cellobiose. But high HPr-Ser-P and low HPr-His-P levels are found in the presence of glucose. The level of HPr-Ser-P in the presence of cellobiose increases in the stationary growth phase; at the same time, the HPr-His-P level drops (but double-labeled P-His-HPr-Ser-P accumulates), and PrfA activity increases. This growth phase reflects the situation where the PTS carbon source is consumed, and the PTS permease(s), which transports cellobiose, probably remains phosphorylated since the phosphate group is no longer transferred to the PTS sugar. When cellobiose together with glycerol (which can freely diffuse into the bacterial cell) is added to MM in equimolar concentrations, the growth kinetics are the same as those in the presence of cellobiose alone. Under these conditions, PrfA activity is also as low as that in the presence of cellobiose alone (data not shown), suggesting that glycerol per se is not an activating factor of PrfA.
In this context, it is interesting that the bilE (lmo1421 and lmo1422) and vip (lmo0320) genes, which were also recently reported as being PrfA regulated (8, 42), are not among the upregulated genes, and bsh (lmo2067), another reported PrfA-regulated gene (14), is only moderately upregulated in the presence of glycerol. A possible explanation for this unexpected observation could be that the regulation of the latter genes requires other transcriptional regulators that are not expressed or not active in the presence of glycerol in addition to active PrfA.
What are the most significant metabolic differences between glucose (or cellobiose)- and glycerol-grown L. monocytogenes cells, and what can we learn from these differences with respect to PrfA modulation?
First, clearly, the cellular concentration of glycerol (and glycerol-3-phosphate) will be higher in glycerol-grown L. monocytogenes cells. A direct role of these metabolites in the modulation of PrfA activity can, however, be ruled out. First, the glpK and glpD mutants, which are defective in glycerol kinase and glycerol-3-P dehydrogenase, respectively, no longer activate PrfA when shifted into a glycerol-containing medium. In both mutants, glycerol could still be taken up and glycerol-3-P could still be produced in the glpD mutant. Second, PrfA activity is strongly inhibited when L. monocytogenes is grown in a glycerol-containing medium when cellobiose is added.
Second, the energy level of L. monocytogenes cells growing in the presence of glycerol seems to be lower than that of cultures in the presence of glucose or cellobiose. We have therefore tested the effects of NAD/NADH, ATP/ADP, fructose-1,6-diphosphate, and PEP on PrfA activity in a PrfA-dependent in vitro transcription system (6, 30). None of these components led to a significant change in PrfA activity (data not shown).
Third, the cellular level of HPr-His-P is high and that of HPr-Ser-P is low in the presence of glycerol, and the levels are quite similar in the presence of cellobiose but opposite in the presence of glucose during the logarithmic growth of L. monocytogenes. These results again rule out a direct role of either of these phosphorylated HPr derivatives, as stated in a recent study (33).
Fourth, HPr-His-P in glycerol-grown cells will lead to the phosphorylation of all EIIA components of the many PTS permeases that are expressed in the presence of glycerol.
This phosphorylation pattern of EIIA components of the expressed PTS and of GlpK in glycerol-grown L. monocytogenes cells is clearly different from the situation in L. monocytogenes cells grown in the presence of PTS sugars. Here, fewer PTS permeases are expressed, and the EIIA components of those PTS permeases involved in the transport of the used carbohydrate will be unphosphorylated, since the phosphate group is completely transferred to the imported carbohydrate. It is therefore intriguing to argue that one or more of the unphosphorylated EIIA components of specific PTS may bind PrfA, thereby inhibiting its activity. The phosphorylation of these EIIA components may then lead to the release of PrfA, which would be active without any further modification. Purified PrfA protein has indeed been shown to be almost as active as purified PrfA* in in vitro transcription assays (32). This hypothesis would be also in line with the recently reported observation that the overexpression of PrfA in L. monocytogenes leads to the inhibition of the PTS-mediated uptake of glucose and cellobiose (31). In this case, one may assume that the binding of excess PrfA to the unphosphorylated EIIA component(s) of PTS mediating transport of these carbohydrates blocks EIIA phosphorylation and hence their uptake.
The fact that PrfA always has high levels of in vitro transcription activity even when isolated from L. monocytogenes cultures, where PrfA is highly inactive in vivo (Q. Luo et al., unpublished data), suggests that the interaction(s) with cellular components or covalent bonds leading to the inhibition of PrfA activity is rather weak, as expected for the two proposed models.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank Tobias Müller and Julia Engelmann (Department of Bioinformatics, University of Würzburg) for helpful discussions with the transcriptional profiling. We are grateful to M. Frosch, A. Schramm, and G. Gerlach (Institute for Hygiene and Microbiology, University of Würzburg) for allowing us to use the microarray facility and for their valuable advice.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB479-B1 and Go-168/27-3), the Network of Excellence/EuroPathoGenomics, and the Fonds der Chemischen Industrie. Q.L. was supported by National Natural Science Foundation of China grant 30500025.
Footnotes
▿
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Abram, F., W. L. Su, M. Wiedmann, K. J. Boor, P. Coote, C. Botting, K. A. Karatzas, and C. P. O'Byrne. 2008. Proteomic analyses of a Listeria monocytogenes mutant lacking σB identify new components of the σB regulon and highlight a role for σB in the utilization of glycerol. Appl. Environ. Microbiol. 74594-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bächler, C., K. Flükiger-Brühwiler, P. Schneider, P. Bähler, and B. Erni. 2005. From ATP as substrate to ADP as coenzyme: functional evolution of the nucleotide binding subunit of dihydroxyacetone kinases. J. Biol. Chem. 28018321-18325. [DOI] [PubMed] [Google Scholar]
- 3.Barabote, R. D., and M. H. Saier, Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69608-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behari, J., and P. Youngman. 1998. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J. Bacteriol. 1806316-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencke, H. M., G. Homuth, H. Ludwig, U. Mader, M. Hecker, and J. Stülke. 2003. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab. Eng. 5133-149. [DOI] [PubMed] [Google Scholar]
- 6.Böckmann, R., C. Dickneite, W. Goebel, and J. Bohne. 2000. PrfA mediates specific binding of RNA polymerase of Listeria monocytogenes to PrfA-dependent virulence gene promoters resulting in a transcriptionally active complex. Mol. Microbiol. 36487-497. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209141-148. [DOI] [PubMed] [Google Scholar]
- 8.Cabanes, D., S. Sousa, A. Cebria, M. Lecuit, F. Garcia-del Portillo, and P. Cossart. 2005. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 242827-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charrier, V., E. Buckley, D. Parsonage, A. Galinier, E. Darbon, M. Jaquinod, E. Forest, J. Deutscher, and A. Claiborne. 1997. Cloning and sequencing of two enterococcal glpK genes and regulation of the encoded glycerol kinases by phosphoenolpyruvate-dependent, phosphotransferase system-catalyzed phosphorylation of a single histidyl residue. J. Biol. Chem. 27214166-14174. [DOI] [PubMed] [Google Scholar]
- 10.Chauvaux, S. 1996. CcpA and HPr(ser-P): mediators of catabolite repression in Bacillus subtilis. Res. Microbiol. 147518-522. [DOI] [PubMed] [Google Scholar]
- 11.Darbon, E., P. Servant, S. Poncet, and J. Deutscher. 2002. Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression. Mol. Microbiol. 431039-1052. [DOI] [PubMed] [Google Scholar]
- 12.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutscher, J., E. Küster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 151049-1053. [DOI] [PubMed] [Google Scholar]
- 14.Dussurget, O., D. Cabanes, P. Dehoux, M. Lecuit, C. Buchrieser, P. Glaser, and P. Cossart. 2002. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 451095-1106. [DOI] [PubMed] [Google Scholar]
- 15.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58587-610. [DOI] [PubMed] [Google Scholar]
- 16.Ermolaeva, S., S. Novella, Y. Vega, M. T. Ripio, M. Scortti, and J. A. Vázquez-Boland. 2004. Negative control of Listeria monocytogenes virulence genes by a diffusible autorepressor. Mol. Microbiol. 52601-611. [DOI] [PubMed] [Google Scholar]
- 17.Erni, B., C. Siebold, S. Christen, A. Srinivas, A. Oberholzer, and U. Baumann. 2006. Small substrate, big surprise: fold, function and phylogeny of dihydroxyacetone kinases. Cell. Mol. Life Sci. 63890-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galinier, A., J. Haiech, M. C. Kilhoffer, M. Jaquinod, J. Stülke, J. Deutscher, and I. Martin-Verstraete. 1997. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc. Natl. Acad. Sci. USA 948439-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbreth, S. E., A. K. Benson, and R. W. Hutkins. 2004. Catabolite repression and virulence gene expression in Listeria monocytogenes. Curr. Microbiol. 4995-98. [DOI] [PubMed] [Google Scholar]
- 20.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294849-852. [DOI] [PubMed] [Google Scholar]
- 21.Goebel, W., S. Müller-Altrock, and J. Kreft. 2006. Regulation of virulence genes in pathogenic Listeria spp., p. 499-506. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, DC.
- 22.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 23.Henkin, T. M. 1996. The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis. FEMS Microbiol. Lett. 1359-15. [DOI] [PubMed] [Google Scholar]
- 24.Hueck, C. J., A. Kraus, D. Schmiedel, and W. Hillen. 1995. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol. Microbiol. 16855-864. [DOI] [PubMed] [Google Scholar]
- 25.Jones, B. E., V. Dossonnet, E. Kuster, W. Hillen, J. Deutscher, and R. E. Klevit. 1997. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J. Biol. Chem. 27226530-26535. [DOI] [PubMed] [Google Scholar]
- 26.Joseph, B., K. Przybilla, C. Stühler, K. Schauer, J. Slaghuis, T. M. Fuchs, and W. Goebel. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreft, J., and J. A. Vázquez-Boland. 2001. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291145-157. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lalic-Mülthaler, M., J. Bohne, and W. Goebel. 2001. In vitro transcription of PrfA-dependent and -independent genes of Listeria monocytogenes. Mol. Microbiol. 42111-120. [DOI] [PubMed] [Google Scholar]
- 30.Luo, Q., M. Rauch, A. K. Marr, S. Müller-Altrock, and W. Goebel. 2004. In vitro transcription of the Listeria monocytogenes virulence genes inlC and mpl reveals overlapping PrfA-dependent and -independent promoters that are differentially activated by GTP. Mol. Microbiol. 5239-52. [DOI] [PubMed] [Google Scholar]
- 31.Marr, A. K., B. Joseph, S. Mertins, R. Ecke, S. Müller-Altrock, and W. Goebel. 2006. Overexpression of PrfA leads to growth inhibition of Listeria monocytogenes in glucose-containing culture media by interfering with glucose uptake. J. Bacteriol. 1883887-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauder, N., R. Ecke, S. Mertins, D. I. Loeffler, G. Seidel, M. Sprehe, W. Hillen, W. Goebel, and S. Müller-Altrock. 2006. Species-specific differences in the activity of PrfA, the key regulator of listerial virulence genes. J. Bacteriol. 1887941-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mertins, S., B. Joseph, M. Goetz, R. Ecke, G. Seidel, M. Sprehe, W. Hillen, W. Goebel, and S. Müller-Altrock. 2007. Interference of components of the phosphoenolpyruvate phosphotransferase system with the central virulence gene regulator PrfA of Listeria monocytogenes. J. Bacteriol. 189473-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 231075-1085. [DOI] [PubMed] [Google Scholar]
- 35.Milohanic, E., P. Glaser, J. Y. Coppée, L. Frangeul, Y. Vega, J. A. Vázquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 471613-1625. [DOI] [PubMed] [Google Scholar]
- 36.Monedero, V., M. J. Gosalbes, and G. Perez-Martinez. 1997. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J. Bacteriol. 1796657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 573046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauch, M. 2003. In vitro Transkription von Virulenzgenen aus Listeria monocytogenes unter der Kontrolle des Transkriptionsregulators PrfA. Bayerische Julius Maximilians Universität Würzburg, Würzburg, Germany.
- 39.Reizer, J., C. Hoischen, F. Titgemeyer, C. Rivolta, R. Rabus, J. Stülke, D. Karamata, M. H. Saier, Jr., and W. Hillen. 1998. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol. Microbiol. 271157-1169. [DOI] [PubMed] [Google Scholar]
- 40.Ripio, M. T., G. Domínguez-Bernal, M. Suárez, K. Brehm, P. Berche, and J. A. Vázquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147371-384. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Sleator, R. D., H. H. Wemekamp-Kamphuis, C. G. Gahan, T. Abee, and C. Hill. 2005. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol. Microbiol. 551183-1195. [DOI] [PubMed] [Google Scholar]
- 43.Sue, D., D. Fink, M. Wiedmann, and K. J. Boor. 2004. SigmaB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology 1503843-3855. [DOI] [PubMed] [Google Scholar]
- 44.Reference deleted.
- 45.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie van Leeuwenhoek 8259-71. [PubMed] [Google Scholar]
- 46.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velge, P., M. Herler, J. Johansson, S. M. Roche, S. Temoin, A. A. Fedorov, P. Gracieux, S. C. Almo, W. Goebel, and P. Cossart. 2007. A naturally occurring mutation K220T in the pleiotropic activator PrfA of Listeria monocytogenes results in a loss of virulence due to decreasing DNA-binding affinity. Microbiology 153995-1005. [DOI] [PubMed] [Google Scholar]
- 49.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wehtje, C., L. Beijer, R. P. Nilsson, and B. Rutberg. 1995. Mutations in the glycerol kinase gene restore the ability of a ptsGHI mutant of Bacillus subtilis to grow on glycerol. Microbiology 1411193-1198. [DOI] [PubMed] [Google Scholar]
- 51.Wuenscher, M. D., S. Köhler, W. Goebel, and T. Chakraborty. 1991. Gene disruption by plasmid integration in Listeria monocytogenes: insertional inactivation of the listeriolysin determinant lisA. Mol. Gen. Genet. 228177-182. [DOI] [PubMed] [Google Scholar]
- 52.Yang, Y. H., S. Dudoit, P. Luu, D. M. Lin, V. Peng, J. Ngai, and T. P. Speed. 2002. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye, J. J., and M. H. Saier, Jr. 1995. Allosteric regulation of the glucose:H+ symporter of Lactobacillus brevis: cooperative binding of glucose and HPr(ser-P). J. Bacteriol. 1771900-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]